Abstract
We identified the human germinal center-associated lymphoma (HGAL) in gene-expression profiling studies of diffuse large B-cell lymphoma (DLBCL). The expression of HGAL correlated with survival in patients with DLBCL. The HGAL gene is the human homolog of M17, a mouse gene expressed specifically in normal germinal center (GC) B cells. We generated a monoclonal antibody against the HGAL protein and show that HGAL is expressed in the cytoplasm of GC lymphocytes and in lymphomas of GC derivation. Among 727 lymphomas tested by immunohistochemistry on tissue microarrays, HGAL staining was found in follicular lymphomas (103 of 107), Burkitt lymphomas (40 of 40), mediastinal large B lymphomas (7 of 8), and in DLBCLs (103 of 151). Most marginal zone lymphomas lacked HGAL staining. Lymphocyte-predominant Hodgkin lymphomas (12 of 17) and, surprisingly, classical Hodgkin lymphomas (78 of 107) were found to be positive. Hierarchical clustering of comparative immunohistologic results in DLBCLs demonstrates that the expression of HGAL is similar to 2 other GC-associated proteins, BCL6 and CD10, but different from 2 markers associated with a non-GC phenotype, MUM1/IRF4 and BCL2. The restricted expression and GC specificity of HGAL protein suggest that it may have an important role in the diagnosis of specific lymphomas, and, potentially in the identification of subtypes associated with different prognoses.
Introduction
Gene expression profiling studies of lymphoid malignancies have led to the discovery of previously unrecognized lymphoma subtypes and novel genes that are beginning to be characterized. Using cDNA microarrays, the most common adult lymphoma, diffuse large B-cell lymphoma (DLBCL), was shown to be composed of at least 2 molecularly distinct subtypes, one with gene expression patterns normally demonstrated by germinal center (GC) B cells (germinal center B-like DLBCL [GCBL]), and the other with expression of genes that are normally induced during activation of peripheral blood B cells (activated B-like DLBCL [ABL]). These subtypes were found to be of clinical significance with the overall survival of GCBL being superior to that of ABL.1 Using clinical data to supervise gene expression patterns, predictive models of outcome in DLBCL have also been postulated.2,3 A supervised statistical method known as significance analysis of microarrays (SAM) was used to generate a rank order of genes that predict outcome in DLBCL from the data derived from previous gene expression studies.1,4 A novel GC-associated expressed sequence tag (EST; IMAGE 814622) that strongly predicted outcome in DLBCL was identified and the corresponding human germinal center–associated lymphoma (HGAL) gene (also known as germinal center expressed transcript-2 or GCET25 ), was cloned and characterized.4 This gene was found to be an interleukin 4 (IL-4)–inducible human homolog of the mouse M17 gene expressed in GCs.4,6 Previous investigation demonstrated that HGAL mRNA is expressed specifically in germinal centers (GCs), spleen, thymus, and GC-derived lymphomas. In addition, expression of HGAL mRNA was found to be an International Prognostic Index (IPI)–independent prognostic factor designating better overall survival in patients with DLBCL.4
Advances in genome-wide screening techniques such as gene expression profiling by cDNA microarrays have necessitated large-scale investigation of protein expression. Tissue microarrays (TMAs) provide an ideal tool for high-throughput protein expression studies.7,8 TMAs are efficacious for validation of gene expression data and for comparative analysis of different immunohistologic stains and tumor subtypes. We have previously described a comprehensive system for large-scale analysis of protein expression by immunohistologic techniques amenable for correlation of gene expression and protein expression data.9 This system combines algorithms such as hierarchic clustering developed for analyzing gene expression data with high-resolution digital imaging with capacity for rapid storage and retrieval of immunohistologic staining results.9
In the current study, we undertook the characterization of HGAL protein expression by a variety of methods. We investigated the subcellular localization of HGAL by immunofluorescence microscopy. We generated a monoclonal antibody against HGAL and have characterized HGAL protein expression in immortalized lymphoma cell lines, normal lymphoid tissue, and 727 non-Hodgkin and Hodgkin lymphomas. Furthermore, we used double immunohistologic staining on tonsil tissue to investigate the colocalization of HGAL protein with BCL6 and CD10 GC B cells. Comparative immunohistologic studies were carried out on 151 DLBCL samples to investigate the expression pattern of the HGAL protein in comparison to GC markers, CD10 and BCL6, and non-GC markers, MUM1/IRF4 and BCL2.
Materials and methods
Generation of monoclonal anti-HGAL antibody
We generated a GST-HGAL construct in pGEX-2T vector (Pharmacia Biotech, Uppsala, Sweden). The GST-HGAL fusion protein, expressed in Rosetta (DE3) pLacI cells (Novagene, Madison, WI), was purified on a solid-phase glutathione column. The resulting protein was approximately 40% pure by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). This protein was used for immunization of mice: 25 to 30 μg total protein was mixed with Freud complete or incomplete adjuvant for the first and 2 subsequent injections, respectively. Injections were given into the footpads of mice at 2-week intervals, followed by 3 injections every 3 days prior to undertaking fusion of draining lymph node or spleen cells to K6H6B5 fusion partner hybridoma cells, as reported previously.10 Enzyme-linked immunosorbent assay using GST-HGAL fusion protein or an unrelated GST fusion protein was used for initial screening of hybridoma supernatants. The secreting hybridoma cells were subcloned by serial dilution and then further screened for specific antibody production by immunoblotting cellular lysates from HGAL-expressing cells (Daudi cells and HeLa cells stably transfected with pcDNA3.1 HGAL construct) and cellular lysates from cells not expressing HGAL (Jurkat and nontransfected HELA cells). Eight distinct hybridomas secreting specific anti-HGAL antibodies were identified and propagated. The antibody chosen for the current study, 1H1 subclone A7, is an IgG2a containing a κ light chain. Ascites was produced in nude mice and partially purified by precipitation with ammonium sulfate. Alternatively, tissue culture supernatant containing the monoclonal was used.
Confocal immunofluorescence microscopy
HeLa cells stably transfected with pcDNA3.1 HGAL-V5 construct were subjected to immunofluorescence assay using fluorescein isothiocyanate (FITC)–conjugated anti-V5 antibody (Invitrogen, Carlsbad, CA) and propidium iodine staining. The slides were analyzed under the Zeiss confocal LSM 510 scanning microscope (Zeiss, Thornwood, NY). Nontransfected HELA cells were used as controls.
HGAL mRNA quantification and Western blotting
HGAL mRNA expression in 6 lymphoma cell lines and in 17 DLBCLs was measured by real-time quantitative reverse transcription–polymerase chain reaction (RT-PCR) as previously reported.4 The cell lines used in this study include 2 cell lines classified as GCB-like (SU-DHL-4, OCI-LY7), 3 classified as non–GCB-like (RCK8, OCILY3, OCILY10) by gene expression analysis,1 one T-cell line, Jurkat, and one Burkitt lymphoma cell line, Raji. Whole-cell extracts for Western blot analysis were prepared by lysing cells (5 × 106) with RIPA buffer (1 × phosphate-buffered saline, 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% SDS, and 10 mM phenylmethylsulfonyl fluoride, 1 mg/mL aprotinin, 100 mM sodium orthovanadate) on ice for 30 minutes. After centrifugation, the supernatant was assayed for protein concentration by BCA assay (Pierce Biotechnology, Rockford, IL). For Western blotting, 20 μg whole-cell lysate was separated on 10% SDS-PAGE, transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA), and probed by anti-HGAL (1H1) and anti–β-actin antibodies (Sigma, St Louis, MO). These antibodies were detected using a goat anti–mouse horseradish peroxidase (HRP)–conjugated antibody (Jackson ImmunoResearch Laboratories, West Grove, PA) and visualized by the Super Signal West Pico Chemiluminescent Substrate kit (Pierce Biotechnology).
Case selection
A total of 727 lymphomas were studied. The lymphoma cases were obtained from the archives of the Departments of Pathology, Stanford University Medical Center, Stanford, CA; Department of Pathology, University of Miami, FL; and Aarhus University Hospital, Aarhus, Denmark. The lymphomas were classified according to the current World Health Organization (WHO) classification scheme.11 These lymphomas were studied by immunohistochemistry on TMAs as well as on whole sections of selected cases wherever detailed morphologic analysis and comparison with other immunohistologic markers was deemed necessary. Institutional Review Board approval was obtained for these studies.
TMA construction
TMAs were constructed using a tissue arrayer (Beecher Instruments, Silver Spring, MD), by a previously described method.7 The cores were selected for TMAs by characteristic morphology based on examination of sections stained with hematoxylin and eosin, without prior knowledge of immunohistologic stains of individual cases. Two cores of 0.6 mm were taken from all normal tissue, non-Hodgkin lymphomas, and plasma cell neoplasms, whereas two 2.0-mm cores were used for Hodgkin lymphomas. Most plasma-cell neoplasms were obtained from bone marrow core biopsies. In addition to lymphoid tumors, the TMAs contained 19 cores from control tissues including 4 normal lymph nodes, 2 normal muscle, 2 melanomas, 2 breast carcinomas, 8 placentas, and 1 normal spleen. Sections of 4-μm thickness were cut from the TMAs and placed on glass slides, which were then baked for 1 hour at 60°C.
Immunohistochemistry
Primary antibodies directed against proteins HGAL, BCL6, CD10, MUM1/IRF4, and BCL2 used in this study and the conditions used for immunohistologic staining are summarized in Table 1. Serial 4-μm sections from paraffin-embedded lymphoid tissue or TMA blocks were deparaffinized in xylene and hydrated in a graded series of alcohol. Heat-induced antigen retrieval was carried out by microwave pretreatment in the appropriate buffer as indicated for each antibody. Detection was carried out using a modified biotin-streptavidin-diaminobenzidine method. Staining for all antibodies was optimized on normal paraffin-embedded tonsil sections. For the HGAL antibody, a tyramide amplification system was used (Dako Catalized Signal Amplification System, Dako, Carpinteria, CA). All single staining results for HGAL were obtained using the tyramide amplification system. Staining in more than 30% of lymphoma cells was scored positive.
Reagents and conditions used for immunohistochemistry
Antibody . | Source . | Dilution . | Pretreatment . | Predominant cell reactivity . | Staining pattern . |
|---|---|---|---|---|---|
| HGAL | * | 1:20 | Citrate | Lymphoid cells | Cytoplasmic |
| BCL6 | 1 | 1:40 | Tris | Lymphoid cells | Nuclear |
| CD10 | 2 | 1:40 | EDTA | Lymphoid cells | Membrane and cytoplasmic |
| MUM1/IRF4 | 1 | 1:50 | EDTA | Lymphoid cells | Nuclear and cytoplasmic |
| BCL2 | 1 | 1:1000 | Tris | Various | Cytoplasmic |
Antibody . | Source . | Dilution . | Pretreatment . | Predominant cell reactivity . | Staining pattern . |
|---|---|---|---|---|---|
| HGAL | * | 1:20 | Citrate | Lymphoid cells | Cytoplasmic |
| BCL6 | 1 | 1:40 | Tris | Lymphoid cells | Nuclear |
| CD10 | 2 | 1:40 | EDTA | Lymphoid cells | Membrane and cytoplasmic |
| MUM1/IRF4 | 1 | 1:50 | EDTA | Lymphoid cells | Nuclear and cytoplasmic |
| BCL2 | 1 | 1:1000 | Tris | Various | Cytoplasmic |
Generation of HGAL monoclonal antibody is described in “Materials and methods.” Source 1 was Dako; 2 was Novocastra (New Castle-upon-Tyne, United Kingdom). Pretreatment for heat-induced antigen retrieval consisted of microwaving with one of the following buffers: citrate (10 mM, pH 6.0, for 10 minutes), Tris (5 mM, pH 10.0 for 20 minutes), or EDTA (1 mM pH 8.0 for 15 minutes)
Double immunohistologic labeling for BCL6 and HGAL proteins was accomplished using the Dako EnVision Doublestain System according to the manufacturer's specifications. Paraffin-embedded tonsil sections of 4 μm were used. After antigen retrieval by microwaving in Tris (tris(hydroxymethyl)aminomethane) buffer (DakoCytomation Target Retrieval Solution), and endogenous peroxidase blockade with 0.03% hydrogen peroxide, the slides were incubated with the first primary antibody, BCL6, at a dilution of 1:10. The slide was rinsed in TBST buffer (Tris-buffered saline with Tween 20, pH 9.0) and incubated with the secondary antibody (labeled polymer-HRP) and liquid diaminobenzidine (DAB) plus chromogen (brown stain). Slides were rinsed in distilled water and incubated with the Doublestain Block reagent followed by incubation with the second primary antibody, HGAL, at a dilution of 1:25. Secondary antibody (labeled polymer-alkaline phosphatase [AP]) with the fast red chromogen (red stain) was next applied. The slides were cover-slipped with an aqueous-based mounting medium (Dako Paramount Aqueous Mounting Medium). A similar method was used for double immunohistologic labeling for HGAL and CD10 proteins. Antigen retrieval was performed by microwaving in EDTA/Tris buffer (20 mM EDTA [ethylenediaminetetraacetic acid]/50 mM Tris, pH 9.0). The first primary antibody CD10 was used at a dilution of 1:20, followed by incubation with labeled polymer-HRP and DAB plus chromogen (brown stain). This step was then followed by incubation with second primary antibody HGAL at a dilution of 1:25 and the secondary antibody labeled polymer-AP with fast red chromogen (red stain).
All hematoxylin-and-eosin and immunohistochemistry images were analyzed using a Nikon Eclipse E1000M microscope (Nikon, Burlingame, CA) equipped with 4×, 10×, 20×, 40×, and 60× objective lenses with numerical apertures ranging from 0.05 to 0.90. Images were photographed with a SPOT RT color camera (Diagnostic Instruments, Sterling Heights, MI) using Ektachrome 100 Plus EPP 135-136 film (Eastman-Kodak, Rochester, NY). The resulting photographs were acquired using a Nikon Superscan 4000ED scanner, and digitized images were processed using Adobe Photoshop CS (Adobe Systems, San Jose, CA).
Large-scale representation of immunohistologic data and hierarchical cluster analysis
The stained lymphoma TMA slides were scanned and stored as high-resolution images using an automated scanner (Bacus Laboratories, Slide Scanner (BLISS) http://www.bacuslabs.com). A total of 1124 TMA images generated from the current study are displayed on the following Web site: http://tma.stanford.edu/tma_portal/hgal. The staining results of 5 antibodies (HGAL, BCL6, CD10, MUM1/IRF4, and BCL2) performed on 151 DLBCL cases were assigned numeric scores based on the following scoring scale: staining in more than 30% of lymphoma cells was scored positive and given the numerical score “2”; lack of staining in more than 30% of lymphoma cells was scored negative and given the numerical score “0.” Stains that were not interpretable due to absence of diagnostic tissue in the core or due to loss of the core during processing were scored equivocal and given the numerical score “1.” The “Deconvoluter” algorithm (custom WBS macro; Excel, Microsoft, Seattle, WA) with appropriate layout for use in the Cluster software12 to allow for hierarchical clustering was used to integrate all immunohistologic staining results using a previously described methodology (http://genome-www.stanford.edu/TMA/).9 The staining data were clustered in such a way that antibodies that reacted most similar to each other across the lymphoma samples were grouped together on one branch of the dendrogram, whereas antibodies with dissimilar reactivity grouped together on a separate branch. Positive staining is represented as red, lack of staining as green, and noninterpretable staining as white.
Results
Subcellular localization of HGAL protein and specificity of anti-HGAL antibody
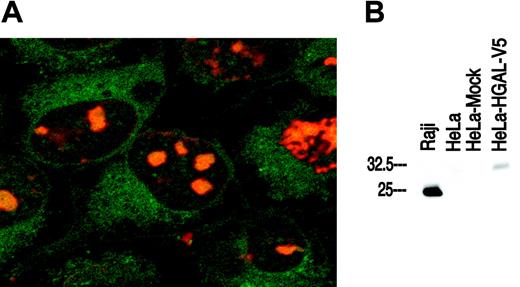
The subcellular localization of the HGAL protein was analyzed in HeLa cells stably transfected with pcDNA3.1 HGAL-V5. Immunofluorescence assay with FITC-conjugated anti-V5 antibody showed that the HGAL protein is localized to the cytoplasm (Figure 1A). No FITC signal was observed in the control nontransfected HeLa cells. Immunofluorescence microscopy with anti-HGAL antibody applied to Raji and SU-DHL4 lymphoma cells fixed on slides following cytospin preparation also demonstrated cytoplasmic localization of HGAL (data not shown). Western blot analysis using the anti-HGAL monoclonal antibody showed expression of a specific band corresponding to the HGAL protein in Raji cells (positive control) and in HeLa cells stably transfected with pcDNA3.1 HGAL-V5 but not in nontransfected or mock-transfected HeLa cells (Figure 1B). The molecular weight of the HGAL-V5 fusion protein is higher than the native HGAL protein in Raji cells.
Correlation of HGAL mRNA and protein expression
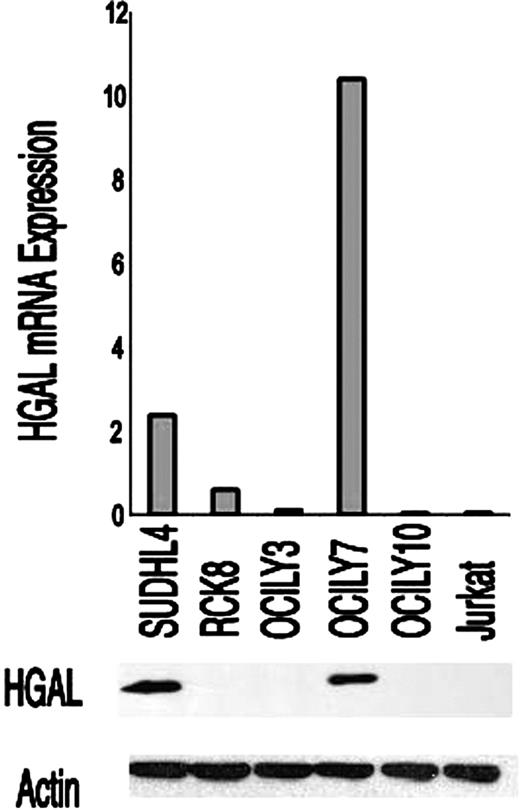
To evaluate the relationship between HGAL mRNA and protein expression, we measured HGAL mRNA by quantitative real-time RT-PCR and HGAL protein expression by Western blot analysis in 6 lymphoma cell lines (SU-DHL4, OCI-LY3, OCI-LY7, OCI-LY10, RCK8, and Jurkat). HGAL mRNA expression was high in the 2 GCB-like DLBCL cell lines (SUDHL4 and OCILY7) and was very low in the remaining cell lines. Similarly, we found HGAL protein expression only in the GCB-like SUDHL4 and OCILY7 cell lines and not in the other cell lines tested (Figure 2). Next, we assessed HGAL mRNA and protein expression in 17 patients with DLBCL in whom both frozen and paraffin tumor specimens were available. An excellent correlation between HGAL mRNA and protein expression was detected in 15 of the 17 specimens (data not shown).
HGAL protein expression in normal lymphoid tissue
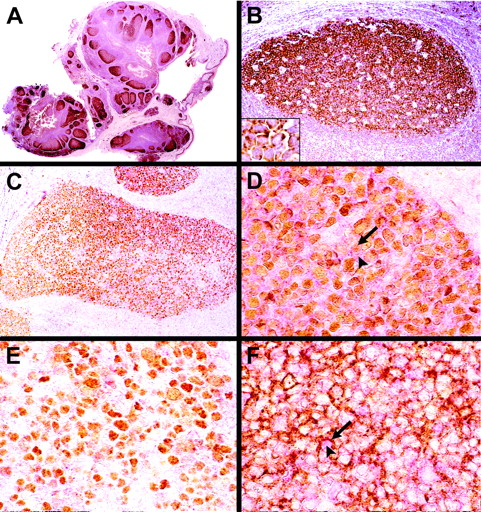
Immunohistochemical staining using the HGAL monoclonal antibody showed that the HGAL protein is specifically expressed in lymphocytes within the GC in normal tonsils and lymph nodes. The staining was localized to the cytoplasm and showed increased intensity within cells of the proliferating pole or dark zone of GCs. Mantle and marginal zones as well as interfollicular and paracortical regions lacked staining (Figure 3A-B). HGAL was also expressed in normal thymus and was predominantly localized to a subset of cells within the medulla with a few scattered cells within the cortex. To assess colocalization of the HGAL protein with 2 other proteins expressed in the GC, BCL6 and CD10, sections of paraffin-embedded tonsil tissue were double stained with antibodies directed against BCL6 and HGAL, and separately with CD10 and HGAL. These studies show that most BCL6+ cells coexpress HGAL, although several BCL6+ cells, especially in the proliferative poles of GCs, lacked staining for HGAL (Figure 3C-E). Similarly, most CD10+ GC cells also showed coexpression of the HGAL protein; however, cells staining for only HGAL or CD10 were also present (Figure 3F).
Localization of HGAL protein and specificity of anti-HGAL monoclonal antibody. (A) Confocal immunofluorescence microscopy using a FITC-conjugated anti-V5 antibody in transfected HeLa cells shows that the HGAL protein is localized to the cytoplasm (green fluorescence). The nuclei are counterstained with an orange Hoechst dye (original magnification, ×1000). (B) Western blot analysis using the anti-HGAL monoclonal antibody shows a specific band corresponding to HGAL protein expression in Raji cells (positive control) and in HeLa cells stably transfected with pcDNA3.1 HGAL-V5 but not in native or mock-transfected HeLa cells.
Localization of HGAL protein and specificity of anti-HGAL monoclonal antibody. (A) Confocal immunofluorescence microscopy using a FITC-conjugated anti-V5 antibody in transfected HeLa cells shows that the HGAL protein is localized to the cytoplasm (green fluorescence). The nuclei are counterstained with an orange Hoechst dye (original magnification, ×1000). (B) Western blot analysis using the anti-HGAL monoclonal antibody shows a specific band corresponding to HGAL protein expression in Raji cells (positive control) and in HeLa cells stably transfected with pcDNA3.1 HGAL-V5 but not in native or mock-transfected HeLa cells.
Correlation between HGAL mRNA and protein expression. HGAL mRNA and protein expression were assessed by real-time RT-PCR and Western blot using the anti-HGAL antibody, respectively, in GC-like (SU-DHL-4, OCI-LY7) and in non–GC-like (RCK8, OCI-LY3, OCI-LY10) DLBCL cell lines and in the Jurkat T-cell line. Actin was used as a loading control.
Correlation between HGAL mRNA and protein expression. HGAL mRNA and protein expression were assessed by real-time RT-PCR and Western blot using the anti-HGAL antibody, respectively, in GC-like (SU-DHL-4, OCI-LY7) and in non–GC-like (RCK8, OCI-LY3, OCI-LY10) DLBCL cell lines and in the Jurkat T-cell line. Actin was used as a loading control.
Immunohistochemical staining with anti-HGAL monoclonal antibody in tonsil tissues. (A-B) GCs are highlighted in a normal tonsil; an increased intensity of staining is seen within cells of the proliferating pole or dark zone of GCs, whereas mantle and marginal zones lack staining. Inset shows a higher magnification of HGAL staining in germinal-center cells. (C) Double immunohistologic labeling for HGAL (red cytoplasmic stain) and BCL6 (brown nuclear stain) are colocalized in a significant number of cells within the GC. (D) A subset of GC cells shows coexpression of BCL6 (arrow) and HGAL (arrowhead). (E) A subset of GC cells located in the proliferative pole of the GC shows staining for BCL6 but not for HGAL. (F) Double immunohistologic labeling for HGAL (red cytoplasmic stain, arrowhead) and CD10 (brown membrane stain, arrow) show that a majority of GC cells coexpress these 2 proteins although cells expressing only HGAL or CD10 are also present. Original magnifications: A, ×30; B and C, ×150; D-F and B inset, ×600.
Immunohistochemical staining with anti-HGAL monoclonal antibody in tonsil tissues. (A-B) GCs are highlighted in a normal tonsil; an increased intensity of staining is seen within cells of the proliferating pole or dark zone of GCs, whereas mantle and marginal zones lack staining. Inset shows a higher magnification of HGAL staining in germinal-center cells. (C) Double immunohistologic labeling for HGAL (red cytoplasmic stain) and BCL6 (brown nuclear stain) are colocalized in a significant number of cells within the GC. (D) A subset of GC cells shows coexpression of BCL6 (arrow) and HGAL (arrowhead). (E) A subset of GC cells located in the proliferative pole of the GC shows staining for BCL6 but not for HGAL. (F) Double immunohistologic labeling for HGAL (red cytoplasmic stain, arrowhead) and CD10 (brown membrane stain, arrow) show that a majority of GC cells coexpress these 2 proteins although cells expressing only HGAL or CD10 are also present. Original magnifications: A, ×30; B and C, ×150; D-F and B inset, ×600.
HGAL protein expression in lymphomas
The lymphoma subtypes tested and the immunohistologic staining results are listed in Table 2. Digital images of all original HGAL-stained TMA cores of the non-Hodgkin and Hodgkin lymphomas and plasma cell neoplasms tested in this paper are shown on the following Web site: http://tma.stanford.edu/tma_portal/hgal.
Immunohistologic analysis of HGAL protein expression in lymphoma subtypes
Lymphoma subtype . | Total positive* . | Percent positive . |
|---|---|---|
| B-cell lymphoma, N = 384 | ||
| Precursor B-lymphoblastic lymphoma | 1/3 | 33 |
| Follicular lymphoma | ||
| Grade 1 | 23/24 | 96 |
| Grade 2 | 21/21 | 100 |
| Grade 3 | 59/62 | 95 |
| Diffuse large B-cell lymphoma | 103/151 | 68 |
| Mediastinal large B-cell lymphoma | 7/8 | 87 |
| Burkitt lymphoma | 40/40 | 100 |
| Extranodal marginal zone lymphoma | 0/22 | 0 |
| Splenic marginal zone lymphoma | 1/8 | 12 |
| Nodal marginal zone lymphoma | 1/18 | 6 |
| Mantle cell lymphoma | 0/7 | 0 |
| Small lymphocytic lymphoma/CLL | 0/8 | 0 |
| Lymphoplasmacytic lymphoma | 0/12 | 0 |
| T-cell lymphoma, N = 24 | ||
| Precursor T-lymphoblastic lymphoma | 0/5 | 0 |
| Peripheral T-cell lymphoma | 0/10 | 0 |
| Anaplastic large cell lymphoma | 0/5 | 0 |
| Nasal-type NK lymphoma | 0/4 | 0 |
| Plasma cell neoplasms, N = 195 | ||
| Multiple myeloma | 2/167 | 1 |
| Plasma cell leukemia | 2/15 | 13 |
| Plasmacytoma | 0/3 | 0 |
| Monoclonal gammopathy | 0/10 | 0 |
| Hodgkin lymphoma, N = 124 | ||
| Lymphocyte predominant | 12/17 | 70 |
| Classical Hodgkin | 78/107 | 73 |
| Nodular sclerosis | 50/77 | 65 |
| Mixed cellularity | 13/22 | 59 |
Lymphoma subtype . | Total positive* . | Percent positive . |
|---|---|---|
| B-cell lymphoma, N = 384 | ||
| Precursor B-lymphoblastic lymphoma | 1/3 | 33 |
| Follicular lymphoma | ||
| Grade 1 | 23/24 | 96 |
| Grade 2 | 21/21 | 100 |
| Grade 3 | 59/62 | 95 |
| Diffuse large B-cell lymphoma | 103/151 | 68 |
| Mediastinal large B-cell lymphoma | 7/8 | 87 |
| Burkitt lymphoma | 40/40 | 100 |
| Extranodal marginal zone lymphoma | 0/22 | 0 |
| Splenic marginal zone lymphoma | 1/8 | 12 |
| Nodal marginal zone lymphoma | 1/18 | 6 |
| Mantle cell lymphoma | 0/7 | 0 |
| Small lymphocytic lymphoma/CLL | 0/8 | 0 |
| Lymphoplasmacytic lymphoma | 0/12 | 0 |
| T-cell lymphoma, N = 24 | ||
| Precursor T-lymphoblastic lymphoma | 0/5 | 0 |
| Peripheral T-cell lymphoma | 0/10 | 0 |
| Anaplastic large cell lymphoma | 0/5 | 0 |
| Nasal-type NK lymphoma | 0/4 | 0 |
| Plasma cell neoplasms, N = 195 | ||
| Multiple myeloma | 2/167 | 1 |
| Plasma cell leukemia | 2/15 | 13 |
| Plasmacytoma | 0/3 | 0 |
| Monoclonal gammopathy | 0/10 | 0 |
| Hodgkin lymphoma, N = 124 | ||
| Lymphocyte predominant | 12/17 | 70 |
| Classical Hodgkin | 78/107 | 73 |
| Nodular sclerosis | 50/77 | 65 |
| Mixed cellularity | 13/22 | 59 |
Cases were scored positive if > 30% of lymphoma cells stained for HGAL
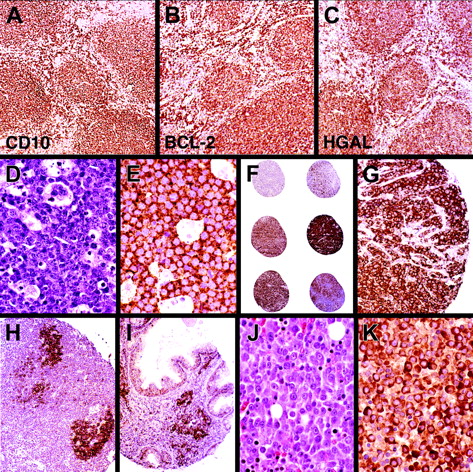
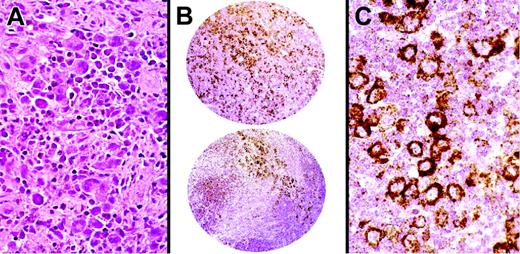
Among 384 B-cell lymphomas, the majority of follicular and mediastinal large B-cell lymphomas and a subset of DLBCLs stained for HGAL. In addition to TMA cores, whole lymph node sections of 5 cases of follicular lymphomas were stained for HGAL, CD10, BCL6, and BCL2 in parallel. The staining pattern for HGAL in follicular lymphomas highlights neoplastic follicles as well as interfollicular neoplastic B cells and is similar to the staining pattern for BCL6, CD10, and BCL2 (Figure 4A-C). All cases of Burkitt lymphomas tested (40 of 40) stained for HGAL (Figure 4D-E). DLBCLs showed a range of staining from strong reactivity in the majority of the lymphoma cells, to staining localized to a subset of lymphoma cells, to absence of staining (Figure 4F-G). Mantle cell and most marginal zone lymphomas lacked staining for HGAL although scoring TMAs especially in cases of extranodal and nodal marginal zone lymphomas was made difficult by entrapped GCs within the infiltrates. Whole sections of 33 cases of marginal zone lymphomas were examined to confirm that all extranodal as well as the majority of nodal and splenic marginal zone lymphomas lacked staining for HGAL (Figure 4H-I). One case each of nodal and splenic marginal zone lymphoma stained for HGAL (Table 2).
A TMA containing 195 plasma cell neoplasms showed rare positive cases including 2 of 167 multiple myelomas and 2 of 15 plasma cell leukemias (Figure 4J-K). These cases uniformly expressed CD138 and did not coexpress CD20, although other plasma cell neoplasms present on this TMA did exhibit CD20 reactivity. The remaining B-cell lymphomas and all T-cell and natural killer (NK) cell lymphomas tested lacked staining for HGAL (Table 2).
A TMA containing 124 Hodgkin lymphomas showed staining for HGAL in 12 of 17 lymphocyte-predominant Hodgkin lymphomas. Interestingly, 78 of 107 classical Hodgkin lymphomas also showed staining for HGAL protein. Subclassification was possible in a subset of these classical Hodgkin lymphomas demonstrating that 50 of 77 nodular sclerosis and 13 of 22 mixed cellularity Hodgkin lymphomas showed staining for HGAL. The staining in all subtypes of Hodgkin lymphomas was localized to the cytoplasm of large atypical cells corresponding to Hodgkin cells (Figure 5).
Comparative immunohistologic studies in DLBCLs
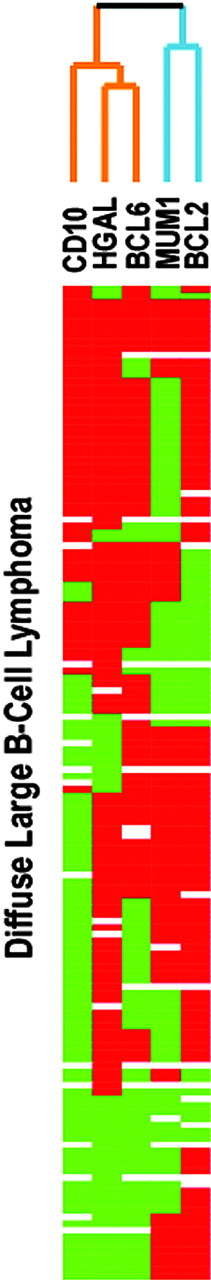
Comparative immunohistologic staining for HGAL and 4 additional antibodies were carried out on 151 cases of DLBCLs where multiple TMA sections were available to perform these studies and the results are summarized in Tables 3 and 4. These 5 antibodies were selected because their corresponding genes had previously been found to contribute to the distinction of prognostic classes of DLBCL in gene expression studies,1,2 and included markers of the GCBL subtype (BCL6, CD10, and HGAL) and the ABL subtype (MUM1 and BCL2). Hierarchical clustering, in a manner similar to that used for gene microarray studies,9 was used to analyze the immunohistologic staining data and the resulting dendrogram is depicted in Figure 6. This dendrogram shows the degree of relatedness between the protein expression patterns detected by the 5 antibodies across the 151 cases of DLBCL, with short branches indicating a high degree of similarity in the staining pattern. The 3 antibodies related to a GC phenotype and whose targets' expression was found to be associated with a better clinical outcome in gene expression studies (HGAL, BCL6, CD10), grouped together on one branch of the dendrogram. On this branch the similarity was closest between HGAL and BCL6 staining. The 2 antibodies associated with an ABL phenotype conferring a worse clinical outcome in gene expression studies (BCL2 and MUM1/IRF4) grouped together on a separate branch.
Immunohistochemical staining with anti-HGAL monoclonal antibody in lymphomas. (A-C) Follicular lymphoma stained with the CD10, BCL2, and anti-HGAL monoclonal antibody highlights neoplastic follicles and interfollicular B cells. (D) Hematoxylin and eosin-stained section of Burkitt lymphoma shows uniform cells admixed with starry sky histiocytes. (E) Burkitt lymphoma cells show staining for HGAL protein. (F) TMA cores of DLBCL show a range of staining from none to weak/partial staining to intense staining of all neoplastic cells. (G) High magnification of a core of a DLBCL shows moderate to strong staining within the cytoplasm. (H-I) Extranodal marginal zone B-cell lymphoma of the lymph node and gastrointestinal tract lacks staining for HGAL although the stain highlights entrapped GCs within the neoplastic infiltrate. (J) Hematoxylin and eosin–stained section of plasma cell myeloma shows sheets of atypical plasma cells with prominent nucleoli. (K) Plasma cell myeloma shows staining for HGAL protein. Original magnifications: A-C, H, and I, ×150; F, ×30; G, ×300; D, E, J, and K, ×600.
Immunohistochemical staining with anti-HGAL monoclonal antibody in lymphomas. (A-C) Follicular lymphoma stained with the CD10, BCL2, and anti-HGAL monoclonal antibody highlights neoplastic follicles and interfollicular B cells. (D) Hematoxylin and eosin-stained section of Burkitt lymphoma shows uniform cells admixed with starry sky histiocytes. (E) Burkitt lymphoma cells show staining for HGAL protein. (F) TMA cores of DLBCL show a range of staining from none to weak/partial staining to intense staining of all neoplastic cells. (G) High magnification of a core of a DLBCL shows moderate to strong staining within the cytoplasm. (H-I) Extranodal marginal zone B-cell lymphoma of the lymph node and gastrointestinal tract lacks staining for HGAL although the stain highlights entrapped GCs within the neoplastic infiltrate. (J) Hematoxylin and eosin–stained section of plasma cell myeloma shows sheets of atypical plasma cells with prominent nucleoli. (K) Plasma cell myeloma shows staining for HGAL protein. Original magnifications: A-C, H, and I, ×150; F, ×30; G, ×300; D, E, J, and K, ×600.
HGAL staining in Hodgkin lymphoma. Immunohistochemical staining for HGAL protein highlights large atypical cells in a case of nodular sclerosis Hodgkin lymphoma on 2.0-mm cores on a TMA. The staining is localized within the cytoplasm of Hodgkin cells. Original magnifications: A and C, ×30; B, ×600.
HGAL staining in Hodgkin lymphoma. Immunohistochemical staining for HGAL protein highlights large atypical cells in a case of nodular sclerosis Hodgkin lymphoma on 2.0-mm cores on a TMA. The staining is localized within the cytoplasm of Hodgkin cells. Original magnifications: A and C, ×30; B, ×600.
Immunohistologic studies in 151 cases of DLBCL
Score . | HGAL . | CD10 . | BCL6 . | MUM1 . | BCL2 . |
|---|---|---|---|---|---|
| Positive | 103 | 54 | 82 | 69 | 101 |
| Negative | 43 | 83 | 57 | 70 | 34 |
| Equivocal | 5 | 14 | 12 | 12 | 16 |
Score . | HGAL . | CD10 . | BCL6 . | MUM1 . | BCL2 . |
|---|---|---|---|---|---|
| Positive | 103 | 54 | 82 | 69 | 101 |
| Negative | 43 | 83 | 57 | 70 | 34 |
| Equivocal | 5 | 14 | 12 | 12 | 16 |
Positive indicates staining in more than 30% of lymphoma cells; negative, lack of staining in more than 30% of lymphoma cells; and equivocal, lack of core for scoring.
Classification by model of Hans
Cannot classify indicates that because the CD10 core was uninformative, the model by Hans et al13 could not be applied to these cases.
NA indicates not applicable.
We used the TMA decision-tree model described by Hans and colleagues to classify our cases into GC and non-GC (NGC) subtypes based on immunohistologic results for CD10, BCL6, and MUM1.13 This model classifies CD10+ and CD10-BCL6+MUM1- cases into the GC subclass. DLBCL cases that exhibit a CD10- BCL6- or a CD10- BCL6+MUM1+ immunophenotype are assigned to the NGC subclass.13 Among the 151 DLBCL cases we analyzed, 68 were classified as belonging to the GC subtype, whereas another 68 were classified as belonging to the NGC subtype. The CD10-stained cores were uninformative in 15 cases and because the decision tree model by Hans et al13 relies on CD10 staining, these cases could not be classified based on this model. Among the 68 GC cases, 61 stained for HGAL and 7 did not. Among 68 NGC cases, 36 stained for HGAL and 32 did not. Thus, a total of 43 of 151 cases showed HGAL staining in a manner dissimilar to what was predicted by this model (Table 4).
Discussion
New markers of GC derivation have become the subject of recent interest due to data from gene expression studies that indicate a link between GC derivation and clinical behavior in DLBCLs.1,2 From these studies, genes/ESTs with high expression in GCs and GC-derived lymphomas have been the focus of subsequent investigations because molecular targets conferring the GC phenotype are likely to be markers of superior outcome in patients with DLBCL. One such gene, HGAL, was found to be a prognostic factor predicting improved survival in a cohort of patients with DLBCL independent from those analyzed in gene expression studies.4 The HGAL gene encodes a 178–amino acid protein lacking a nuclear localization sequence or a transmembrane domain indicating a likely cytoplasmic localization for the HGAL protein. Similar to its mouse homolog M17,6 the presence of a modified immunoreceptor tyrosine-based activation motif also suggested a role for HGAL in B-cell signaling.4
In the current study, we show that the HGAL protein is localized to the cytoplasm as predicted from its sequence analysis.4 We generated a monoclonal antibody to HGAL protein and found that the HGAL protein expression parallels HGAL mRNA expression in cell lines and in 17 cases of DLBCL studied by quantitative RT-PCR analysis.4 Immunohistologic staining shows that HGAL protein is expressed in normal GCs and in GC-derived lymphomas in a survey of normal lymphoid tissue and 727 human lymphomas. Similar to HGAL mRNA, HGAL protein was found in follicular lymphoma, Burkitt lymphoma, lymphocyte-predominant Hodgkin lymphoma, and a subset of DLBCL. The expression of HGAL mRNA4 and protein in the normal thymus suggested that mediastinal large B-cell lymphoma considered to be of thymic B-cell origin was likely to express HGAL protein. We found that 7 of 8 cases tested in this study stained for HGAL. Interestingly, the single case that did not stain for HGAL also lacked staining for BCL6 and CD10. Because we analyzed large numbers of cases, the few HGAL+ non–GC-derived lymphomas that stained for HGAL attests to the remarkable GC specificity of HGAL protein expression. A small subset of lymphomas not considered to be of GC derivation also showed staining for HGAL and included one precursor B-acute lymphoblastic lymphoma, one splenic and one nodal marginal zone lymphoma, and 4 plasma cell neoplasms. This expression pattern may be due to aberrant antigenic expression not uncommonly found in neoplastic states and although a normal counterpart is postulated for many lymphomas, antigenic expression shows significant overlap among different types of lymphomas.11,14 The GC specificity of HGAL protein expression is thus amenable for exploitation in the diagnosis of certain lymphoma subtypes. For example, the lack of staining for HGAL in extranodal and the majority of nodal marginal zone lymphomas may be useful in differentiating marginal zone lymphomas from follicular lymphomas. This distinction is particularly relevant in cases where follicular colonization by marginal zone lymphoma cells and disruption of the GC make separating these entities from one another and from reactive proliferations by routine immunohistologic staining difficult. Although BCL6 protein expression is a useful marker in this setting, staining for BCL6 by a subset of T cells often complicates interpretation especially because reactive proliferations may have increased numbers of T cells. The lack of staining for HGAL by reactive T cells engenders a situation where neoplastic GC versus non-GC cells may be better separated from one another in a background devoid of T-cell staining. Furthermore, because nodal marginal zone lymphomas are thought to arise from histogenetically heterogeneous subgroups of marginal zone cells,15 it is not surprising that a subset of these lymphomas express GC markers such as BCL6 and HGAL. However, together with morphologic features and other differentially expressed immunohistologic markers such as CD10 and coexpression of CD43, HGAL staining is likely to be useful in separating follicular from marginal zone lymphomas.
The lymphomas of GC derivation incorporate entities as diverse as follicular lymphoma, Burkitt lymphoma, and lymphocyte-predominant Hodgkin lymphoma. Although evidence indicates that lymphocyte-predominant Hodgkin lymphoma is derived from highly mutated GC B cells,16,17 classical Hodgkin lymphoma shows significant differences in gene expression, transcriptional regulation, and intracellular signaling from lymphocyte-predominant Hodgkin lymphoma and from other B-cell lymphomas.18-21 Recent gene expression profiling studies have also shown that there is drastic loss of B lineage-specific gene expression in classical Hodgkin lymphoma.22 Our finding that HGAL protein is expressed in a subset of classical Hodgkin lymphoma suggests that there are exceptions to this extensive down-regulation of B lineage-specific genes and that HGAL expression supports the hypothesis that at least a subgroup of classical Hodgkin lymphoma may be derived from GC B cells. Moreover, it is likely that the GC incorporates subsets of cells with a range of gene and protein expression patterns that allow for more than one “germinal center profile” to be ascribed to the GC.
Hierarchical clustering of protein expression data. The expression patterns of 5 proteins, HGAL, CD10, BCL6, MUM1/IRF4 (MUM1), and BCL2, predicted to be associated with distinct subtypes of DLBCL based on the cell of origin by gene expression profiling studies are shown. Positive staining is indicated as red and the lack of staining as green. Stains that were not interpretable are indicated in white. The branching pattern of the dendrogram reflects similarities in the patterns of reactivity of the antibodies, with short branches denoting a high degree of similarity in expression pattern of the cognate protein across 151 DLBCL cases. HGAL clusters with BCL6 and CD10, associated with a GC phenotype, on one branch of the dendrogram (indicated in orange), whereas MUM1 and BCL2, associated with an ABL phenotype, cluster on a separate branch (indicated in blue).
Hierarchical clustering of protein expression data. The expression patterns of 5 proteins, HGAL, CD10, BCL6, MUM1/IRF4 (MUM1), and BCL2, predicted to be associated with distinct subtypes of DLBCL based on the cell of origin by gene expression profiling studies are shown. Positive staining is indicated as red and the lack of staining as green. Stains that were not interpretable are indicated in white. The branching pattern of the dendrogram reflects similarities in the patterns of reactivity of the antibodies, with short branches denoting a high degree of similarity in expression pattern of the cognate protein across 151 DLBCL cases. HGAL clusters with BCL6 and CD10, associated with a GC phenotype, on one branch of the dendrogram (indicated in orange), whereas MUM1 and BCL2, associated with an ABL phenotype, cluster on a separate branch (indicated in blue).
Predictive models of survival in DLBCL using clinical outcome data to supervise the discovery of genes resulted in the identification of non-overlapping candidate genes by 2 groups.2,3 These differences, attributed to differences in the types of gene arrays and the analysis platforms used in these investigations, underscore the complexity of outcome prediction in an extremely heterogeneous disease. Although gene expression signatures can be used to predict the prognosis in patients with DLBCL, the prohibitive expense and technical constraints have limited the utility of this genome-scale methodology. A recent quantitative real-time PCR study of 36 genes whose expression had been reported to predict survival in DLBCL was undertaken in 66 patients with DLBCL.23 The expression of each of these genes in samples of lymphoma was correlated to overall survival independent of the IPI. The following genes were the strongest predictors: LMO2, BCL6, FN1, CCND2, SCYA3, and BCL2. These 6 genes were used to develop a multivariate model that could predict survival in patients with DLBCL.23 Previous gene expression analyses had identified HGAL as a strong predictor of outcome in patients with DLBCL,1,2 which was confirmed by the recent RT-PCR analysis of the candidate 36 genes.23 This latter study showed that HGAL mRNA was one of the top 10 candidate molecules predicting improved survival in patients with DLBCL, although only the strongest 6 genes were included in the gene predictive model due to the need for simplicity and clinical utility of the model.23 An additional consideration is the regulation of HGAL by another GC marker, BCL6, which may result in the abrogation of any independent predictive power asserted by HGAL mRNA expression alone. Support for this hypothesis comes from BCL6-immortalized p53-deficient mouse cell lines that exhibit a GC phenotype in culture. These cells express M17, the mouse homolog of HGAL, although freshly isolated B cells do not, indicating that BCL6, a postulated transcriptional repressor, activates GC-specific genes by repression of B-cell differentiation.24 Although models based on mRNA expression may be useful for prediction of prognosis in patients with DLBCL, examination of mRNA expression requires fresh or frozen tissue samples, which limits the applicability of this method in routine practice in the community.
Several recent studies have focused on the analysis of protein expression of select markers by immunohistologic studies in patients with DLBCL, in an effort to define immunophenotypic profiles that better identify risk groups. This approach is worth-while as illustrated in a report of protein products of genes involved in chromosomal alterations in lymphoma and leukemia where immunohistochemistry was necessary to discern differences not appreciable or amenable for study by mRNA expression alone.25 Protein expression studies in DLBCL, however, have yielded conflicting results.26-29 Lastly, a study comparing immunohistochemical data from 142 DLBCLs previously analyzed by gene expression profiling found that BCL6 or CD10 protein expression conferred a superior survival similar to data from cDNA microarrays.13 Our selection of target proteins was based on findings from gene microarrays,1,2 and included HGAL and 4 others that were identified as contributing to subclassification of DLBCLs in the gene microarray studies. Hierarchical cluster analysis of the staining results obtained with the 5 antibodies showed that antibodies directed against genes conferring a GCBL phenotype (HGAL, CD10, and BCL6) clustered together on one branch of the dendrogram, whereas the antibodies directed against genes conferring the ABL phenotype (MUM-1 and BCL2) clustered together on a separate branch. In addition, this dendrogram shows that HGAL protein expression was most closely related to BCL6 protein expression. The segregation of HGAL protein expression on a branch of the dendrogram with BCL6 and CD10 attests to the similarity of HGAL protein expression with markers characteristic of the GCBL phenotype, which is clearly distinct from the expression of the cognate proteins of genes associated with an ABL phenotype. To correlate our findings with those of Hans et al,13 we used the immunohistologic TMA decision tree described in that report to classify our cases into GC and non-GC categories based on CD10, BCL6, and MUM1 staining, with the expectation that the cases assigned to the GCB subtype would show HGAL staining, whereas the non-GC cases would lack HGAL staining. However, among the 151 DLBCL cases we studied, 43 cases showed a different HGAL staining result than was expected from the model. These findings suggest that assigning GC derivation based on the expression of a few proteins may not necessarily allow accurate classification of DLBCL cases into GC and non-GC categories and that the robustness of this subclassification may improve with the addition of markers such as HGAL. Collectively, although investigations from several institutions, including our own, have rigorously explored the clinicopathologic and molecular diversity of DLBCL, there is presently no consensus as to which model best describes the heterogeneity of DLBCL or offers the greatest clinical utility.
In conclusion, our studies using the anti-HGAL monoclonal antibody we generated indicate that the HGAL protein and mRNA expression show correlation in that they are both expressed in GCs and predominantly in GC-derived lymphomas. The correlation between HGAL mRNA and protein expression suggests that HGAL protein expression may be the preferred method of analysis because of its accessibility in routine clinical practice. A larger cohort of patients from multiple institutions carefully controlled for clinical parameters and therapeutic interventions are necessary for further study and rigorous statistical analysis to dissect whether HGAL protein expression may function as a robust marker in predicting outcome in patients with DLBCL. Given the recent data that the addition of the anti-CD20 monoclonal antibody rituximab to CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) chemotherapy for DLBCL may abrogate the predictive power of markers such as BCL230 and BCL6,31 it is of particular importance to examine HGAL protein expression in patients carefully controlled for this and other differences in treatment modalities.
Prepublished online as Blood First Edition Paper, January 27, 2005; DOI 10.1182/blood-2004-08-3112.
Supported by grant CA 34233 from the National Institutes of Health, Bethesda, MD.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Robert Marinelli, Department of Biochemistry, Stanford University School of Medicine, for creating the Web portal containing the HGAL images from tissue microarray staining data linked to this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal