Abstract
Adeno-associated viral (AAV) vectors have been successfully used for therapeutic expression of systemic transgene products (such as factor IX or erythropoietin) following in vivo administration to skeletal muscle of animal models of inherited hematologic disorders. However, an immune response may be initiated if the transgene product represents a neoantigen. Here, we use ovalbumin (OVA) as a model antigen and demonstrate immune-mediated elimination of expression on muscle-directed AAV-2 gene transfer. Administration to immune competent mice resulted in transient systemic OVA expression. Within 10 days, OVA-specific T-helper cells had been activated in draining lymph nodes, an inflammatory immune response ensued, and OVA-expressing muscle fibers were destroyed by a cytotoxic CD8+ T-cell response. Use of a muscle-specific promoter did not prevent this immune response. Adoptively transferred CD4+ cells transgenic for a T-cell receptor specific to OVA peptide-major histocompatibility complex class II showed antigen-specific, vector dose-dependent proliferation confined to the draining lymph nodes of AAV-OVA–transduced muscle within 5 days after gene transfer and subsequently participated in lymphocytic infiltration of transduced muscle. This study documents that a local immune response limits sustained expression of a secreted protein in muscle gene transfer, a finding that may have consequences for design of clinical protocols.
Introduction
Gene replacement therapy attempts to restore the function of the defective gene product through sustained therapeutic transgene expression. However, depending on the nature of the mutation in the defective gene (eg, a deletion or nonsense mutation), the therapeutic gene product may represent a nonself protein to the host and thus may be subject to an immune response, which can eliminate transgene expression through antibody-mediated or cellular mechanisms. Therefore, understanding mechanisms of immune activation is of great clinical significance.
Adeno-associated viral (AAV) vectors have been extensively tested for therapeutic gene delivery by means of in vivo gene transfer to nondividing target cells such as muscle fibers and hepatocytes.1 AAV serotype 2 vectors are derived from a nonpathogenic, replication-deficient parvovirus with an approximate 4.7-kb single-stranded DNA genome.2 These vectors do not contain viral-coding sequences and can be manufactured in a helper virus-free system. Skeletal muscle is an attractive target for gene transfer because of its abundance and easy accessibility, thereby allowing gene transfer with relatively noninvasive procedures. Muscle fibers are capable of expressing and secreting biologically active gene products that are normally not synthesized by this tissue.3 However, sustained systemic expression of several therapeutic proteins has been limited by a neutralizing antibody response after intramuscular administration of recombinant AAV. Examples include coagulation factor IX (FIX) in treatment of hemophilia B, α1-antitrypsin, and erythropoietin.4-6 These responses were typically observed if a neo-antigen was expressed such as a human protein in a mouse or a species-specific transgene product in the context of a gene deletion or other type of null mutation.7-9 Therefore, only subjects with missense mutations in the F9 gene were enrolled in a phase 1 clinical trial based on intramuscular administration of AAV-FIX vector.10 Anti-FIX formation in the context of muscle gene transfer is the result of an adaptive CD4+ T-helper cell-dependent immune response that is not observed in Rag-1–deficient or CD4-deficient mice and that may be blocked by transient immune suppression in immunocompetent animals.5,8,11 Of interest, hepatic AAV-mediated expression of the same gene products was often sustained and has been shown to induce immune tolerance to FIX and α-galactosidase.7,12-17
Adaptive immune responses after gene transfer to skeletal muscle have been shown to require antigen presentation to T cells by bone marrow-derived professional antigen-presenting cells (APCs).18 Antigen presentation by dendritic cells is thought to be crucial for initiation of a primary immune response. In contrast to many other viral vectors, AAV vectors often fail to induce a cytotoxic T-lymphocyte (CTL) response to the transgene product, which is likely due to inefficiency of the vector to productively infect dendritic cells in vivo and to elicit a potent innate immune response, thereby failing to provide efficient major histocompatibility complex (MHC) class I antigen presentation and activation signals.19-21 Nonetheless, CTL responses to certain transgene products (ovalbumin [OVA], herpes simplex virus glycoproteins A and D, and membrane-associated β-galactosidase) have been described after intramuscular injection of AAV vector.22-24
To study immunologic consequences of in vivo AAV-mediated gene transfer for systemic protein delivery, we performed AAV-OVA gene transfer to BALB/c mice transgenic for DO11.10 T-cell receptor (TCR). This TCR (encoded by rearranged VA13 and VB8.2 genes) is specific for a chicken OVA peptide, amino acids 323 to 339, presented by the MHC class II molecule I-Ad to CD4+ T cells. DO11.10 TCR+CD4+ cells can be identified by flow cytometry using dual antibody stain (antimurine CD4 and clonotypic KJ1-26 monoclonal antibody specific to the DO11.10 TCR).25 Administration of hepatic AAV vector resulted in sustained systemic OVA expression and evidence for CD4+ T-cell tolerance to the transgene product through anergy and deletion mechanisms.26 Here, we use this model to investigate lymphocyte responses to a secreted transgene product in muscle-directed AAV gene transfer. Transgene expression was eliminated within 1 month by a local immune response in the transduced muscle. Activation of transgene product-specific T cells in draining lymph nodes (LNs) of injected skeletal muscle severely limited transgene expression.
Materials and methods
AAV vector construction and production
The OVA cDNA was cloned as a 1.8-kb EcoRI fragment into pAAV-CMV-cFIX replacing canine FIX cDNA.27 The resulting expression cassette (flanked by AAV serotype 2 inverted terminal repeats [ITRs]) contains the cytomegalovirus (CMV) immediate early (IE) enhancer/promoter, a chimeric intron with CMV splice donor and β-globin splice acceptor sites, OVA cDNA, and human growth hormone polyadenylation signal. In a second construct (AAV-C5/12-OVA), the CMV enhancer/promoter was replaced by the muscle-specific synthetic C5-12 promoter obtained from Valentis (Burlingame, CA).28 AAV-CMV-green fluorescent protein (GFP) and AAV-null vectors (no transgene product) were provided by Avigen (Alameda, CA).29 AAV vectors were produced in a helper virus-free system by triple transfection of HEK-293 cells, and viral particles purified from cell lysate by repeated CsCl density gradient centrifugation as published.30 Vector titers were quantified by dot-blot hybridization as previously described.4 Viral genomes were packaged in AAV serotype 2 (AAV-2) capsid.
Animal experiments
Wild-type BALB/c and C57BL/6 mice, CD8-deficient mice, and BALB/c mice transgenic for rearranged DO11.10 TCR were purchased from Jackson Laboratory (Bar Harbor, ME). Male mice at the age of 4 to 6 weeks were used in the study. For injection into muscle, a small incision in the skin of the left hind limb was made, and viral vector was injected with a Hamilton syringe into quadriceps (50 μL) and tibialis (25 μL) anterior muscles.31 Plasma samples were collected by retro-orbital bleed using heparinized capillary tubes. For adoptive T-cell transfer, spleens were harvested from DO11.10 transgenic BALB/c mice, pooled, and processed to produce single-cell suspensions.26 CD4+ T cells were further purified by magnetic cell sorting (Miltenyi Biotec, Auburn, CA). Purified CD4+ T cells were labeled with 5-(and-6)-carboxyfluorescein diacetate succinmidyl ester (CFSE flow kit; Renovar, Madison, WI) and injected intravenously into naïve BALB/c recipient mice (5 × 106 CD4+ cells/mouse). After 24 hours, contralateral legs were injected intramuscularly with AAV-CMV-OVA or AAV-null vector. Spleens and LNs were harvested 5 days later for flow cytometry.
Assays for systemic OVA levels and antibody formation
Plasma levels of the OVA antigen were measured by an OVA-specific enzyme-linked immunosorbent assay (ELISA). Microtiter plates were coated with affinity-purified rabbit anti-OVA (1:2000 dilution; Chemicon, Temecula, CA) and detected with rabbit anti-OVA conjugated to horseradish peroxidase (HRP; 1:2000 dilution; Rockland Immunochemicals, Gilbertsville, PA). Immunocapture assay for determination and quantitation of anti-OVA immunoglobulin subclasses (IgG1, IgG2a, IgG2b) were performed as published using antibodies from Roche Molecular Biochemicals (Indianapolis, IN).8,11 OVA protein for ELISA standards and for antibody capture and immunoglobulin standards were from Sigma (St Louis, MO).
Histochemical analyses
Mouse muscle tissue was snap-frozen in liquid nitrogen-cooled isopentene.31 Cryosections of muscle tissue were fixed with 3% paraformaldehyde (PFA) for 10 minutes at room temperature (RT), followed by permeabilization with methanol for 10 minutes. Tissue sections were blocked with 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) plus 10% goat serum for 90 minutes at RT. Anti-CD8β (PharMingen, San Diego, CA) in a dilution of 1:100 and anti-OVA (Chemicon) in a dilution of 1:10 were applied in 1% BSA for 90 minutes, followed by secondary antibody conjugated to fluorescein isothiocyanate (FITC; PharMingen) for CD8 stain and tetramethylrhodamine isothiocyanate (TRITC) for OVA stain (1:100 dilution; Caltag, Burlingame, CA). Fluorescence microscopy and light microscopy were performed with a Nikon E800 microscope (Nikon, Tokyo, Japan). Images were captured with a Cool Snap-Pro camera and analyzed with Image Pro-Plus software (both from Media Cybernetics, Silver Spring, MD). For light microscopy, a Plan Apo 10 ×/0.45 objective was used; for microscopy with epifluorescent light, a Plan Fluor 20 ×/0.45 or 40 ×/0.75 objective was used. To assess inflammation, tissue morphology, and OVA expression, a total of 10 AAV-transduced muscles and 10 control muscles were analyzed per time point when the animals were humanely killed (20-30 serial cross-sections/muscle). For intracellular interferon γ (IFN-γ) staining, FITC-conjugated anti–mouse IFN-γ (PharMingen) was used at a dilution of 1:50. To detect CD4+TCR+ cells in tissue sections, FITC-conjugated rat anti–mouse CD4 (Caltag) was used at a dilution of 1:100, and biotin-conjugated KJ1-26 clonotypic antibody (Caltag) was applied at a dilution of 1:50. The secondary antibody for DO11.10 TCR staining was rhodamine-conjugated antibiotin (1:100 dilution; BD Bioscience, San Jose, CA). Cryosections were also stained with hematoxylin and eosin.
Flow cytometry
Cells were isolated from the spleen and LNs following standard protocol. Viable cells were stained with FITC-conjugated rat anti–mouse CD4, phycoerythrin (PE)–conjugated hamster antimouse CD69 (BD Bioscience) and PE-cyanine 5.5 (Cy5.5)–conjugated KJ1-26 (mouse monoclonal antibody to DO11.10 TCR; Caltag). Isotype controls were as follows: rat IgG1-FITC (Caltag), hamster IgG-PE (BD Biosciences), and mouse IgG2a PE-Cy5.5 (Caltag). Flow cytometry was performed on FACSCalibur (Becton Dickinson, Mountain View, CA) and analyzed with the CellQuest program.
In vitro cytokine release and proliferation assays
Lymphocytes isolated from spleen and LNs (inguinal and popliteal nodes) were isolated and purified following standard procedure. Cells (5 × 106/well) were cultured in 6-well plates in Dulbecco modified Eagle medium (DMEM; supplemented with 2% heat-inactivated fetal calf serum and 10-6 M 2-mercaptoethanol) with and without OVA stimulation (100 μg/mL). All lymphocyte cultures were incubated at 37°C at 10% CO2. Cell-free supernatants were harvested at 72 hours. Secreted cytokines (interleukin 2 [IL-2], IFN-γ, or IL-10) were measured by ELISA using antibodies and protocols provided by the manufacturer (BD Bioscience), with recombinant mouse cytokines serving as standard. To determine OVA-specific lymphocyte proliferation, splenocytes or LN cells were incubated in 96-well plates (quadruplicate, 5 × 105 cells/well) for 24 hours with or without 100 μg OVA/mL media, and then pulsed with 3H-thymidine for 12 hours. Thymidine uptake was measured with a MicoBeta scintillation counter (Wallac, Turku, Finland).
ELISPOT assay
This assay was done following the instructions of the manufacturer (PharMingen). Briefly, enzyme-linked immunospot assay (ELISPOT) plates (BD Bioscience) were coated with capture antibody against mouse IFN-γ or IL-4 and blocked with RPMI 1640 plus 10% fetal bovine serum (FBS). Cells were inoculated at 2-fold serial dilutions starting with 1 × 106 cells/well in RPMI 1640/10 medium, and cultured for 3 days (IFN-γ) or 5 days (IL-4). Spots were developed after incubation with secondary antibody conjugated to HRP followed by addition of 3-amino-9-ethylcarbazole solution. Plates were analyzed with an ImmunoSpot analyzer (Hightech Instruments, Edgemont, PA).
In vitro CTL assay
Splenocytes and LN cells from C57BL/6 mice were isolated 10 and 30 days after intramuscular injection with AAV-CMV-OVA and restimulated in vitro for 6 days using irradiated (6000 rad) OVA-expressing E.G7-OVA cells as feeder cells.22 Culture media were RPMI 1640 containing 10% FBS, 1 mM sodium pyruvate, MEM nonessential amino acids, 50 μM 2-mercaptoethanol, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 5 U/mL recombinant human IL-2 (rhIL-2). Subsequently, these OVA-stimulated effector cells were mixed with E.G7-OVA target cells or EL-4 target cells (mock control). E.G7-OVA cells are G418-resistant, stably OVA-transfected cells derived from C57BL/6 lymphoma cell line EL-4 (both cell lines were obtained from American Type Culture Collection, Manassas, VA). Specific lysis of target cells was determined with the CytoTox96 Non-Radioactive Cytotoxicity Assay from Promega (Madison, WI).
Results
AAV-mediated OVA expression derived from murine skeletal muscle is transient
AAV-CMV-OVA vector was injected into quadriceps and tibialis anterior muscles of the left hind limb of BALB/c mice at doses of 1 × 1011 vector genomes (vg) or 1 × 1012 vg/animal. Systemic OVA expression was monitored by ELISA on plasma samples. Data from Figure 1A demonstrate that OVA expression was dependent on vector dose and was transient. Circulating levels up to 30 ng/mL were detectable only at 2 weeks after gene delivery but declined to undetectable levels by week 4. Virtually identical appearance and loss of transgene expression were obtained in BALB/c mice transgenic for DO11.10 TCR (Figure 1C). Because systemic transgene expression was undetectable by week 4 without evidence for antibody to OVA at this time (Figure 1B,D, and data not shown), we suspected a cellular immune response. At a dose of 1 × 1012 vg/mouse, IgG1 antibody to OVA was measured at low titer by 5 to 6 weeks in both strains (Figure 1B,D).
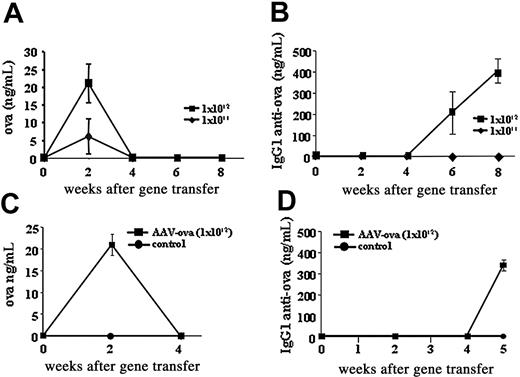
OVA expression and antibody formation to OVA. Systemic OVA expression and antibody formation to OVA after intramuscular injection of AAV-CMV-OVA into wild-type BALB/c mice (A-B) or BALB/c mice transgenic for DO11.10 TCR (C-D). (A-B) Mice received doses of 1 × 1011 vg/animal (♦) or 1 × 1012 vg/animal (▪). (C-D) Mice received doses of 1 × 1012 vg/animal (▪) or were naive controls (•). All data points represent average of 5 animals ± SD. Panels A and C show plasma levels of OVA as a function of time after vector administration; panels B and D indicate levels of IgG1 anti-OVA as a function of time after vector administration.

OVA expression and antibody formation to OVA. Systemic OVA expression and antibody formation to OVA after intramuscular injection of AAV-CMV-OVA into wild-type BALB/c mice (A-B) or BALB/c mice transgenic for DO11.10 TCR (C-D). (A-B) Mice received doses of 1 × 1011 vg/animal (♦) or 1 × 1012 vg/animal (▪). (C-D) Mice received doses of 1 × 1012 vg/animal (▪) or were naive controls (•). All data points represent average of 5 animals ± SD. Panels A and C show plasma levels of OVA as a function of time after vector administration; panels B and D indicate levels of IgG1 anti-OVA as a function of time after vector administration.
Destruction of muscle fibers eliminates expression
To test the hypothesis that CTL responses to OVA have been generated, we killed DO11.10 transgenic mice at days 10 and 30 after intramuscular injection of 1 × 1012 vg/animal (5 mice/time point) and analyzed cross-sections of muscle tissue by immunohistochemistry. As shown in Figure 2A-B, there was a strong inflammatory reaction in AAV-CMV-OVA–injected muscle at day 10. Substantial inflammatory infiltrate was seen after hematoxylin and eosin staining. Tissue damage was evident, whereas tissue sections from the noninjected leg showed normal morphology (Figure 2C). Immunofluorescence staining for OVA-expressing muscle fibers was positive at day 10, but not at day 30 (Figure 2A-B,J). Although a few areas showed OVA-expressing fibers at day 10 without cellular infiltrate (Figure 2G), most OVA-expressing fibers (> 70%) were surrounded by CD8+ cells. A representative micrograph is shown in Figure 2H. At day 30, strong inflammatory infiltration was still observed in tissue sections of transduced muscle. Some level of CD8+ cellular infiltrate remained, whereas no OVA expression was detectable at this time point (Figure 2J). An example of dead muscle fibers surrounded by inflammatory cells is shown in Figure 2D. Active muscle fiber regeneration (characterized by emergence of fibers with central nuclei) was seen at both time points, which was extensive at day 30 (Figure 2E). These observations provide strong evidence that OVA expression evoked a CTL response, which caused muscle fiber destruction and disappearance of OVA expression. Intramuscular administration of AAV-CMV-GFP (1 × 1012 vg/animal) resulted in sustained GFP expression without inflammation in DO11.10 TCR transgenic BALB/c mice (Figure 2Q-R). This finding indicates that immune responses on AAV-CMV-OVA injection were antigen-specific and were directed to the transgene product rather than viral antigens.
Use of a muscle-specific promoter for transgene expression did not prevent the inflammatory response in transduced muscle, which again was associated with a CD8+ cell infiltrate at the site of transgene expression (Figure 2T-U). The synthetic C5-12 promoter used in these experiments is entirely composed of muscle-specific enhancer/promoter elements and had been shown by others to be myocyte-specific in a transgenic animal model.32
CD4+ T-cell activation in draining LNs
To further study T-cell activation, we analyzed OVA-specific T-cell responses by various techniques. Lymphocytes from spleen and inguinal and popliteal LNs were analyzed by flow cytometry. As shown in Figure 3G, we measured a 2-fold increase in the proportion of CD4+TCR+ cells expressing the activation marker CD69 at day 10 after gene transfer in draining LNs, but not in nondraining LNs or spleens (compared with cells from noninjected controls). These data are consistent with the hypothesis that immune activation initiated locally from the draining LNs of injected muscles.
On activation, CD4+ T cells provide help for activation of B cells or CD8+ T cells, depending on patterns of cytokine expression.11,33 Lymphocytes isolated from draining LNs at day 10 secreted higher amounts of IL-2 and IFN-γ (but not IL-10) on in vitro stimulation with OVA (Figure 3D-F), whereas cells from nondraining LNs secreted cytokines at levels lower than controls (IL-2, IL-10, and IFN-γ; Figure 3D-F). OVA-specific cytokine secretion from splenocytes was similar to controls (IL-2, IFN-γ)or reduced (IL-10; Figure 3A-C). Taken together, these data demonstrate local CD4+ T-cell activation resulting in a Th1 response early after gene transfer.
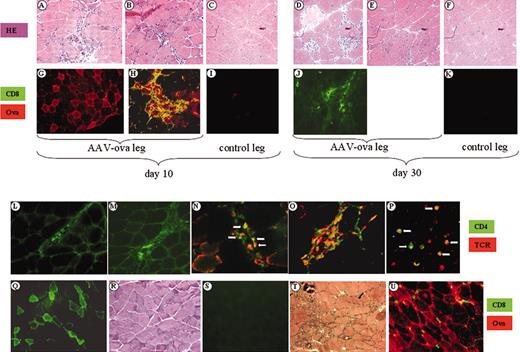
Histochemical and immunochemical analyses of skeletal muscle from DO11.10 TCR transgenic BALB/c mice. Muscle cross-sections were taken at day 10 (A-C,G-I,L,N) or day 30 (D-F,J,K,M,O) after intramuscular injection of AAV-CMV-OVA (1 × 1012 vg/mouse) from injected legs (A-B,D-E,G-H,J,L-O) and contralateral noninjected legs (C,F,I,K). Panels A-F represent muscle sections stained with hematoxylin and eosin (HE); panels G-K represent dual immunofluorescence stain for OVA (red stain, TRITC label) and CD8 (green stain, FITC label). Sections of injected leg muscle were also stained for IFN-γ–expressing cells (panel L, day 10, and panel M, day 30). Dual antibody stain for CD4 (green stain, FITC label) and DO11.10 TCR (red stain, TRITC label) are shown in panels N-P. Panels N and O are vector-injected muscle, days 10 and 30, respectively. Dual positive cells are shown by arrows in panels N and P, and are abundant in panel O. Panel P shows CD4/TCR stain on lymphocytes from DO11.10 TCR transgenic BALB/c mice. Original magnifications × 100 (A-K), × 200 (L-O), and × 400 (P). (Q-S) Muscle sections from animals 30 days after AAV-CMV-GFP (1 × 1012 vg/mouse) administration. GFP expression is shown for injected (Q) and contralateral noninjected (S) muscles. (R) Hematoxylin and eosin stain of AAV-CMV-GFP–injected muscle. (U-T) Muscle injected with AAV-C5/12-OVA, day 10 after gene transfer. Panel T is hematoxylin and eosin stain; panel U is immunofluorescence stain for CD8 (green) and OVA (red). Original magnification × 200 (Q-U).

Histochemical and immunochemical analyses of skeletal muscle from DO11.10 TCR transgenic BALB/c mice. Muscle cross-sections were taken at day 10 (A-C,G-I,L,N) or day 30 (D-F,J,K,M,O) after intramuscular injection of AAV-CMV-OVA (1 × 1012 vg/mouse) from injected legs (A-B,D-E,G-H,J,L-O) and contralateral noninjected legs (C,F,I,K). Panels A-F represent muscle sections stained with hematoxylin and eosin (HE); panels G-K represent dual immunofluorescence stain for OVA (red stain, TRITC label) and CD8 (green stain, FITC label). Sections of injected leg muscle were also stained for IFN-γ–expressing cells (panel L, day 10, and panel M, day 30). Dual antibody stain for CD4 (green stain, FITC label) and DO11.10 TCR (red stain, TRITC label) are shown in panels N-P. Panels N and O are vector-injected muscle, days 10 and 30, respectively. Dual positive cells are shown by arrows in panels N and P, and are abundant in panel O. Panel P shows CD4/TCR stain on lymphocytes from DO11.10 TCR transgenic BALB/c mice. Original magnifications × 100 (A-K), × 200 (L-O), and × 400 (P). (Q-S) Muscle sections from animals 30 days after AAV-CMV-GFP (1 × 1012 vg/mouse) administration. GFP expression is shown for injected (Q) and contralateral noninjected (S) muscles. (R) Hematoxylin and eosin stain of AAV-CMV-GFP–injected muscle. (U-T) Muscle injected with AAV-C5/12-OVA, day 10 after gene transfer. Panel T is hematoxylin and eosin stain; panel U is immunofluorescence stain for CD8 (green) and OVA (red). Original magnification × 200 (Q-U).
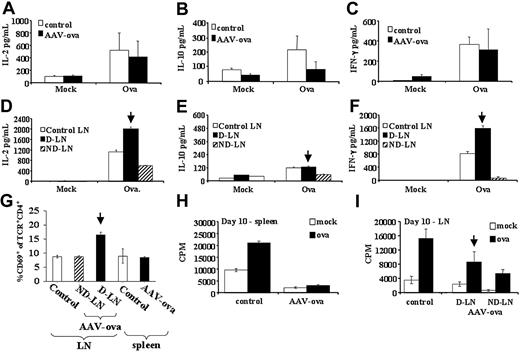
In vitro cytokine secretion, activation, and proliferation by lymphocyte cultures from DO11.10 TCR transgenic BALB/c mice at 10 days after vector administration. Splenocytes or LN cells from naive controls or AAV-CMV-OVA intramuscularly injected mice were cultured in 6-well plates (5 × 106 cells/well) for 3 days and stimulated with 100 μg/mL OVA or mock stimulated. Conditioned media were collected at day 3 of cell culture and analyzed by cytokine-specific ELISA for IL-2 (A,D), IL-10 (B,E), and IFN-γ (C,F). (A-C) Splenocyte cultures. (G) Summary of percent CD69+ of CD4+TCR+ cells for lymphoid organs of naive controls and vector-injected mice as determined by flow cytometry. Spleens from each animal were processed and analyzed individually. Results are average (5/group) ± SD. Results from LN cells represent average ± SD for 3 counts by flow cytometry with each count representing LN cells pooled from 1 to 2 animals. Splenocytes (H) and LN cells (I) were also cultured (5 × 105 cells/well in 96-well plates) overnight with OVA stimulation (100 μL/mL) or without OVA stimulation (mock), and pulsed with 3H-thymidine for an additional 12 hours. Results for 3H-thymidine incorporation are shown in counts per minute (CPM). All cell cultures were set up in quadruplicate. LNs were inguinal and popliteal LNs. D-LN indicates draining LNs of vector-injected leg muscle; ND-LN, nondraining LNs of noninjected contralateral leg. Results from D-LNs are marked by vertical arrow. Spleens from each animal were processed and analyzed individually. Results are average (5/group) ± SD. Results from LN cells represent average ± SD for 3 wells with each well representing LN cells pooled from 1 to 2 animals.

In vitro cytokine secretion, activation, and proliferation by lymphocyte cultures from DO11.10 TCR transgenic BALB/c mice at 10 days after vector administration. Splenocytes or LN cells from naive controls or AAV-CMV-OVA intramuscularly injected mice were cultured in 6-well plates (5 × 106 cells/well) for 3 days and stimulated with 100 μg/mL OVA or mock stimulated. Conditioned media were collected at day 3 of cell culture and analyzed by cytokine-specific ELISA for IL-2 (A,D), IL-10 (B,E), and IFN-γ (C,F). (A-C) Splenocyte cultures. (G) Summary of percent CD69+ of CD4+TCR+ cells for lymphoid organs of naive controls and vector-injected mice as determined by flow cytometry. Spleens from each animal were processed and analyzed individually. Results are average (5/group) ± SD. Results from LN cells represent average ± SD for 3 counts by flow cytometry with each count representing LN cells pooled from 1 to 2 animals. Splenocytes (H) and LN cells (I) were also cultured (5 × 105 cells/well in 96-well plates) overnight with OVA stimulation (100 μL/mL) or without OVA stimulation (mock), and pulsed with 3H-thymidine for an additional 12 hours. Results for 3H-thymidine incorporation are shown in counts per minute (CPM). All cell cultures were set up in quadruplicate. LNs were inguinal and popliteal LNs. D-LN indicates draining LNs of vector-injected leg muscle; ND-LN, nondraining LNs of noninjected contralateral leg. Results from D-LNs are marked by vertical arrow. Spleens from each animal were processed and analyzed individually. Results are average (5/group) ± SD. Results from LN cells represent average ± SD for 3 wells with each well representing LN cells pooled from 1 to 2 animals.
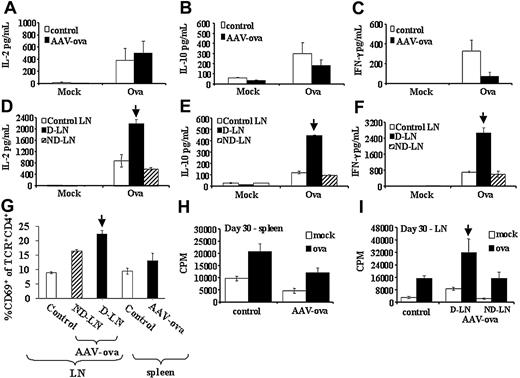
In vitro cytokine secretion, activation, and proliferation by lymphocyte cultures from DO11.10 TCR transgenic BALB/c mice at 30 days after vector administration. Splenocytes or LN cells from naive controls or AAV-CMV-OVA intramuscularly injected mice were cultured in 6-well plates (5 × 106 cells/well) for 3 days and stimulated with 100 μg/mL OVA or mock stimulated. Conditioned media were collected at day 3 of cell culture and analyzed by cytokine-specific ELISA for IL-2 (A,D), IL-10 (B,E), and IFN-γ (C,F). (A-C) Splenocyte cultures. (D-F) LN cell cultures. (G) Summary of percentage CD69+ of CD4+TCR+ cells for lymphoid organs of naive controls and vector-injected mice as determined by flow cytometry. Splenocytes (H) and LN cells (I) were also cultured (5 × 105 cells/well in 96-well plates) overnight with OVA stimulation (100 μL/mL) or without OVA stimulation (mock), and pulsed with 3H-thymidine for an additional 12 hours. Results for 3H-thymidine incorporation are shown in CPM. LNs were inguinal and popliteal LNs. D-LN indicates draining LNs of vector-injected leg muscle; ND-LN, nondraining LN of noninjected contralateral leg. Results from D-LNs are marked by vertical arrow. All experimental conditions for panels A-I (including sizes per group and determination of average ± SD) were as described for Figure 3.

In vitro cytokine secretion, activation, and proliferation by lymphocyte cultures from DO11.10 TCR transgenic BALB/c mice at 30 days after vector administration. Splenocytes or LN cells from naive controls or AAV-CMV-OVA intramuscularly injected mice were cultured in 6-well plates (5 × 106 cells/well) for 3 days and stimulated with 100 μg/mL OVA or mock stimulated. Conditioned media were collected at day 3 of cell culture and analyzed by cytokine-specific ELISA for IL-2 (A,D), IL-10 (B,E), and IFN-γ (C,F). (A-C) Splenocyte cultures. (D-F) LN cell cultures. (G) Summary of percentage CD69+ of CD4+TCR+ cells for lymphoid organs of naive controls and vector-injected mice as determined by flow cytometry. Splenocytes (H) and LN cells (I) were also cultured (5 × 105 cells/well in 96-well plates) overnight with OVA stimulation (100 μL/mL) or without OVA stimulation (mock), and pulsed with 3H-thymidine for an additional 12 hours. Results for 3H-thymidine incorporation are shown in CPM. LNs were inguinal and popliteal LNs. D-LN indicates draining LNs of vector-injected leg muscle; ND-LN, nondraining LN of noninjected contralateral leg. Results from D-LNs are marked by vertical arrow. All experimental conditions for panels A-I (including sizes per group and determination of average ± SD) were as described for Figure 3.
At day 30, cells from draining and nondraining LNs as well as splenocytes had increased numbers of CD4+TCR+CD69+ cells, with draining LNs showing a further increase in CD4+TCR+CD69+ cells (3-fold of controls, Figure 4G). At this time point, cells from draining LNs showed increased IL-2, IL-10, and IFN-γ secretion on in vitro stimulation with OVA (Figure 4D-F), suggesting Th1 and Th2 responses at this time point. IL-10 and IFN-γ secretion remained reduced for splenocytes (Figure 4A-C) but returned to levels of controls in nondraining LNs by day 30.
Consistent with cytokine release data, ELISPOT assays showed an increase in IFN-γ–secreting cells in draining LNs at days 10 and 30 (Figure 5C,G). The numbers of cells secreting IL-4 in response to stimulation with OVA increased substantially in draining LNs and spleens (and to lesser extent in nondraining LNs) at day 10 (Figure 5B,D). IL-4 responses largely declined by day 30, but remained somewhat elevated in draining LNs, consistent with an increase in IL-10 secretion by draining LN cells at day 30.
Taken together and consistent with inflammatory reactions, these data demonstrate a predominantly Th1-driven local immune response, with some level of Th2 immunity. To further correlate in vitro data on Th1 responses with in vivo data on an inflammatory reaction in injected muscles, we performed additional immunostaining and found infiltrates of CD4+TCR+ cells as well as of cells producing IFN-γ cytokine (Figure 2L-O).33
We also measured in vitro lymphocyte proliferation for draining LN cells and splenocytes of AAV-CMV-OVA–injected DO11.10 mice. At day 10 after gene transfer, splenocytes failed to proliferate to OVA, cells from nondraining LNs showed substantially reduced proliferation, and cells from draining LNs had mildly reduced proliferative responses (Figure 3H-I). At day 30 after vector administration, OVA-specific proliferation was similar for nondraining LN cells and splenocytes compared with controls, whereas OVA-specific proliferation was increased for draining LN cells (Figure 4H-I).
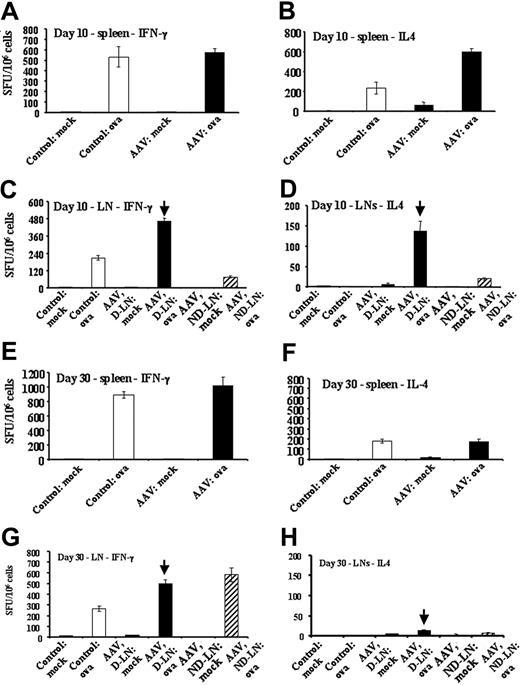
Quantitation of OVA-specific cytokine responses in DO11.10 TCR transgenic BALB/c mice by ELISPOT. Mice were naive controls or had been injected intramuscularly with AAV-CMV-OVA vector (3 × 1012 vg/animal). Panels A, C, E, and G show numbers of IFN-γ–secreting cells as spot-forming units (SFUs) per 106 cells after mock or OVA stimulation. Panels B, D, F, and H show numbers of IL-4–secreting cells as SFUs per 106 cells after mock or OVA stimulation. (A-B) Splenocytes, day 10 after gene transfer. (C-D) LN cells, day 10. (E-F) Splenocytes, day 30. (G-H) LN cells, day 30 after gene transfer. LNs were inguinal and popliteal LNs. D-LN indicates draining LNs of vector-injected leg muscle; ND-LN, nondraining LNs of noninjected contralateral leg. Results from D-LNs are marked by vertical arrow. All ELISPOT cultures were set up in quadruplicate. Spleens from each animal were processed and analyzed individually. LN cells were pooled for experimental groups (5 animals/group). Results are average of quadruplicate measurement ± SD.

Quantitation of OVA-specific cytokine responses in DO11.10 TCR transgenic BALB/c mice by ELISPOT. Mice were naive controls or had been injected intramuscularly with AAV-CMV-OVA vector (3 × 1012 vg/animal). Panels A, C, E, and G show numbers of IFN-γ–secreting cells as spot-forming units (SFUs) per 106 cells after mock or OVA stimulation. Panels B, D, F, and H show numbers of IL-4–secreting cells as SFUs per 106 cells after mock or OVA stimulation. (A-B) Splenocytes, day 10 after gene transfer. (C-D) LN cells, day 10. (E-F) Splenocytes, day 30. (G-H) LN cells, day 30 after gene transfer. LNs were inguinal and popliteal LNs. D-LN indicates draining LNs of vector-injected leg muscle; ND-LN, nondraining LNs of noninjected contralateral leg. Results from D-LNs are marked by vertical arrow. All ELISPOT cultures were set up in quadruplicate. Spleens from each animal were processed and analyzed individually. LN cells were pooled for experimental groups (5 animals/group). Results are average of quadruplicate measurement ± SD.
Induction of CD8+ CTLs to OVA
These presented data pointed toward destruction of AAV-CMV-OVA–transduced muscle fibers by a cytotoxic CD8+ T-lymphocyte response to OVA. This was studied in more detail by intramuscular administration of AAV-CMV-OVA vector (1 × 1012 vg/animal) in wild-type or CD8-deficient C57BL/6 mice. Similar to studies in BALB/c mice, systemic OVA expression was transient in wild-type mice, although somewhat more prolonged in C57BL/6 compared with BALB/c mice (Figure 6A). Only 1 of 4 wild-type mice injected intramuscularly with vector formed antibody to OVA during the course of the experiment (data not shown), whereas all animals lost systemic expression by 6 weeks (Figure 6A). Again, sections of muscle tissue showed an inflammatory reaction, CD8+ cell infiltrate, and muscle regeneration (Figure 6B left column). In vitro CTL assay using MHC class I-compatible target cells demonstrated a cytolytic response to OVA-expressing cells (Figure 6C-E). CTL responses were also induced when AAV-C5/12 vector containing a muscle-specific promoter was injected, albeit with a 4- to 8-fold lower in vitro cytolytic activity compared with the CMV-driven construct (Figure 6F-G). In contrast with wild-type mice, CD8-deficient C57BL/6 mice showed sustained systemic OVA expression and lack of inflammation after intramuscular administration of AAV-CMV-OVA, supporting the interpretation that CD8+ T-cell activation was required for muscle fiber destruction (Figure 6A-B right columns). In contrast to T-helper cell responses, CTLs were detected in spleens in addition to draining LNs.
Muscle gene transfer induces transgene product-specific T-cell proliferation in the draining LNs
Although the DO11.10 transgenic mouse strain is a powerful model to study mechanisms of tolerance induction, these animals are not optimal for studies on development of immune responses. First, the large number of CD4+ T cells with a TCR for OVA results in competition for the antigen and thus in inefficient T-cell activation.34 Second, an immune response is typically characterized by a limited number of T cells that become activated and subsequently proliferate in vivo. A superior approach has been developed by Jenkins et al and involves adoptive transfer of CD4+ T cells from DO11.10 TCR transgenic mice to wild-type BALB/c mice.34,35 In our gene transfer system, we adoptively transferred CFSE dyelabeled CD4+ cells from DO11.10 transgenic mice to BALB/c mice, which after 24 hours received intramuscular injections of equal amounts of AAV-CMV-OVA and AAV-null vector (no transgene expressed) into contralateral legs (Figure 7A). Vector doses were 1 × 1012, 3 × 1012, and 1 × 1013 vg/leg. After 5 days, presence of donated CFSE+ cells in draining LNs (AAV-CMV-OVA leg), nondraining LNs (AAV-null vector leg), and spleen was demonstrated by flow cytometry (∼0.2% of total splenocytes and 0.3%-0.4% of LN cells were CFSE+, data not shown). CFSE is a fluorescent dye that is equally partitioned during cell division. Fluorescence intensity is reduced by 50% during each cell division. At all 3 vector doses, no evidence for proliferation (< 1% cell division) of the donated cells was obtained in nondraining LNs and spleens (Figure 7B and data not shown). As expected, about 70% of donated cells expressed the DO11.10 TCR (Figure 7B lower right panel).26 In contrast, DO11.10 TCR+ cells (but not TCR- cells) had proliferated in vivo in the draining LNs in a vector dose-dependent fashion (Figure 7C). Of CFSE+TCR+ cells, 14% to 68% had been proliferating (1-5 cell divisions), depending on vector dose. These percentages may somewhat underestimate the number of proliferating cells because CFSE dye was too dilute in cells with more than 5 divisions for detection above background in our assay. No T-cell proliferation was observed in draining LNs when vector was injected that contained the OVA cDNA in antisense orientation (data not shown). By day 10, an inflammatory reaction was evident in AAV-CMV-OVA–transduced, but not in AAV-null vector-transduced muscle, with higher vector doses yielding more severe inflammation (Figure 7D). Inflammatory infiltrate included CD8+ and IFN-γ+ cells (Figure 7E-F). Furthermore, CD4+ cellular infiltrates included CD4+TCR+ cells (Figure 7G), suggesting that adoptively transferred OVA-specific T cells were activated in the draining LNs and subsequently participated in the inflammatory response that targeted AAV-CMV-OVA–transduced muscle.
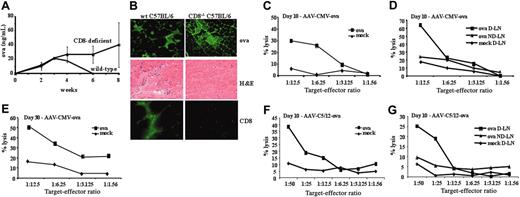
Muscle-directed AAV-OVA gene transfer in C57BL/6 mice. (A) Systemic OVA levels as a function of time after intramuscular injection of AAV-CMV-OVA vector (1 × 1012 vg/animal) in wild-type (♦) or CD8-deficient (▪) C57BL/6 mice (4/group). (B) Analysis of muscle sections (day 21 after gene transfer). Panels in left column are from wild-type mice; panels in right column are from CD8-deficient mice. Shown are immunofluorescence stain for OVA expression (top row, green stain, FITC label), hematoxylin and eosin stain (middle row), and immunofluorescence stain for CD8 (green stain, FITC label, bottom row). (C-F) In vitro assay for cytotoxic T-lymphocyte responses to OVA in wild-type C57BL/6 mice. Shown are percentage lysis of MHC I-compatible, OVA-expressing E.G7-OVA target cells (ova; ▪) and EL-4 mock targets (mock; ♦) as a function of effector-target cell ratio. (C,E) Effector cells were splenocytes from mice 10 or 30 days after intramuscular administration of 1 × 1012 vg AAV-CMV-OVA. (D) Effector cells were LN cells (draining, D-LN, or nondraining, ND-LN) from mice 10 days after intramuscular administration of 1 × 1012 vg AAV-CMV-OVA. (F-G) Effector cells were splenocytes (F) or LN cells (G) from mice 10 days after intramuscular administration of AAV-C5/12-OVA vector.

Muscle-directed AAV-OVA gene transfer in C57BL/6 mice. (A) Systemic OVA levels as a function of time after intramuscular injection of AAV-CMV-OVA vector (1 × 1012 vg/animal) in wild-type (♦) or CD8-deficient (▪) C57BL/6 mice (4/group). (B) Analysis of muscle sections (day 21 after gene transfer). Panels in left column are from wild-type mice; panels in right column are from CD8-deficient mice. Shown are immunofluorescence stain for OVA expression (top row, green stain, FITC label), hematoxylin and eosin stain (middle row), and immunofluorescence stain for CD8 (green stain, FITC label, bottom row). (C-F) In vitro assay for cytotoxic T-lymphocyte responses to OVA in wild-type C57BL/6 mice. Shown are percentage lysis of MHC I-compatible, OVA-expressing E.G7-OVA target cells (ova; ▪) and EL-4 mock targets (mock; ♦) as a function of effector-target cell ratio. (C,E) Effector cells were splenocytes from mice 10 or 30 days after intramuscular administration of 1 × 1012 vg AAV-CMV-OVA. (D) Effector cells were LN cells (draining, D-LN, or nondraining, ND-LN) from mice 10 days after intramuscular administration of 1 × 1012 vg AAV-CMV-OVA. (F-G) Effector cells were splenocytes (F) or LN cells (G) from mice 10 days after intramuscular administration of AAV-C5/12-OVA vector.
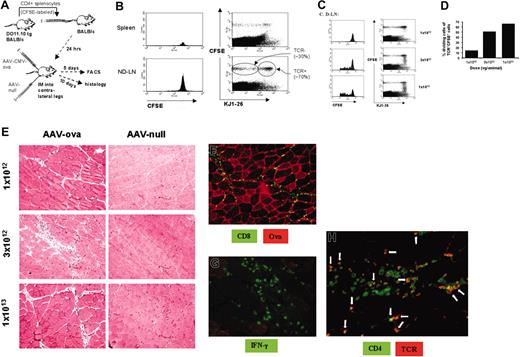
Gene transfer into BALB/c mice that had received adoptive transfer of CFSE-labeled CD4+ splenocytes from 4 DO11.10 TCR mice. (A) Experimental strategy. Mice were injected intramuscularly with equal doses of AAV-CMV-OVA and AAV-null vector into contralateral legs 24 hours after adoptive T-cell transfer. (B) Flow cytometry to detect CFSE-stained cells (histograms, left column) and CFSE and KJ1-26 (DO11.10 TCR expression) dual-stained cells (scatter graphs, right column) in spleens (top row) and nondraining LNs (ND-LN, ie, LNs of AAV-null injected leg; bottom row). Note that about 70% of CFSE+ cells were TCR+, and about 30% of CFSE+ cells were TCR-. Analysis shown here was done 5 days after injection of 1 × 1013 vg/leg. Each graph represents pooled cells from experimental animals. (C) Flow cytometry to detect CFSE-stained cells (histograms, left column) and CFSE and KJ1-26 (DO11.10 TCR expression) dual-stained cells (scatter graphs, right column) in LNs draining AAV-CMV-OVA–injected leg (D-LN) as a function of vector dose 5 days after gene transfer. Note a vector dose-dependent increase in cells with decreased CFSE fluorescence intensity indicating in vivo proliferation of TCR+ cells. (D) Estimate of percentage TCR+ cells that had undergone cell division in the draining LNs as a function of vector dose. (E) Hematoxylin and eosin stain of muscle tissue 10 days after AAV-CMV-OVA (left column) or AAV-null (right column) administration. Shown are representative sections for the 3 different vector doses. AAV-CMV-OVA–transduced muscle was also analyzed (day 10) for OVA expression and CD8+ cellular infiltrate (F), for infiltrate of IFN-γ–expressing cells (G), and for CD4+ and DO11.10 TCR+ cellular infiltrate (H). Arrows in panel H depict examples of CD4+TCR+ dual positive cells (yellow to orange color). Original magnification, × 100 (E-F), × 200 (G), and × 400 (H).

Gene transfer into BALB/c mice that had received adoptive transfer of CFSE-labeled CD4+ splenocytes from 4 DO11.10 TCR mice. (A) Experimental strategy. Mice were injected intramuscularly with equal doses of AAV-CMV-OVA and AAV-null vector into contralateral legs 24 hours after adoptive T-cell transfer. (B) Flow cytometry to detect CFSE-stained cells (histograms, left column) and CFSE and KJ1-26 (DO11.10 TCR expression) dual-stained cells (scatter graphs, right column) in spleens (top row) and nondraining LNs (ND-LN, ie, LNs of AAV-null injected leg; bottom row). Note that about 70% of CFSE+ cells were TCR+, and about 30% of CFSE+ cells were TCR-. Analysis shown here was done 5 days after injection of 1 × 1013 vg/leg. Each graph represents pooled cells from experimental animals. (C) Flow cytometry to detect CFSE-stained cells (histograms, left column) and CFSE and KJ1-26 (DO11.10 TCR expression) dual-stained cells (scatter graphs, right column) in LNs draining AAV-CMV-OVA–injected leg (D-LN) as a function of vector dose 5 days after gene transfer. Note a vector dose-dependent increase in cells with decreased CFSE fluorescence intensity indicating in vivo proliferation of TCR+ cells. (D) Estimate of percentage TCR+ cells that had undergone cell division in the draining LNs as a function of vector dose. (E) Hematoxylin and eosin stain of muscle tissue 10 days after AAV-CMV-OVA (left column) or AAV-null (right column) administration. Shown are representative sections for the 3 different vector doses. AAV-CMV-OVA–transduced muscle was also analyzed (day 10) for OVA expression and CD8+ cellular infiltrate (F), for infiltrate of IFN-γ–expressing cells (G), and for CD4+ and DO11.10 TCR+ cellular infiltrate (H). Arrows in panel H depict examples of CD4+TCR+ dual positive cells (yellow to orange color). Original magnification, × 100 (E-F), × 200 (G), and × 400 (H).
Discussion
In animal models, AAV2-mediated F9 gene transfer to the liver often resulted in sustained expression even if the transgene product represented a neo-antigen such as human FIX in mice or nonhuman primates, canine FIX in hemophilia B dogs with an F9 nonsense mutation, or murine FIX in hemophilia B mice with a large FIX gene deletion.7,12,15,16,36 Sustained hepatocyte-derived transgene expression has been shown to be associated with FIX-specific tolerance induction.15 In contrast, intramuscular administration of AAV2 vector expressing these transgene products in the identical strains of animals invariably caused a neutralizing antibody response to FIX, which has been shown to require T-cell help.5,36,11 Similar immune responses have been described to several other secreted transgene products in AAV gene transfer to skeletal muscle. We sought to develop a model system that will allow identification of immunologic mechanisms underlying these observations.
Following OVA gene transfer to mice transgenic for OVA-specific DO11.10 TCR, we can physically identify CD4+ T cells with TCR specific to transgene product-derived peptide-MHC class II complex.37 Furthermore, this model mimics therapeutic gene transfer in several key aspects. Vector dose-dependent systemic expression of OVA is obtained after AAV gene transfer. For the hepatic route, we observed sustained expression associated with induction of OVA-specific CD4+ T-cell tolerance.26 Here, we report a dramatically different outcome of AAV-OVA gene transfer in the same model system. Administration of vector to skeletal muscle induced a destructive inflammatory immune response that ultimately eliminated transgene expression. Activation of CD4+ T cells and of CD8+ CTLs was evident. Despite systemic delivery of OVA, we found a local immune response to be largely responsible for the failure to achieve sustained expression. This response was specific to the transgene product and was characterized by activation of OVA-specific CD4+ T cells in the draining LNs of the AAV-OVA–transduced muscle, resulting in vector dose-dependent in vivo T-cell proliferation, OVA-specific cytokine production reflective of Th1 and Th2 responses, and generation of a cellular infiltrate in muscle tissue.
As discussed, DO11.10 TCR transgenic mice contain a large number of antigen-specific CD4+ T cells and are therefore not ideally suited for the study of initiation of an immune response.34 Yet, kinetics of rise and loss of transgene expression were identical in wild-type and TCR transgenic BALB/c mice. Transgenic mice were clearly capable of generating an immune response that destroyed the transduced muscle fibers. However, despite evidence for T-cell activation in draining LNs of transgenic mice by day 10, T-cell proliferation in the in vitro assay was not elevated until later time points, which can be explained by competition for antigen and signaling, resulting in a more heterogeneous response compared with the physiologic situation, where antigen-specific T cells are limited in numbers.34 Therefore, definitive answers to the question of how transgene product-specific T cells are activated required an adoptive T-cell transfer model. The experimental design, summarized in Figure 7, allowed us to demonstrate activation of transgene product-specific CD4+ T cells in the draining LNs of the transduced tissue and provides us with a powerful tool to track responses to a vector-encoded antigen in vivo. One can now pinpoint where T-cell activation occurs and where these cells traffic subsequently.
In vitro CTL assays and experiments in CD8-deficient mice support the conclusion that activation of cytotoxic CD8+ T cells was responsible for destruction of transduced muscle fibers. Furthermore, our study and data by others indicate that immune responses limiting expression (in animals lacking memory T cells to AAV) were directed to the transgene product rather than AAV capsid antigens.38 The gradual rise of transgene expression over several weeks that is typically observed for AAV vector may be responsible for constant recruitment of CD8+ T cells and other inflammatory cells to the site of transgene expression during the first month after gene transfer, thereby causing prolonged inflammation. Activation of CD8+ T-cell responses has been shown to be dependent on CD4+ T-cell help, and cellular destruction of target cells in vivo is thought to require up-regulation of MHC class I expression by IFN-γ secretion.33 In vitro T-helper cell assays indicated activation of Th1 cells and secretion of IFN-γ in the early response to OVA (day 10), which intensified over time (day 30). These in vitro responses to OVA correlated with in vivo responses (activation of CD4+TCR+ cells as measured by flow cytometry, infiltration of transduced muscle by CD4+TCR+ cell in addition to CD8+ cells, and presence of IFN-γ–secreting cells in cellular infiltrate). Activation of Th2 responses may be responsible for IgG1 anti-OVA formation in animals that were followed for longer periods of time.
An obvious difference between the immune response described here against OVA and responses to FIX, erythropoietin, or α1-antitrypsin transgene products is rapid elimination of muscle fibers by a CD8+ T-cell response. FIX and other mentioned transgene products are primarily removed by antibodies, that is, a B cell-mediated response, which is often noninflammatory.5,6,11 However, inflammatory CD8+ T-cell responses can be observed in muscle-directed AAV-FIX gene transfer as well (L.W. and R.W.H., unpublished results, August 2004). It has been proposed that lack of synthesis of the transgene product by APCs, which are unproductively transduced by AAV vector, contributes to inefficient CTL induction.20,21 In contrast, OVA can be efficiently reprocessed by dendritic cells following uptake from the environment, which results in MHC class I presentation to CD8+ T cells through a cross-priming mechanism.39 This interpretation is further supported by our results with the myocyte-specific promoter. Similarly, T-cell activation to AAV-encoded influenza virus hemagglutinin (HA, a membrane-bound protein) has been reported in the context of muscle-directed AAV gene transfer despite inability to demonstrate transgene expression in dendritic cells.20 The sum of several published studies suggests certain patterns for T-cell responses as a function of the transgene product in AAV gene transfer to muscle. Cytoplasmic proteins such as β-galactosidase or GFP are typically not associated with T-cell responses, which is likely owing to ignorance.21 CD4+ T- and B-cell responses may be observed to systemic proteins (characterized by efficient secretion and high solubility) such as FIX.11 In contrast, potent CTL response proteins expressed on the cell surface such as HA and membrane-associated β-galactosidase, have been described.20,23 Of interest, activation of CD8+ T cells to OVA by cross-priming is 50 000-fold more efficient for cell-associated OVA than for soluble OVA.40 We therefore assume that OVA protein associated with muscle cell surfaces rather than circulating OVA was responsible for CTL activation in our study. It remains unclear at this point whether and how interactions between cell membrane surfaces of transduced muscle fibers and dendritic cells result in antigen uptake and processing.
Our data, supported by recent findings with a FIX transgene, suggest a model for T-cell activation on AAV-mediated, muscle-directed gene transfer of a secreted protein, in which a local immune response in the muscle leads to antigen presentation in the draining LNs and activation of CD4+ T cells of Th1 and Th2 subsets.41 Depending on processing of the antigen, activation of CD8+ T cells or B cells generates effector cells that neutralize transgene expression by cytotoxic or antibody-mediated mechanisms. This model is consistent with data in hemophilia B dogs that suggested T- and B-cell activation at high levels of local FIX production in skeletal muscle, an observation that prompted use of limited AAV-FIX vector doses per site of intramuscular injection in a clinical trial.10,42 Furthermore, these data suggest that analyses of lymphocyte responses solely based on use of splenocytes are insufficient to characterize immune responses to the transgene product in transduction of skeletal muscle.
Others have shown that very high levels of systemic FIX transgene expression achieved by administration of high-dose AAV-1 vector may not cause or at least down-regulate an immune response.43,44 Of interest, our study shows transient decreases in T-cell proliferation and cytokine release (IL-2, IL-10, and IFN-γ), and a transient increase in IL-4 production in spleens and nondraining LNs of DO11.10 transgenic mice after muscle-directed OVA gene transfer. We propose that systemic protein delivery, similar to intravenous injection of antigen, may lead to tolerogenic antigen presentation at sites outside the transduced muscle. Additional experiments will be necessary to test this hypothesis.
There are substantial differences in T-cell activation between hepatic and muscle-directed routes of AAV vector administration, which appears to be largely independent of the promoter used for transgene expression but may be influenced by levels of the transgene product.7,9,12,15,26,44 These observations are consistent with the notion that during T-cell activation, the immune system relies on signals that are provided by tissues.45 Therefore, the target tissue plays a crucial role in T-cell activation during AAV-mediated transfer of a gene encoding a secreted protein that represents a neo-antigen. In contrast to the capacity of the liver to promote tolerance induction and sustained systemic transgene expression, a local immune response is initiated in transduced skeletal muscle. Generation of antigen-specific CD4+ T-helper cell responses combined with CD8+ T-cell or B-cell activation ultimately eliminates transgene expression, which represents a severe limitation to use of skeletal muscle for systemic therapeutic protein delivery.
Prepublished online as Blood First Edition Paper, February 15, 2005; DOI 10.1182/blood-2004-03-0848.
Supported by National Institutes of Health grant R01 AI/HL51390-01 (R.W.H.) and training grant T32HL07439 (E.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Ovalbumin cDNA was a kind gift from Dr N. Shastri. The authors thank Y.-L. Liu, F. Mingozzi, M. Erne, M. Cooper, and J. Watkins, as well as the Flow Cytometry Core Facility of the University of Pennsylvania Cancer Center for technical assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal