Abstract
The AML1-CBFβ transcription factor complex is the most frequent target of specific chromosome translocations in acute myeloid leukemia (AML). The promyelocytic leukemia (PML) gene is also frequently involved in AML-associated translocation. Here we report that a specific isoform PML I forms a complex with AML1. PML I was able to recruit AML1 and coactivator p300 in PML nuclear bodies and enhance the AML1-mediated transcription in the presence of p300. A specific C-terminal region of PML I and a C-terminal region of AML1 were found to be required for both their association and colocalization in the nuclear bodies. Overexpression of PML I stimulates myeloid cells to differentiate. These results suggest that PML I could act as a mediator for AML1 and its coactivator p300/CBP to assemble into functional complexes and, consequently, activate AML1-dependent transcription and myeloid cell differentiation. (Blood. 2005;105:292-300)
Introduction
Chromosome translocations are frequently detected in 50% to 70% of human leukemia. The AML1 (CBFA2/PEBP2αB/RUNX1) gene is the most frequent target of leukemia-associated chromosome translocations.1 The AML1 gene was first cloned at the breakpoint of chromosome 21 in t(8;21) translocation, which is frequently found in the M2 subtype of acute myeloid leukemia (AML).2 So far, it has been reported that AML1 is disrupted by other translocations such as t(3;21),3,4 t(12;21),5,6 and t(16;21).7,8 AML1 also has been found to be mutated in familial platelet disorder (FPD) associated with a predisposition to leukemia9 and in sporadic cases of AML and myelodysplastic syndrome (MDS) without chromosome translocations.10-12 AML1 protein forms a heterodimer with CBFβ and binds to the specific DNA sequence TGT/cGGT13,14 to regulate the expression of a number of hematopoietic genes. Both AML1 and CBFβ are essential for the development of all lineages of definitive hematopoiesis.15-19 AML1 is hypothesized to act as an organizing factor that facilitates the assembly of transcriptional activation complexes. In this regard, AML1 could synergize with other transcription factors such as AP-1,20 C/EBPa,21 c-Myb,22 PU1,23 and Ets-124 to activate transcription. Furthermore, AML1 cooperates with coactivators such as YAP,25 ALY,26 Ear-2,27 p300/CREB-binding protein (CBP),28 and MOZ.29 On the other hand, AML1 has been reported to function as a transcriptional repressor of some target genes via its interaction with corepressors like TLE1,30,31 SIN3, and NCoR.32 Notably, the genes encoding some of the AML1-interacting proteins are also the targets of leukemia-associated chromosome translocations. In particular, CBFβ is disrupted in inv(16)33 ; p300 is disrupted in t(8;22)34,35 and t(11; 22)36 ; CBP is disrupted in t(8;16)37 and t(11;16)38-40 ; and MOZ is disrupted in t(8;16),37 inv(8),41 and t(8;22).34,35 Thus, understanding the contexts in which AML1 acts in cooperation with multiple factors is necessary in order to elucidate the regulatory function of AML1 in hematopoiesis and the mechanisms of leukemogenesis caused by chromosome translocations that disrupt AML1 and its cofactors.
The promyelocytic leukemia (PML) gene is involved in the chromosome translocation t(15;17), which occurs in most cases of the acute promyelocytic leukemia (APL) M3 subtype of AML.42-47 Targeted disruption of the PML locus demonstrated that PML could control the differentiation of hematopoietic progenitors, cell proliferation, and tumorigenesis.48 PML coordinates tumor suppressive functions such as induction of apoptosis, growth arrest, and cellular senescence.49 PML concentrates in speckled subnuclear structures, termed PML nuclear bodies (NBs)/ND10/PODs, together with many proteins (Zhong et al50 ). The proteins that can colocalize with PML into NBs include Sp100, p53, pRb, Daxx, and CBP, which suggests that PML could modulate, at least in part from the NBs, a variety of nuclear processes such as gene expression and genome stabilization. PML can modulate transcription by recruiting transcription factors such as p53 to NBs. Recruitment of p53 to the PML-NBs was shown to accompany Ras- and PML-induced senescence and p53-dependent apoptosis.51-53 Although PML-/- mice have been shown to have remarked reduction of granulocyte and monocytes in their peripheral blood and bone marrow,48 the current understanding of the function of PML is not enough to explain the deficiency in myeloid hematopoiesis. Multiple isoforms of PML are generated as a result of alternative splicing54 (Jensen et al55 ). Whether these isoforms possess the specific functions remains largely unexplored.
In this study, we found that the 2 major leukemia-associated proteins, AML1 and PML, can function in the same complex. A specific PML isoform could interact with AML1 transcription factor, target AML1 into nuclear bodies together with its coactivator p300, enhance AML1-mediated transcription, and stimulate differentiation of myeloid cells. Our findings suggest cooperation of AML1 and PML in hematopoiesis.
Materials and methods
Cells and retrovirus
Murine myeloid progenitor L-G cells were cultured in RPMI medium supplemented with 10% fetal calf serum (FCS), recombinant mouse interleukin-3 (IL-3) (0.1 ng/mL) and 50 μM β-mercaptoethanol. To monitor the growth and differentiation of myeloid cells in response to granulocyte colony-stimulating factor (G-CSF), L-G cells were maintained in the medium with G-CSF (5 ng/mL) instead of IL-3. Every day, viable cells were counted using a Coulter counter (Beckman Coulter, Hialeah, FL). To evaluate the cell differentiation, cells were stained with May-Gruenwald and Giemsa solutions. BOSC23 and SaOS2 cells were maintained in Dulbecco modified Eagle medium (DMEM) supplemented with 10% FCS. To produce the retrovirus, BOSC23 cells were transfected with LNCX-derived vectors by the calcium phosphate precipitation method, and the culture supernatants were recuperated 48 hours after transfection. For infection, L-G cells were incubated in the culture supernatant of BOSC23 transfectants with 4 μg/mL polybrene for 24 hours and then selected with antibiotic G418 (1 mg/mL).
Plasmids
The AML1 expression vectors pLNCX-FLAG-AML1a and pLNCX-FLAG-AML1b have been previously described.28
PML isoforms. The PML expression vector pCMV-HA-PML, which was a generous gift from Dr A. Kakizuka (Kyoto University),44 carries the PML transcript corresponding to human PML VI. To construct the expression vector for other human PML isoforms I-V, we isolated cDNA fragments encoding the specific 5′-terminal region of these isoforms from the cDNA pool of HL60 cells by reverse transcriptase-polymerase chain reaction (RT-PCR) using the up-primer 5′-ATCCAAGAAAGCCAGCCCAGAG-3′ (1208-1229) common to all, and the down primers 5′-TGGGAATGCTGTGATGGGTG-3′ (2749-2730), 5′-ACTAAATTAGAAAGGGGTGGGG-3′ (1975-1953), and 5-AGCACAGCTTGGGACTCAATGC-3′ (1865-1844), specific to PML I (M79462), PML IV (X63131), and PML V (M79463), respectively, and the 5′-GGTGTTGGCTGCAAGGTTACAAG-3′ (2527-2505) that was shared by isoforms II (M79464) and III (S50913). These fragments that represented the specific C-termini of the individual isoforms were replaced with specific sequences of PML VI on the expression vector pCMV-HA-PMLVI by using appropriate enzymes. The sequences of these constructs were checked by DNA sequencing. Subsequently, full-length cDNA of the PML isoforms I-VI were subcloned into a retrovirus-derived LNCX vector by using HindIII and NotI restriction enzymes.
PML I mutants. We introduced point mutations into PML I by the method of site-specific mutagenesis using overlapping extension PCR. To create unSUMOylated PML protein, which could not be SUMOylated, we substituted 3 lysine residues at codon 65, 160, and 490, which were found be modified by SUMO-156 into arginine (AAG65Lys-AGG65Arg, AAG160Lys-AGG160Arg, and AAG490Lys-AGG490Arg, bold letters indicate substituted nucleotides). Deletion mutants of PML I were generated by using appropriate restriction enzymes.
mCCP1-luc reporter. Mouse mCCP1 promoter (-243 to +27) was isolated by PCR and subcloned between the NheI and HindIII sites of the pGL3-Basic Vector (Promega, Madison, WI).
Identification of proteins in the AML1b complex
We purified AML1b complex and identified peptides in the AML1b complex as previously described.29 Briefly, cell lysates were prepared from L-G cell infectants that stably expressed AML1b tagged with FLAG peptide in lysis buffer (250 mM NaCl, 20 mM sodium phosphate pH 7.0, 30 mM sodium pyrophosphate, 10 mM NaF, 0.1% NP-40, 5 mM dithiothreitol (DTT), 1 mM phenylmethyl sulforyl fluoride [PMSF]) supplied with Complete protease inhibitor (Roche, Mannheim, Germany), and then cleared by ultracentrifugation with a Hitachi RP50T2 rotor (Hitachi, Tokyo, Japan) at 4°C, 81 000 g (30 000 rpm) for 30 minutes. The supernatant was incubated with anti-FLAG antibody conjugated agarose beads (Sigma, St Louis, MO) and rotated at 4°C for 8 to 10 hours. After extensive wash with lysis buffer, the absorbed proteins were eluted from agarose beads with FLAG peptide in lysis buffer (0.2 mg/mL) for 1 hour. The eluents were concentrated by using a filtration device (Vivaspin, 10K-PES; Sartorius, Hannover, Germany) and separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were stained with Coomassie brilliant blue, excised, destained with 25 mM ammonium bicarbonate and 50% acetonitrile, dried, digested with sequence grade modified trypsin (Promega) in 50 mM Tris [tris(hydroxymethyl) aminomethane] pH7.6, extracted with 5% trifluoroacetic acid (TFA), 50% acetonitrile, and subjected to liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS) analysis.
Immunoprecipitation, immunoblotting, and antibodies
Cell lysates were prepared in lysis buffer (250 mM NaCl, 20 mM sodium phosphate pH 7.0, 30 mM sodium pyrophosphate, 10 mM NaF, 0.1% Nonidet P-40 (NP-40), 5 mM DTT, 1 mM phenylmethyl sulfonyl fluoride [PMSF]) supplied with complete protease inhibitor (Roche). After removal of cell debris by ultracentrifugation with a Beckman TLA55 rotor (Beckman Coulter, Marseilles, France) at 4°C and 72 000 g (40 000 rpm) for 30 minutes, the supernatant was incubated with anti-FLAG antibody-conjugated agarose beads (Sigma) and slightly rotated at 4°C for 8 to 10 hours. The absorbed beads were extensively washed with lysis buffer. Precipitated proteins were eluted from the beads by 200 μL of lysis buffer containing the FLAG peptide at a final concentration of 2 mg/mL. The eluate was concentrated by using filtration devices (Vivaspin 10K-PES; Sartorius) and dissolved with the same volume of 2 × SDS-PAGE sample buffer (125 mM Tris-HCl, 4% SDS, 10% β-mercaptoethanol) and then subjected to SDS-PAGE. When immunoprecipitation was not performed, total protein lysates were prepared in 2 × SDS-PAGE sample buffer. Antibodies were detected by chemiluminescence using enhanced chemiluminescence (ECL) plus Detection Reagents (Amersham Biosciences, Buckinghamshire, United Kingdom). Primary antibodies were anti-FLAG (M2) (Sigma), anti-HA (3F10) (Roche), anti-human AML rabbit polyclonal,28 anti-human PML rabbit polyclonal (PML001) and mouse monoclonal (1B9) (MBL), anti-mouse PML rabbit polyclonal,48 and anti-p300 rabbit polyclonal (N15) (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies were used in this study.
Immunofluorescence analysis
SaOS2 cells were plated at 1 × 105 per chamber on 4 chamber vessels (BD Falcon, Bedford, MA), the day before transfection. Plasmid DNA was transfected by using Lipofectamine2000 reagent (Invitrogen, Carlsbad, CA) according to the supplier's instructions. Four hours after transfection, the medium was refreshed, and cells were incubated at 37°C for a further 36 hours for gene expression. Before staining, transfectants were washed with phosphate-buffered saline (PBS). Cells were fixed with 4% paraformaldehyde in PBS for 10 minutes and washed twice in PBS. Next, cells were permeabilized with 0.1% Triton-X 100 in PBS for 3 minutes, washed 4 times with PBS and blocked with 10% FCS in PBS for 2 hours at room temperature. Cells were incubated with primary antibodies at a final concentration of 2 μg/mL in PBS containing 3% bovine serum albumin (BSA) at 4°C for 10 hours. Primary antibodies were revealed by a 4-hour incubation with appropriate fluorescence-conjugated secondary antibodies (Santa Cruz Biotechnology) in a 100-fold dilution in PBS containing 3% BSA. For double staining, cells were incubated with the 2 antibodies under the same conditions. Slides were mounted in mounting liquid Vectashield containing 0.4 mg/mL 4′,6-diamidino-2-phenylindole (DAPI) to visualize nuclear DNA (Vector Laboratories, Burlingame, CA). The images under fluorescence microscope (Olympus, Melville, NY) at magnification × 60 were captured with a CoolSNAP-HQ CCD camera (Roper Scientific, Ottobrunn, Germany).
Luciferase assay
Wild-type or PmL-/- mouse embryo fibroblasts (MEF) cells48 were transfected by the calcium phosphate precipitation method in 24-well plates, and luciferase activity was assayed 48 hours later using a luminometer Lumat LB9507 (Berthold, Bad Wildbad, Germany) according to the manufacturer's protocol (Promega). Results of reporter assays represent the average values for relative luciferase activity generated from 3 independent experiments that were normalized using the activity of the enzyme from pRL-CMV as an internal control.
Results
AML1b forms complex with a specific isoform PML I
We previously purified the AML1 complexes from a cell lysate of L-G cells expressing a FLAG-tagged full-length AML1 (AML1b) or a splicing variant lacking a C-terminal transcriptional activation domain (AML1a)29 (Figure 1A). Mass spectrometry analysis indicated that the 125-kDa protein in the AML1b complex contained the sequences that are found in murine promyelocytic leukemia (PML) protein (Figure 1B). To confirm this interaction, we performed immunoblot analysis using anti-mouse PML antibody and found that PML was certainly contained in the AML1b fraction (Figure 1C). To test interaction of endogenous AML1 and PML, cell lysates were prepared from K562 myeloid cells and then immunoprecipitated with anti-human PML monoclonal antibody (1B9). Immunoblot analysis using anti-human AML1 rabbit polyclonal antibody indicated that the immunoprecipitates obtained with anti human PML monoclonal antibody (1B9) contained AML1, whereas the immunoprecipitates with control IgG did not contain AML1 (Figure 1D). These results suggested that the PML protein might be a binding factor of AML1b.
PML I is a part of the AML1 complex. (A) Purification of the AML1b complex. The complexes were purified from cell lysates prepared from L-G infectants carrying empty vector (mock), stably expressing FLAG-tagged AML1a (AML1a, a splicing variant lacking the C-terminal transcriptional regulatory domain), and FLAG-tagged full-length AML1b proteins (AML1b). The complexes were immunoprecipitated on anti-FLAG antibody-conjugated agarose, and the bound materials were eluted with the epitope peptide. Proteins were resolved by SDS-PAGE and visualized by silver staining. The proteins were identified by mass analysis. (B) The amino acid sequences of peptides derived from the 125-kDa protein specific to the fraction purified from the FLAG-AML1b expressing cells. The protein was identified as PML (accession number NM178087). (C) Detection of PML protein in the AML1 complex. The purified AML1 complexes were analyzed by immunoblot with anti-mouse PML antibody (rabbit polyclonal, Wang et al, 1998). (D) Interaction of endogenous AML1 and PML proteins. Cell lysates were prepared from 5 × 107 K562 cells and then were immunoprecipitated with anti-human PML monoclonal antibody 1B9 (MBL) or control IgG. The immunoprecipitates were analyzed by immunoblot with anti-mouse AML1 rabbit polyclonal antibody.
PML I is a part of the AML1 complex. (A) Purification of the AML1b complex. The complexes were purified from cell lysates prepared from L-G infectants carrying empty vector (mock), stably expressing FLAG-tagged AML1a (AML1a, a splicing variant lacking the C-terminal transcriptional regulatory domain), and FLAG-tagged full-length AML1b proteins (AML1b). The complexes were immunoprecipitated on anti-FLAG antibody-conjugated agarose, and the bound materials were eluted with the epitope peptide. Proteins were resolved by SDS-PAGE and visualized by silver staining. The proteins were identified by mass analysis. (B) The amino acid sequences of peptides derived from the 125-kDa protein specific to the fraction purified from the FLAG-AML1b expressing cells. The protein was identified as PML (accession number NM178087). (C) Detection of PML protein in the AML1 complex. The purified AML1 complexes were analyzed by immunoblot with anti-mouse PML antibody (rabbit polyclonal, Wang et al, 1998). (D) Interaction of endogenous AML1 and PML proteins. Cell lysates were prepared from 5 × 107 K562 cells and then were immunoprecipitated with anti-human PML monoclonal antibody 1B9 (MBL) or control IgG. The immunoprecipitates were analyzed by immunoblot with anti-mouse AML1 rabbit polyclonal antibody.
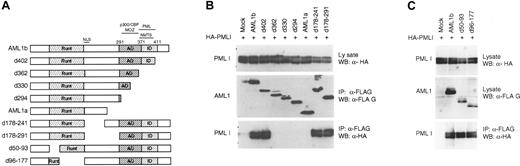
Multiple isoforms of PML proteins are generated by alternative usage of 3′ exons.55 To verify the possible association between AML1b and PML, the FLAG-tagged major isoforms PML I-VI (Figure 2A) were cotransfected with HA-AML1b into BOSC23 cells. Cell lysates were immunoprecipitated with anti-FLAG antibody. Immunoblot analysis of the immunoprecipitate (IP) samples showed that only PML I could interact with AML1b (Figure 2B). These results indicated that PML I is one of the components in the multiple protein complex assembled by AML1b.
PML I isoform specifically interacts with AML1b. (A) Schematic representation of the PML protein and genomic structure of various PML transcripts. The PML protein contains the RBCC motif that consists of the RING finger domain (RING), the 2 B boxes (B1 and B2), and the helical coiled-coil region. The proline rich region (P) and serine-proline-rich region (S-P) have been thought to contain the putative phosphorylation sites.44 SUMOylation sites at lysines 65, 160, and 49056 are indicated. The PML gene consists of 9 exons (shaded boxes) with exon 7 divided into exons 7a and 7b, and the introns being retained in several transcripts as a consequence of alternative splicing (black boxes).54 The nomenclature for PML isoforms proposed by Jensen55 was used in this study. The alternative usage of 3′ exons generates multiple transcripts I-VI. In the case of PML III and PML VI, the encoding regions in which frame-shift occurred as a result of the retained intron are indicated by the gray color. The total number of amino acids for each isoform is given on the right side. (B) The interaction with AML1b is specific to the PML I isoform. BOSC23 cells were cotransfected with HA-tagged AML1b and together either with control vector (mock) or FLAG-tagged PML isoforms (I-VI). The expression of AML1 in the lysates of transfectants was detected by immunoblotting using anti-HA (3F10) antibody (top). The lysates of transfectants were immunoprecipitated with anti-FLAG (M2) antibody. The immunoprecipitates were analyzed by immunoblotting using anti-FLAG (M2) (middle) and anti-HA (3F10) antibodies (bottom). (C) Identification of regions of PML I required for interaction with AML1b. BOSC23 cells were cotransfected with FLAG-AML1b and either with control vector (mock) or constructs of PML I as indicated. The expression of PML I in the lysates of transfectants were detected by immunoblotting using anti-HA antibody (3F10) (top). The lysate of transfectants were immunoprecipitated with anti-FLAG (M2) antibody and were analyzed by immunoblotting using anti-FLAG (M2) (middle) and anti-HA (3F10) antibodies (bottom).
PML I isoform specifically interacts with AML1b. (A) Schematic representation of the PML protein and genomic structure of various PML transcripts. The PML protein contains the RBCC motif that consists of the RING finger domain (RING), the 2 B boxes (B1 and B2), and the helical coiled-coil region. The proline rich region (P) and serine-proline-rich region (S-P) have been thought to contain the putative phosphorylation sites.44 SUMOylation sites at lysines 65, 160, and 49056 are indicated. The PML gene consists of 9 exons (shaded boxes) with exon 7 divided into exons 7a and 7b, and the introns being retained in several transcripts as a consequence of alternative splicing (black boxes).54 The nomenclature for PML isoforms proposed by Jensen55 was used in this study. The alternative usage of 3′ exons generates multiple transcripts I-VI. In the case of PML III and PML VI, the encoding regions in which frame-shift occurred as a result of the retained intron are indicated by the gray color. The total number of amino acids for each isoform is given on the right side. (B) The interaction with AML1b is specific to the PML I isoform. BOSC23 cells were cotransfected with HA-tagged AML1b and together either with control vector (mock) or FLAG-tagged PML isoforms (I-VI). The expression of AML1 in the lysates of transfectants was detected by immunoblotting using anti-HA (3F10) antibody (top). The lysates of transfectants were immunoprecipitated with anti-FLAG (M2) antibody. The immunoprecipitates were analyzed by immunoblotting using anti-FLAG (M2) (middle) and anti-HA (3F10) antibodies (bottom). (C) Identification of regions of PML I required for interaction with AML1b. BOSC23 cells were cotransfected with FLAG-AML1b and either with control vector (mock) or constructs of PML I as indicated. The expression of PML I in the lysates of transfectants were detected by immunoblotting using anti-HA antibody (3F10) (top). The lysate of transfectants were immunoprecipitated with anti-FLAG (M2) antibody and were analyzed by immunoblotting using anti-FLAG (M2) (middle) and anti-HA (3F10) antibodies (bottom).
AML1b binding domain is located within the specific C-terminus of PML I
Given that PML I differs from other isoforms in its C-terminus, we focused on this region to determine the AML1 binding site. Two kinds of C-terminal deletion mutants (mutant d851 and d592) were constructed. Since the leucine zipper in some proteins has been demonstrated to be essential for protein-protein interaction,57 we constructed a mutant lacking the region (dL-Z) in order to investigate whether this domain is required for the interaction of PML I with AML1b. IP-Western blot analysis indicated that deletion of the region from the C-terminal end to amino acid 851 did not affect the PML-AML1b interaction (Figure 2C). Further truncation to residue 592 resulted in the loss of association with AML1b. In addition, without the leucine zipper, mutant dL-Z still bound to AML1b. Thus, the AML1b interacting domain was found expanding from codon 592 to codon 851, which resides within the specific C-terminal region of PML I.
PML-interacting domain overlaps the nuclear matrix signal of AML1b
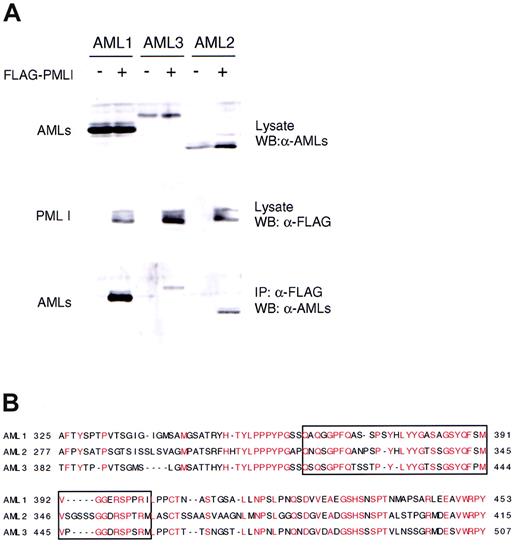
To determine the domains in AML1 that are required for interaction with PML I, we performed IP-Western analysis using a series of deletion mutants of AML1b (Figure 3A). Removal of 78 amino acids of AML1b from the C-terminal end did not affect the interaction with PML I (Figure 3B). However, further deletion up to codon 362 resulted in loss of the interaction. Truncation of amino acids 178-241 or 178-291 did not eliminate the interaction with PML I. Deletion of the Runt domain, which has been demonstrated to be essential for the association of AML1 with other proteins such as CBFβ, did not affect the binding to PML I (Figure 3C). Thus, the PML I interacting site is located between amino acids 362 and 402 within the C-terminal region of AML1b. This region was demonstrated to be indispensable for the function of AML1 in transcription regulation, myeloid cell differentiation, and hematopoiesis,28,58,59 and it overlaps with the nuclear matrix targeting signal (NMTS)60 (Figure 3A). Interestingly, 2 other members of the RUNX family, AML2 and AML3, could bind to PML I (Figure 4A). The comparison performed to examine for the presence of identical amino acids revealed the region in AML1, which we identified to be essential for the interaction with PML I, is highly conserved in AML2 and AML3 (Figure 4B).
PML I interacting domain is located at the C-terminal of AML1. (A) Schematic diagram of the structure of AML1 deletion mutants. The Runt, activation, and inhibition domains are indicated as Runt, AD, and ID, respectively. Nuclear import signal (NLS) and nuclear matrix targeting signal (NMTS) are shown. (B) Identification of regions of AML1b required for interaction with PML I. BOSC23 cells were cotransfected with PML I and control vector (mock) or AML1 constructions as indicated. The expression of PML I in the lysates of transfectants was detected by immunoblotting using anti-HA (3F10) antibody (top). The lysates of transfectants were immunoprecipitated with anti-FLAG (M2) antibody. The immunoprecipitates were analyzed by immunoblotting using anti-FLAG (M2) (middle) and anti-HA (3F10) antibodies (bottom). (C) The Runt domain is dispensable for the interaction of AML 1 with PML I. BOSC23 cells were cotransfected with PML I and full-length AML1b or deletion mutants of AML1 partially lacking Runt domain as indicated. The expression of PML I (top) and AML1 (middle) proteins in the cell lysates was detected with anti-HA antibody and anti-FLAG antibody, respectively. The immunoprecipitates with anti-FLAG antibody were analyzed using anti-HA antibody (lower).
PML I interacting domain is located at the C-terminal of AML1. (A) Schematic diagram of the structure of AML1 deletion mutants. The Runt, activation, and inhibition domains are indicated as Runt, AD, and ID, respectively. Nuclear import signal (NLS) and nuclear matrix targeting signal (NMTS) are shown. (B) Identification of regions of AML1b required for interaction with PML I. BOSC23 cells were cotransfected with PML I and control vector (mock) or AML1 constructions as indicated. The expression of PML I in the lysates of transfectants was detected by immunoblotting using anti-HA (3F10) antibody (top). The lysates of transfectants were immunoprecipitated with anti-FLAG (M2) antibody. The immunoprecipitates were analyzed by immunoblotting using anti-FLAG (M2) (middle) and anti-HA (3F10) antibodies (bottom). (C) The Runt domain is dispensable for the interaction of AML 1 with PML I. BOSC23 cells were cotransfected with PML I and full-length AML1b or deletion mutants of AML1 partially lacking Runt domain as indicated. The expression of PML I (top) and AML1 (middle) proteins in the cell lysates was detected with anti-HA antibody and anti-FLAG antibody, respectively. The immunoprecipitates with anti-FLAG antibody were analyzed using anti-HA antibody (lower).
PML could interact with 2 other members of RUNX family AML2 and AML3. (A) BOSC23 cells were cotransfected with PML I and AMLs as indicated. The expression of AML proteins (upper) and PML I (middle) in the lysate of transfectants was analyzed by immunoblotting and detected with anti-AML antibody28 mixed with anti-AML3 antibody (Upstate Biotechnology, Charlottesville, VA) and anti-FLAG (M2) antibody, respectively. The lysate of transfectants were immunoprecipitated with anti-FLAG (M2) antibody, analyzed by immunoblotting, and probed with a mixture of anti-AML1 antibodies (lower). (B) Comparison of amino acid sequences for the C-terminal of AML1, AML2, and AML3. Identical amino acids are indicated in red. The boxed region corresponds to the PML I interacting domain of AML1.
PML could interact with 2 other members of RUNX family AML2 and AML3. (A) BOSC23 cells were cotransfected with PML I and AMLs as indicated. The expression of AML proteins (upper) and PML I (middle) in the lysate of transfectants was analyzed by immunoblotting and detected with anti-AML antibody28 mixed with anti-AML3 antibody (Upstate Biotechnology, Charlottesville, VA) and anti-FLAG (M2) antibody, respectively. The lysate of transfectants were immunoprecipitated with anti-FLAG (M2) antibody, analyzed by immunoblotting, and probed with a mixture of anti-AML1 antibodies (lower). (B) Comparison of amino acid sequences for the C-terminal of AML1, AML2, and AML3. Identical amino acids are indicated in red. The boxed region corresponds to the PML I interacting domain of AML1.
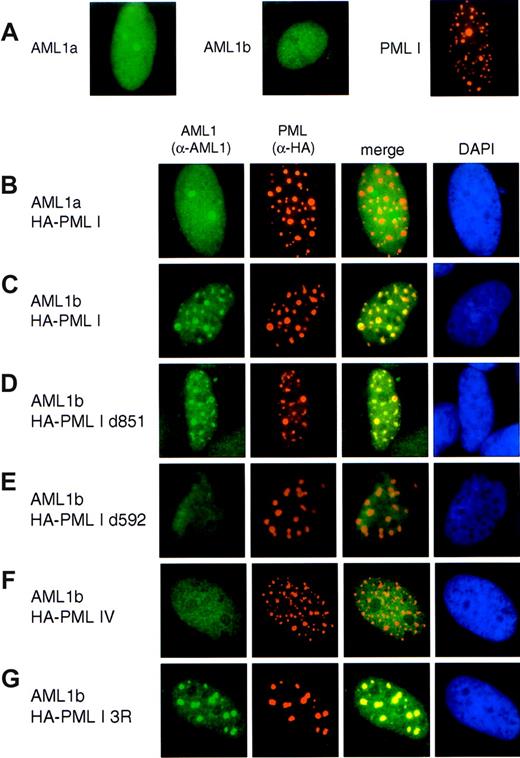
PML I targets AML1b into nuclear bodies
The association with PML was demonstrated to be essential for the recruitment of pRb, CBP, Daxx, and p53 into NBs,61-64 prompting us to examine whether AML1b, when binds to PML I, could accumulate into NBs. In order to determine the subnuclear localization of AML1 proteins in relation to PML, double immunofluorescent staining of transiently coexpressed AML1 and PML was performed. AML1 proteins were coexpressed with HA-tagged PML proteins in human osteosarcoma (SaOS2) cells, and location of the expressed proteins were detected by anti-AML1 or anti-HA antibodies, respectively. Without cotransfection with PML I, both AML1a and AML1b were nuclear diffuse (Figure 5A). Being coexpressed with PML I, AML1b was localized to small dotlike structures (Figure 5C). These structures coincided with PML I NBs, as revealed by the merged image, implying that AML1b and PML I colocalized in NBs. In contrast, AML1a remained diffuse in the nuclei (Figure 5B). These results suggest that binding to PML I is essential for the accumulation of AML1b into nuclear bodies, since AML1a does not contain a binding domain for PML I. To further clarify this possibility, we investigated the subnuclear distribution of AML1b when coexpressed with PML I deletion mutants d851 or d592. As we expected, coexpressed AML1b was sequestered to NBs containing deletion mutant d851 (Figure 5D), but not to the one formed by mutant d592 (Figure 5E). Additionally, we could not find colocalization between AML1b and PML IV (Figure 5F), which is one of the PML isoforms unable to associate with AML1b. Lacking SUMO1 modification, PML I 3R forms several aggregates. AML1 b was found together with PML I 3R in these structures (Figure 5G).
PML I recruits AML1b into nuclear bodies. (A) SaOS2 cells were transfected with LNCX-FLAG-AML1a, LNCX-FLAG-AML1b, or LNCX-HA-PML I. (B,C) AML1b, not AML1a, is recruited into NBs. SaOS2 cells were cotransfected with LNCX-FLAG-AML1a (B) or LNCX-FLAG-AML1b (C) together with LNCX-HA-PMLI. (D,E) Localization of AML1b and PML I mutants. SaOS2 cells were cotransfected with LNCX-FLAG-AML1b together with either LNCX-HA-PMLI d851 (D) or LNCX-HA-PMLI d592 (E). (F) AML1b was not colocalized with PML IV into NBs. SaOS2 cells were cotransfected with LNCX-FLAG-AML1b and LNCX-HA-PML IV. (G) AML1b is presented in the aggregates formed by PML I 3R. SaOS2 cells were cotransfected with LNCX-FLAG-AML1b and LNCX-HA-PML I 3R. In all experiments, AML1 was stained with anti-human AML rabbit polyclonal antibody, as shown by the green color, and PML was stained with anti-HA antibody (3F10), as shown by the orange color. Merging of the 2 colors results in a yellow signal, indicating the colocalization of 2 proteins. Nuclear staining with DAPI is shown by the blue color.
PML I recruits AML1b into nuclear bodies. (A) SaOS2 cells were transfected with LNCX-FLAG-AML1a, LNCX-FLAG-AML1b, or LNCX-HA-PML I. (B,C) AML1b, not AML1a, is recruited into NBs. SaOS2 cells were cotransfected with LNCX-FLAG-AML1a (B) or LNCX-FLAG-AML1b (C) together with LNCX-HA-PMLI. (D,E) Localization of AML1b and PML I mutants. SaOS2 cells were cotransfected with LNCX-FLAG-AML1b together with either LNCX-HA-PMLI d851 (D) or LNCX-HA-PMLI d592 (E). (F) AML1b was not colocalized with PML IV into NBs. SaOS2 cells were cotransfected with LNCX-FLAG-AML1b and LNCX-HA-PML IV. (G) AML1b is presented in the aggregates formed by PML I 3R. SaOS2 cells were cotransfected with LNCX-FLAG-AML1b and LNCX-HA-PML I 3R. In all experiments, AML1 was stained with anti-human AML rabbit polyclonal antibody, as shown by the green color, and PML was stained with anti-HA antibody (3F10), as shown by the orange color. Merging of the 2 colors results in a yellow signal, indicating the colocalization of 2 proteins. Nuclear staining with DAPI is shown by the blue color.
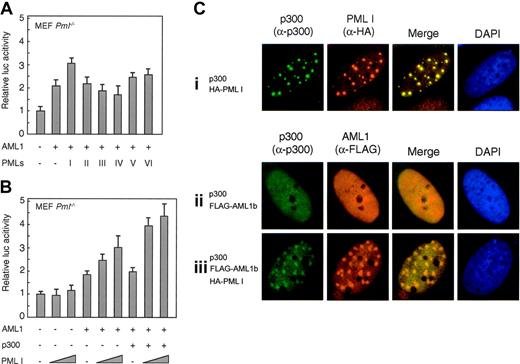
PML I stimulates cooperation of AML1 and p300
The effect of PML proteins on AML1-dependent transcription was investigated using the reporter gene under the control of the mCCP1 promoter, which contains 2AML1-binding sites.65 Figure 6A shows that among PML isoforms I-VI, only PML I significantly activates AML1-dependent transcription activity of the mCCP1 promoter. The stimulation by PML I was strongly enhanced when p300, which could function as a coactivator of AML1, was transfected together with PML I (Figure 6B). These results indicated that PML I could facilitate functional cooperation of AML1b and p300 in transcription activation. We therefore investigated the localization of AML1b and p300 in relation to PML I by immunostaining. When p300 and PML I were cotransfected into SaOS2 cells, they colocalized in PML-NBs (Figure 6Ci). Without PML cotransfection, both p300 and AML1b were nuclear diffuse (Figure 6Cii). In contrast, coexpression of PML I facilitated the accumulation of these proteins together in small dotlike structures (Figure 6Ciii). Thus, PML I could recruit both AML1 and its cofactor p300 to the same nuclear bodies. By this way, the functional cooperation of AML1 and p300 proteins might be enhanced.
PML I stimulates cooperation of AML1 and p300. (A) PML I specifically enhanced AML1-induced transcription. The effect of PML proteins on AML1-dependent transcription was investigated using the reporter gene under the control of mCCP1 promoter. PmL-/- MEF cells were spread in 24-well plates and transfected with 50 ng of reporter gene, 200 ng of pLNCX-AML1b, 30 ng of empty vector or PML isoforms as indicated, and 2 ng of pRL-CMV. (B) PML I stimulates the cooperated transcription activity of AML 1 and p300. PmL-/- MEF cells were spread in 24-well plates and transfected with 50 ng of CCP1-luc, 200 ng of pLNCX-AML1b (lanes 4-9), 250 ng of pCMV-p300 (lanes 6-9), and either 30 ng (lanes 2, 5, 8) or 100 ng (lanes 3, 6, 9) of pLNCX-PML I and 2 ng of pRL-CMV. Luciferase activity was assayed as described in the Experimental Procedure. (C) PML I recruits AML1b and p300 into nuclear bodies. SaOS2 cells were cotransfected with pCMV-p300 (i-iii) together with LNCX-FLAG-AML1b (ii, iii) and LNCX-HA-PMLI (i, iii). p300 stained with anti-p300 rabbit polyclonal antibody (N15) is shown by the green color, and PML (i) stained with anti-HA antibody (3F10) and AML1 (ii, iii) stained with anti-FLAG antibody (M2) are shown by orange color. Merging of the 2 colors results in a yellow signal, indicating the colocalization of 2 proteins. Nuclear staining with DAPI is shown by the blue color.
PML I stimulates cooperation of AML1 and p300. (A) PML I specifically enhanced AML1-induced transcription. The effect of PML proteins on AML1-dependent transcription was investigated using the reporter gene under the control of mCCP1 promoter. PmL-/- MEF cells were spread in 24-well plates and transfected with 50 ng of reporter gene, 200 ng of pLNCX-AML1b, 30 ng of empty vector or PML isoforms as indicated, and 2 ng of pRL-CMV. (B) PML I stimulates the cooperated transcription activity of AML 1 and p300. PmL-/- MEF cells were spread in 24-well plates and transfected with 50 ng of CCP1-luc, 200 ng of pLNCX-AML1b (lanes 4-9), 250 ng of pCMV-p300 (lanes 6-9), and either 30 ng (lanes 2, 5, 8) or 100 ng (lanes 3, 6, 9) of pLNCX-PML I and 2 ng of pRL-CMV. Luciferase activity was assayed as described in the Experimental Procedure. (C) PML I recruits AML1b and p300 into nuclear bodies. SaOS2 cells were cotransfected with pCMV-p300 (i-iii) together with LNCX-FLAG-AML1b (ii, iii) and LNCX-HA-PMLI (i, iii). p300 stained with anti-p300 rabbit polyclonal antibody (N15) is shown by the green color, and PML (i) stained with anti-HA antibody (3F10) and AML1 (ii, iii) stained with anti-FLAG antibody (M2) are shown by orange color. Merging of the 2 colors results in a yellow signal, indicating the colocalization of 2 proteins. Nuclear staining with DAPI is shown by the blue color.
PML I stimulates G-CSF-induced differentiation of myeloid cells
L-G is an IL-3-dependent myeloid precursor cell line that can be differentiated into mature neutrophils in response to G-CSF.66 AML1b strongly stimulates the G-CSF-induced differentiation of L-G cells.28,67 Therefore, we investigated whether PML I could elicit any effect on the proliferation and differentiation of L-G cells. To this end, we infected L-G cells with a retrovirus encoding PML I and followed up the growth and morphological changes of the infectants cultured in the medium containing G-CSF (5 ng/mL) (Figure 7). As expected, the overexpression of PML I wild-type inhibited the cell proliferation (Figure 7B) and conferred upon the L-G cells advantages for maturation into neutrophils. At day 6, most PML I wild-type infectants differentiated into mature neutrophils, whereas control cells did not (Figures 7C, E). The cells expressing the 3R mutant that lacks all SUMOylation sites proliferated exponentially (Figure 7B) and remained in its immature/intermediate status until day 8, when control cells differentiated into neutrophils (Figures 7D, F). Altogether, these results indicate that PML could be involved in the regulation of myeloid differentiation and that SUMO-1 modification also are essential for the function of PML I.
PML I inhibits the proliferation and stimulates the differentiation of murine myeloid progenitor cells in response to G-CSF. (A) Expression of PML protein in L-G infectants. Cell lysates were prepared in SDS-PAGE sample buffer and then analyzed by Western blot using anti-HA antibody. Subfraction of PML protein modified by SUMO-1 is indicated. (B) The L-G infectants were cultured in the presence of 5 ng/mL G-CSF. The relative number of viable cells is shown. (C,D) Morphology of L-G infectants exposed to G-CSF for 6 days (C) and 8 days (D) were tested by staining with May-Gruenwald and Giemsa solutions. (E,F) Percentage of L-G infectants differentiated by G-CSF at day 6 (E) and day 8 (F). Immature includes myeloblasts and promyelocytes; intermediate, myelocytes and metamyelocytes; and mature, band cells and segmented neutrophil cells.
PML I inhibits the proliferation and stimulates the differentiation of murine myeloid progenitor cells in response to G-CSF. (A) Expression of PML protein in L-G infectants. Cell lysates were prepared in SDS-PAGE sample buffer and then analyzed by Western blot using anti-HA antibody. Subfraction of PML protein modified by SUMO-1 is indicated. (B) The L-G infectants were cultured in the presence of 5 ng/mL G-CSF. The relative number of viable cells is shown. (C,D) Morphology of L-G infectants exposed to G-CSF for 6 days (C) and 8 days (D) were tested by staining with May-Gruenwald and Giemsa solutions. (E,F) Percentage of L-G infectants differentiated by G-CSF at day 6 (E) and day 8 (F). Immature includes myeloblasts and promyelocytes; intermediate, myelocytes and metamyelocytes; and mature, band cells and segmented neutrophil cells.
Discussion
AML1 forms a complex with specific PML I isoform
We previously purified AML1 complexes from murine progenitor L-G cells that stably expressed AML1a or AML1b proteins. Multiple proteins were copurified with AML1b, but they were absent from both the mock-purified fraction and AML1a complex, suggesting that they could interact specifically with AML1b. Mass spectrometry revealed the presence of PML protein in the purified AML1b complex. It is known that alternative usage of 3′ exons of the PML locus results in generation of several isoforms that have a specific C-terminus. Our results showed that only PML I is contained in the AML1b complex. Analysis of peptides derived from the PML protein in the AML1b complex detected 4 fragments, 2 of which are contained in the common region and 2 others that are in the specific C-terminal region of PML I. No specific sequence for other isoforms was detected. The molecular mass of the PML protein in the purified AML1b complex is about 125 kDa, which corresponds to that of PML I, the largest isoform among identified isoforms of PML. Using IP-Western analysis, we found that among the major isoforms PML I-VI, only PML I could be coprecipitated with AML1b. The specific association of PML I with AML1b suggests that their interaction might be mediated through the C-terminal region of PML I. Indeed, deletion analysis mapped the binding domain for AML1b to this region of PML I. Taken together, we concluded that the specific isoform PML I is a part of a multiple complex of AML1b. It has been reported that p53 could specifically interact with PML IV isoform.64 This and our findings suggest that each of the PML isoforms might play important roles in regulation of factor-specific transcription.
Localization of AML1 in PML nuclear bodies
The PML nuclear body represents a specialization of the protein-based architecture and exists as an entity that is independent of either DNA or RNA.68 An increasing number of transcription factors and coactivators that have been found to reside together with PML in nuclear bodies give support for the role of this structure in transcription regulation. In this study, we demonstrated that AML1b is colocalized with PML I in nuclear bodies. This accumulation required the interaction between AML1b and PML I, since the respective binding domains in both AML1b and PML I are essential for recruitment of AML1b into the nuclear bodies. We previously found that p300 histone acetyltransferases can function as coactivators of AML1b.28 Here we showed that PML I can recruit both AML1b and p300 coactivator to the same nuclear bodies and enhance their cooperation in the activation of transcription.
The PML nuclear body is a stationary structure that associates with the nuclear matrix. It has been reported that AML1 localizes within the nucleus to punctuate foci that associate with the nuclear matrix.58,60 Targeting AML1 to these sites involves at least 2 trafficking signals, one of which mediates nuclear import-nuclear localization signal (NLS) and the other promotes association with nuclear matrix-nuclear matrix targeting signal (NMTS).60 In our study, we found that NMTS of AML1 is required for both interaction with PML and localization in nuclear matrix-associated PML bodies, suggesting that PML I could modulate distribution of AML1 in the nuclear matrix.
A subset of AML1 was shown to colocalize with RNA polymerase II at the site of ongoing transcription.69 Given that the C-terminus of AML1 mediates association with the nuclear matrix and its interaction with transcriptional cofactors such as p300/CBP28 and MOZ,29 AML1 might assemble into macromolecular complexes with coregulatory proteins at nuclear matrix-associated sites. Recently, observation of living cells showed that AML1 continuously and rapidly shuttles into and out of the dynamic yet spatially stable foci.70 The C-terminal region of AML1 that mediates association with other cofactors and the nuclear matrix appears to reduce the mobility of this protein. Thus, dynamic association of AML1 to stationary subnuclear foci might be essential for AML1 to assemble complexes with regulatory cofactors. Several proteins have different potentials to localize within PML nuclear bodies. PML and Sp100 are relatively immobile in the nucleoplasm and reside within the PML nuclear bodies, which suggests that they play a structural role in the integrity of these domains. In contrast, CBP, a transcriptional cofactor, moves relatively rapidly into and out of the PML nuclear bodies.68 From these data, taken together, we propose that stationary PML nuclear bodies serve as a scaffold for the formation of the reversible complex between AML1 and cofactors such as p300/CBP. This model might be applied to complexes of the other PML-interacting transcription factors such as p53, AP1, and SP1 since these transcription factors also use p300/CBP as coactivators.
Functional implication of AML1-PML I interaction
In this study, we mapped the PML I binding site to the portion between residues 363 and 402 in the C-terminal of AML1b. Previously, this domain and the p300 binding site (residues 295 to 362) were both demonstrated to be essential for the stimulatory effect of AML1b on G-CSF-induced differentiation of the myeloid progenitor L-G cells.28 In this study, we showed that PML I could stimulate the L-G cells to mature into neutrophils in response to G-CSF (Figure 7). However, this effect of PML I could not be observed in the cell expressing AML1-MTG8 (data not shown), a dominant negative form of AML1, which represses AML1-dependent transcription.71 This suggests that the ability of PML I to regulate cell differentiation might be mediated via functional AML1 proteins. To further clarify this possibility, the effect of PML I on differentiation of cells, in which gene targeting or RNAi suppresses the expression of AML1 proteins, needs to be investigated.
Mice lacking either AML1 or its heterodimeric partner CBFβ are deficient in definitive hematopoiesis.15 PmL-/- mice also have an impaired capacity for terminal maturation of their myeloid cells.48 The comparison of the phenotype of these mice suggests that PML is not always essential for the function of AML1, but it is involved in the development of myeloid cells that is regulated by AML1. Both AML1 and PML are frequent targets of chromosome abnormalities that are found in acute myeloid leukemias. AML1 interacting proteins, which have been identified so far (including CBFβ, CBP/p300, MOZ, and PML), all are the target of leukemia-associated chromosome translocations. The fact that about 50% of patients with acute leukemia harbor chromosome abnormalities or mutations that disrupt AML1 and/or its binding factors suggests that instability of AML1 complex might be a common mechanism shared by distinct myeloid malignancies and underscores the importance of this complex in hematopoiesis and leukemogenesis.
Prepublished online as Blood First Edition Paper, August 26, 2004; DOI 10.1182/blood-2004-03-1185.
Supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology; and by the Program for Promotion of Fundamental Studies in Health Sciences of the Organization for Pharmaceutical Safety and Research of Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr A. Kakizuka for human PML VI cDNA, Dr T. Okuda for AML2 and AML3 cDNAs, Dr S. Nakamura and Dr H. Ichikawa for mCCP1-luciferase construct, and Dr David Baltimore for BOSC23 cells.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal