Abstract
We have engineered an anti-CD20–interleukin 2 (IL-2) immunocytokine (ICK) based on the Leu16 anti-CD20 antibody and have deimmunized both the variable (V) regions as well as the junction between the heavy (H) chain constant region and IL-2. Mutations were made to remove potential T-cell epitopes identified by in silico binding to major histocompatibility complex (MHC) class II molecules. The resulting immunocytokine, DI-Leu16-IL-2, retained full anti-CD20 activity as assessed by fluorescence-activated cell-sorting (FACS) analysis, and had enhanced antibody-dependent cellular cytotoxicity (ADCC) effector function relative to the DI-Leu16 antibody or control anti-CD20 antibody (rituximab). In a severe combined immunodeficient (SCID) mouse model of disseminated, residual lymphoma, anti-CD20–IL-2 immunocytokines based on Leu16 were far more effective at a dose of 0.25 mg/kg than anti-CD20 antibody given at 25/mg/kg, despite a shorter half-life of the ICK. Anti-CD20–IL-2 was also far more effective than a control ICK targeted to an antigen with greatly reduced expression on Daudi tumor cells, or various combinations of anti-CD20 antibodies and IL-2. Antitumor activity of DI-Leu16-IL-2 was shown to partially but not entirely depend on Fc receptor (R) binding, suggesting that ADCC and targeting of IL-2 both play roles in the mechanism of tumor clearance. Based on these animal models, DI-Leu16-IL-2 could offer therapeutic potential for patients with CD20 positive lymphoma. Clinical trials are currently under development.
Introduction
Non-Hodgkin lymphomas (NHLs) continue to present a significant clinical challenge in cancer management. Approximately 55 000 new cases are diagnosed in the United States each year and NHLs rank fifth overall in cancer incidence and mortality. Standard therapies include single-agent combination chemotherapy with or without stem cell transplantation and radiation therapy. High-dose chemotherapy with autologous or allogeneic hematopoietic stem cell transplantation is primarily reserved for patients with multiple relapsed disease. The majority of NHLs are of B-cell origin and more than 90% express the leukocyte antigen CD20, making these malignancies an attractive target for antibody therapy. The CD20 antigen is an integral transmembrane protein expressed by cells in the B lineage from B-cell precursors through mature B cells, but not plasma cells.1 This 33-37 kDa phosphoprotein, thought to play a role in calcium conductance, is not internalized, downmodulated, or shed, making it an ideal target for exogenous antibody-based therapies.2 Unmodified anti-CD20 murine or chimeric antibodies have been demonstrated to be effective in inducing regression of B-cell lymphomas.3 The chimeric anti-CD20 antibody, C2B8 (Rituxan [rituximab]; Biogen, Cambridge, MA), has been extensively evaluated in patients with recurrent B-cell lymphoma,4-6 leading to Federal Drug Administration (FDA) licensure. In the multi-institutional pivotal phase 2 trial of 166 patients with relapsed follicular lymphoma (FL), an overall response rate was 48%, with 6% complete response. The median time to progression for responders was 13 months. The treatment was well tolerated and most common adverse events were fever, chills, and headache related to the first infusion.7
Although the clinical efficacy of C2B8 allowed rapid approval, the mechanism of action is still not well understood.8 Multiple hypotheses have been generated: induction of apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), phagocytosis, complement-mediated cytotoxicity, and cross-priming of CD8+ cytotoxic T cells.9,10 It is interesting to note in this context that combinations of rituximab with interleukin-2 (IL-2), a potent stimulator of both T cells and natural killer (NK) cells mediating ADCC, have shown promising results in both preclinical models and in the clinic.11,12
Despite the success of anti-CD20 naked antibody therapy, most patients eventually relapse. Radioimmune therapy directed against CD20 (Zevalin [tositumomab]; Corixa, Seattle, WA, and Glaxo-SmithKline, Middlesex, United Kingdom; and Bexxar [ibritumomab tituxetan]; Biogen) is one approach to improving the clinical outcome in the relapse setting following rituximab therapy. Still, other alternative approaches to augment the activity of antibody-based therapies should be considered, especially ones that do not result in bone marrow suppression as is seen with radioimmune therapy. One potential approach is the use of anti-CD20 antibodies to deliver cytokines to the tumor microenvironment. Tumor-targeted antibody-cytokine fusion proteins (immunocytokines [ICKs]) have potent antitumor activity in several preclinical tumor models, especially in the setting of minimal residual disease.13 In all cases examined, IL-2–based immunocytokines have shown far better antitumor activity than the combination of antibody and IL-2, and successful treatment in immune-competent mice usually leads to long-term resistance to subsequent tumor challenge. Two IL-2–based immunocytokines are currently in early-stage clinical development for treatment of epithelial-14 or neuroectodermal-derived cancers15 and have shown potent in vivo biological activity over a wide dose range. In some cases, patients treated in the minimal residual disease setting have remained disease-free for extended periods of time after only 2 courses of treatment.15
The strong preclinical data and preliminary clinical results with other IL-2–based immunocytokines have encouraged us to apply this technology to the lymphoma setting where a minimal residual disease setting is possible in a large majority of patients following C2B8 therapy. Specifically, we have genetically engineered the variable (V) regions of the mouse Leu16 anti-CD2016 antibody, which binds to the same Ala-X-Pro motif as rituximab (and several other anti-CD20 antibodies) and causes distribution of CD20 into the Triton X–insoluble fraction of lipid rafts, but does not cause homotypic cell aggregation.17 The antibody was humanized by first removing potential helper T-cell epitopes (deimmunization) and second, by combining these V regions with human light (L) chain and heavy (H) chain constant regions. The H chain is fused genetically at the C terminus to the mature sequence of human IL-2, resulting in a whole antibody-IL-2 fusion protein designated DI-Leu16-IL-2. In these studies we show that we have preserved the binding of the deimmunized V regions and characterize both in vitro and in vivo antitumor activity against the human Daudi Burkitt lymphoma in a severe combined immmunodeficient (SCID) mouse model of disseminated disease. Results show that this approach is more effective than treatment with much higher doses of naked anti-CD20 antibody, combinations of antibody and free IL-2, or a control, nontargeted immunocytokine.
Materials and methods
Animals and cell lines
BALB/c and BALB/c SCID/SCID mice (6 to 8 weeks old) were purchased from Taconic Farms (Germantown, NY). The NS/0 cell line was obtained from the European Collection of Animal Cell Cultures (ECACC, Salisbury, United Kingdom). The human Daudi Burkitt lymphoma line was obtained from the American Type Culture Collection (Rockville, MD).
Recombinant antibodies and fusion proteins
The V regions of the murine Leu16 antibody were constructed by ligation of oligonucleotide duplex synthesized according to the published sequences.16 The V region sequences of both the H and L chain were deimmunized by Biovation (Aberdeen, Scotland, United Kingdom) according to their proprietary peptide-threading technology. Briefly, sequences were analyzed in silico as consecutive potential 13-mer peptides for their binding affinity to human class II major histocompatibility complex (MHC) molecules. Peptides predicted to have a high affinity were modified in their anchor residues using conservative amino acid substitutions in order to reduce the affinity to acceptable levels. Modified V regions were tested for antigen-binding activity by immunofluorescent staining and fluorescence-activated cell-sorting (FACS) analysis of CD20+ Daudi lymphoma cells.
Recombinant antibodies were constructed by inserting the mouse or deimmunized V regions into a vector containing both the H and L chain human constant regions, essentially as described previously.18 IL-2-based immunocytokines were constructed by inserting the V regions into a vector containing the human L chain constant region and the human H chain constant regions-IL-2 fusion, as described earlier.19
Both antibodies and immunocytokines were expressed in NS/0 cells by transfection and selection of producer cell clones as described.19 Proteins were purified by binding to and elution from protein A Sepharose, followed by diafiltration into phosphate-buffered saline (PBS).
Antigen binding assay
About 1 × 106 Daudi lymphoma cells were incubated with varying concentrations of antibodies and immunocytokines in a final volume of 0.2 mL. After incubation on ice for 30 minutes, cells were washed twice with PBS–bovine serum albumin (BSA), and a fluorescein isothiocyanate (FITC)–conjugated antihuman immunoglobulin G (IgG) Fc antibody (Ab) F(ab′)2 (Jackson ImmunoResearch, West Grove, PA) was added. Incubation was continued for an additional 30 minutes on ice. After 2 washes with PBS-BSA, the cells were analyzed by flow cytometry. A negative control immunocytokine, 425-IL-2, with specificity for epidermal growth factor receptor (EGFR)22 expressed at only low levels on these cells, was analyzed in the same manner and compared with DI-Leu16-IL-2.
IL-2 bioactivity
The IL-2 activity of the immunocytokine was assayed using 3 different cell lines. The standard T-cell proliferation assay using the mouse CTLL-2 cell line expressing the high-affinity IL-2 receptor was performed as described.21 In the second assay, TF-1β (provided by Paul Sondel, University of Wisconsin) was used. The TF-1β cell line is a human immature precursor erythroid TF-1 cell line transfected to express the human IL-2 receptor beta chain.23 Briefly, washed TF-1β cells in log phase (10 000 cells/well) were incubated with IL-2 for 32 hours. 3H-thymidine was then added and the incubation continued for an additional 16 hours. The cells were then harvested from the wells with water onto glass microfiber filter plates and radioactivity was measured by liquid scintillation counting. In the third assay, Kit 225 (K6), a human adult T-cell lymphoma cell line expressing both the high- and intermediate-affinity IL-2 receptors (provided by Angus Grant, EMD Pharmaceuticals, Durham, NC) was used.24 Briefly, washed Kit-225 cells that had been starved for 3 days in serum-free AIM-V medium (Life Technologies, Rockville, MD, no. 12055-091; 15 000 cells/well) were incubated with IL-2 for 32 hours. 3H-thymidine was then added and the incubation continued for an additional 16 hours. The cells were harvested and radioactivity measured as described for the assay using TF-1β cells. An effective dose causing 50% inhibition (ED50) value for each protein with respect to cell proliferation was obtained from plotting a dose response curve and identifying the protein concentration that resulted in half-maximal response.
Pharmacokinetic analysis
BALB/c mice were injected with 25 μg of an immunocytokine in a volume of 0.2 mL in the tail vein using a slow push. At various time points, small blood samples were taken by retro-orbital bleeding and collected in tubes coated with heparin to prevent clotting. After centrifugation to remove the cells, the plasma was assayed by capture with anti–human IgG H and L chains antisera and detection with an anti–human IL-2 antibody. Results were normalized to the initial concentration in the serum of each mouse taken immediately after injection.
Effector activity of parental antibodies and IL-2 immunocytokines
ADCC was determined in a 4-hour assay using 51Cr-labeled Daudi target cells and human peripheral blood mononuclear cells (PBMCs) as effectors (E/T = 100).25 For determination of complement-dependent cytotoxicity (CDC) activity, 51Cr-labeled Daudi cells were incubated for 1 hour with human plasma (diluted 1 in 8) as a source of complement. Percentage of specific lysis was calculated by subtracting the background radioactivity from the experimental values, dividing by the total releasable radioactivity obtained by detergent lysis, and multiplying by 100.
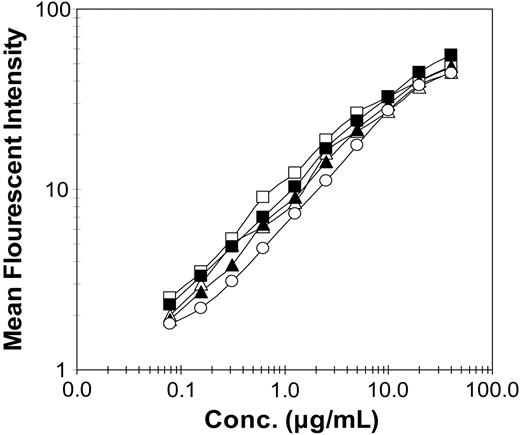
Antigen binding activity of recombinant antibodies and immunocytokines. Relative binding of Daudi cell-surface antigen was measured by FACS analysis following incubation with varying concentrations of recombinant proteins. Proteins included DI-Leu16-IL-2 (▪), DI-Leu16 antibody (□), chLeu16-IL-2 (▴), chLeu16 antibody (▵), and enzymatically deglycosylated DI-Leu16-IL-2 (○).
Antigen binding activity of recombinant antibodies and immunocytokines. Relative binding of Daudi cell-surface antigen was measured by FACS analysis following incubation with varying concentrations of recombinant proteins. Proteins included DI-Leu16-IL-2 (▪), DI-Leu16 antibody (□), chLeu16-IL-2 (▴), chLeu16 antibody (▵), and enzymatically deglycosylated DI-Leu16-IL-2 (○).
Tumor models
SCID mice (9 animals per group) were injected intravenously with 5 × 106 CD20+ Daudi lymphoma cells (day 0) followed by intravenous injection of immunocytokines (5 daily doses of 5 or 20 μg) or control antibody (500 μg every other day for a total of 3 doses) beginning on day 7. A low-targeting control immunocytokine 425-IL-2, specific for EGFR, was used at the high dose to define activity due primarily to the increased half-life of IL-2. Results are reported as general health (eg, paralysis), which preceded death by 10 to 14 days, and in some cases, survival of mice.
In the SCID model reconstituted with human B cells, mice (6 animals per group) were injected intravenously with 5 × 106 CD20+ Daudi lymphoma cells on day 0 and 4.5 × 107 human PBMCs on day 5. One group of mice received PBS only; 1 group received antibody only (500 μg on days 7, 9, and 11); 1 group received immunocytokine only (20 μg on days 11-15); and 1 group received the combination of antibody (500 μg on days 7, 9, and 11) and immunocytokine (20 μg on days 11-15). All mice were checked for the presence of human antibodies in their serum by anti–human IgG enzyme-linked immunosorbent assay (ELISA) on days 21 and 34.
Results
Deimmunization of Leu16 anti-CD20 antibody
Peptide-threading analysis of peptides binding to class II MHC molecules and subsequent mutagenesis was used to generate deimmunized V regions with the potential for reduced immunogenicity in patients. A total of 15 changes were made in the 2 V regions including 8 in the heavy variable (VH) and 7 in the light variable (VL). All of these were in the framework residues and none were within the complementarity-determining regions (CDRs). These V regions were used to generate whole antibodies or whole antibody IL-2 fusion proteins (immunocytokines) by expression in transfected NS/0 myeloma cells and purification from conditioned cell-culture media. The quality of the proteins was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and size-exclusion chromatography and all proteins were found to be more than 95% pure and more than 90% monomeric (data not shown). Increasing concentrations of the purified proteins were used to immunostain CD20-expressing Daudi lymphoma cells and results were analyzed by flow cytometry. Deimmunization was found to have little or no effect on antigen binding (Figure 1) for either the antibody (DI-Leu16) or the immunocytokine (DI-Leu16-IL-2), relative to the chimeric forms containing the original mouse V region and the same human C regions. Binding was also similar to C2B8 (rituximab).
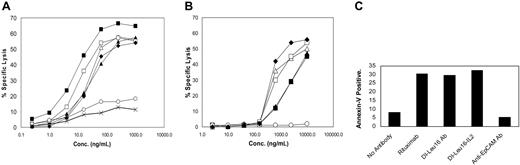
ADCC, CDC, and proapoptotic activities of recombinant antibodies and immunocytokines. (A) The ability of resting human PBMCs to mediate the specific lysis of CD20+ Daudi lymphoma cells was measured in a 4-hour ADCC assay as described in “Materials and methods.” Proteins included DI-Leu16-IL-2 (▪), DI-Leu16 antibody (□), rituximab (♦), chLeu16-IL-2 (▴), chLeu16 antibody (▵), enzymatically deglycosylated DI-Leu16-IL-2 (○) and a nontargeting IL-2 immunocytokine (×). (B) CDC activity was measured as described in “Materials and methods” using the same proteins as in panel A. (C) Early apoptosis of Daudi cells induced by the indicated antibodies or immunocytokine (50 μg/mL) was measured by Annexin-V staining and FACS analysis. The anti-EpCAM humanized antibody, huKS-1/4, was used as a nonbinding IgG1 antibody control.
ADCC, CDC, and proapoptotic activities of recombinant antibodies and immunocytokines. (A) The ability of resting human PBMCs to mediate the specific lysis of CD20+ Daudi lymphoma cells was measured in a 4-hour ADCC assay as described in “Materials and methods.” Proteins included DI-Leu16-IL-2 (▪), DI-Leu16 antibody (□), rituximab (♦), chLeu16-IL-2 (▴), chLeu16 antibody (▵), enzymatically deglycosylated DI-Leu16-IL-2 (○) and a nontargeting IL-2 immunocytokine (×). (B) CDC activity was measured as described in “Materials and methods” using the same proteins as in panel A. (C) Early apoptosis of Daudi cells induced by the indicated antibodies or immunocytokine (50 μg/mL) was measured by Annexin-V staining and FACS analysis. The anti-EpCAM humanized antibody, huKS-1/4, was used as a nonbinding IgG1 antibody control.
Biological characterization
The DI-Leu16 antibody and DI-Leu16-IL-2 immunocytokine were characterized further for effector functions—ADCC and CDC— using Daudi lymphoma cells as targets. As reported in earlier studies,26 the ADCC activity of the IL-2 immunocytokine was generally higher than the parent DI-Leu16 antibody (Figure 2A), likely due to higher affinity to Fc receptor (R). Although it is difficult to determine if this increase is significant, due to variations between effector cells from different individual donors, at least the fusion of IL-2 has not compromised the activity of the antibody. This potent ADCC was found to be completely dependent on the N-terminal glycosylation of the H chain since treatment of DI-Leu16-IL-2 with N-glycanase completely abrogated this activity. Previous reports have shown the importance of this N-glycosylation in maintaining the structure of the Fc region of antibodies and the ability to bind FcR.27 CDC activity, on the other hand, was greatly reduced as a consequence of fusing IL-2 to the C-terminus of the H chain (Figure 2B). A similar effect was reported earlier with an anti–ganglioside GD2 immunocytokine.26 The abilities of the deimmunized Leu16 antibody and immunocytokine to induce apoptosis of CD20+ Daudi cells, as assessed by annexin-V staining, were also very similar to each other and to the rituximab control (Figure 2C).
The IL-2 activity of the immunocytokine was tested using 3 different cell lines: the standard mouse CTLL-2 T-cell line expressing high-affinity IL-2R, as well as 2 human cell lines expressing both high and intermediate receptors (Kit225) or just the intermediate receptor (TF-1β). While the activity of DI-Leu16-IL-2 was somewhat reduced, relative to control IL-2, for the ability to induce the proliferation of mouse CTLL-2 cells, the ability to stimulate proliferation of the human cell lines was virtually identical to the IL-2 standard (Table 1). This activity corresponds to approximately 3 × 106 IU/mg of immunocytokine or 18 × 106 IU/mg of the IL-2 component. The lowest activity for both the human IL-2 standard and DI-Leu16-IL-2 was seen using purified mouse NK cells. There was also a 20-fold lower activity for the immunocytokine compared with the free IL-2 molecule. Another IL-2 immunocytokine, huKS-IL-2, also showed lower activity than the free IL-2 but not quite as low as DI-Leu16-IL-2.
IL-2 bioactivity using human or mouse cells or cell lines
. | Mouse T-cell CTLL-2 ED50, ng/mL . | Mouse NK cells ED50, ng/mL . | Human PBMCs ED50, ng/mL . | Human T-cell Kit-225 ED50, ng/mL . | Intermediate, IL-2R cell TF-1β ED50, ng/mL . |
|---|---|---|---|---|---|
| HuKS-IL-2 | 1.71 | 172.0 | 2.09 | 0.08 | 0.51 |
| Human rIL-2 | 0.65 | 26.0 | 1.51 | 0.07 | 0.71 |
| DI-Leu16-IL-2 | 1.94 | 549.0 | 2.03 | 0.05 | 1.15 |
| Degly-DI-Leu16-IL-2 | 3.30 | NT | 3.42 | 0.09 | 1.99 |
. | Mouse T-cell CTLL-2 ED50, ng/mL . | Mouse NK cells ED50, ng/mL . | Human PBMCs ED50, ng/mL . | Human T-cell Kit-225 ED50, ng/mL . | Intermediate, IL-2R cell TF-1β ED50, ng/mL . |
|---|---|---|---|---|---|
| HuKS-IL-2 | 1.71 | 172.0 | 2.09 | 0.08 | 0.51 |
| Human rIL-2 | 0.65 | 26.0 | 1.51 | 0.07 | 0.71 |
| DI-Leu16-IL-2 | 1.94 | 549.0 | 2.03 | 0.05 | 1.15 |
| Degly-DI-Leu16-IL-2 | 3.30 | NT | 3.42 | 0.09 | 1.99 |
ED50 values are the average of at least 4 determinations except for mouse NK cells (2 assays) and were determined as described in “Materials and methods.”
NT indicates not tested.
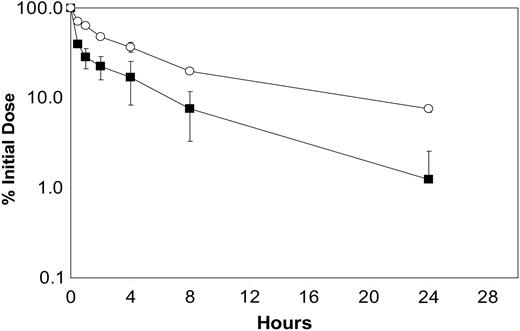
The pharmacokinetic properties of immunocytokines are reported to be dependent to some extent on uptake by FcR-bearing cells26 as well as intracellular proteolysis.19 In the current DI-Leu16-IL-2 construct we have maintained FcR binding and ADCC activity by using a Cγ1 H chain isotype but have incorporated a modified linker region between the H chain and IL-2 in order to reduce intracellular proteolysis. As reported earlier for the huKS-IL-2 immunocytokine, a Lys to Ala change at the junction region resulted in a more favorable pharmacokinetic profile following intravenous administration, especially during the distribution α phase. The contribution of FcR binding was examined by testing the enzymatically deglycosylated DI-Leu16-IL-2 and comparing its pharmacokinetic profile to the fully glycosylated molecule (Figure 3). Results indicate that loss of FcR binding improved the pharmacokinetic behavior somewhat, indicating that both cell uptake and intracellular degradation play a role in the distribution, recycling, and eventual clearance of this class of molecule.
Antitumor activity of DI-Leu16-IL-2 against Daudi metastases in SCID mice
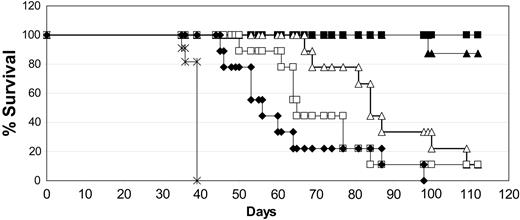
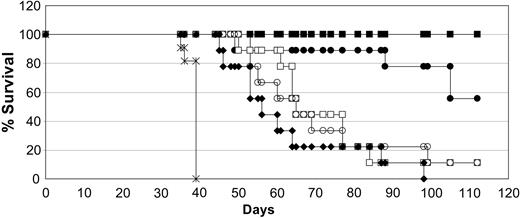
The conditions we have used for testing antitumor activity are detailed in “Materials and methods.” After intravenous injection of SCID mice we observed that the first disease symptom is hind-leg paralysis in all mice, followed by outgrowth of solid tumors in many visceral organs as well as in the brain and skin. Death occurs in all mice approximately 10 days following the first signs of paralysis. This consistent pattern led us to switch endpoints from survival to first appearance of disease (paralysis) after our initial studies. Animals scored as disease-free had no observable symptoms or signs of tumor burden. In the first antitumor experiments, the injection of Daudi cells into SCID mice resulted in extensive disseminated disease leading to paralysis of all mice by day 30. Treatment was delayed until day 7 to ensure the tumor cells had engrafted. We compared low and moderate doses of both the chimeric and DI-Leu16-IL-2 immunocytokines to high-dose rituximab using 5 daily doses of the immunocytokines and 3 alternate day doses of the antibody. This schedule was chosen because of the much longer circulating half-life of the antibody (several days) compared with the immunocytokines (approximately 8 hours). Under these conditions, rituximab (25 mg/kg × 3) extended the 50% survival of tumor-bearing mice from 39 to 56 days, relative to the PBS control (Figure 4). The low-dose chimeric and DI-Leu16-IL-2 groups (0.25 mg/kg × 5) had similar survival curves as the high-dose rituximab (50% survival at 64 days). The groups treated with the moderate doses of the immunocytokines (1 mg/kg × 5) showed a dramatic increase in survival with no mouse deaths in the DI-Leu16-IL-2 group at the termination of the experiment (day 110) and only 1 of 8 mice dead in the chLeu16-IL-2 group. Thus, the deimmunized V regions of the Leu16 antibody were as effective as those of the murine Leu16 antibody, in the context of an IL-2–based immunocytokine, for the treatment of disseminated lymphoma in SCID mice.
Pharmacokinetics of DI-Leu16-IL-2 before and after deglycosylation. A time-concentration analysis was performed following a moderate intravenous dose of each immunocytokine. Serum concentrations were determined by an ELISA that detects the intact forms for the native (▪) and deglycosylated proteins (○).
Pharmacokinetics of DI-Leu16-IL-2 before and after deglycosylation. A time-concentration analysis was performed following a moderate intravenous dose of each immunocytokine. Serum concentrations were determined by an ELISA that detects the intact forms for the native (▪) and deglycosylated proteins (○).
In the same experiment we also tested the contribution of antibody effector function on antitumor activity using the enzymatically deglycosylated DI-Leu16-IL-2, shown above to have lost ADCC activity. As shown in Figure 5, a significant portion of the antitumor activity was preserved despite the loss of ADCC activity, at least for the early time points. At later time points, a marked difference between the intact and deglycosylated forms was observed in the higher dose group. Therefore, while ADCC appears to play a role in this model, a good deal of the antitumor activity in this model can be attributed to targeted delivery of IL-2 to the tumor alone. To support this further, we tested another IL-2 immunocytokine targeting EGFR,22 which is expressed at only very low levels (Figure 6B) on this cell line, and found that even the higher dose (1 mg/kg), given 5 consecutive days, had far less antitumor activity in this model than the same dose of the immunocytokines targeting CD20 (Figure 6A). The EGFR-targeted immunocytokine also had significantly less activity than the same dose of DI-Leu16-IL-2 given only twice, 3 days apart (days 7 and 10), or at a 4-fold lower dose given over 5 days. These results demonstrate the importance of specific tumor-cell targeting for antitumor activity, especially for this system where shed antigen is not present.
The chimeric and deimmunized forms of Leu16-IL-2 have similar antitumor activity. SCID mice were injected with Daudi lymphoma cells and treated with the indicated antibody or immunocytokine beginning 7 days later. Treatments included PBS only (×; on days 7-11); rituximab (♦; 25 mg/kg on days 7, 9, and 11); medium-dose DI-Leu16-IL-2 (▪; 1 mg/kg on days 7-11); low-dose DI-Leu16-IL-2 (□; 0.25 mg/kg on days 7-11); medium-dose chLeu16-IL-2 (▴; 1 mg/kg on days 7-11); and low-dose chLeu16-IL-2 (▵; 0.25 mg/kg on days 7-11). The higher dose groups of both the chimeric and DI forms of Leu16-IL-2 were significantly different from all other groups (P < .005 or less). The lower doses were not significantly different from each other but the chLeu16-IL-2 group was significantly different from the rituximab control (P < .0025). All treatment groups were significantly different from the PBS control (P < .025 or less).
The chimeric and deimmunized forms of Leu16-IL-2 have similar antitumor activity. SCID mice were injected with Daudi lymphoma cells and treated with the indicated antibody or immunocytokine beginning 7 days later. Treatments included PBS only (×; on days 7-11); rituximab (♦; 25 mg/kg on days 7, 9, and 11); medium-dose DI-Leu16-IL-2 (▪; 1 mg/kg on days 7-11); low-dose DI-Leu16-IL-2 (□; 0.25 mg/kg on days 7-11); medium-dose chLeu16-IL-2 (▴; 1 mg/kg on days 7-11); and low-dose chLeu16-IL-2 (▵; 0.25 mg/kg on days 7-11). The higher dose groups of both the chimeric and DI forms of Leu16-IL-2 were significantly different from all other groups (P < .005 or less). The lower doses were not significantly different from each other but the chLeu16-IL-2 group was significantly different from the rituximab control (P < .0025). All treatment groups were significantly different from the PBS control (P < .025 or less).
Loss of ADCC activity has only a partial effect on antitumor activity mediated by DI-Leu16-IL-2. Two different doses of native and enzymatically deglycosylated DI-Leu16-IL-2 were used to treat SCID mice beginning 7 days after injection of Daudi lymphoma cells. Treatments included PBS only (×; on days 7-11); rituximab (♦; 25 mg/kg on days 7, 9, and 11); medium-dose DI-Leu16-IL-2 (▪; 1 mg/kg on days 7-11); low-dose DI-Leu16-IL-2 (□; 0.25 mg/kg on days 7-11); medium-dose deglycosylated DI-Leu16-IL-2 (•; 1 mg/kg on days 7-11); and low-dose deglycosylated DI-Leu16-IL-2 (○; 0.25 mg/kg on days 7-11). Both high-dose groups of DI-Leu16-IL-2, with and without deglycosylation, were significantly different from all other groups (P < .005 or less), but were not significantly different from each other. The low-dose groups of these molecules were not significantly different from each other or from the rituximab group, but were significantly different from the PBS control (P < .0025 or less).
Loss of ADCC activity has only a partial effect on antitumor activity mediated by DI-Leu16-IL-2. Two different doses of native and enzymatically deglycosylated DI-Leu16-IL-2 were used to treat SCID mice beginning 7 days after injection of Daudi lymphoma cells. Treatments included PBS only (×; on days 7-11); rituximab (♦; 25 mg/kg on days 7, 9, and 11); medium-dose DI-Leu16-IL-2 (▪; 1 mg/kg on days 7-11); low-dose DI-Leu16-IL-2 (□; 0.25 mg/kg on days 7-11); medium-dose deglycosylated DI-Leu16-IL-2 (•; 1 mg/kg on days 7-11); and low-dose deglycosylated DI-Leu16-IL-2 (○; 0.25 mg/kg on days 7-11). Both high-dose groups of DI-Leu16-IL-2, with and without deglycosylation, were significantly different from all other groups (P < .005 or less), but were not significantly different from each other. The low-dose groups of these molecules were not significantly different from each other or from the rituximab group, but were significantly different from the PBS control (P < .0025 or less).
Antitumor activity of separate antibody and IL-2 components.
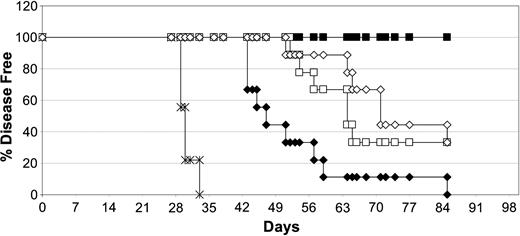
Clinical trials combining rituximab and IL-2 have already shown increased response rates of the combination over the single agent treatment.11 We tested this combination in the same Daudi lymphoma model using 2 different approaches and compared it with treatment with DI-Leu16-IL-2. In the first case, we dosed animals intravenously for 5 consecutive days with the equivalent molar amounts of DI-Leu16 antibody and IL-2 contained in 20 μg of DI-Leu16-IL-2. In the second case, we used a more optimized protocol using high doses of rituximab (25 mg/kg) and of IL-2 (10 μg) given subcutaneously every other day for 3 doses. This dosing regimen would ensure high levels of antibody as well as a sustained IL-2 activation due to the depot effect of subcutaneous administration. Surprisingly, results indicate that the 2 combination protocols resulted in roughly the same degree of antitumor activity, with 50% survival of 63 days (Figure 7). Treatment of mice with IL-2 alone (no anti-CD20 antibody) delayed the appearance of disease only slightly in this model and was not significantly different from the PBS control group (not shown). Treatment with the equivalent amount of DI-Leu16-IL-2 immunocytokine, used in the low-dose combination group, resulted in long-term survival of all mice. This is particularly noteworthy since the groups treated with the separate antibody and IL-2 are exposed to antibody for a much longer time than DI-Leu16-IL-2 due to the antibody's much longer half-life.
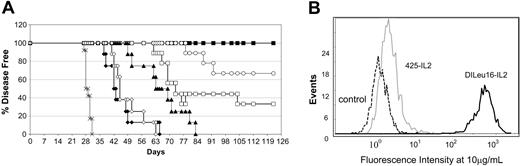
Antigen specificity is important for optimal antitumor activity. The role of tumor cell targeting was tested by comparing the activity of DI-Leu16-IL-2 and another immunocytokine with binding specificity for EGFR, a molecule expressed at only low levels on Daudi lymphoma cells. Experimental conditions were the same as in Figures 4 and 5 except for the indicated changes in dosing. (A) Treatments included PBS only (×; on days 7-11); rituximab (♦; 25 mg/kg on days 7, 9, and 11); DI-Leu16 antibody (⋄; 25 mg/kg on days 7, 9, and 11); medium-dose DI-Leu16-IL-2 (▪; 1 mg/kg on days 7-11); reduced-dose DI-Leu16-IL-2 (○; 1 mg/kg on days 7 and 10); low-dose DI-Leu16-IL-2 (□; 0.25 mg/kg on days 7-11); and medium-dose anti-EGFR–IL-2 (▴; 1 mg/kg on days 7-11). Results were scored as disease-free survival. (B) FACS analysis of Daudi lymphoma cells using either anti-EGFR antibody or anti-CD20 as the primary unlabeled antibodies. Labeled antihuman Fc antibody was used for detection. The low level of EGFR expression was confirmed by PCR analysis (data not shown). All treatment groups were significantly different from the PBS control (P < .005 or less) but the difference between the groups treated with either rituximab or DI-Leu16 antibody, or the groups treated for different numbers of days with the higher dose of DI-Leu16-IL-2 were not. The group treated with the anti-EGFR–IL-2 immunocytokine was significantly different from the antibody treatment groups (P < .005) as well as the low dose of DI-Leu16-IL-2 (P < .05).
Antigen specificity is important for optimal antitumor activity. The role of tumor cell targeting was tested by comparing the activity of DI-Leu16-IL-2 and another immunocytokine with binding specificity for EGFR, a molecule expressed at only low levels on Daudi lymphoma cells. Experimental conditions were the same as in Figures 4 and 5 except for the indicated changes in dosing. (A) Treatments included PBS only (×; on days 7-11); rituximab (♦; 25 mg/kg on days 7, 9, and 11); DI-Leu16 antibody (⋄; 25 mg/kg on days 7, 9, and 11); medium-dose DI-Leu16-IL-2 (▪; 1 mg/kg on days 7-11); reduced-dose DI-Leu16-IL-2 (○; 1 mg/kg on days 7 and 10); low-dose DI-Leu16-IL-2 (□; 0.25 mg/kg on days 7-11); and medium-dose anti-EGFR–IL-2 (▴; 1 mg/kg on days 7-11). Results were scored as disease-free survival. (B) FACS analysis of Daudi lymphoma cells using either anti-EGFR antibody or anti-CD20 as the primary unlabeled antibodies. Labeled antihuman Fc antibody was used for detection. The low level of EGFR expression was confirmed by PCR analysis (data not shown). All treatment groups were significantly different from the PBS control (P < .005 or less) but the difference between the groups treated with either rituximab or DI-Leu16 antibody, or the groups treated for different numbers of days with the higher dose of DI-Leu16-IL-2 were not. The group treated with the anti-EGFR–IL-2 immunocytokine was significantly different from the antibody treatment groups (P < .005) as well as the low dose of DI-Leu16-IL-2 (P < .05).
DI-Leu16-IL-2 is more potent than the sum of its parts or higher doses of anti-CD20 antibody and free IL-2. Antitumor activities of medium-dose DI-Leu16-IL-2 (▪; 20 μg/mouse on days 7-11) and the corresponding doses of the individual antibody and IL-2 components (□; 16.7 μg DI-Leu16 and 3.3 μg IL-2 by intravenous on days 7-11) were compared to high-dose rituximab and subcutaneous IL-2 (⋄; 500 μg rituximab on day 7 and 10 μg IL-2 on days 7, 9, and 11), rituximab alone (♦; 500 μg on day 7), or PBS control (×). All treatment groups were significantly different from the PBS control and from each other (P < .05 or less) except for the 2 groups treated with both antibody and either intravenous or subcutaneous IL-2. These were not significantly different from each other.
DI-Leu16-IL-2 is more potent than the sum of its parts or higher doses of anti-CD20 antibody and free IL-2. Antitumor activities of medium-dose DI-Leu16-IL-2 (▪; 20 μg/mouse on days 7-11) and the corresponding doses of the individual antibody and IL-2 components (□; 16.7 μg DI-Leu16 and 3.3 μg IL-2 by intravenous on days 7-11) were compared to high-dose rituximab and subcutaneous IL-2 (⋄; 500 μg rituximab on day 7 and 10 μg IL-2 on days 7, 9, and 11), rituximab alone (♦; 500 μg on day 7), or PBS control (×). All treatment groups were significantly different from the PBS control and from each other (P < .05 or less) except for the 2 groups treated with both antibody and either intravenous or subcutaneous IL-2. These were not significantly different from each other.
Furthermore, the amounts of IL-2 used for comparison were based on mass and not on IL-2 activity units. As shown in Table 1, free rIL-2 is approximately 3-fold more active than the equivalent molar amount of IL-2 contained in DI-Leu16-IL-2 when measured with a mouse cell line expressing the high-affinity IL-2R. For immune cells in the SCID mice expressing only the intermediate IL-2R, this difference is more than 10-fold in favor of free rIL-2.
Antitumor activity in the presence of CD20+ human B cells.
Targeting CD20 on B lymphoma cells is complicated by the fact that the antigen is expressed on normal B cells. Thus, therapy involves the targeted depletion of tumor cells in the background of a vast number of normal B cells. Since IL-2 immunocytokine dosing is likely to be limited by the toxicity of the IL-2 component, it is unlikely that the high doses required for normal B-cell depletion by the naked antibody could be used for DI-Leu16-IL-2. Combination treatment is a likely clinical approach in which DI-Leu16-IL-2 therapy would follow rituximab treatment to first debulk both CD20+ tumor cells and normal B cells.
In an attempt to create a more informative tumor model we injected mice with PBMCs containing human B cells and then compared monotherapy with rituximab or DI-Leu16-IL-2 as well as the combination in which the antibody is given as a single dose at day 7, followed by a course of therapy with DI-Leu16-IL-2 beginning on day 11. A second set of mice were not implanted with human PBMCs but received the same treatment regimens. Confirmation that B cells had been implanted was obtained by measuring levels of human IgG in all mice groups. Data in Table 2 show that mice receiving human PBMCs all had levels of human IgG of more than 500 μg/mL demonstrating efficient grafting. Antibody levels at day 21 show dramatic decreases in all treatment groups, including monotherapy with DI-Leu16-IL-2; however, the IgG level increased slightly by day 34, indicating continued production by B cells. Treatment with rituximab or the combination, on the other hand, resulted in elimination of human antibody production by day 34. The remaining levels detected in the blood of mice in both rituximab- and combination-treated groups were clearly rituximab itself, since the same levels were seen in the corresponding groups that were not implanted with PBMCs.
Human antibody production in SCID mice implanted with human PBMCs
. | Day 21 . | . | Day 34 . | . | ||
|---|---|---|---|---|---|---|
| Treatment group . | - B cells . | + B cells . | - B cells . | + B cells . | ||
| PBS | NT | > 500 | 0 | > 500 | ||
| DI-Leu16-IL-2 (days 11-15) | NT | 64.1 ± 25.1 | 0.83 | 88.3 ± 36.5 | ||
| Rituximab (day 7) | NT | 14.3 ± 0.25 | 9.1 | 6.2 ± 0.62 | ||
| Combination | NT | 9.5 ± 0.45 | 6.0 | 5.8 ± 0.27 | ||
. | Day 21 . | . | Day 34 . | . | ||
|---|---|---|---|---|---|---|
| Treatment group . | - B cells . | + B cells . | - B cells . | + B cells . | ||
| PBS | NT | > 500 | 0 | > 500 | ||
| DI-Leu16-IL-2 (days 11-15) | NT | 64.1 ± 25.1 | 0.83 | 88.3 ± 36.5 | ||
| Rituximab (day 7) | NT | 14.3 ± 0.25 | 9.1 | 6.2 ± 0.62 | ||
| Combination | NT | 9.5 ± 0.45 | 6.0 | 5.8 ± 0.27 | ||
Human antibodies were quantitated by anti-IgG ELISA and represent the average values and standard errors of all 6 animals per group in μg/mL. Antibodies detected in the - B-cell group show the contribution of rituximab and DI-Leu16-IL-2 (both human IgG) to the total circulating antibody. NT indicates not tested.
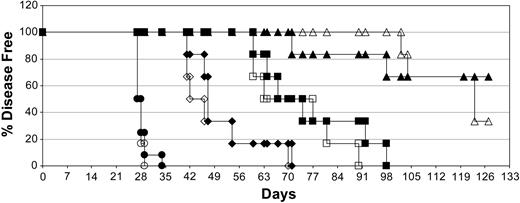
Results also showed that the antitumor activities of all treatment groups were not significantly affected by the presence of CD20+ human B cells (Figure 8). This may be due in part to the ability of DI-Leu16-IL-2 alone to eliminate the majority of implanted B cells, as well as tumor cells. The level of activity of DI-Leu16-IL-2 in this model was markedly reduced compared with earlier experiments due to the delay in initiating treatment (day 11 vs day 7); however, it should be noted that only a single course of treatment was used to compare different molecules and additional cycles of treatment would be used clinically. We also found that the combination of a single dose of rituximab, followed by a single course of DI-Leu16-IL-2, had at least additive antitumor activity with the majority of mice remaining disease free at the end of the experiment (day 120).
Discussion
We have shown for the first time that an IL-2–based immunocytokine, targeted to CD20, can effectively target and eradicate disseminated human lymphoma in a SCID mouse model. This was achieved under conditions where even much higher doses of an anti-CD20 antibody, rituximab, only delayed disease onset. Even the addition of free IL-2 to animals receiving high doses of rituximab were not as effective as the DI-Leu16-IL-2 immunocytokine. The diminished antitumor effect was seen using 2 very different combinations of rituximab and IL-2: the first representing the same molar amounts of antibody and IL-2 as DI-Leu16-IL-2 (16.7 μg + 3.3 μg, respectively), and the second representing much higher amounts of antibody (500 μg and 10 μg IL-2). Increased doses of the antibody do not improve disease outcome, even with effector cell activation by IL-2, whereas treatment with much lower doses of DI-Leu16-IL-2 much more effectively suppressed disease. Therefore, the mechanism of tumor-cell killing by DI-Leu16-IL-2 may not simply be due to effector cell activation and subsequent ADCC.
The presence of normal anti-CD20+ B cells does not diminish the antitumor activity of DI-Leu16-IL-2. The effect of prior implantation of normal human CD20+ B cells was tested in the same SCID/Daudi lymphoma model as described in “Materials and methods.” Groups of mice were treated with a single high dose of rituximab (diamonds; 25 mg/kg on day 7), a medium dose of DI-Leu16-IL-2 (squares; 1 mg/kg on days 11-15), the combination of both dosing regimens (triangles; rituximab on day 7 followed by DI-Leu16-IL-2 on days 11-15) or PBS alone (circles) on all dosing days. Half of the groups were injected intravenously with 4.5 × 107 PBMCs on day 5 (open symbols) or received only PBS (filled symbols). B-cell engraftment was confirmed by measuring human antibody levels in the serum of all mice. All treatment groups were significantly different from the PBS control (P < .005 or less) and all different treatment modalities were significantly different from each other (P < .01 or less). There were no significant differences between groups receiving the same treatment but with or without human B-cell implantation.
The presence of normal anti-CD20+ B cells does not diminish the antitumor activity of DI-Leu16-IL-2. The effect of prior implantation of normal human CD20+ B cells was tested in the same SCID/Daudi lymphoma model as described in “Materials and methods.” Groups of mice were treated with a single high dose of rituximab (diamonds; 25 mg/kg on day 7), a medium dose of DI-Leu16-IL-2 (squares; 1 mg/kg on days 11-15), the combination of both dosing regimens (triangles; rituximab on day 7 followed by DI-Leu16-IL-2 on days 11-15) or PBS alone (circles) on all dosing days. Half of the groups were injected intravenously with 4.5 × 107 PBMCs on day 5 (open symbols) or received only PBS (filled symbols). B-cell engraftment was confirmed by measuring human antibody levels in the serum of all mice. All treatment groups were significantly different from the PBS control (P < .005 or less) and all different treatment modalities were significantly different from each other (P < .01 or less). There were no significant differences between groups receiving the same treatment but with or without human B-cell implantation.
ADCC was further evaluated by comparing the effect of deglycosylation on antitumor activity. In this case we showed that enzymatic removal of the single N-linked glycan resulted in an immunocytokine with no ADCC activity but otherwise similar properties of the parent molecule. The effect of decreasing ADCC was a reduction of antitumor response but clearly there was significant antitumor activity independent of this effector mechanism. The nature of this activity appears to be associated with specific targeting of IL-2 and merits further study. This is especially true since this model provides no potential for T-cell responses, the cells shown in many earlier studies to be the major effector-cell population responding to IL-2–based immunocytokines.13 We are currently working to develop a syngeneic model to examine the potential contribution that T cells can make to the antitumor activity of DI-Leu16-IL-2. This model will also allow the investigation of the potential of the ICK to contribute significantly to vaccination effects when used alone or in combination with other vaccine approaches.
The tumor model described in this report has been useful in testing the activity of this novel immunocytokine but obviously does not represent the clinical situation in which a large number of normal B cells express the antigen targeted by the molecule. We attempted to provide a reservoir of human B cells to serve as decoys by implanting human PBMCs prior to initiating treatment. The conditions used for cell transfer were effective enough to result in sustained human antibody production in all mice. Under these conditions, there was no interference of the human B cells with respect to the antitumor activity of DI-Leu16-IL-2. In fact, the relatively low dose of the immunocytokine was surprisingly effective at reducing the antibody titers, although not as effective as high dose rituximab, raising the possibility that the normal B cells are not as sensitive as the lymphoma to the effects of the ICK.
Nonetheless, we would expect that the most effective use of DI-Leu16-IL-2 will be for the treatment of residual disease following standard rituximab treatment. To this end we tested the combination of both molecules and found that antitumor activity was at least additive. It should be mentioned that the lower efficacy of DI-Leu16-IL-2 seen in this experiment, likely reflecting incomplete killing of tumor cells, was due to the delay of treatment until 11 days after tumor implantation. These conditions were used so that combination effects could be observed and DI-Leu16-IL-2 alone would not eradicate the tumor. We also used just a single dosing cycle and observed animals for an extended period. Clinically we would expect to use repeat dosing cycles in combination with rituximab at 3- to 4-week intervals. Such a protocol has the potential of generating antibodies against the immunocytokine, especially since it is physically linked to a potent immune stimulant, IL-2. We have already reported anti-immunocytokine responses in patients treated with another molecule, hu14.18-IL-2,15 although these responses had no adverse clinical effects. In order to avoid this problem, we have attempted to deimmunize the Leu16 antibody V regions by identification and removal of potential helper T-cell epitopes. This process resulted in an antibody that retained full antigen-binding activity. It will be important to determine whether the deimmunization will effectively eliminate anti-ICK responses in humans.
Recently, a human anti-CD20 antibody has been reported with increased in vitro activity compared with rituximab.28,29 The increased activity was apparently due to increased complement activation that was linked to the accumulation of the CD20 complex into lipid rafts. The ability of the anti-CD20–IL-2 molecule to cause similar effects is under investigation although the parent Leu-16 has been shown by others to cause CD20 to enter the Triton X–insoluble fraction. Animal models and human studies have suggested that the preponderant antitumor effect is mediated through Fc receptors,30-33 while complement activation has been linked to infusion related toxicities of rituximab compared with anti-B1, a murine IgG1 which does not fix complement well.29,34 The diminished complement activity seen with the ICK should confer a favorable therapeutic ratio for the humanized anti-CD20–IL-2 protein.
Prepublished online as Blood First Edition Paper, February 3, 2005; DOI 10.1182/blood-2004-09-3533.
Several of the authors (S.D.G., K.-M.L., Y.L., S.W., and F.C.) are employed by companies (EMD Lexigen Research Center and Biovation) whose potential product was studied in the present work. Both companies are associates of Merck KGaA of Darmstadt, Germany.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal