Abstract
Kaposi sarcoma (KS) is a multifocal angioproliferative neoplasm strictly dependent on angiogenic growth factors and cytokines and invariably associated with infection by the Kaposi sarcoma–associated herpesvirus (KSHV or HHV8). A G protein–coupled receptor encoded by KSHV (vGPCR) is able to initiate KS-like tumors when targeted to the vascular endothelium of mice. Analogous to human KS, vGPCR sarcomagenesis involves the paracrine secretion of angiogenic growth factors and proinflammatory molecules from vGPCR-expressing cells. Here we demonstrate that vGPCR up-regulates expression and secretion of critical KS cytokines by stimulating key transcription factors, including nuclear factor–κB (NF-κB), activator protein-1 (AP-1), and nuclear factor of activated T cells (NFAT), through the activation of the small G protein Rac1. Inhibition of Rac1 blocked vGPCR-induced transcription and secretion of KS cytokines, including interleukin-6 (IL-6), IL-8, and growth-regulated oncogene α (GROα), in vitro and reduced vGPCR tumorigenesis in vivo. Moreover, endothelial-specific infection with the constitutively active Rac1QL induced vascular lesions in mice that were remarkably similar to early vGPCR experimental lesions. These results identify Rac1 as a key mediator of vGPCR paracrine neoplasia, suggesting that this small G protein and its downstream effectors may represent suitable therapeutic targets for the treatment of KS.
Introduction
Kaposi sarcoma (KS) was originally identified as a rare benign skin tumor usually affecting the lower extremities of elderly Mediterranean men.1 Today, KS is the most frequent tumor arising in HIV-infected patients and has recently emerged as the most prevalent cancer in parts of the developing world. AIDS-associated KS can be an aggressive disease with fungating and exophytic tumors that can invade the subcutaneous and surrounding tissue.2 Oral mucosa, lymph node, and visceral organ involvement is not uncommon. The clinical management of AIDS-related KS has proven to be challenging. Although recent advances in the elucidation of its pathogenesis are uncovering many potential therapeutic targets, AIDS-KS still remains an incurable disease.3
In 1994, Chang et al4 detected DNA sequences from a then unknown human γ-herpesvirus in KS tissue. The newly identified viral agent, the Kaposi sarcoma–associated herpesvirus (KSHV or HHV8), belongs to the genus Rhadinovirus, and its genomic structure is most similar to the closely related lymphotropic γ-herpesviruses Epstein Barr virus (EBV) and herpesvirus saimiri (HVS).5 KSHV has been found to be associated with all 4 forms of KS (classic, iatrogenic, endemic, and AIDS related) in addition to 2 lymphoproliferative disorders: primary effusion lymphoma (PEL) and multicentric Castleman disease (MCD).6 In KS, it is believed that KSHV infects and transforms endothelial cells, thereby generating the KS tumor (or spindle) cell.7 The KS spindle cell is the driving force of KS lesions, producing a variety of angiogenic cytokines and growth factors that provoke the inflammatory and neovascular responses characteristic of this unusual neoplasm.8
Using an endothelial cell–specific retroviral gene transfer system to systematically examine the contribution of each KSHV-encoded oncogene to the initiation of KS, we have previously observed that the KSHV-encoded G protein–coupled receptor (vGPCR) is able to initiate KS-like tumors in mice.9 These observations are aligned with recent reports supporting the remarkable sarcomagenic potential of vGPCR when expressed under the control of other promoters using conventional transgenic animal models.10,11 Surprisingly, receptor-positive cells are very rare in the tumors found in these animal models,9 analogous to the expression pattern observed in primary human KS tissue,12 suggesting that vGPCR-expressing cells could contribute to the pathogenesis of KS through paracrine mechanisms.
Similar to the KS spindle cell, vGPCR-transformed endothelial cells elaborate angiogenic growth factors, chemokines, and cytokines, which recruit and transform bystander endothelial cells and may provoke immune cell infiltration.9-11,13-18 In this regard, vGPCR can activate the transcription factors nuclear factor–κB (NF-κB) and activator protein-1 (AP-1), thereby inducing expression of endogenous NF-κB– and AP-1–dependent cytokines (interleukin-1 [IL-1], tumor necrosis factor-α [TNF-α], IL-6), chemokines (IL-8 and monocyte chemoattractant protein-1 [MCP-1]), and growth factors (basic fibroblast growth factor [bFGF]).16,19-21 However, it is still unclear as to the nature of the signaling molecules involved in the activation of gene transcription by this viral receptor.
Because small guanosine triphosphate (GTP)–binding proteins represent critical links between GPCRs and nuclear transcription factors,22 we set out to determine if members of the Rho family of small guanosine triphosphatases (GTPases) could play a role in vGPCR-induced transcriptional regulation. We found that the small GTP-binding protein Rac1 is potently activated in vGPCR-expressing endothelial cells. Indeed, preventing the activation of Rac1 by vGPCR blocked the stimulation of key transcription factors, including NF-κB, AP-1, and nuclear factor of activated T cells (NFAT), resulting in the inhibition of cytokine secretion in vitro and vGPCR sarcomagenesis in vivo. Moreover, endothelial infection with a retrovirus expressing a constitutively active Rac1 was sufficient to promote endothelial cell transformation. Together, these results implicate Rac1 as a key player in the initiation and progression of Kaposi sarcomagenesis through its stimulation of cytokine secretion and thus identify this small GTP-binding protein and its downstream effectors as novel molecular targets in the development of new anti-KS strategies.
Materials and methods
Expression plasmids and reagents
The expression plasmids for vGPCR and its mutants, vGPCR R143Q (R143Q) and vGPCR R143A (R143A), and for HA-Akt and enhanced green fluorescent protein (EGFP) have been previously described.23,24 The expression plasmids for Rac1WT, Rac1QL, RacN17, Cdc42QL, RhoQL, TIAM1 C1199, Gα13, 5 × κB-LUC, AP-1–luciferase (LUC), and IκBαA32A36 have been described elsewhere.25,26 Cyclic adenosine mono-phosphate [cAMP]-response element binding protein (CREB)–LUC and NFAT-LUC reporter plasmids were obtained from Stratagene (La Jolla, CA). The reporter vectors pIL6-CAT and pIL6-mut κB-CAT were kindly provided by Towia A. Libermann.27 pCEFL AU1 DH/PH domain from PDZ-Rho-GEF was described elsewhere.28 pCMV6 Myc PAKN and PAKN2L were generously provided by Jeffrey Field29 and subcloned into the pCEFL AU1 expression vector. The bicistronic constructs vGPCR-I-GFP, vGPCR-I-RacN17, and vGPCR-I-PAKN were obtained by first inserting the internal ribosome entry site (IRES) from Clontech Technologies (Palo Alto, CA) into the pCEFL expression vector by polymerase chain reaction (PCR) to create pCEFL IRES. vGPCR, EGFP, RacN17, and PAKN were subsequently cloned into pCEFL IRES. RCAS-EGFP and RCAS-vGPCR were previously described.9 HA-Rac1QL was subcloned into the replication-competent avian leukosis virus (ALV) long terminal repeat with splice acceptor (RCAS) vector as a PacI-NotI insert. Recombinant human IL-8 was purchased from Peprotech (Rocky Hill, NJ).
Cell lines and transfections
Immortalized murine endothelial cells (SVECs), 293T cells, and COS-7 cells were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and penicillin/streptomycin. DF1 chicken fibroblasts were maintained in DMEM with high glucose supplemented with 10% FBS and penicillin/streptomycin. NIH 3T3 cells were grown in DMEM supplemented with 10% calf serum (CS) and penicillin/streptomycin. Transfection was performed using Fugene Reagent (Roche Applied Science, Indianapolis, IN) (SVECs), Lipofectamine Plus reagent (Invitrogen, Carlsbad, CA) (COS-7, 293T, and NIH 3T3 cells), and Superfect (Qiagen, Valencia, CA) (DF1), according to the manufacturer's protocol. SVEC stable cell lines were obtained by stable transfection of the corresponding pCEFL-derived plasmids, as has been previously described.9
Mouse strains
Generation and characterization of the TIE2-tva transgenic mouse line has been described elsewhere.9 Transgenic mice were generated in FVB/N mice using standard techniques. Genotypes were determined by Southern blotting and by PCR with tail DNA. The athymic (nu/nu) nude females were purchased from Harlan Sprague Dawley (Indianapolis, IN).
Enzyme-linked immunosorbent assay (ELISA)
Conditioned media from transiently transfected 293T cells or SVEC stable cell lines were prepared by incubating subconfluent cultured cells for 24 hours in DMEM without supplements. Harvested conditioned media were then filtered through a 0.22-μm low protein-binding polyethylsulfonate membrane filter. Quantitation of cytokine levels was performed by Pierce Biotechnologies (Rockford, IL) using Searchlight technology.
Reporter gene assay
Cells were transfected with different expression plasmids together with 0.1 μg pIL6-CAT, pIL6-mut κB-CAT, 5 × κB-LUC, AP-1-LUC, CREB-LUC, or NFAT-LUC and 0.01 μg pRL-null (expressing the enzyme Renilla luciferase from Renilla reniformis) as an internal control. In all cases, the total amount of plasmid DNA was adjusted with pcDNAIII–β-galactosidase (pcDNAIII–β-gal). Firefly and Renilla luciferase activities present in cellular lysates were assayed using the Dual-Luciferase Reporter System (Promega, Madison, WI), and light emission was quantitated using the Microliter Plate luminometer as specified by the manufacturer (DINEX Tech, Chantilly, VA). Chloramphenicol acetyl transferase (CAT) activity was assayed as described previously.30
Rho and Rac1 guanine nucleotide exchange assays
Rho or Rac1 activity in cultured cells was assessed by a modified method described elsewhere.28,31 Briefly, cells were transfected with the indicated plasmids, and after serum starvation for 24 hours cells were lysed at 4°C in a buffer containing 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.4, 0.1 M NaCl, 1% Triton X-100, 10 mM EGTA (ethylene glycol tetraacetic acid), 40 mM glycerophosphate, 20 mM MgCl2, 1 mM Na3VO4, 1 mM dithiothreitol, 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride. Lysates were incubated with glutathione S-transferase (GST)–rhotekin–Rho binding domain (RBD) (Rho assay) or GST-PAK1 Cdc42/Rac interactive binding (CRIB) domain (Rac assay) previously bound to glutathione-Sepharose beads and washed 4 times with lysis buffer. Associated GTP-bound forms of Rho or Rac1 were released with protein loading buffer and revealed by Western blot analysis using a monoclonal antibody against RhoA (26C4) (Santa Cruz Biotechnology, Santa Cruz, CA) or against Rac1 (BD Transduction Laboratories, San Jose, CA).
Western blots and immunofluorescence
Western blots and immunofluorescence were performed as previously described.9 Immunodetection of the AU5 epitope was used to identify the expression of AU5-tagged vGPCR and vGPCR R143A.
Establishment of tumor allografts in athymic nu/nu mice
Athymic (nu/nu) nude mice, 8 weeks of age and weighing 22 to 24 g, were housed in appropriate sterile filter-capped cages and fed and watered ad libitum. All animal studies were carried out using the appropriate National Institutes of Health (NIH) animal care and user protocol. SVEC stable cell lines were used to induce tumors in athymic mice as previously described.9 Briefly, exponentially growing cells were harvested, washed, resuspended in DMEM, and 5 × 105 viable cells were transplanted subcutaneously into the right flank of athymic mice. The animals were monitored twice weekly for tumor formation. For analysis, tumor weight was determined as previously described,9 whereby tumor volume (LW2/2, where L and W represent longest length and shortest width of the tumor) was converted to weight. Results of animal experiments were expressed as mean ± SEM. At the end of the study period, animals were humanely killed.
Cells were visualized using an Axioplhot 2 microscope (Zeiss, Jena, Germany) using Axioplan2 software (Zeiss) under 20 × (Figures 4 and 6) or 63 × (Figures 1 and 2) original magnification. Images were captured and processed using a SPOT camera and software (Diagnostic Instruments, Sterling Heights, MI).
Coexpression of vGPCR with dominant negative Rac constructs using an IRES system. (A) Schematic representation of the bicistronic constructs encoding the vGPCR and GFP, RacN17, or PAKN. (B) Immunofluorescence demonstrating coexpression both of vGPCR and GFP, RacN17, or PAKN in COS-7 cells transfected with IRES constructs. (C) COS-7 cells coexpressing both vGPCR and RacN17 or PAKN fail to stimulate NF-κB or induce IL-6 transcription. 293T cells coexpressing both vGPCR and RacN17 or PAKN fail to induce IL-6 secretion. Data represent the mean ± SEM of triplicate samples from a typical experiment and are expressed as percent of luciferase activity, CAT activity, or IL-6 secretion levels with respect to vGPCR-I-GFP–expressing cells.
Coexpression of vGPCR with dominant negative Rac constructs using an IRES system. (A) Schematic representation of the bicistronic constructs encoding the vGPCR and GFP, RacN17, or PAKN. (B) Immunofluorescence demonstrating coexpression both of vGPCR and GFP, RacN17, or PAKN in COS-7 cells transfected with IRES constructs. (C) COS-7 cells coexpressing both vGPCR and RacN17 or PAKN fail to stimulate NF-κB or induce IL-6 transcription. 293T cells coexpressing both vGPCR and RacN17 or PAKN fail to induce IL-6 secretion. Data represent the mean ± SEM of triplicate samples from a typical experiment and are expressed as percent of luciferase activity, CAT activity, or IL-6 secretion levels with respect to vGPCR-I-GFP–expressing cells.
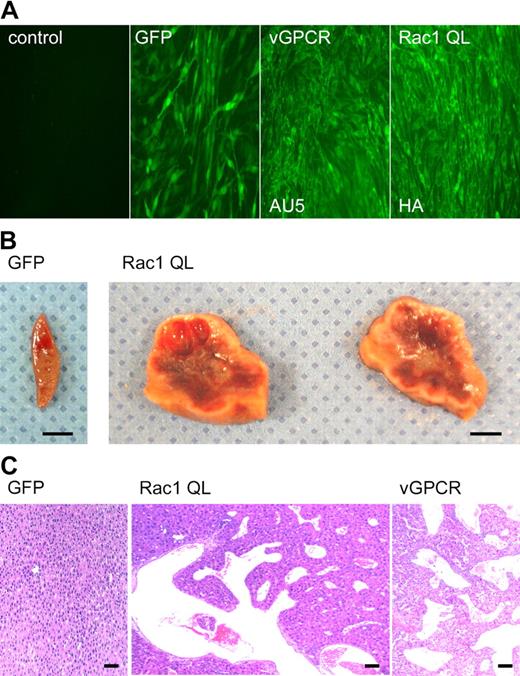
Angioproliferative lesions in RCAS-racQL–injected TIE2-tva mice closely resemble early vGPCR experimental KS lesions. (A) Immunofluorescence of permissive (DF1) chicken fibroblasts infected with RCAS alone (control), RCAS-GFP, RCAS-vGPCR, or RCAS-racQL virus, respectively. Expression of vGPCR or RacQL was detected by staining against AU5 or hemagglutinin (HA) tags, respectively. (B) Representative vascular lesions found in liver from 2 TIE2-tva mice injected with RCAS-racQL (107 IU). Liver from a littermate control injected with RCAS-GFP showed no lesions. Scale bar, 5 mm. (C) Representative hematoxylin and eosin (H&E) staining of liver reveals benign angiectasias similar to those seen in early vGPCR experimental KS lesions. Sections initially photographed at magnification × 40. Scale bar, 50 μm.
Angioproliferative lesions in RCAS-racQL–injected TIE2-tva mice closely resemble early vGPCR experimental KS lesions. (A) Immunofluorescence of permissive (DF1) chicken fibroblasts infected with RCAS alone (control), RCAS-GFP, RCAS-vGPCR, or RCAS-racQL virus, respectively. Expression of vGPCR or RacQL was detected by staining against AU5 or hemagglutinin (HA) tags, respectively. (B) Representative vascular lesions found in liver from 2 TIE2-tva mice injected with RCAS-racQL (107 IU). Liver from a littermate control injected with RCAS-GFP showed no lesions. Scale bar, 5 mm. (C) Representative hematoxylin and eosin (H&E) staining of liver reveals benign angiectasias similar to those seen in early vGPCR experimental KS lesions. Sections initially photographed at magnification × 40. Scale bar, 50 μm.
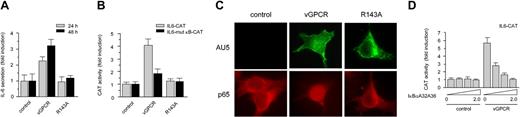
vGPCR induces IL-6 secretion by stimulating the transcription factor NF-κB. (A) Secretion of IL-6 in media conditioned by 293T cells expressing GFP (control), vGPCR (vGPCR), or the inactive mutant vGPCR R143A (R143A). (B) Transcriptional activation of the IL6 promoter induced by vGPCR is dependent on the κB site in COS-7 cells. (C) Expression of vGPCR, but not vGPCR R143A (R143A) or GFP (control), in COS-7 cells induces the translocation of RelA (p65) to the nucleus. (D) Inhibition of vGPCR induction of pIL6-CAT by overexpression of increasing concentrations of the NF-κB inhibitor, IκBαA32A36, in COS-7 cells. Data represent the mean ± SEM of triplicate samples from a typical experiment and are expressed as fold induction with respect to control.
vGPCR induces IL-6 secretion by stimulating the transcription factor NF-κB. (A) Secretion of IL-6 in media conditioned by 293T cells expressing GFP (control), vGPCR (vGPCR), or the inactive mutant vGPCR R143A (R143A). (B) Transcriptional activation of the IL6 promoter induced by vGPCR is dependent on the κB site in COS-7 cells. (C) Expression of vGPCR, but not vGPCR R143A (R143A) or GFP (control), in COS-7 cells induces the translocation of RelA (p65) to the nucleus. (D) Inhibition of vGPCR induction of pIL6-CAT by overexpression of increasing concentrations of the NF-κB inhibitor, IκBαA32A36, in COS-7 cells. Data represent the mean ± SEM of triplicate samples from a typical experiment and are expressed as fold induction with respect to control.
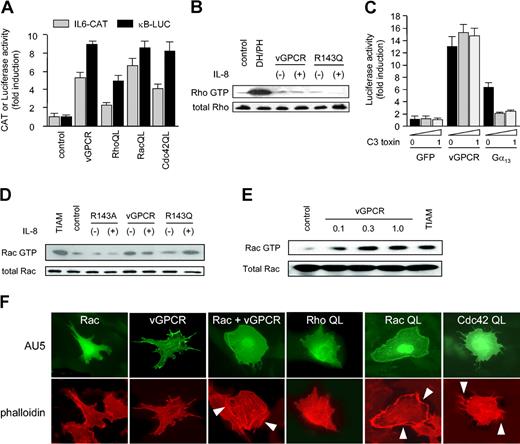
vGPCR induces activation of Rac but not Rho. (A) Activated mutants of all 3 small G proteins (RhoA, Rac1, and Cdc42) can induce NF-κB activation and IL-6 transcription, similar to vGPCR, in COS-7 cells. (B) 293T cells expressing vGPCR or vGPCR R143Q (R143Q) did not demonstrate elevated levels of active Rho in the absence (-) or presence (+) of 50 nM IL-8 (1 minute) with respect to GFP-expressing cells (control). Similar results were obtained using longer exposure to agonist. The DH/PH domain of PDZ-Rho-GEF was used as a positive control. (C) Transcriptional activation through the κB site by vGPCR in COS-7 cells is not inhibited by treatment with C3 toxin. Gα13 was used as a positive control for C3 toxin effects. Data in panels A and C represent the mean ± SEM of triplicate samples from a typical experiment, expressed as fold induction with respect to control. (D) 293T cells expressing vGPCR had elevated levels of active Rac in the absence (-) or presence (+) of 50 nM IL-8 (1 minute). Agonist-dependent vGPCR mutant (R143Q) only induced activation of Rac in presence of IL-8. The constitutively active Rac GEF, truncated TIAM (TIAM1 C1199), was used as a positive control. (E-F) PAE cells transfected with vGPCR and Rac WT had elevated levels of active Rac (E) and demonstrated Rac-like morphology (F). Cells were fixed and stained with phalloidin-specific antibodies to label the actin cytoskeleton. Arrows indicate membrane ruffling (vGPCR and RacQL) or filopodia (Cdc42QL). Pictures are representative of 3 independent experiments. PAE cells transfected with expression vectors for RhoAQL, Rac1QL, or Cdc42QL were used as controls.
vGPCR induces activation of Rac but not Rho. (A) Activated mutants of all 3 small G proteins (RhoA, Rac1, and Cdc42) can induce NF-κB activation and IL-6 transcription, similar to vGPCR, in COS-7 cells. (B) 293T cells expressing vGPCR or vGPCR R143Q (R143Q) did not demonstrate elevated levels of active Rho in the absence (-) or presence (+) of 50 nM IL-8 (1 minute) with respect to GFP-expressing cells (control). Similar results were obtained using longer exposure to agonist. The DH/PH domain of PDZ-Rho-GEF was used as a positive control. (C) Transcriptional activation through the κB site by vGPCR in COS-7 cells is not inhibited by treatment with C3 toxin. Gα13 was used as a positive control for C3 toxin effects. Data in panels A and C represent the mean ± SEM of triplicate samples from a typical experiment, expressed as fold induction with respect to control. (D) 293T cells expressing vGPCR had elevated levels of active Rac in the absence (-) or presence (+) of 50 nM IL-8 (1 minute). Agonist-dependent vGPCR mutant (R143Q) only induced activation of Rac in presence of IL-8. The constitutively active Rac GEF, truncated TIAM (TIAM1 C1199), was used as a positive control. (E-F) PAE cells transfected with vGPCR and Rac WT had elevated levels of active Rac (E) and demonstrated Rac-like morphology (F). Cells were fixed and stained with phalloidin-specific antibodies to label the actin cytoskeleton. Arrows indicate membrane ruffling (vGPCR and RacQL) or filopodia (Cdc42QL). Pictures are representative of 3 independent experiments. PAE cells transfected with expression vectors for RhoAQL, Rac1QL, or Cdc42QL were used as controls.
Preparation of virus and infection of TIE2-tva mice
DF1 cells were transfected with RCAS vectors to produce recombinant viruses. Viral stocks were collected, titered, and injected intraperitoneally into 5-day old littermates (100 μL per mouse) at the indicated viral load as previously described.9
Histology
Murine tissues were fixed in 4% paraformaldehyde, 1 × phosphate-buffered saline (PBS) for 36 hours, transferred to 70% ethanol/PBS, and embedded in paraffin. Slides were stained with hematoxylin and eosin, dehydrated, and mounted with Permount (Fisher Scientific, Fair Lawn, NJ).
Results
vGPCR induces IL-6 secretion by stimulating transcription from a κB-responsive element
The KSHV vGPCR is unique among candidate KSHV oncogenes in that it has been consistently shown to initiate KS-like tumors in mice.9-11 Similar to KS spindle cells, vGPCR-transformed endothelial cells elaborate angiogenic growth factors, chemokines, and cytokines that likely recruit and transform bystander endothelial cells through a paracrine mechanism.9-11,13-18 Among the cytokines elaborated by the KS spindle cell, emerging evidence suggests that interleukin-6 (IL-6) may play an essential role in Kaposi sarcomagenesis. IL-6 serves as an autocrine growth factor for cultured AIDS-KS cells and appears to induce endothelial cell proliferation in KS through a paracrine mechanism.32-34 Recent evidence further indicates that an IL-6 promoter polymorphism resulting in enhanced IL-6 production is associated with an increased lifetime risk of development of KS in men infected with HIV, thus supporting the direct clinical relevance of IL-6 to human KS development.35 To explore the nature of vGPCR's contribution to KS spindle cell function, we examined if vGPCR could induce the secretion of IL-6. To this end, conditioned media from 293T cells transiently transfected with vGPCR were collected and assayed for the presence of this cytokine. The secretion of IL-6 by vGPCR-expressing cells was 3-fold that of control cells (Figure 1A). Conversely, an inactive mutant of the receptor (R143A)24 did not show significant induction of IL-6 secretion (Figure 1A).
To determine whether this enhanced steady-state level of IL-6 secretion in cells expressing vGPCR results from an increase in IL-6 transcription, we transfected COS-7 cells with vGPCR along with the pIL6-CAT reporter plasmid containing the full-length IL-6 promoter.27 Expression of vGPCR but not the inactive vGPCR mutant, R143A, potently stimulated transcription from the promoter of IL-6 (Figure 1B), suggesting that up-regulation of IL-6 secretion in vGPCR-expressing cells may result from the transcriptional activation of the corresponding gene. Because it had been previously shown that the vGPCR can activate the transcriptional regulator nuclear factor κB (NF-κB),16,19-21 we next examined the ability of this viral receptor to up-regulate transcription from an IL-6 promoter construct containing a mutation in the κB-responsive element (pIL6-mut κB-CAT).27 Inactivation of the κB site significantly diminished the induction of IL-6 transcription by vGPCR (Figure 1B), consistent with a critical role for NF-κB in vGPCR stimulation of IL-6 transcription. Indeed, vGPCR expression potently induced RelA (p65) nuclear translocation (Figure 1C). Moreover, expression of a mutant IκB, IκBαA32A36, that cannot be phosphorylated and thus acts as an NF-κB inhibitor potently inhibited vGPCR stimulation of the IL-6 promoter in a dose-dependent manner (Figure 1D). Taken together, these observations demonstrate that the stimulation of IL-6 transcription and secretion by vGPCR requires the activation of NF-κB. Because κB response elements are found in the promoters of many cytokines,36 these observations further suggested that the activation of NF-κB may be a general mechanism whereby this viral receptor stimulates transcription of other cellular chemokines.16,19-21,37 Thus, vGPCR induction of IL-6 transcription and secretion may provide a model system to explore the mechanism whereby vGPCR regulates NF-κB activation.
vGPCR activates the small G protein Rac1 but not RhoA
Members of the Rho family of small GTPases have been shown to be critical links between G protein–coupled receptors and transcription regulation.22 The Rho family forms a large subgroup within the Ras superfamily of GTP-binding proteins and regulates a wide spectrum of cellular functions, including actin cytoskeleton reorganization, endocytosis and exocytosis, transcription activation, stimulation of DNA synthesis, and/or translational regulation.38 Because members of this family of small G proteins have previously been shown to stimulate NF-κB,25,39 we first tested the ability of activated forms of RhoA, Rac1, and Cdc42 to induce transcription from the IL-6 promoter. Activated forms of all 3 small G proteins induced transcription from the IL-6 promoter and from an isolated κB-responsive element, similar to vGPCR (Figure 2A).
Of note, it has previously been reported that vGPCR may stimulate NF-κB through Gα12/13 activation of a Rho guanine nucleotide exchange factor (GEF), p115-RhoGEF, and subsequently RhoA.21 We therefore examined if expression of vGPCR could lead to the activation of RhoA. To this end, 293T cells were transfected with vGPCR and assayed for Rho GTP levels. Surprisingly, expression of vGPCR did not cause Rho activation (Figure 2B), although the DH/PH domain of a Rho exchange factor, PDZ-Rho-GEF, activated Rho potently. Inhibition of Rho with C3 toxin also failed to affect the vGPCR stimulation of NF-κB (Figure 2C). Moreover, coexpression of dominant negative constructs blocking the 3 known Rho GEFs connecting members of Gα12/13 to Rho activation (PDZ-Rho-GEF, p115-Rho-GEF, and LARG)28,31,40,41 also failed to affect NF-κB activation by vGPCR (results not shown). Conversely, both the C3 toxin and the dominant negative Rho GEFs potently inhibited NF-κB activation by an activated mutant of Gα13, which signals to the nucleus through RhoA42 (Figure 2C and results not shown). Collectively, this suggested that vGPCR-induced activation of NF-κB is unlikely to be mediated by RhoA.
To explore whether other members of the Rho family of small GTPases could mediate the stimulation of NF-κB by vGPCR, we next examined if expression of vGPCR could lead to the activation of Rac1. We found that 293T cells expressing vGPCR showed elevated levels of Rac1-GTP compared with control cells (Figure 2D). Induction of Rac1 by vGPCR was similar to Rac1 activation by the constitutively active truncated mutant of a Rac GEF, TIAM1 C1199. To confirm that Rac1 activation in vGPCR-expressing cells was not a consequence of paracrine secretions, we used an agonist-dependent vGPCR mutant (R143Q), which is active only in the presence of ligand.24 This agonist-dependent mutant induced Rac1 activation within only 1 minute after IL-8 stimulation (Figure 2D), suggesting that vGPCR directly stimulates a pathway leading to Rac1 activation. In contrast, cells expressing the inactive vGPCR mutant R143A, which served as a negative control, showed no activation of Rac1.
We then examined Rac GTP levels in porcine aortic endothelial (PAE) cells transiently transfected with vGPCR to verify if endothelial cells expressing vGPCR also show increased levels of activated Rac1. Expression of vGPCR potently induced Rac activation in endothelial cells in a dose-dependent manner (Figure 2E). Moreover, PAE cells expressing vGPCR and wild-type (WT) Rac presented a Rac-like morphology, exhibiting membrane ruffling and lamellipodia (Figure 2F). Together, these results demonstrate that vGPCR potently induces Rac1 activity, suggesting that this GTPase could play a role in vGPCR-induced activation of the NF-κB transcription factor.
Inhibition of Rac activity blocks vGPCR-induced activation of key transcription factors and prevents the secretion of critical KS cytokines
We next set out to determine if the Rac GTPase links this viral GPCR to NF-κB activation. To this end, we used 2 well-characterized dominant negative mutants, RacN17 and a truncated p21-activated kinase (PAK) mutant (PAKN), to inhibit Rac function.43,44 Coexpression of vGPCR with either RacN17 or PAKN potently inhibited vGPCR-induced activation of NF-κB (Figure 3A). Conversely, transfection of a mutant of PAKN, which cannot bind to (or inhibit) Rac1 (mPAKN),29 did not affect the stimulation of the κB-LUC reporter by vGPCR, confirming the specificity of the inhibition observed using these dominant negative constructs. Indeed, inhibition of Rac1 function also impaired vGPCR-induced nuclear translocation of RelA(p65) (results not shown), supporting the role of Rac1 in the vGPCR stimulation of this transcriptional modulator. Taken together, these results suggest that Rac1 mediates vGPCR stimulation of NF-κB.
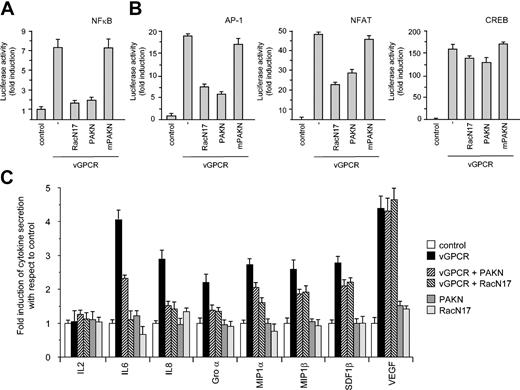
Rac1 activation is involved in transcriptional regulation and cytokine secretion. (A) Dominant negative RacN17 and Rac inhibitor PAKN block vGPCR stimulation of a κB-responsive element in COS-7 cells. (B) Dominant negative RacN17 and Rac inhibitor PAKN also block vGPCR stimulation of AP-1 and NF-AT but not CREB-responsive element in COS-7 cells. Data represent the mean ± SEM of triplicate samples from a typical experiment and are expressed as fold induction with respect to control. (C) Dominant negative RacN17 and Rac inhibitor PAKN block secretion of cytokines in conditioned media from transiently transfected 293T cells.
Rac1 activation is involved in transcriptional regulation and cytokine secretion. (A) Dominant negative RacN17 and Rac inhibitor PAKN block vGPCR stimulation of a κB-responsive element in COS-7 cells. (B) Dominant negative RacN17 and Rac inhibitor PAKN also block vGPCR stimulation of AP-1 and NF-AT but not CREB-responsive element in COS-7 cells. Data represent the mean ± SEM of triplicate samples from a typical experiment and are expressed as fold induction with respect to control. (C) Dominant negative RacN17 and Rac inhibitor PAKN block secretion of cytokines in conditioned media from transiently transfected 293T cells.
The potent inhibition of NF-κB by blocking vGPCR activation of Rac1 prompted us to explore whether activation of Rac1 may be a general mechanism whereby vGPCR up-regulates the activity of other transcription factors. In this regard, Rac1 has previously been shown to potently activate several transcription factors that are known to be stimulated by vGPCR, including AP-1, CREB, and NFAT.45 We therefore set out to determine if Rac1 could also link vGPCR to these transcription factors. Coexpression of vGPCR with RacN17 or PAKN inhibited vGPCR-induced activation of AP-1 and NFAT but had no effect on vGPCR activation of CREB (Figure 3B). Because NF-κB, AP-1, and NFAT response elements are found in the promoter region of critical KS cytokines, we next examined the consequence of inhibiting Rac1 activation on the secretion of KS cytokines and growth factors. For these experiments, conditioned media from 293T cells coexpressing vGPCR with RacN17 or PAKN were assayed for the presence of cytokines and growth factors previously implicated in Kaposi sarcomagenesis. Blocking Rac1 activation potently inhibited secretion of several cytokines, including IL-6, IL-8, and growth-regulated oncogene α (GROα) (Figure 3C). Other cytokines were either only partially inhibited (eg, macrophage inflammatory protein-1α [MIP-1α] and β and stromal-derived factor-1β [SDF-1β]) or unaffected (eg, vascular endothelial growth factor [VEGF]) (Figure 3C). Of note, the secretion of several inflammatory cytokines (eg, IL-1β, IL-2, and TNF-α) previously shown to be stimulated in vGPCR-expressing B cells was not up-regulated (results not shown), consistent with a cell type specificity in the cytokine secretion profile provoked by vGPCR.46 Collectively, these results are aligned with a key role for Rac1 in vGPCR-induced secretion of cellular cytokines.
Inhibition of Rac blocks vGPCR-induced tumorigenesis in vivo
The observation that Rac1 is required for vGPCR-induced secretion of critical KS cytokines prompted us to explore the role of this GTPase in vGPCR-induced tumorigenesis in vivo. To coexpress vGPCR and 1 of the 2 Rac inhibitors, we first engineered bicistronic constructs expressing vGPCR along with GFP, RacN17, or PAKN using an internal ribosomal entry site (IRES) system (Figure 4A), thus ensuring the expression of both encoded genes in target cells. Both vGPCR and the second gene (GFP, RacN17, or PAKN) encoded by each bicistronic construct could be readily detected in COS-7 cells transfected with each corresponding construct (Figure 4B). To confirm that the expression of the Rac inhibitors was sufficient to block Rac-mediated vGPCR signaling, COS-7 cells expressing each construct were assayed for NF-κB activation and IL-6 transcription and secretion. Both vGPCR-I-RacN17 and vGPCR-I-PAKN were unable to activate NF-κB and could not induce IL-6 transcription or secretion when compared with vGPCR-I-GFP (Figure 4C).
Endothelial cell lines stably expressing the vGPCR bicistronic constructs were then established (EC lines), and expression of the encoded proteins was verified by Western blot (Figure 5A). Conditioned media from the EC lines were collected and assayed for cytokine secretion. ELISA analysis of conditioned media from the EC-vGPCR cell line revealed elevated levels of IL-6, KC (the IL-8/GROα murine homolog), and SDF-1 (Figure 5B), confirming that endothelial cells expressing vGPCR secrete elevated levels of key KS cytokines. However, the secretion of inflammatory cytokines (eg, IL-1β, IL-2, and TNF-α) was not detected (results not shown). ELISAs of conditioned media from endothelial cell lines expressing vGPCR along with RacN17 or PAKN, encoded by the 2 bicistronic constructs, vGPCR-I-RacN17 (EC-vGPCR-I-RacN17) or vGPCR-I-PAKN (EC-vGPCR-I-PAKN), revealed a remarkable decrease in the secretion of IL-6 and KC and partial inhibition of SDF-1 (Figure 5B), consistent with a role for Rac1 activation in vGPCR stimulation of cytokine secretion in endothelial cells.
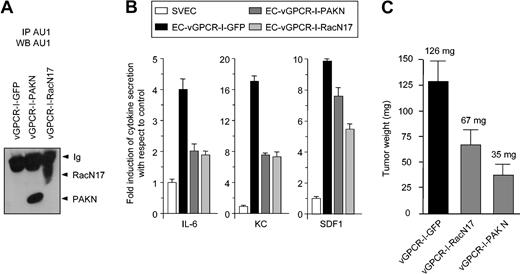
Inhibition of Rac1 blocks vGPCR tumorigenesis. (A) Western blot demonstrating expression of RacN17 or PAKN in immortalized endothelial cells (SVECs) stably expressing bicistronic constructs (EC-vGPCR-I-GFP, EC-vGPCR-I-PAKN, or EC-vGPCR-I-RacN17). (B) EC-vGPCR-I-PAKN and EC-vGPCR-I-RacN17 show reduced secretion of cytokines with respect to EC-vGPCR-I-GFP. (C) EC-vGPCR-I-GFP, EC-vGPCR-I-PAKN, or EC-vGPCR-I-RacN17 was used to generate tumors in 8-week-old athymic nu/nu mice. Mice were killed after 8 weeks. Tumors formed from EC-vGPCR-I-GFP were significantly larger than those formed from EC-vGPCR-I-PAKN or EC-vGPCR-I-RacN17. Data represent the mean ± SEM of 10 tumors from a typical experiment. Average tumor weight is indicated.
Inhibition of Rac1 blocks vGPCR tumorigenesis. (A) Western blot demonstrating expression of RacN17 or PAKN in immortalized endothelial cells (SVECs) stably expressing bicistronic constructs (EC-vGPCR-I-GFP, EC-vGPCR-I-PAKN, or EC-vGPCR-I-RacN17). (B) EC-vGPCR-I-PAKN and EC-vGPCR-I-RacN17 show reduced secretion of cytokines with respect to EC-vGPCR-I-GFP. (C) EC-vGPCR-I-GFP, EC-vGPCR-I-PAKN, or EC-vGPCR-I-RacN17 was used to generate tumors in 8-week-old athymic nu/nu mice. Mice were killed after 8 weeks. Tumors formed from EC-vGPCR-I-GFP were significantly larger than those formed from EC-vGPCR-I-PAKN or EC-vGPCR-I-RacN17. Data represent the mean ± SEM of 10 tumors from a typical experiment. Average tumor weight is indicated.
When EC lines were used to generate tumors in 8-week-old athymic nu/nu mice, the EC-vGPCR-I-GFP cell line formed tumors within 6 weeks of injection (Figure 5C), similar to the EC-vGPCR cell line.9 However, EC-vGPCR-I-RacN17 and EC-vGPCR-I-PAKN had significantly diminished abilities to form tumors in vivo compared with EC-vGPCR-I-GFP cells (Figure 5C). The reduced ability of EC-vGPCR-I-RacN17 and EC-vGPCR-I-PAKN to form tumors in nude mice correlated with the reduction in cytokine secretion by these cells. Collectively these results provide in vivo evidence that vGPCR tumorigenesis requires Rac1 activation.
Endothelial-specific expression of constitutively active Rac1 induces vascular lesions in mice similar to early experimental vGPCR lesions
Initiation of endothelial cell transformation by vGPCR appears to involve paracrine secretions of proangiogenic and proinflammatory molecules by vGPCR-expressing cells.13,15,19 Because Rac1 appears to play a critical role in vGPCR paracrine tumorigenesis, we set out to determine if constitutive activation of Rac1 was sufficient to induce endothelial cell transformation in vivo. To this end, we took advantage of the recently developed endothelial cell–specific retroviral gene transfer (TIE2-tva) system.9,24 Only mammalian cells engineered to express the transgene for the avian leukosis virus (ALV) receptor, TVA,47,48 can be transduced by infection with ALV, thus enabling the somatic introduction of multiple genes in vivo in a tissue-specific manner. TIE2-tva transgenic mice express the tva transgene specifically in endothelial cells and are therefore susceptible to endothelial-specific infection using ALV-derived vectors. We prepared an ALV-derived (replication-competent ALV long terminal repeat with splice acceptor or RCAS) vector containing an activated form of Rac1 (RCAS-rac1QL)or EGFP (RCAS-EGFP) and confirmed expression in DF1 (chicken fibroblast) packaging cells (Figure 6A). TIE2-tva transgenic mice were then infected with high-titer (107 IU) virus. RCAS-rac1QL–infected animals killed 12 months after injection grossly demonstrated multiple hemorrhagic vascular lesions in the liver (Figure 6B), whereas TIE2-tva mice infected with high-titer (107 IU) RCAS-EGFP showed no gross pathology or histopathology for up to 18 months following injection (Figure 6B and results not shown). Histologic examination of RCAS-rac1QL–infected animals revealed benign angiectasias in the liver, spleen, and lungs similar to early vGPCR-induced lesions (Figure 6C) and consistent with a role for Rac1 in vGPCR paracrine neoplasia.
Discussion
Kaposi sarcoma is a multifocal neovascular tumor characterized histologically by proliferating spindle cells, angiogenesis, erythrocyte-replete vascular slits, profuse edema, and a variable inflammatory cell infiltrate. The dominant cell of KS lesions, the spindle cell, elaborates a variety of proinflammatory and angiogenic factors and is considered the driving force in KS lesions.8 Of note, the role of the KS spindle cell in paracrine-driven tumorigenesis is not without precedent. A similar function has previously been attributed to Reed-Sternberg cells in Hodgkin lymphoma.49,50 While mutations leading to dysregulation of NF-κB activity have been implicated in the genesis of Hodgkin lymphoma,51 emerging evidence suggests that KSHV-encoded genes may play an analogous role in promoting the stimulation of paracrine secretions in KS. Current efforts are now focused on identifying the KSHV gene(s) responsible for this unique model of paracrine cell transformation.
KSHV latent genes are expressed in most spindle cells in late KS lesions, and significant evidence suggests that they participate in KS paracrine neoplasia.52 For example, the induction of IL-6 secretion in KSHV-infected cells has previously been attributed—at least in part—to the activation of the IL-6 promoter by KSHV-encoded genes, including the latency-associated nuclear antigen 1 (LANA1), Rta, and vFlip.53-56 Indeed, vFlip has been shown to up-regulate the activity of 2 pluripotent transcription factors, NF-κB and AP-1,53 and activation of NF-κB by vFlip appears to be essential for the survival of KSHV-infected PEL cell lines.57 Collectively, these results strongly suggest that KSHV latent genes may play an important role in supporting KS paracrine neoplasia in established KS lesions.
In this regard, using a recently developed endothelial-specific retroviral gene transfer (TIE2-tva) system, we have previously shown that endothelial expression of KSHV latent genes may not be sufficient to initiate KS.9 Conversely, accumulating evidence points to a critical role for a KSHV lytic gene, vGPCR, in Kaposi sarcomagenesis.58 In 3 different transgenic animal models, expression of vGPCR induced sarcomas that were remarkably similar to human KS lesions and that had a unique predilection for the skin.9-11 Moreover, vGPCR tumorigenesis appears to be driven by a paracrine mechanism involving the secretion of key KS cytokines. This striking congruence between vGPCR oncogenesis and human KS spindle cell sarcomagenesis prompted us to explore the molecular mechanism whereby vGPCR promotes paracrine neoplasia.
We show here that vGPCR induces the activation of critical transcription factors, including NF-κB, AP-1, and NFAT, which in turn appears to lead to the secretion of a number of KS cytokines. We further observed that activation of the Rac1 GTPase plays an essential role in the ability of vGPCR to stimulate these transcription factors and that this facilitates the secretion of key KS cytokines by vGPCR-expressing cells. Indeed, endothelial cells expressing vGPCR that were rendered unable to efficiently activate Rac1 were significantly less tumorigenic than vGPCR-expressing endothelial cells in which Rac1 activation was not prevented, aligned with a critical role for Rac1 in vGPCR tumorigenesis. This also prompted us to explore the biologic consequence of the constitutive activation of this small GTPase in vivo. Using the TIE2-tva retroviral gene transfer system, we demonstrate that constitutive activation of Rac1 alone proved sufficient to initiate endothelial cell transformation in mice. Vascular lesions initiated by Rac1 activation were remarkably similar to those found in early vGPCR experimental lesions. Together, these observations implicate this small GTP-binding protein in the initiation of vGPCR tumorigenesis likely by promoting paracrine neoplasia.
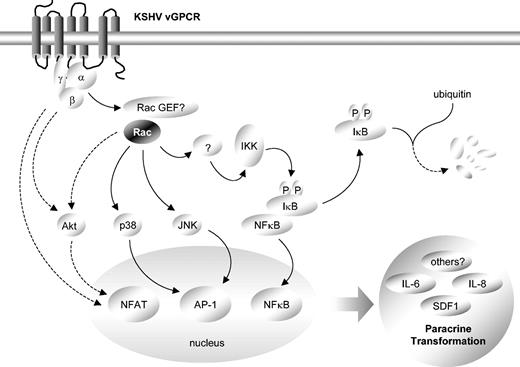
vGPCR transcriptional up-regulation has previously been shown to involve the activation of a number of intracellular kinase cascades,9,13-17 some of which may be mediated by Rac1. Indeed, inhibition of Rac1 abolished the ability of vGPCR to activate both Jun N-terminal kinase (JNK) and p38 and partially inhibited its ability to stimulate Akt without affecting extracellular signal-regulated kinase (ERK) activation (results not shown). Collectively, these results are consistent with a scenario in which vGPCR stimulates the Rac1-dependent activation of both JNK and p38, and thus of AP-1, while vGPCR activation of NFAT may be only partially dependent on Rac1 activation and Akt, with other (Rac1-independent) vGPCR-initiated signaling pathways also contributing to the up-regulation of this transcription factor.17 vGPCR activation of NF-κB is strictly dependent on Rac1, although the precise mechanism whereby this small GTPase regulates the IκB/NF-κB signaling system still remains unclear (Figure 7).
Schematic representation of critical role of Rac1 in vGPCR paracrine neoplasia. Constitutive stimulation of Rac1 by vGPCR likely leads to phosphorylation of IκB. Phosphorylation targets IκB for ubiquitin-mediated degradation, releasing NF-κB, which then translocates to the nucleus to promote cytokine transcription. Concurrently, Rac1 activation of JNK and p38 may facilitate AP-1–dependent transcription while both Rac1-dependent and -independent signaling pathways may lead to the activation of Akt and the transcription factor NFAT. Transcriptional activation by Rac1 ultimately drives vGPCR paracrine neoplasia.
Schematic representation of critical role of Rac1 in vGPCR paracrine neoplasia. Constitutive stimulation of Rac1 by vGPCR likely leads to phosphorylation of IκB. Phosphorylation targets IκB for ubiquitin-mediated degradation, releasing NF-κB, which then translocates to the nucleus to promote cytokine transcription. Concurrently, Rac1 activation of JNK and p38 may facilitate AP-1–dependent transcription while both Rac1-dependent and -independent signaling pathways may lead to the activation of Akt and the transcription factor NFAT. Transcriptional activation by Rac1 ultimately drives vGPCR paracrine neoplasia.
Because emerging evidence suggests that small GTPases of the Rho family are critical for the malignant progression as well as for the invasiveness and metastatic potential of tumor cells,59 the activation of Rac1 by vGPCR may further influence the aggressiveness of KS tumors. Indeed, Rac1 activation in endothelial cells expressing vGPCR leads to the formation of membrane ruffles and lamellipodia that are actin-based structures commonly observed in migrating cells in vitro60 and are likely to play a role in tumor cell invasion in vivo.61 Rac1 can also promote the formation of integrin-containing adhesion complexes, which mediate attachment to the extracellular matrix, and may also modify the strength of cadherin-mediated cell-cell adhesions, thus enabling cells to detach from the tumor mass.62 In addition, Rho proteins also regulate expression of metalloproteinases, phospholipid metabolism, and have been implicated in various vesicular transport events, all of which may further impact tumor cell invasiveness.63 Thus, it is reasonable to suspect that Rac1 may play an important role as part of the still poorly understood molecular mechanisms underlying the aggressive nature of invasive KS.
However, activated alleles of Rac1 promoted the formation of vascular lesions that failed to progress into the sheets of spindle-shaped tumor cells and erythrocyte-replete vascular slits characteristic of late vGPCR experimental tumors and human KS. Thus, additional vGPCR-regulated (and likely Rac1-independent) signaling pathways may be further required for progression to full vGPCR-initiated sarcomagenesis. Nonetheless, our present findings indicate that Rac1 provides a critical connection between vGPCR and the secretion of inflammatory and angiogenic growth factors, suggesting that this small GTPase and its recently identified downstream effectors may represent suitable molecular targets for the development of rationally designed therapies targeting the initiation of KSHV-induced paracrine neoplasia.
Prepublished online as Blood First Edition Paper, July 1, 2004; DOI 10.1182/blood-2003-12-4436.
Supported by National Institutes of Health (NIH) grant RO-1 AI46145-01A2 and a grant from the Department of Defense, BC972195 (E.T.S.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to M. Kriete and the Veterinary Resources Core Facility (National Institute of Dental and Craniofacial Research [NIDCR]) for assistance with the animal care and Science Applications International Corporation (SAIC) Frederick for tissue preparation.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal