Abstract
This case report is on a 40-year-old male patient with chronic myeloid leukemia (CML) receiving an allogeneic hematopoietic stem cell transplantation (HSCT) in first chronic phase from an HLA-identical sibling brother. He suffered from alopecia universalis occurring 11 years previously. The alopecia involved all body hair, including eyebrows and eyelashes. Between day 40 and day 55 after transplantation, hair started to grow on the chin, eyelashes, and on the top of his head. Immunosuppression was stopped at 6 months because of cytogenetic relapse and incomplete donor chimerism with some renewed hair loss. He returned to full donor chimerism with mild chronic graft-versus-host disease and continued hair growth. With 2 years of follow-up he has remained in continuous remission. Chimerism analyses of hair follicles did not show any donor alleles. Alopecia universalis is probably a chronic autoimmune disorder, curable with replacement of the immune system with an allogeneic HSCT. (Blood. 2005;105:426-427)
Introduction
Alopecia universalis is defined as nonscarring loss of all body hair, characterized by sudden onset independent of age and sex. The cause is unknown. Genetic, environmental, and individual etiologic factors are discussed. Its association with other autoimmune disorders renders an autoimmune pathogenesis very likely, the targeted antigen being private to the hair follicle. Histology is characterized by peribulbar lymphocyte or eosinophilic infiltration. There is no established treatment, and hair loss is usually definitive. We present a case of alopecia universalis of 12 years' duration, with hair growth after allogeneic hematopoietic stem cell transplantation (HSCT) for leukemia.
Case report
A 40-year-old patient was referred for hematopoietic stem cell transplantation from his HLA-identical brother to treat BCR/ABL-positive chronic myeloid leukemia (CML) in chronic phase, diagnosed 5 months previously. Bone marrow was hypercellular without signs of acceleration. Initial treatment was with hydroxyurea.
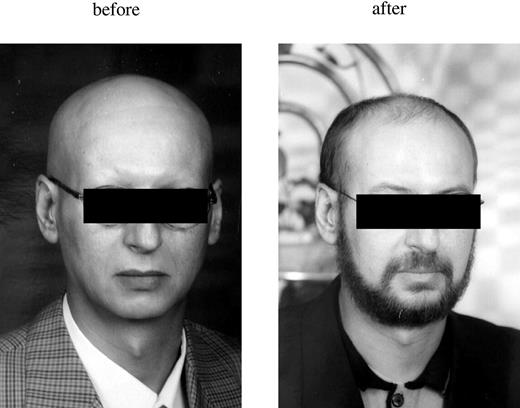
The patient's history revealed total body hair loss, including eyebrows and eyelashes, occurring suddenly 12 years previously (Figure 1), diagnosed as alopecia universalis. At that time he was healthy, denied exposures to toxins, and ascribed alopecia to stress associated with his mother being ill. The family history was negative for autoimmune disease and leukemia. He was positive for the alopecia universalis-associated HLA class II antigen DQB1*0301.
Hair growth. The left panel shows hair growth prior to the patient having been diagnosed with CML; the right panel shows hair growth 3 months after HSCT. Hair growth after transplantation includes the top of the head, as well as the beard and eyebrows.
Hair growth. The left panel shows hair growth prior to the patient having been diagnosed with CML; the right panel shows hair growth 3 months after HSCT. Hair growth after transplantation includes the top of the head, as well as the beard and eyebrows.
He underwent conditioning with 120 mg/kg cyclophosphamide and 12 Gy fractionated total body irradiation and received a non-T-cell-depleted allogeneic peripheral blood stem cell graft (6.29 × 106 CD34 cells per kilogram) from his HLA-identical brother. Graft-versus-host disease (GvHD) prophylaxis was with cyclosporine and short-course methotrexate. The early posttransplantation course was uneventful; he engrafted with more than 0.5 × 109/L neutrophils on day +21, and there were no infectious complications and no signs of GvHD. On day +100 after transplantation he was in good health with normal blood counts; the leukemia was in complete hematologic and cytogenetic remission, and chimerism studies showed the blood cells to be of 100% donor origin.
Remarkably, on day +40 hair started to grow on his upper lip and chin. Eyebrows and eyelashes grew back. On day +55 hair started to grow on the top of his head and on day +80 hair grew on his chest (Figure 1). Chimerism studies of hair follicles showed them to be completely of recipient origin.
Briefly, 50 hairs including follicles were pulled in sterile conditions from the scalp using phosphate-buffered saline (PBS)-rinsed forceps and collected in a 50 mL Falcon tube, according to the methodology of the Institute for Legal Medicine. DNA was extracted with the QIAamp DNA Micro Kit (Qiagen, Hilden, Germany). For the amplification with a multiplex short tandem repeat (STR) polymerase chain reaction (PCR), the AmpFlSTR Profiler (Applied Biosystems, Weiterstadt, Germany) STR multiplex PCR amplification kit, amplifying 9 different STR loci and the Amelogenin locus, discriminating X and Y chromosomes, was used. PCR fragments were separated by capillary electrophoresis on an ABI Prism 310 Genetic Analyzer (Applied Biosystems). Fragment size and peaks were analyzed using the Genescan Analysis Software (Applied Biosystems). Informative peaks (ie, loci different in recipient and donor) were used for calculation of the donor proportion.1 Sensitivity for detecting a minor population of alleles is 1% to 3% in peripheral blood and 1 of 50 hairs.
On day +180 a cytogenetic relapse was diagnosed with mixed hematopoietic chimerism. Some of the hair that had grown back started to fall out again. Histology of a scalp biopsy showed sparse peribulbar lymphocytic infiltrates. Immunosuppression with cyclosporine was stopped, and he was started on imatinib. He developed mild biopsy-proven chronic GvHD limited to the liver (elevated liver function tests [LFTs]), spontaneously regressing without specific treatment. He returned to full donor chimerism and BCR/ABL negativity. Imatinib was stopped 2 years after transplantation. He remains in complete hematologic, cytogenetic, and molecular remission with full donor hematopoietic chimerism and with a scalp of hair as shown in Figure 1. Repeated chimerism analyses of his hair follicles did not show any donor alleles.
Discussion
This case demonstrates that long-lasting alopecia universalis may recover completely after allogeneic HSCT. This supports the concept of an autoimmune pathogenesis of alopecia universalis. Intense immunosuppression combined with replacement of the immune system by donor cells can induce regrowth of body hair. Long-lasting alopecia universalis is considered to be irreversible, and there is no established treatment. Alopecia areata and, more rarely, alopecia universalis may respond to immunosuppressive or immunomodulatory treatment, but no confirmed treatment exists. This case report, supplemented by similar findings in a case after autologous HSCT,2 implies that the pattern of hair loss in alopecia universalis must be due to reversible inhibition of hair growth without complete destruction of hair follicles.
The antigen in alopecia universalis is not defined, and it is currently unknown to what degree cellular and/or humoral immunity against hair follicle antigens are involved. The association of alopecia universalis with other types of autoimmune disease, the identification of hair follicle-specific autoantibodies in animal models, the ability to induce alopecia in an animal model by transfer of skin from affected to naive individuals, the induction of disease by transfer of lymphocytes to human skin grafted to severe combined immunodeficiency (SCID) mice, and inhibiting hair loss by removal of T lymphocytes or treatment with antibodies restricting mobility of CD8+ cells all suggest that alopecia universalis is a tissue-restricted autoimmune disease.3-7 The association of alopecia universalis with human leukocyte antigens (HLAs), specifically with DRB1*0401 and DQB1*0301, has been described.8 The multifactorial nature of this disease, with genetic predisposition representing just one aspect, is made evident by the absence of alopecia in the patient's brother, who shares the same HLA haplotypes.
We performed chimerism studies of hair follicles because of the possibility of pluripotent stem cells from the donor contributing to hair growth in the recipient. Interest has been stimulated by reports of donor cell microchimerism in liver and gut biopsies of allogeneic stem cell transplant recipients.9 We found no evidence of donor origin in the hair follicles examined.10 The PCR approach used is possibly sensitive to contamination by blood cells. We have minimized these risks by careful washing. Contamination can be excluded in this case by the fact that we found no donor alleles in the hair analyzed.
The mechanism of response is unknown. The conditioning regimen is highly immunosuppressive and might have induced a remission of alopecia universalis in its own right. Replacing the recipient's immune system by the allogeneic graft might restore normal lymphocyte ontogeny. Posttransplantation immunosuppressive therapy with cyclosporine might also contribute. The early reappearance of alopecia during a phase of transient mixed chimerism and the stability of response over 2 years after establishing full donor chimerism favors a concept of eradication of autoreactive cells. This observation is in line with other reports of response to immunosuppressive therapy.11-13
HSCT is currently under investigation as a treatment for severe autoimmune disease. Allogeneic and, more frequently, autologous HSCT is used. Durable responses have been reported in patients receiving allogeneic and autologous HSCT. These observations as well as this case report all suggest the possibility of treating autoimmune diseases by eradication of autoreactive cells. This possibly could be achieved via high-dose immunoablation or a graft-versus-host immunity effect.13-17
In conclusion, complete recovery of alopecia universalis after allogeneic HSCT adds evidence to the autoimmune disease hypothesis of alopecia. Moreover, this case shows alopecia universalis to be a reversible condition.
Prepublished online as Blood First Edition Paper, April 8, 2004; DOI 10.1182/blood-2004-01-0136.
Supported in part by the Swiss National Research Foundation grant NF 32-52756.97 and the Horten Foundation.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal