Abstract
Genetic modification of dendritic-cell (DC) function is an attractive approach to treat disease, either using mature DCs (mDCs) to immunize patients, or immature DCs (iDCs) to induce tolerance. Viral vectors are efficient at transducing DCs, and we have investigated the effect of transduction with a variety of viral vectors on the phenotype and function of DCs. Adenovirus (Ad), human immunodeficiency virus (HIV), equine anemia virus (EIAV), and Moloney murine leukemia virus (MMLV) all up-regulate costimulatory molecules and major histocompatibility complex (MHC) class II expression on DCs, as well as, in the case of Ad and lentiviral vectors, inducing production of Th1 and proinflammatory cytokines. Following transduction there is activation of double-stranded (ds) RNA-triggered pathways resulting in interferon (IFN) α/β production. In addition, the function of virally infected DCs is altered; iDCs have an increased, and mDCs a decreased, ability to stimulate a mixed lymphocyte reaction (MLR). Viral transduction of mDCs results in up-regulation of the indoleamine 2,3-dioxygenase (IDO) enzyme, which down-regulates T-cell responsiveness. Inhibition of IDO restores the ability of mDCs to stimulate an MLR, indicating that IDO is responsible for the modulation of mDC function. These data have important implications for the use of viral vectors in the transduction of DCs.
Introduction
Dendritic cells (DCs) have long been known to be the most important antigen-presenting cell for priming T-helper cells.1 It is now also clear that DCs are involved in establishing tolerance to self antigens and nonpathogenic foreign antigens. DCs located in the periphery internalize both self and nonself antigens and migrate to lymph nodes.2,3 Depending on their state of maturation, the stimulatory signals they have received, and the antigens they are presenting, DCs can activate the lymphocytes to respond to the antigen, or to induce tolerance to the presented antigen.1
The ability of DCs to induce immunity or tolerance is a consequence of a number of factors. These include the expression of costimulatory molecules on their surface (such as CD40, CD80, and 86) and the secretion of cytokines.3,4 In recent years the importance of the expression of the indoleamine 2, 3-dioxygenase enzyme (IDO) by DCs has been recognized.5 This enzyme catabolizes tryptophan, which is essential for lymphocyte function, and has an important role in immunomodulation in sites such as the placenta, where it prevents T-cell-mediated rejection of allogeneic fetuses.6 DCs that express high levels of IDO cannot activate T-cell responses, and so are capable of inducing tolerance to antigens that they express. Interferon γ (IFN-γ) is a powerful inducer of IDO activity in DCs. Recently, it has been shown that cytotoxic T-lymphocyte-associated antigen 4-immunoglobulin (CTLA4-Ig)7 and surface bound CTLA48 act to up-regulate IDO expression in DCs, possibly by increasing IFN-γ production.
This ability of DCs to induce either tolerance or immunity offers considerable opportunities for their use in therapy. Thus activated DCs (referred to as mature DCs [mDCs]) can be used to induce immunity to pathogens or tumor-related antigens, while immature DCs (iDCs), which are tolerogenic, may have a role in controlling autoimmune disease or transplantation. Such strategies may also involve the genetic modification of DCs, either to express an antigen of interest or to modulate the function of the cells.
At present the most efficient method for genetic modification of DCs is to use viral vectors, though there are a number of nonviral strategies that are being developed. In particular, adenoviral (Ad) or lentiviral vectors have all been shown to be effective in transducing DCs. However, the transduction of DCs by these vectors may be associated with alterations in the phenotype and function of the cells.
Viruses can activate iDCs to become mDCs by a number of pathways,9-12 in particular through the presence of double-stranded (ds) RNA in virally infected cells. Perhaps the best-characterized pathways are the coupled 2-5A synthetase/RNase L and the protein kinase R (PKR) pathways.13 The 2-5A synthetase/RNase L system is composed of the 2′-5′ oligoadenylate synthetase (OAS) family of dsRNA-dependent enzymes and dormant, cytosolic RNAase L. dsRNA-activated 2′-5′ OAS synthesizes 2′-5′-linked oligoadenylate (2-5A) that specifically binds and activates RNase L. Activated RNase L cleaves diverse RNA substrates, thus inhibiting cellular and viral protein synthesis. Constitutively expressed PKR is activated by autophosphorylation initiated by direct binding of dsRNA. A major substrate of PKR is the α-subunit of eukaryotic translation initiation factor 2 (EIF-2α). PKR-phosphorylated EIF-2α cannot be recycled, thereby reducing the rate of translation initiation. Activated PKR also phosphorylates IκB, resulting in activation of nuclear factor κ B (NFκB) and the resulting transcription of IFN-α/β and other IFN-α/β-stimulated genes with antiviral activity. In addition to these well-characterized pathways, antiviral activity has also been attributed to other proteins induced by IFN-α/β and/or dsRNA, including myxovirus resistance protein (Mx),14 adenosine deaminase (ADAR1),15 interferon-stimulating gene 12 (ISG12),16 ISG20,17 and ubiquitin-like protein ISG.18
In this study we compare the effect of different viral vectors on the phenotype and function of iDCs and mDCs. In general terms, viral vectors that are successful at transducing DCs also activate the cells, as determined both by alterations in the surface phenotype and secretion of cytokines. The vectors activate the dsRNA-triggered 2-5A synthetase/RNase L and PKR pathways, resulting in antiviral IFN-α/β production. DCs transduced with these vectors have an altered phenotype and function, with iDCs becoming capable of stimulating a mixed lymphocyte response (MLR), but mDCs having a reduced ability to stimulate allogeneic T cells. This later effect is a consequence of up-regulation of IDO expression following transduction. These data have important consequences for the therapeutic application of DCs that have been genetically modified using viral vectors.
Materials and methods
DC preparation and cultures
Human DCs were generated from peripheral blood monocytes by treatment with granulocyte-macrophage colony stimulating factor (GM-CSF) and interleukin-4 (IL-4) as described.19 Peripheral blood mononuclear cells were isolated from buffy coat preparation of healthy donors by Ficoll-Hypaque centrifugation (1.077 g/cm3) followed by plastic adherence to enrich monocytes. The nonadherent cell fraction was used for T-cell isolation as described below. To obtain iDCs, adherent cells were cultured in X-VIVO 10 medium (BioWhittaker, Cambridge, United Kingdom) containing 10% human serum (Sigma, Poole, United Kingdom), 2 mM l-glutamine (Invitrogen, Paisley, United Kingdom), 100 U/mL penicillin and streptomycin (Invitrogen) in the presence of GM-CSF (20 ng/mL; Firstlink, Birmingham, United Kingdom) and IL-4 (20 ng/mL; Firstlink) at 37°C in 5% CO2 atmosphere for 5 to 10 days. DCs were matured (to give mDCs) in 20 ng/mL tumor necrosis factor α (TNF-α; PeproTech, London, United Kingdom), 20 ng/mL IL-1β (PeproTech), 20 ng/mL lipopolysaccharide (LPS; Sigma), 10 ng/mL prostaglandin E2 (PGE2; Sigma) for 48 hours.
Adenovirus production
The E1a-, partial E1b-, partial E3-adenovirus serotype 5 (Ad) vector Ad0 (a kind gift from Dr M. J. A. Wood, Human Anatomy, University of Oxford, United Kingdom) and AdGFP (carrying the enhanced green fluorescence protein-1; Clontech, Palo Alto, CA) were amplified and titered using a standard plaque assay on 293 cells as previously described.20 For transduction, 104 cells were incubated with adenovirus vectors (Ad0 or AdGFP at various multiplicities of infection [MOIs]) in 100 μL optiMEM I (Invitrogen) for 2 to 3 hours, at which time the volume was increased to 0.5 mL by addition of DC culture medium.
Lentivirus production
The HIV- and EIAV-based constructs were propagated by a 3 plasmid cotransfection technique in 293T cells as described elsewhere.21 All plasmids were a kind gift of Oxford Biomedica (Oxford, United Kingdom). Transfections were performed using polyethylenimine (PEI; Sigma) on 9-cm tissue-culture plates. The 293T cells were transfected at a confluency of approximately 70%. For each plate to be transfected, a DNA mixture consisting of 16 μg of an EIAV/HIV gag/pol-expressing plasmid (pONY3.1),18 8 μg of a viral envelope-expressing plasmid (pRV67),19 16 μg of the vector constructs pH7G (HIV) or pSMART2G (EIAV),22 were added to 2.5 mL serum-free Dulbecco modified Eagle medium (DMEM; Figure 1A). A 10-mM PEI solution was diluted 1:1000 in serum-free DMEM, of which 100 μL was added to 2.4 mL serum-free DMEM. The DNA and PEI solutions were mixed vigorously by vortexing for 30 seconds and then kept for 15 minutes at room temperature to allow complex formation. A quantity of 5 mL of the DNA/PEI solution was added to each 9-cm plate and left at 37°C for 3.5 hours; this was then replaced with full tissue-culture medium. At 48 hours after transfection, supernatant was collected, cleared by low-speed centrifugation (1200g, 5 minutes) and filtered through 0.45-μm filters. Vector particles were then concentrated by ultracentrifugation at 50 000g for 120 minutes at 4°C. The virus pellet was resuspended on ice in serum-free DMEM, snap-frozen, and kept in liquid nitrogen. The viruses were titrated on D17 cells, which were seeded on 24-well plates at 3 × 104 cells per well and incubated at 37°C overnight. Viral stocks were serially diluted 10-fold (10-1 to 10-5) in medium, and then 250 μL of each dilution was added to wells in duplicate. Titres determined were of the order of 1 × 105 to 2 × 108 infectious particle units/mL. The transduction of DCs was performed as previously described.23
MMLV production
293T24 and the amphotropic “Phoenix” packaging25 cell lines were cocultured at a relative ratio of 1:1 (1.5 × 106 cells seeded in 25-cm2 flasks on the day before transfection). Infectious viral particles were generated by calcium phosphate transfection26 of packaging cell lines with either LZRS-EGFP,27 pFB-hrGFP (Strategene, Cambridge, United Kingdom), or pBullet-EGFP28 (Figure 1A). Supernatants were harvested 48 hours after transfection. The collected viral supernatants were filtered through a 0.45-μm pore size filter and stored at -80°C for further use. For transduction, viral supernatants were cultured with various cells at different MOIs in the presence or absence of polybrene (Sigma). As described previously,29 stock viruses were titred by infecting 293T and/or HeLa cells with serial 1:10 dilutions of virus and analyzed for enhanced green fluorescent protein ([e]GFP) expression at 5 days after infection.
Flow cytometry
The phenotype of transfected or untransfected DCs was assessed by flow cytometry 5 days after transduction. These times were determined in preliminary experiments when the maximal expression of transgenes was seen. Cell staining was performed using mouse antibody (Ab) conjugated with allophycocyanin (APC) or primary Ab followed by goat anti-mouse APC, as previously described.30 Flow cytometric analysis of all cells was performed using the following mouse monoclonal Abs (all from Caltag, Silverstone, United Kingdom, unless stated otherwise): 3.9 (anti-CD11c); BA-8 (anti-CD14; Santa Cruz, San Diego, CA); HB14 (anti-CD40); MEM-233 (anti-CD80; Serotec, Oxford, United Kingdom); HB15e (anti-CD83); UB63 (anti-CD86); TU149 (anti-MHC class I); CR3/43 (anti-HLADR; Dako, Glostrup, Denmark); and 3D2 (anti-ICOS L [inducible costimulator-ligand]; Neomarker, Fremont, CA).
Ad is the most efficient vector to transduce DCs. (A) We used Ad, HIV, EIAV, and 3 MMLV constructs to generate replication-deficient Ad, HIV, EIAV, and MMLV viruses encoding (e)GFP. ITR indicates inverted terminal repeat; CMV, cytomegalovirus; LTR, long terminal repeat; cPPT, central polypurine tract cis acitve sequence; VGV, vesicular stomatitis virus; IRES, internal ribosomal entry site; and MCS, multiple cloning site. (B) The DCs were transduced with Ad, EIAV, or HIV at the MOI indicated either as iDCs (top row) or following stimulation with 20 ng/mL TNF-α, 20 ng/mL IL-1β, 20 ng/mL LPS, and 10 ng/mL PGE2 for 48 hours (bottom row). The transfection efficiency was assessed after 5 days using flow cytometry to measure (e)GFP expression. Results are expressed as the mean ± standard deviation of triplicate determinations. (C) The ability of the 3 MMLV retroviral supernatants to infect iDCs was tested either in the presence (right column) or absence (left column) of polybrene. The transfection efficiency was assessed after 2 to 3 days using flow cytometry to measure (e)GFP expression. Percentages of (e)GFP-positive and -negative cells are shown at the bottom-left corner of each flow cytometry plot.
Ad is the most efficient vector to transduce DCs. (A) We used Ad, HIV, EIAV, and 3 MMLV constructs to generate replication-deficient Ad, HIV, EIAV, and MMLV viruses encoding (e)GFP. ITR indicates inverted terminal repeat; CMV, cytomegalovirus; LTR, long terminal repeat; cPPT, central polypurine tract cis acitve sequence; VGV, vesicular stomatitis virus; IRES, internal ribosomal entry site; and MCS, multiple cloning site. (B) The DCs were transduced with Ad, EIAV, or HIV at the MOI indicated either as iDCs (top row) or following stimulation with 20 ng/mL TNF-α, 20 ng/mL IL-1β, 20 ng/mL LPS, and 10 ng/mL PGE2 for 48 hours (bottom row). The transfection efficiency was assessed after 5 days using flow cytometry to measure (e)GFP expression. Results are expressed as the mean ± standard deviation of triplicate determinations. (C) The ability of the 3 MMLV retroviral supernatants to infect iDCs was tested either in the presence (right column) or absence (left column) of polybrene. The transfection efficiency was assessed after 2 to 3 days using flow cytometry to measure (e)GFP expression. Percentages of (e)GFP-positive and -negative cells are shown at the bottom-left corner of each flow cytometry plot.
Assessment of (e)GFP reporter-gene expression
Following transfection, (e)GFP reporter-gene (Clontech, Palo Alto, CA) expression was determined using flow cytometry or an inverted fluorescent microscope as previously described.30
ELISA
In order to determine cytokine production, enzyme-linked immunosorbent assays (ELISAs) for IFN-γ, IL-4, IL-10, and IL-12 were carried out as previously described.31 IL-1β, IL-6, IL-8, and TNF-α were measured using an ELISA kit from R&D Systems Europe (Oxon, United Kingdom). Supernatants were obtained from the culture 4 days after transduction.
Allogeneic mixed lymphocyte reactions
Allogeneic T cells were isolated and purified using previously described protocols.32 Accessory cell contamination was assessed by measuring T-cell proliferation in the presence of 1 μg/mL phytohemagglutinin (PHA) in a 48-hour assay. The purified cells (1 × 105/well) were cultured in 96-well U-bottom plates in the presence of 104 irradiated (3000 rads from 137Cs source except with IDO experiments) transfected or untransfected allogeneic DCs as stimulators for 5 days. Proliferation was measured by a 16-hour pulse with 3H-thymidine (10 μL; ∼5 μCi/mL [∼1.85 × 105 Bq]; Amersham Pharmacia Biotech, Little Chalfont, United Kingdom). When appropriate, the IDO inhibitor, 500 μM 1-methyl-dl-tryptophan (Sigma) was added to the cultures.
RT-PCR assay and PCR Southern blotting
Following transduction, the DCs were isolated with magnetic beads (CD11c). Reverse transcriptase-polymerase chain reaction (RT-PCR) assays were carried out using paired primers, annealing temperatures, and protocols that have been previously described.33 The following paired primers were used to detect 2-5 OAS-1: ATG CCA TTG ACA TCA TCT GTG G (sense) CTC ACC AGC AGA ATC CAG GAG C (antisense); IRF-1: CAG GAC TTG GAG TGT GAG CAG G (sense), CAG CCA GTG ACA GCG AGA CC (antisense); IFNα2: TAC ACT GAA CTC TAC CAG CAG C (sense), ATG TAT TCT GTAATC AGG TTG C (antisense); IFNβ1: ACT GCC TCA AGG ACA GGA TG (sense), AGC CAG GAG GTT CTC AAC AA (antisense); β-actin: ATC TGG CAC CAC ACC TTC TAC AAT GAG (sense), CGT GGT GGT GAA GCT GTA GCC GCG GCT C (antisense); and IDO: ACT ACA AGA ATG GCA CAC GC (sense), TTG CCA AGA CAC AGT CTG CA (antisense). General hybridization protocols were carried out as described34 using the following probes: 5′-GAT CAT CTC ACA GAC CAC AAA TG-3′ and 5′-GCT ATC CCT GTA CGC CTC TG-3 for IDO and β-actin, respectively.
Western blots
Cell lysates were prepared and Western blotted as described.33,35,36 The blots were probed with monoclonal antibodies against β-actin (Sigma),33 phospho-PKR and phospho-EIF-2α (Biosource, Camarillo, CA), phospho-IκBα and phospho-PI3 Kinase (Cell Signaling Technology, Beverly, MA), and IDO (Chemicon International, Chandlers Ford, United Kingdom).
Measurement of l-kynurenine
The biologic activity of IDO was evaluated by measuring the levels of tryptophan metabolite, l-kynurenine, present in conditioned medium derived from the transduced cells. The amount of l-kynurenine was measured as previously described.37 Briefly, about 2 mL tissue-culture medium was collected from 5 × 105 cells 72 hours after transfection, and proteins were precipitated by trichloroacetic acid and resuspended in 0.5 mL dimethyl-paminobenzaldehyde in HCl (Sigma) for 10 minutes at room temperature. Absorption of resultant solution was measured at 490 nm by spectrophotometer; purified kynurenine (Sigma; 0-100 μM) was used as a standard.
Bioassay for IFN type I
HL116 cells were seeded at 3.5 × 104 per well in a 96-well flat-bottom plate. This cell line is derived from the human fibrocarcinoma HT1080 cells and contains a luciferase cDNA under the control of the IFN-inducible promoter derived from the human 6-16 gene.38 After 24 hours of incubation at 37°C, supernatant were discarded and samples for testing were added to a final volume of 100 μL in duplicate. Two-fold dilutions of a standard (IFN-α2a; Hoffman-LaRoche, Welwyn Garden City, United Kingdom) were added to the plate in duplicate (from 100 U/mL). The plates were incubated for 6 hours at 37°C. Supernatants were then discarded and the cells were lysed with Lysis Buffer (Promega, Southampton, United Kingdom). The plates were then frozen at -80°C, thawed when ready to be analyzed, and samples were transferred to a white-bottom plate. Luminescence was measured from the reaction between the luciferase induced by IFNs and 50 μL Luciferin (Promega) dispensed by an automated luminometer (Ascent; Thermo Labsystems, Helsinki, Finland). The data were then transferred to computer for analysis by the Cytafzal software.
Reproducibility and statistical analysis
Statistical evaluation of data was performed with Student t test for simple comparison between 2 means. For multiple comparisons, results were analyzed by analysis of variance (ANOVA). P less than .05 was considered statistically significant. All data shown are representative of at least 3 experiments.
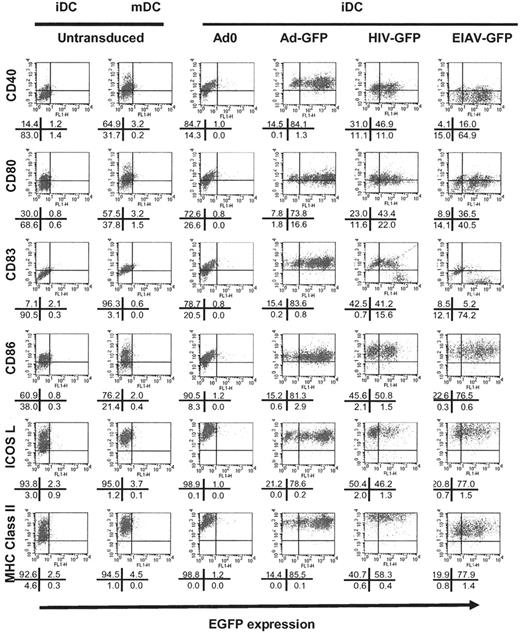
Change in phenotype of iDCs following viral transduction. Following transduction of iDCs with Ad0 (third column; MOI 500), AdGFP (fourth column; MOI 500), HIV-GFP (fifth column; MOI 500), or EIAV-GFP (sixth column; MOI 500), the phenotype of the cells was analyzed using 2-color flow cytometry for the expression of (e)GFP (x axis) and the surface marker indicated (y axis). As a control, untransfected cells were either unstimulated (iDCs, first column) or activated with 20 ng/mL TNF-α, 20 ng/mL IL-1β, 20 ng/mL LPS, and 10 ng/mL PGE2 for 48 hours (mDCs, second column). The results shown are representative of 3 experiments. The percentage of cells in each quadrant of the flow cytometry profiles is shown in the diagram beneath each profile.
Change in phenotype of iDCs following viral transduction. Following transduction of iDCs with Ad0 (third column; MOI 500), AdGFP (fourth column; MOI 500), HIV-GFP (fifth column; MOI 500), or EIAV-GFP (sixth column; MOI 500), the phenotype of the cells was analyzed using 2-color flow cytometry for the expression of (e)GFP (x axis) and the surface marker indicated (y axis). As a control, untransfected cells were either unstimulated (iDCs, first column) or activated with 20 ng/mL TNF-α, 20 ng/mL IL-1β, 20 ng/mL LPS, and 10 ng/mL PGE2 for 48 hours (mDCs, second column). The results shown are representative of 3 experiments. The percentage of cells in each quadrant of the flow cytometry profiles is shown in the diagram beneath each profile.
Results
Adenoviral vectors are the most efficient at transducing human DCs
In order to compare the ability of different viral vectors to transduce DCs, we exposed either iDCs or mDCs (matured by treatment with TNF-α, IL-1β, LPS, and PGE2) to Ad, EIAV, HIV, or MMLV vectors encoding (e)GFP at different MOIs (Figure 1A). The transfection efficiency was then determined by flow cytometry. As shown in Figure 1B, Ad, EIAV, and HIV were all capable of transducing DCs, in each case showing a reduced ability to transduce mDCs when compared with iDCs. Ad was the most efficient vector, showing higher transfection efficiencies at lower MOIs. In contrast, we saw poor transfection of monocyte-derived DCs with 3 different MMLV vectors, with less than 10% of iDCs in the presence of polybrene and approximately 0.2% of iDCs in the absence of polybrene expressing the marker gene (Figure 1C).
Changes in phenotype of iDCs following viral transduction
In order to determine the effect of transduction with Ad and lentiviral vectors on the expression of surface markers, we assessed the phenotype of cells 4 days after transduction of iDCs with the vectors. As shown in Figure 2 and Figure S1 (available on the Blood website; see the Supplemental Figures link at the top of the online article), transduction of iDCs with Ad vectors resulted in marked up-regulation of CD83, MHC class II and class I, and the costimulatory molecules CD40 and CD80. There was also some up-regulation of ICOS L and CD86. The cells therefore adopted a phenotype similar to that of mDCs, as has been previously reported.12,39 This was seen both with vectors encoding GFP and with a control vector (Ad0) containing no insert, indicating that this up-regulation is not a consequence of GFP expression. There was no augmentation of the expression of these molecules following exposure of mDCs to adenoviral particles (data not shown).
Following transduction of lentiviral vectors, CD83, MHC class II, and costimulatory molecules on both iDCs (Figure 2) and mDCs were up-regulated (data not shown).
As might be expected from their poor ability to transduce DCs, MMLV resulted in a limited activation of iDCs but not of mDCs (data not shown). Incubation of DCs in medium/buffers used to prepare viruses did not affect the phenotype of the cells (data not shown).
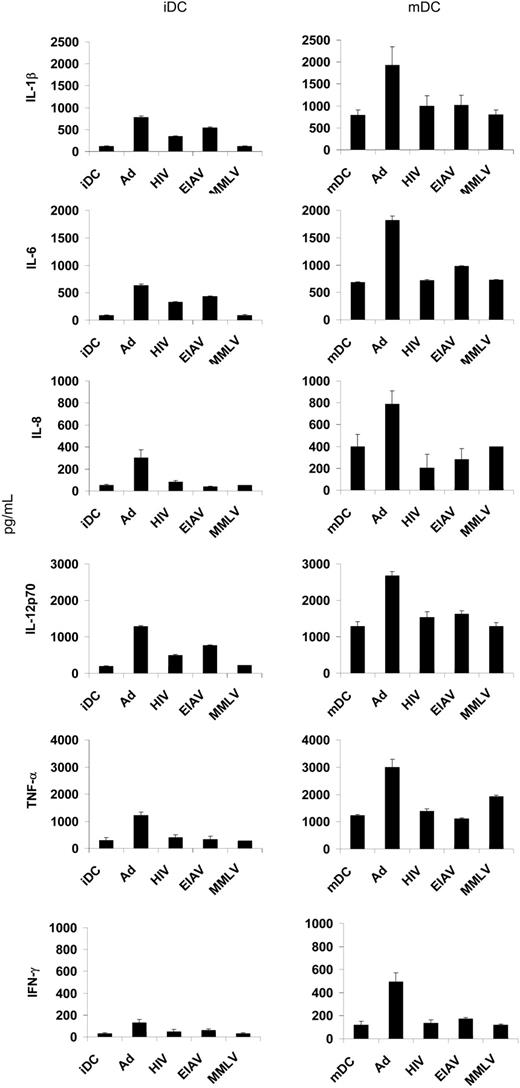
Th1-type and proinflammatory cytokine production following viral transduction of DCs
It has previously been reported that transduction of DCs with Ad vectors results in the secretion of Th1 cytokines IL-12 and IFN-γ.12 We saw a similar up-regulation of these cytokines, as well as enhanced secretion of IL-1β and IL-6, in both iDCs and mDCs (Figure 3). Transduction of DCs with lentiviral vectors resulted in an increase in secretion of IL-1β, IL-6, and IL-12 in iDCs (though less than that seen with Ad), with no effect on mDCs. There was no significant change in the secretion profile of DCs following transduction with MMLV.
Immunostimulatory capacity of DCs is altered following viral transduction
In order to test the functional consequences of viral transduction of DCs, we incubated either iDCs or mDCs with viral vectors at various MOIs, and then used them as stimulators in an MLR. As expected, untransduced iDCs were poor at stimulating an MLR; however, when they were transduced with Ad their ability to activate allogeneic T cells was markedly increased (Figure 4), as previously reported.40 These differences were significant at an MOI of 500. These data are consistent with our observations on the up-regulation of MHC class II and costimulatory molecules on iDCs following Ad transduction. Conversely, mDCs are potent stimulators of the MLR; however, when transduced with Ad their immunostimulatory capabilities are reduced. The effect of lentiviral or MMLV transduction on the function of iDCs and mDCs was less obvious, though there was some increase in the capacity of iDCs to stimulate an MLR, and a reduced response to mDCs, at a high MOI.
Activation of IFN-α/β antiviral pathways in virally transduced DCs
DCs have been shown to produce type I IFN in response to viral infection by a pathway that is initiated by dsRNA and is partially dependent on the cytosolic dsRNA-binding enzyme protein kinase R.9 Alternatively, they may be activated through the 2-5A synthetase/RNase L pathway. We therefore determined whether these pathways are activated in DCs following transduction with viral vectors. We show that Ad, EIAV, HIV, and MMLV triggered theproduction of type I IFN at mRNA (Figure 5) and protein levels (Figure S2) and IFN-γ at the protein level (Figure 3), together with up-regulation of 2-5 OAS and interferon regulatory factor 1 (IRF-1) at mRNA levels (2-5A synthetase/RNase L pathway) (Figure 5). We also saw phosphorylation of PKR and its downstream substrate phosphorylated EIF-2α (Figure 5), indicative of PKR pathway activation.
Th1 and proinflammatory cytokine production following viral vector transduction. DCs that were either unstimulated (iDCs) or activated (mDCs) were transduced with various viral vectors (MOI 500) on day 5 or untransduced and the supernatants collected on day 10. The levels of IL-1β, IL-6, IL-8, IL-12, TNF-α, and IFN-γ were determined using ELISA. The results are the mean of triplicate wells plus or minus the standard deviation (SD).
Th1 and proinflammatory cytokine production following viral vector transduction. DCs that were either unstimulated (iDCs) or activated (mDCs) were transduced with various viral vectors (MOI 500) on day 5 or untransduced and the supernatants collected on day 10. The levels of IL-1β, IL-6, IL-8, IL-12, TNF-α, and IFN-γ were determined using ELISA. The results are the mean of triplicate wells plus or minus the standard deviation (SD).
A recent report demonstrated that murine bone marrow-derived DCs showed activation of the P13 kinase/Akt pathway following Ad infection.41 The mechanism responsible for Ad-mediated activation and maturation of DCs was attributed to PI3 kinase-mediated induction of TNF-α.41 Consistent with this, we saw up-regulation of PI3 kinase (Figure 5) and TNF-α production (Figure 3) in human monocyte-derived DCs following Ad transduction.
Viral vectors have a profound effect on MLR. The functional consequence of viral transduction was tested in MLRs to evaluate the capacity of 104 DCs to stimulate 105 allogeneic T cells. DCs (either iDCs, ▪, or mDCs, □) were transduced with Ad, EIAV, HIV, or MMLV at the MOI indicated and then used as stimulators of allogeneic T cells in an MLR. 3H-thymidine incorporation was analyzed on day 5. The results are shown as the mean plus or minus SD. *P was less than .05 when ANOVA analysis and simple Student t test were carried out to compare multiple means and 2-pairing means, respectively.
Viral vectors have a profound effect on MLR. The functional consequence of viral transduction was tested in MLRs to evaluate the capacity of 104 DCs to stimulate 105 allogeneic T cells. DCs (either iDCs, ▪, or mDCs, □) were transduced with Ad, EIAV, HIV, or MMLV at the MOI indicated and then used as stimulators of allogeneic T cells in an MLR. 3H-thymidine incorporation was analyzed on day 5. The results are shown as the mean plus or minus SD. *P was less than .05 when ANOVA analysis and simple Student t test were carried out to compare multiple means and 2-pairing means, respectively.
These and other pathways result in activation of the NFκB pathway, which is central to DC activation, as indicated by increases in phosphorylated IκB (Figure 5). All the viral preparation medium did not activate any of the signaling pathways tested (data not shown).
Up-regulation of IDO in mDCs transduced with adenoviral vectors
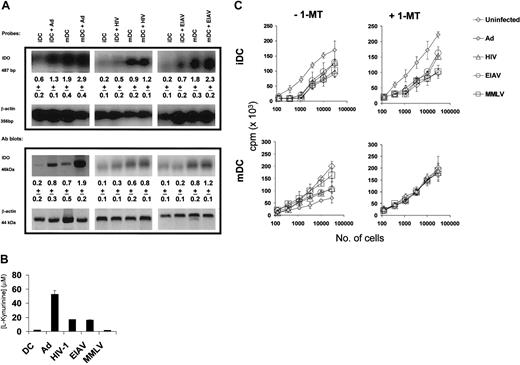
While the increased expression of costimulatory and other molecules following transduction with viral vectors can explain the increased stimulatory capacity of these cells, it is less clear why virally transduced mDCs show a reduced ability to activate allogeneic T cells. One possibility is that viral transduction might result in an increase in the expression of the tryptophan catabolizing enzyme (IDO). This up-regulation may occur as a result of the production of IFN-γ by DCs. As shown in Figure 6A, both iDCs and mDCs transduced with Ad show an increased expression of IDO as determined both by Southern blotting of an RT-PCR and by Western blot analysis of protein levels.
The IDO activity was confirmed by the measurement of l-kynurenine, the breakdown product of tryptophan (Figure 6B). There was also a slight increase in IDO expression following transduction with HIV or EIAV vectors.
In order to determine if the IDO expression was responsible for the functional differences seen following viral transduction, iDCs or mDCs were transduced with viral vectors and then used as stimulators in an MLR. The MLR was carried out in the presence and absence of 1-methyl tryptophan (1-MT), a competitive inhibitor of IDO. As shown in Figure 6C, iDCs transduced with Ad (and to a lesser extent HIV and EIAV) showed an increased ability to stimulate an MLR when compared with untransduced iDCs. This was unaffected by the addition of 1-MT to the cultures. On the other hand, mDCs transduced with Ad (or HIV or EIAV) showed a reduced ability to stimulate an MLR. This reduction in the immunostimulatory capacity of the transduced mDCs was reversed by addition of 1-MT, indicating that it was due to the activity of IDO.
Discussion
Given the central role of DCs in both the innate and adaptive immune systems, genetic manipulation of DCs is a promising therapeutic strategy. However, in the design of any such approach it is important to appreciate the effect of the gene therapy vector on the phenotype and function of the DC. To that end we have therefore compared the changes in phenotype and function of DCs following transduction with Ad, EAIV, HIV, and MMLV vectors.
As reported previously, both Ad vectors39 and lentiviral vectors23,42 are efficient at the transduction of DCs, with Ad vectors being somewhat more efficient. MMLV, on the other hand, was very inefficient at transducing human monocyte-derived DCs, in contrast to data obtained with CD34+-derived DCs.43-47 This difference presumably reflects the different proliferative capacities of the 2 types of DCs.
In common with other published data,11,40,48 we observed up-regulation of the expression of CD83, MHC class II, and costimulatory molecules by iDCs following transduction with Ad vectors. We also saw an increase in the secretion of Th1 proinflammatory cytokines by both iDCs and mDCs. We saw an up-regulation of IL-12 secretion following Ad transduction; this is in contrast to published data that indicate that Ad transduction of DCs does not result in an increase in IL-12, though it is capable of increasing the secretion in response to CD40 crosslinking.11 This difference may relate to differences in the techniques used to generate the DCs or in the timing of secretion (we assessed IL-12 production on day 5 rather than day 211 ). A similar up-regulation of Th1 cytokines has also been seen in murine DCs following Ad transduction.48 This production of Th1 cytokines has important implications in the use of genetically modified DCs in vivo.
Viral vectors activate dsRNA-triggered antiviral and PI3 kinase/Akt pathways. Following viral infection (MOI 500), iDCs (left column) and mDCs (right column; activated with 20 ng/mL TNF-α, 20 ng/mL IL-1β, 20 ng/mL LPS, and 10 ng/mL PGE2 for 48 hours) were analyzed. Five days after transduction, the DCs were analyzed by Western blotting for expression of phosphorylated PKR, phosphorylated EIF-2α, phosphorylated IκBα, and phosphorylated PI3 kinase as well as β-actin. In addition, RT-PCR was used to analyze mRNA levels of 2′-5′-OAS, IRF-1, IFN-α and -β, and β-actin.
Viral vectors activate dsRNA-triggered antiviral and PI3 kinase/Akt pathways. Following viral infection (MOI 500), iDCs (left column) and mDCs (right column; activated with 20 ng/mL TNF-α, 20 ng/mL IL-1β, 20 ng/mL LPS, and 10 ng/mL PGE2 for 48 hours) were analyzed. Five days after transduction, the DCs were analyzed by Western blotting for expression of phosphorylated PKR, phosphorylated EIF-2α, phosphorylated IκBα, and phosphorylated PI3 kinase as well as β-actin. In addition, RT-PCR was used to analyze mRNA levels of 2′-5′-OAS, IRF-1, IFN-α and -β, and β-actin.
Viral vector up-regulation of IDO has a significant consequence on MLR. (A) Five days after viral transduction of either iDCs or mDCs (activated with 20 ng/mL TNF-α, 20 ng/mL IL-1β, 20 ng/mL LPS, and 10 ng/mL PGE2 for 48 hours) with viral vectors, the cells were analyzed for expression of IDO using RT-PCR Southern and Western blots. The density of the IDO bands (as a ratio to the β-actin levels) are shown below each band, as the mean plus or minus SD of 3 experiments. (B) The activity of IDO was determined by measurement of the concentration of tryptophan breakdown product l-kynurenine in the supernatant of virally transduced cells. (C) In order to determine the functional effect of IDO expression, we used variable numbers of virally transduced iDCs (top row) or mDCs (bottom row) as stimulators in an allogeneic MLR performed in the presence (right column) or the absence (left column) of 500 μM 1-MT, an IDO inhibitor. 3H-thymidine incorporation was determined on day 5. The results are the mean plus or minus SD of triplicate cultures.
Viral vector up-regulation of IDO has a significant consequence on MLR. (A) Five days after viral transduction of either iDCs or mDCs (activated with 20 ng/mL TNF-α, 20 ng/mL IL-1β, 20 ng/mL LPS, and 10 ng/mL PGE2 for 48 hours) with viral vectors, the cells were analyzed for expression of IDO using RT-PCR Southern and Western blots. The density of the IDO bands (as a ratio to the β-actin levels) are shown below each band, as the mean plus or minus SD of 3 experiments. (B) The activity of IDO was determined by measurement of the concentration of tryptophan breakdown product l-kynurenine in the supernatant of virally transduced cells. (C) In order to determine the functional effect of IDO expression, we used variable numbers of virally transduced iDCs (top row) or mDCs (bottom row) as stimulators in an allogeneic MLR performed in the presence (right column) or the absence (left column) of 500 μM 1-MT, an IDO inhibitor. 3H-thymidine incorporation was determined on day 5. The results are the mean plus or minus SD of triplicate cultures.
Similar, though less marked, iDC activation (in terms of cell-surface phenotype and cytokine secretion) was also seen following transduction with EAIV- and HIV-based lentiviral vectors. However, no increase in secretion of any of the cytokines tested was seen in mDCs following lentiviral transduction. These data are in contrast to one report that indicates HIV-based vectors do not activate DCs23 ; however, it should be noted that in our hands changes in DC phenotype were only seen at high MOIs.
The main pathway involved in the activation of iDCs to mDCs is that mediated through NFκB.49 However, there are several potential ways in which NFκB can be activated. In response to viral infection, DCs can be activated in response to dsRNA. This response is partially dependent on the cytosolic dsRNA-binding enzyme protein kinase R and does not require signaling through toll-like receptor 3 (TLR3), a surface receptor for dsRNA.9 Alternatively, plasmacytoid DCs have been shown to respond to wild-type influenza virus by a pathway that requires endosomal recognition of single-stranded RNA virus through TLR7- and MyD88-mediated signaling.10 In this report, we show that viral vectors activated the 2-5A synthetase/RNase L and the protein kinase R (PKR) pathway, resulting in production of Th1 and proinflammatory cytokines as well as IFN-α and -β.
Ad-mediated activation and maturation of DCs was recently attributed to the high levels of TNF-α expression by murine bone marrow-derived DCs, comparable to levels observed with LPS exposure.41 Ad-induced TNF-α production was found to be necessary for DC maturation but was not dependent on the MyD88 signaling pathway. It was shown to be dependent on signaling by phosphoinositide-3-OH kinase (PI3K), as determined by wortmannin and LY294 002 blocking experiments. It was proposed that integrin-mediated PI3K induction of NFκB activates an autocrine TNF-α pathway required for DC maturation in response to Ad. While our observations in human DCs are consistent with this, in as much as we saw the activation of PI3 kinase and high production of TNF-α following Ad infection, our data indicate that activation of dsRNA-triggered antiviral pathways may also be important.
The phenotypic changes seen following viral transduction are reflected in functional alterations. Thus, as might be expected from the up-regulation of costimulatory molecules and MHC class II expression, iDCs transduced with Ad or lentiviral vectors showed an increased ability to act as stimulators in an MLR. In contrast, and somewhat surprisingly, mDCs that have been transduced with Ad show a reduced ability to stimulate an MLR. This has been reported previously,39 and it has been suggested that this effect is due to viral immunodominance and/or the expression of immunomodulatory viral proteins.39 However, we have shown that DCs respond to Ad transduction by up-regulation of the IDO enzyme, which is responsible for tryptophan catabolism. IDO has an important role in regulating the immune response, for example at the maternal-fetal interface.6 Increasingly, the expression of IDO by DCs, for example following crosslinking of CD80/86 with CTLA4-Ig, and the consequent release of IFN-γ, is recognized as being an important factor in immunoregulation.5,7 IDO down-regulates the T cells by depletion of tryptophan, essential for lymphocyte function, and possibly by the action of the metabolites such as kynurenine, which can have a profound effect on T-cell viability.5 We have shown that not only is IDO up-regulated in DCs following transduction with viral vectors but that in mDCs the use of the IDO inhibitor 1-MT restores the capacity of these cells to stimulate an MLR.
Similar up-regulation of IDO has been reported following infection of DCs with intracellular pathogens such as Listeria monocytogenes or Chlamydiae.50 In addition, IDO up-regulation has been seen in vivo following lung infection with influenza virus51 and LPS.52 It is interesting to speculate why IDO is up-regulated in DCs on infection. It may simply reflect a consequence of a pathway designed to prevent an overzealous immune response to infection. Alternatively, IDO may participate in feedback pathways that serve to regulate the induction of the immune response.
Up-regulation of IDO was seen in iDCs following Ad transduction, yet these cells were capable of stimulating an MLR and their function was not noticeably affected by addition of 1-MT. The action of IDO is affected by other molecules; for example, IL-6 has been reported to inhibit the function of IDO.53 It is therefore possible that in iDCs factors such as IL-6 serve to inhibit IDO while in Ad-transduced DCs, which show a massive up-regulation of IDO, these factors are overwhelmed.
In conclusion, we have shown that transduction with viral vectors, in particular Ad and lentiviral vectors, has dramatic consequences for DCs in terms of their phenotype, the activation pathways induced, and their function. Of particular interest in the context of immunomodulation is the activation of IDO, which in mDCs blocks their ability to stimulate T-cell responses. These data are important not only in the context of genetic manipulation of DCs for immunotherapy, but also in our understanding of the response of DCs to viral infection.
Prepublished online as Blood First Edition Paper, January 25, 2005; DOI 10.1182/blood-2004-10-3880.
Supported by research training fellowships and grants from the Medical Research Council (London, United Kingdom) and the Royal College of Surgeons Edinburgh (P.H.T.); the Gertrud-Kusen-Foundation, Hamburg, Germany (S.C.B.); the TFC Frost Charitable Trust (J.C.M.; Escher, United Kingdom), and the Leukaemia Research Fund (London, United Kingdom) (S.A.X. and H.J.S.). A.G. is a BBSRC (Biotechnology and Biological Sciences Research Council, Swindon, United Kingdom) Research Development Fellow.
An Inside Blood analysis of this article appears at the front of the issue.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors would like to thank Oxford Biomedica for providing the EIAV and HIV vectors.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal