Abstract
Emerging evidence to support the use of endothelial progenitor cells (EPCs) for angiogenic therapies or as biomarkers to assess cardiovascular disease risk and progression is compelling. However, there is no uniform definition of an EPC, which makes interpretation of these studies difficult. Although hallmarks of stem and progenitor cells are their ability to proliferate and to give rise to functional progeny, EPCs are primarily defined by the expression of cell-surface antigens. Here, using adult peripheral and umbilical cord blood, we describe an approach that identifies a novel hierarchy of EPCs based on their clonogenic and proliferative potential, analogous to the hematopoietic cell system. In fact, some EPCs form replatable colonies when deposited at the single-cell level. Using this approach, we also identify a previously unrecognized population of EPCs in cord blood that can achieve at least 100 population doublings, replate into at least secondary and tertiary colonies, and retain high levels of telomerase activity. Thus, these studies describe a clonogenic method to define a hierarchy of EPCs based on their proliferative potential, and they identify a unique population of high proliferative potential-endothelial colony-forming cells (HPP-ECFCs) in human umbilical cord blood. (Blood. 2004;104:2752-2760)
Introduction
Endothelial progenitor cells (EPCs) may potentially be used for angiogenic therapies or as biomarkers to assess cardiovascular disease risk.1-6 Currently, there is no uniform definition of an EPC.1,2 Although hallmarks of stem and progenitor cells are their ability to proliferate and to give rise to functional progeny, EPCs are defined primarily by the expression of cell-surface antigens.1,2 Thus, given the potential usefulness of EPCs, developing clonogenic assays to better define EPCs is a priority.
Hematopoietic and endothelial progenitor cells share a number of cell-surface markers in the developing yolk sac and embryo, and genetic disruption of numerous genes affects hematopoietic and endothelial cell development, suggesting they originate from a common precursor, the hemangioblast.7-10 Although hierarchies of hematopoietic stem and progenitor cells have been well established (based on differences in clonogenic and proliferative potential11 ), evidence to support similar hierarchies of stem and progenitor cells (based on differences in proliferative potential) for the endothelial lineage has not been established.
In the current study, we describe an approach that identifies a novel hierarchy of EPCs isolated from adult peripheral and umbilical cord blood based on their clonogenic and proliferative potential, analogous to the hematopoietic cell system. Using this approach, we also identify a unique population of high proliferative potential-endothelial colony-forming cells (HPP-ECFCs) in human cord blood that can achieve at least 100 population doublings (PDs), replate into at least secondary and tertiary colonies, and retain high levels of telomerase activity.
Patients, materials, and methods
Adult peripheral and umbilical cord blood samples
Blood samples (50-100 mL) were collected in citrate phosphate dextrose (CPD) solution from 18 healthy human volunteers (11 men, 7 women; age range, 22-50 years). Human umbilical cord blood samples (20-70 mL) from 13 healthy newborns (7 boys, 6 girls; gestational age range, 38-40 weeks) were collected in CPD solution. The Institutional Review Board at the Indiana University School of Medicine approved all protocols, and informed consent was obtained from all adult donors and parents of newborns.
Buffy coat cell preparation
Human mononuclear cells (MNCs) were obtained as previously described, with minor modifications.12 Blood (20-100 mL) was diluted 1:1 with Hanks balanced salt solution (HBSS; Invitrogen, Grand Island, NY) and was overlaid onto an equivalent volume of Histopaque 1077 (ICN, Costa Mesa, CA). Cells were centrifuged for 30 minutes at room temperature at 740g. MNCs were isolated and washed 3 times with EBM-2 medium (Cambrex, Walkersville, MD) supplemented with 10% fetal bovine serum (FBS; Hyclone, Logan, UT), 2% penicillin/streptomycin (Invitrogen), and 0.25 μg/mL amphotericin B (Invitrogen) (complete EGM-2 medium).
Culture of adult peripheral and umbilical cord blood endothelial cells
MNCs were resuspended in 12 mL complete EGM-2 medium. Cells were seeded onto 3 separate wells of a 6-well tissue culture plate precoated with type 1 rat tail collagen (BD Biosciences, Bedford, MA) at 37°C, 5% CO2, in a humidified incubator. After 24 hours of culture, nonadherent cells and debris were aspirated, adherent cells were washed once with complete EGM-2 medium, and complete EGM-2 medium was added to each well. Medium was changed daily for 7 days and then every other day until the first passage.
Colonies of endothelial cells appeared between 5 and 22 days of culture and were identified as well-circumscribed monolayers of cobblestone-appearing cells (Figure 1C). Endothelial cell colonies (ECCs) were enumerated by visual inspection using an inverted microscope (Olympus, Lake Success, NY) under 40 × magnification. Endothelial cells derived from the ECCs were released from the original tissue culture plates, resuspended in complete EGM-2 media, and plated onto 75-cm2 tissue culture flasks coated with type 1 rat tail collagen for further passage.
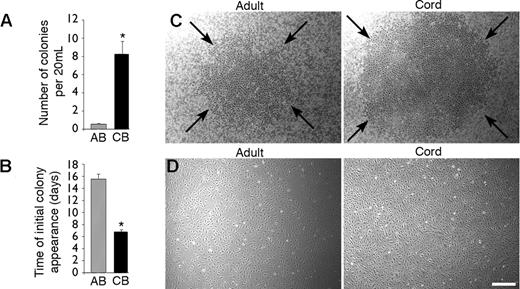
Isolation of endothelial progenitor cell colonies derived from adult peripheral and umbilical cord blood. (A) Number of EPC-derived ECCs isolated per 20 mL adult peripheral blood (AB) and umbilical cord blood (CB). Results represent the mean ± SEM ECCs of 18 independent experiments for adult donors and 13 independent experiments for cord blood samples. *P < .0001 by Student paired t test. (B) Time of initial EPC-derived ECC appearance after culture initiation from equivalent volumes of AB and CB blood. Results represent the mean ± SEM number of days before initial ECC appearance of 18 independent experiments for AB donors and 13 independent experiments for CB donors. *P < .0001 by Student paired t test. (C) Representative photomicrographs (50 × magnification) of EPC-derived ECCs from mononuclear cells isolated from AB (day 17) and CB (day 9). Arrows outline a representative ECC from cord and adult blood. Similar colonies were observed from 18 other adult samples and 13 other cord blood donors. (D) Representative photomicrographs (50 × magnification) of cell monolayers derived from either adult or cord blood EPC-derived ECCs. Similar cell monolayers were observed after initial passage of EPC-derived ECCs harvested from 18 other adult donors and 13 other cord blood samples. Scale bar represents 100 μm.
Isolation of endothelial progenitor cell colonies derived from adult peripheral and umbilical cord blood. (A) Number of EPC-derived ECCs isolated per 20 mL adult peripheral blood (AB) and umbilical cord blood (CB). Results represent the mean ± SEM ECCs of 18 independent experiments for adult donors and 13 independent experiments for cord blood samples. *P < .0001 by Student paired t test. (B) Time of initial EPC-derived ECC appearance after culture initiation from equivalent volumes of AB and CB blood. Results represent the mean ± SEM number of days before initial ECC appearance of 18 independent experiments for AB donors and 13 independent experiments for CB donors. *P < .0001 by Student paired t test. (C) Representative photomicrographs (50 × magnification) of EPC-derived ECCs from mononuclear cells isolated from AB (day 17) and CB (day 9). Arrows outline a representative ECC from cord and adult blood. Similar colonies were observed from 18 other adult samples and 13 other cord blood donors. (D) Representative photomicrographs (50 × magnification) of cell monolayers derived from either adult or cord blood EPC-derived ECCs. Similar cell monolayers were observed after initial passage of EPC-derived ECCs harvested from 18 other adult donors and 13 other cord blood samples. Scale bar represents 100 μm.
Growth kinetics and estimate of replicative capacity of adult peripheral and cord blood EPC-derived endothelial cells
At first passage, endothelial cells were enumerated using a trypan blue exclusion assay (Sigma, St Louis, MO). At each subsequent passage, cells were enumerated for calculation of a growth kinetic curve, population-doubling times (PDTs), and cumulative population-doubling levels (CPDLs). The number of PDs occurring between passages was calculated according to the equation: PD = log2 (CH/CS), where CH is the number of viable cells at harvest and CS is the number of cells seeded. The sum of all previous PDs determined the CPDL at each passage. The PDT was derived using the time interval between cell seeding and harvest divided by the number of PDs for that passage.
Matrigel assays and uptake of DiI-acetylated-low-density lipoprotein
Matrigel assays were performed as previously described with minor modifications.13 Early-passage (2-3) ECC-derived endothelial cells were seeded onto 96-well tissue culture plates coated with 30 μL Matrigel (BD Biosciences) at a cell density of 5000 to 20 000 cells per well. Cells were observed every 2 hours by visual microscopy with an inverted microscope at 40 × magnification for capillary-like formation.
To assess the ability of endothelial cells to incorporate DiI-acetylated-low-density lipoprotein (DiI-Ac-LDL), attached cells were incubated with 10 μg/mL DiI-Ac-LDL (Biomedical Technologies, Stoughton, MA) in complete EGM-2 media for 4 hours at 37°C. Cells were washed 3 times and stained with 1.5 μg/mL of 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI; Sigma). Cells were examined for uptake of Dil-Ac-LDL using a Zeiss LSM510-Meta confocal microscopy system (Zeiss, Thornwood, NY) with a 40 × C-Apochromat/1.2 NA water immersion lens. Dil-Ac-LDL was excited with the 543-nm line of a He-Ne laser, and DAPI was excited by a tunable titanium-sapphire laser at 800 nm. Images were acquired with the manufacturer's software, and images were assembled in Adobe Photoshop CS version 8.0.
Immunophenotyping of endothelial cells
Early-passage (1-2) ECC-derived endothelial cells (5 × 105) were incubated at 4°C for 30 to 60 minutes with varying concentrations of the primary or isotype control antibody, as outlined below in 100 μL phosphate-buffered saline (PBS) and 2% fetal bovine serum (FBS), washed 3 times, and analyzed by fluorescence-activated cell sorting (FACS) (Becton Dickinson, San Diego, CA). We used primary murine monoclonal antibodies against human CD31 conjugated to fluorescein isothiocyanate (FITC) (all BD PharMingen, San Diego, CA, unless otherwise indicated), human CD34 conjugated to allophycocyanin (APC), human CD14 conjugated to FITC, human CD45 conjugated to FITC, human CD117 conjugated to APC, human CD146 conjugated to phycoerythrin (PE), human AC133 conjugated to PE (Miltenyi Biotec, Auburn, CA), human CD141 conjugated to FITC (Cymbus Biotechnology, Chandlers Ford, United Kingdom), human CD105 conjugated to Alexa Fluor 647 (Molecular Probes, Eugene, OR), and human CD144 conjugated to Alexa Fluor 647.
Serum-starved endothelial cells were stimulated with either 10 ng/mL recombinant human interleukin-1 (rhIL-1) (Peprotech, Rocky Hill, NJ) or 10 ng/mL recombinant human tumor necrosis factor-α (rhTNF-α) (Peprotech) for 4 hours at 37°C to examine cell-surface expression of vascular cell adhesion molecule 1 (VCAM-1). After stimulation, cells were incubated with a primary antibody against human VCAM-1 conjugated to FITC. For isotype controls for immunophenotyping and VCAM-1 expression, we used the following antibodies: mouse IgG2aκ conjugated to FITC, mouse IgG1κ conjugated to FITC, mouse IgG1κ conjugated to PE, and mouse IgG1κ conjugated to APC.
To detect cell-surface expression of von Willebrand factor (VWF) and flk-1, cells were fixed in acetone for 10 minutes at room temperature, washed, and permeabilized for 30 minutes with PBS, 3% nonfat dry milk, and 0.1% Triton X-100 (Sigma). We used 2 μg/mL of a primary antibody directed against human VWF (DAKO, Carpinteria, CA) and a biotinylated primary antibody directed against human flk-1 (Sigma) for detection. The secondary antibody used for VWF was a goat antirabbit antibody conjugated to FITC, and the secondary antibody used for flk-1 was streptavidin conjugated to APC. For the isotype control for VWF, we used rabbit immunoglobulin primary antibody (DAKO) with antirabbit immunoglobulin secondary antibody conjugated to FITC. For the isotype control for flk-1, we used biotinylated mouse IgG1κ (BD PharMingen) with streptavidin APC secondary antibody.
Thymidine incorporation assays
ECC-derived endothelial cells were deprived of growth factors and were cultured in EBM-2 media supplemented with 5% FBS for 24 hours, then plated in 6-well tissue culture dishes precoated with type 1 collagen and cultured for 16 hours in EBM-2 media supplemented with 1% FBS. Cells were then cultured in EBM-2 without serum for another 8 hours to ensure quiescence. Cells were stimulated in EBM-2 media supplemented with 10% FBS with 25 ng/mL recombinant human vascular endothelial growth factor (rhVEGF) (Peprotech), 25 ng/mL recombinant human basic fibroblast growth factor (rhbFGF) (Peprotech), or no growth factors in a 37°C, 5% CO2, humidified incubator. These cells with or without growth factors were cultured under for 16 hours, and 1 μCi (0.037 MBq) tritiated thymidine (Perkin Elmer Life Sciences Products, Boston, MA) was added 5 hours before harvest. Cells were lysed with 0.1 N sodium hydroxide for 1 hour. Lysates were collected into 5 mL liquid scintillant (Fisher Scientific, St Louis, MO), and β emission was measured. Assays were performed in triplicate.
Telomerase activity assay
Telomerase activity was measured by the telomeric amplification protocol (TRAP) as previously described14 using the TRAP-eze telomerase detection kit (Chemicon, Temecula, CA). Cell lysates from 103 cells were used for each assay. The PCR product and a 6-base pair incremental ladder were electrophoresed on a 12.5% nondenaturing polyacrylamide gel and visualized by SYBR gold staining (Molecular Probes, Eugene, OR).
Generation of GALV-pseudotyped MFG-EGFP
MFG-enhanced green fluorescence protein (MGF-EGFP) retrovirus vector expressing EGFP under the control of the Moloney murine leukemia virus long terminal repeat (LTR) was used as previously described.15 Supernatant from MFG-EGFP clone 5, with a titer of 0.5-1 × 106 infectious U/mL, was used for all experiments.
Retroviral transduction of endothelial cells
Early-passage (1-2) ECC-derived endothelial cells were transduced with MGF-EGFP supernatant in nontissue culture plates coated with 5 μg/cm2 fibronectin CH-296 (Takara Shuzo, Otsu, Japan). Cells were infected with retrovirus supernatant diluted 1:1 with complete EGM-2 for 4 hours on 2 consecutive days with a change of complete EGM-2 media for overnight incubation. After the second round of infection, cells were harvested, counted, and analyzed for EGFP expression using fluorescence cytometry.
Single-cell assays
Early passage (1-2) ECC-derived endothelial cells, transduced with the MFG-EGFP retrovirus, were sorted by fluorescence cytometry for EGFP expression. The FACS Vantage Sorter (Becton Dickinson) was used to place one single EGFP+ endothelial cell/well of a 96-well flat-bottom tissue culture plate precoated with type 1 collagen containing 200 μL complete EGM-2 media. Individual wells were examined under a fluorescence microscope at 50 × magnification to ensure that only one cell had been placed into each well. Cells were cultured at 37°C, 5% CO2, in a humidified incubator. Media were changed every 4 days, and fresh complete EGM-2 media were added. At day 14, each well was examined for the growth of endothelial cells. We scored as positive those wells in which 2 or more endothelial cells were identified under a fluorescence microscope at 100 × magnification. To enumerate the number of cells per well, we counted cells by visual inspection with a fluorescence microscope at 100 × magnification (fewer than 50 cells per well), or we trypsinized the cells and counted them with a hemacytometer (more than 50 cells per well). Individual wells (14 days after culture initiation from a single cell) containing more than 50 cells were trypsinized and subcultured to a 24-well tissue culture dish coated with type 1 collagen. Four days later, the media were aspirated and replaced with 500 μL fresh complete EGM-2 media. On day 7, wells were examined for colony growth or cell confluence by visual inspection. Cells were then trypsinized, counted, and subcultured in a 6-well tissue culture plate precoated with type 1 collagen. After 7 days of culture in a 6-well plate, we randomly selected 10 to 12 confluent wells for long-term passage in T75 flasks. For each sample passaged, we calculated the PDT and CPDL as described above.
Phase contrast and fluorescence imaging
Adherent ECs were imaged in PBS or complete EGM-2 medium. Phase contrast (Figures 1C-D and 3) and fluorescence (Figures 2D, 5E, 6B, and 7) images were collected using a Zeiss Axiovert 2 inverted microscope with a 5× CP-ACHROMAT/0.12 NA objective. Images were acquired using a SPOT RT color camera (Diagnostic Instruments, Sterling Heights, MI) with the manufacturer's software. Composite images were assembled in Adobe Photoshop version 8.0.
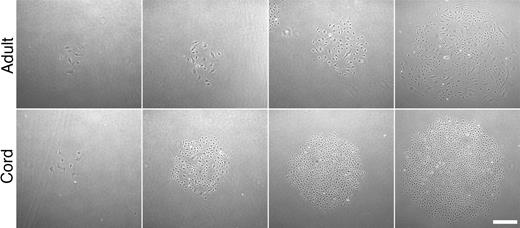
Representative photomicrographs of secondary colonies. Colonies formed 7 days after adult and cord blood EPC-derived endothelial cells were plated at low cell density (50 × magnification). Scale bar represents 100 μm.
Representative photomicrographs of secondary colonies. Colonies formed 7 days after adult and cord blood EPC-derived endothelial cells were plated at low cell density (50 × magnification). Scale bar represents 100 μm.
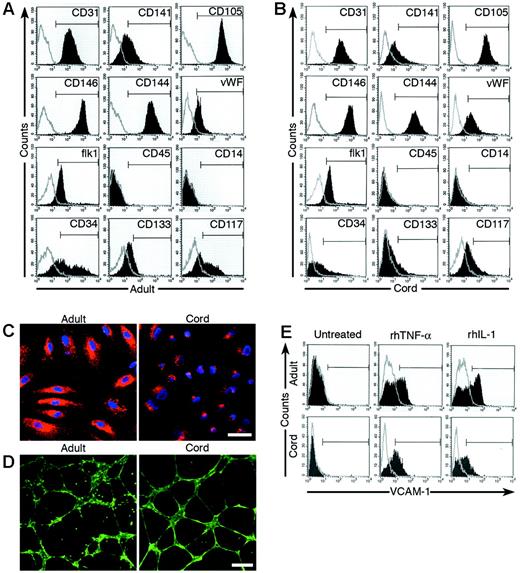
Phenotypic and functional analysis of adult and cord blood EPC-derived endothelial cells. (A-B) Immunophenotyping of cell monolayers derived from adult peripheral blood (AB; panel A) or umbilical cord blood (CB; panel B) EPC-derived ECCs by fluorescence cytometry. Cord blood and adult EPC-derived ECCs express CD31, CD141, CD105, CD146, CD144, VWF, and Flk-1 but do not express CD45 and CD14. Some cord blood and adult cells express CD34, CD133, and CD117. Shown are representative data from 18 independent experiments using different adult cell monolayers and 13 independent experiments using different cord blood cell monolayers with similar results. Isotype controls are overlaid in a gray line on each histogram for each surface antigen tested. (C) Adult and cord blood EPC-derived endothelial cells incorporate DiI-Ac-LDL (50 × magnification). A representative photomicrograph is shown for adult and cord blood EPC-derived endothelial cells, which have taken up DiI-Ac-LDL (red) and have also stained with DAPI (blue). Shown are representative data from 18 independent experiments using different adult cell monolayers and 13 independent experiments using different cord blood cell monolayers with similar results. Scale bar represents 100 μm. (D) Adult and cord blood EPC-derived endothelial cells expressing EGFP were plated in Matrigel for the formation of capillary-like structures (50 × magnification). Shown are representative data from 18 independent experiments using different adult cell monolayers and 13 independent experiments using different cord blood cell monolayers with similar results. Scale bar represents 100 μm. (E) Adult and cord blood EPC-derived endothelial cells up-regulate the cell-surface expression of VCAM-1 in response to either rhTNF-α or rhIL-1. Shown are representative data from 18 independent experiments using different adult cell monolayers and 13 independent experiments using different cord blood cell monolayers with similar results. The isotype control for VCAM-1 is overlaid in a gray line on each histogram.
Phenotypic and functional analysis of adult and cord blood EPC-derived endothelial cells. (A-B) Immunophenotyping of cell monolayers derived from adult peripheral blood (AB; panel A) or umbilical cord blood (CB; panel B) EPC-derived ECCs by fluorescence cytometry. Cord blood and adult EPC-derived ECCs express CD31, CD141, CD105, CD146, CD144, VWF, and Flk-1 but do not express CD45 and CD14. Some cord blood and adult cells express CD34, CD133, and CD117. Shown are representative data from 18 independent experiments using different adult cell monolayers and 13 independent experiments using different cord blood cell monolayers with similar results. Isotype controls are overlaid in a gray line on each histogram for each surface antigen tested. (C) Adult and cord blood EPC-derived endothelial cells incorporate DiI-Ac-LDL (50 × magnification). A representative photomicrograph is shown for adult and cord blood EPC-derived endothelial cells, which have taken up DiI-Ac-LDL (red) and have also stained with DAPI (blue). Shown are representative data from 18 independent experiments using different adult cell monolayers and 13 independent experiments using different cord blood cell monolayers with similar results. Scale bar represents 100 μm. (D) Adult and cord blood EPC-derived endothelial cells expressing EGFP were plated in Matrigel for the formation of capillary-like structures (50 × magnification). Shown are representative data from 18 independent experiments using different adult cell monolayers and 13 independent experiments using different cord blood cell monolayers with similar results. Scale bar represents 100 μm. (E) Adult and cord blood EPC-derived endothelial cells up-regulate the cell-surface expression of VCAM-1 in response to either rhTNF-α or rhIL-1. Shown are representative data from 18 independent experiments using different adult cell monolayers and 13 independent experiments using different cord blood cell monolayers with similar results. The isotype control for VCAM-1 is overlaid in a gray line on each histogram.
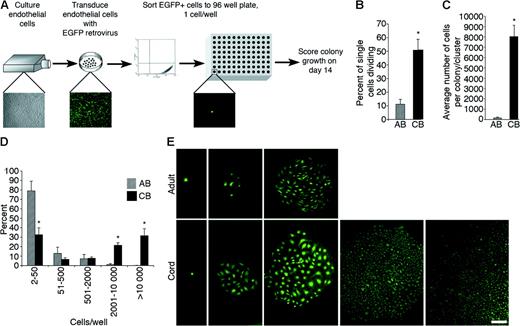
Quantitation of the clonogenic and proliferative potential of single cord blood and adult endothelial cells derived from EPC colonies. (A) Schematic of single-cell assays using endothelial cells derived from adult or cord EPC colonies. (B) The percentage of single adult peripheral blood (AB) or umbilical cord blood (CB) EPC-derived endothelial cells undergoing at least 1 cell division after 14 days of culture. Results represent the average ± SEM of 5 independent experiments using single endothelial cells derived from different donors. *P < .01 by Student paired t test. (C) Average number of cell progeny derived from a single adult (AB) or cord (CB) EPC-derived endothelial cell after 14 days in culture. Results represent the average ± SEM of 5 independent experiments using single endothelial cells derived from different donors. *P < .01 by Student paired t test. (D) Number of cell progeny derived from a single adult (AB) or cord (CB) EPC-derived endothelial cell in an individual well after 14 days of culture. Results represent the average ± SEM of 5 independent experiments. *P < .01 by Student paired t test. (E) Representative photomicrographs (50 × magnification) of the different endothelial cell clusters (fewer than 50 cells) or colonies (more than 50 cells) derived from a single cord blood or adult EPC-derived endothelial cell. Results are representative of 4 other independent experiments using cells from different donors. Scale bar represents 100 μm.
Quantitation of the clonogenic and proliferative potential of single cord blood and adult endothelial cells derived from EPC colonies. (A) Schematic of single-cell assays using endothelial cells derived from adult or cord EPC colonies. (B) The percentage of single adult peripheral blood (AB) or umbilical cord blood (CB) EPC-derived endothelial cells undergoing at least 1 cell division after 14 days of culture. Results represent the average ± SEM of 5 independent experiments using single endothelial cells derived from different donors. *P < .01 by Student paired t test. (C) Average number of cell progeny derived from a single adult (AB) or cord (CB) EPC-derived endothelial cell after 14 days in culture. Results represent the average ± SEM of 5 independent experiments using single endothelial cells derived from different donors. *P < .01 by Student paired t test. (D) Number of cell progeny derived from a single adult (AB) or cord (CB) EPC-derived endothelial cell in an individual well after 14 days of culture. Results represent the average ± SEM of 5 independent experiments. *P < .01 by Student paired t test. (E) Representative photomicrographs (50 × magnification) of the different endothelial cell clusters (fewer than 50 cells) or colonies (more than 50 cells) derived from a single cord blood or adult EPC-derived endothelial cell. Results are representative of 4 other independent experiments using cells from different donors. Scale bar represents 100 μm.
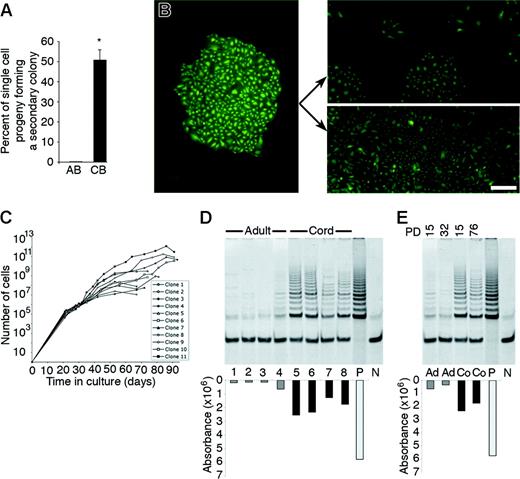
Replating potential and long-term culture of the cell progeny derived from a single cord blood or adult EPC-derived endothelial cell. (A) Percentage of the cell progeny derived from a single adult peripheral blood (AB) or umbilical cord blood (CB) EPC-derived endothelial cell, which formed secondary colonies or rapidly grew to cell confluence after 7 days of culture in a 24-well tissue culture plate. Results represent the mean ± SEM of 4 independent experiments using cells derived from 4 different donors. *P < .01 by Student paired t test. (B) A representative photomicrograph (50 × magnification) of the primary colony (left panel) or the secondary endothelial cell colonies or confluent cell monolayers (right panels) derived from the cell progeny of a single plated cord blood EPC-derived endothelial cell in a 24-well plate after 7 days in culture. Arrows indicate either secondary colonies (top right panel) or a confluent monolayer of ECs (bottom right panel) derived from the primary colony. Scale bar represents 100 μm. (C) Growth kinetics of the cell progeny of 11 single-plated EPC-derived endothelial cells isolated from 3 different cord blood donors in long-term culture. ▪ indicates the total number of cells at each passage. (D) Telomerase activity of early-passage adult (lanes 1-4) and cord (lanes 5-8) blood EPC-derived endothelial cells isolated from different donors. Adult and cord cells were tested at a CPDL of 15. P indicates telomerase activity in HeLa cells, which were used as a positive control, and N indicates a negative control. (E) Comparison of telomerase activity of early- and late-passage adult (Ad) and cord (Co) blood EPC-derived endothelial cells. PD indicates the cumulative population doubling level of the cells tested. P indicates telomerase activity in HeLa cells, which were used as a positive control. N indicates a negative control. Three other experiments using early- and late-passage cord blood and adult EPC-derived endothelial cells from 3 different donors showed similar results.
Replating potential and long-term culture of the cell progeny derived from a single cord blood or adult EPC-derived endothelial cell. (A) Percentage of the cell progeny derived from a single adult peripheral blood (AB) or umbilical cord blood (CB) EPC-derived endothelial cell, which formed secondary colonies or rapidly grew to cell confluence after 7 days of culture in a 24-well tissue culture plate. Results represent the mean ± SEM of 4 independent experiments using cells derived from 4 different donors. *P < .01 by Student paired t test. (B) A representative photomicrograph (50 × magnification) of the primary colony (left panel) or the secondary endothelial cell colonies or confluent cell monolayers (right panels) derived from the cell progeny of a single plated cord blood EPC-derived endothelial cell in a 24-well plate after 7 days in culture. Arrows indicate either secondary colonies (top right panel) or a confluent monolayer of ECs (bottom right panel) derived from the primary colony. Scale bar represents 100 μm. (C) Growth kinetics of the cell progeny of 11 single-plated EPC-derived endothelial cells isolated from 3 different cord blood donors in long-term culture. ▪ indicates the total number of cells at each passage. (D) Telomerase activity of early-passage adult (lanes 1-4) and cord (lanes 5-8) blood EPC-derived endothelial cells isolated from different donors. Adult and cord cells were tested at a CPDL of 15. P indicates telomerase activity in HeLa cells, which were used as a positive control, and N indicates a negative control. (E) Comparison of telomerase activity of early- and late-passage adult (Ad) and cord (Co) blood EPC-derived endothelial cells. PD indicates the cumulative population doubling level of the cells tested. P indicates telomerase activity in HeLa cells, which were used as a positive control. N indicates a negative control. Three other experiments using early- and late-passage cord blood and adult EPC-derived endothelial cells from 3 different donors showed similar results.
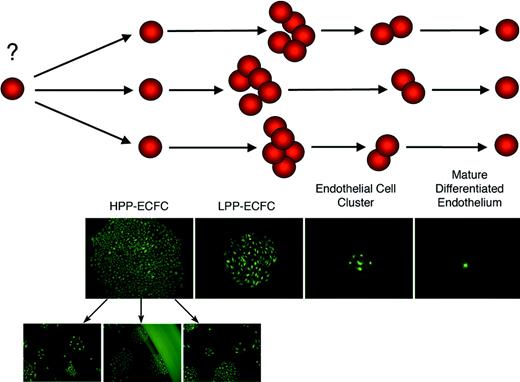
Model of an endothelial progenitor cell hierarchy based on the proliferative and clonogenic potential of discrete populations of progenitor cells. HPP-ECFCs are large colonies that form secondary and tertiary colonies on replating. HPP-ECFCs give rise to all subsequent stages of endothelial progenitors in addition to replating into secondary HPP-ECFCs. LPP-ECFCs form colonies that contain more than 50 cells but do not form secondary colonies or LPP-ECFCs on replating. Endothelial cell clusters can arise from a single cell but contain fewer than 50 cells that are typically larger than the smaller cells found in HPP-ECFC and LPP-ECFC colonies. Mature, terminally differentiated endothelial cells do not divide.
Model of an endothelial progenitor cell hierarchy based on the proliferative and clonogenic potential of discrete populations of progenitor cells. HPP-ECFCs are large colonies that form secondary and tertiary colonies on replating. HPP-ECFCs give rise to all subsequent stages of endothelial progenitors in addition to replating into secondary HPP-ECFCs. LPP-ECFCs form colonies that contain more than 50 cells but do not form secondary colonies or LPP-ECFCs on replating. Endothelial cell clusters can arise from a single cell but contain fewer than 50 cells that are typically larger than the smaller cells found in HPP-ECFC and LPP-ECFC colonies. Mature, terminally differentiated endothelial cells do not divide.
Results
Characterization of EPC-derived ECCs from adult peripheral and cord blood
EPCs have been isolated as cell colonies and expanded ex vivo from adult peripheral and umbilical cord blood MNCs.1,2,16-22 However, it is unknown whether a hierarchy of EPCs exists that can be discriminated by the clonogenic and proliferative potential of individual cells as in the hematopoietic cell system. To isolate EPCs to address this question, we harvested MNCs from healthy adults and umbilical cords of full-term infants and observed for ECC formation. Interestingly, the number of ECCs per equivalent blood volume was increased 15-fold in cord blood compared with adult samples, and the cord blood-derived colonies emerged in culture 1 week earlier than in adult colonies (Figure 1A-B). Further, cord blood colonies consistently appeared larger than adult colonies (Figure 1C). Thus, while isolating ECCs to address our experimental question, we detected distinct differences in the size, frequency, and time of appearance between adult and cord blood ECCs. These observations suggested that cord blood ECCs may be derived from a different population of progenitors than those previously identified as adult EPCs.
Before testing the proliferative and clonogenic potential of EPC-forming cord blood and adult ECCs, we verified that the cell progeny derived from cord blood and adult ECCs were not contaminated with other cell types. This is important because earlier studies have shown that the cell progeny derived from some EPC-derived colonies isolated from adult and cord blood MNCs contain cells that express the hematopoiesis-specific cell-surface antigen CD45.1,18,23 After initial ECC passage, monolayers of spindle-shaped cells formed that had a cobblestone morphology (Figure 1D). Immunophenotyping revealed that cord blood and adult cells expressed endothelial cell-surface antigens CD31, CD141, CD105, CD146, CD144, VWF, and flk-1 (Figure 2A-B). Importantly, neither cell population expressed the hematopoietic cell-specific surface antigens CD45 and CD14 (Figure 2A-B). However, a small percentage of cord blood and adult cells did express CD34, AC133, and CD117, cell-surface antigens previously identified on endothelial and hematopoietic progenitor cells1 (Figure 2A-B). Nevertheless, plating the EPC-derived endothelial cells that expressed CD34, AC133, or CD117 failed to give rise to hematopoietic colony-forming cells in methylcellulose assays, demonstrating that these cells do not have hematopoietic activity (data not shown).
We next tested whether the ECC-derived cells would incorporate DiI-acetylated low-density lipoprotein (DiI-Ac-LDL), form capillary-like structures in Matrigel, and up-regulate VCAM-1 in response to either rhTNF-α or rhIL-1 stimulation, which are characteristic of endothelial cells.13 To better visualize whether cord blood or adult endothelial cells form capillary-like structures in Matrigel, early-passage (1-2) cord and adult cells derived from the initial ECCs were transduced with a retrovirus encoding EGFP and were selected for EGFP expression. Cord blood and adult cells subcultured from the adherent colonies uniformly incorporated DiI-Ac-LDL, formed capillary-like structures in Matrigel, and up-regulated VCAM-1 in response to rhTNF-α or rhIL-1 stimulation (Figure 2C-E). However, the time course for cord blood cells to form capillary-like structures in Matrigel was significantly reduced compared with that of adult cells (data not shown). Thus, these studies confirm that the cell progeny derived from cord and adult ECCs were endothelial in origin and were not contaminated with hematopoietic cells.
Growth kinetics of EPC-derived cord blood and adult endothelial cells
Progenitor cells of different lineages are defined and distinguished by their clonogenic and proliferative potential. Given the differences in cord blood and adult EPC-derived ECC formation, we compared the proliferative kinetics of EPC-derived cord blood and adult endothelial cells. We initially plated cells derived from cord blood and adult ECCs at limiting cell dilutions to test whether the cells would form secondary colonies and grow to confluence. Interestingly, the cell progeny derived from adult and cord blood EPC-derived colonies formed secondary cell colonies of various sizes before growing to confluence (Figure 3). However, colonies derived from cord blood EPC-derived cell progeny were consistently larger and contained smaller cells than adult colonies (Figure 3).
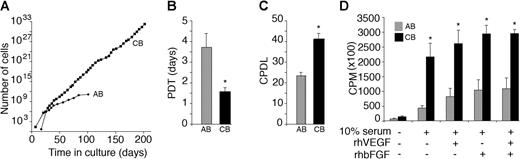
Cells were serially passaged to determine the proliferative potential of EPC-derived cord blood and adult endothelial cells. Remarkably, cord blood EPC-derived cells could be expanded for at least 100 PDs without obvious signs of senescence. In contrast, adult EPC-derived cells could be passaged for only 20 to 30 PDs, which is consistent with prior studies16,19 (Figure 4A). To quantitate and compare the proliferative kinetics of cord blood and adult EPC-derived cells, we calculated PDTs and CPDLs during a defined time in culture (60 days). There was a 2.5-fold decrease in the PDT and a 1.5-fold increase in the CPDLs of cord blood EPC-derived cells compared with adult EPC-derived cells (Figure 4B-C). PDTs and CPDLs of adult EPCs were similar to those of 2 recent reports that tested the proliferative kinetics of EPC-derived cells isolated from healthy adult donors.16,19
Growth kinetics of the endothelial cell progeny derived from cord and adult endothelial progenitor cell colonies. (A) Ex vivo expansion of adult peripheral blood (AB) and umbilical cord blood (CB) EPC-derived endothelial cells harvested from mononuclear cells. ▪ indicates the total cell number at each passage. Cells uniformly expressed the endothelial cell surface antigens shown in Figure 2A-B and not the hematopoietic cell-specific antigens, CD45 and CD14, at each passage (data not shown). A representative growth curve for cord blood and adult EPC-derived endothelial cells is shown. Eleven other cord blood and adult endothelial cell monolayers derived from different donors showed similar growth kinetics. (B-C) PDTs (B) and CPDLs (C) of cord (CB) and adult (AB) EPC-derived endothelial cells during 60 days of culture. Results represent the mean ± SEM PDTs and CPDLs of 6 independent experiments. *P < .01 by Student paired t test. (D) DNA synthesis of cord (CB) and adult (AB) EPC-derived endothelial cells. Early-passage (1-2) cord blood EPC-derived endothelial cells demonstrate increased DNA synthesis in response to 10% FBS, rhVEGF, and rhbFGF compared with adult cells. Results represent the average of 4 independent experiments using endothelial cells derived from different donors. *P < .01 by Student paired t test.
Growth kinetics of the endothelial cell progeny derived from cord and adult endothelial progenitor cell colonies. (A) Ex vivo expansion of adult peripheral blood (AB) and umbilical cord blood (CB) EPC-derived endothelial cells harvested from mononuclear cells. ▪ indicates the total cell number at each passage. Cells uniformly expressed the endothelial cell surface antigens shown in Figure 2A-B and not the hematopoietic cell-specific antigens, CD45 and CD14, at each passage (data not shown). A representative growth curve for cord blood and adult EPC-derived endothelial cells is shown. Eleven other cord blood and adult endothelial cell monolayers derived from different donors showed similar growth kinetics. (B-C) PDTs (B) and CPDLs (C) of cord (CB) and adult (AB) EPC-derived endothelial cells during 60 days of culture. Results represent the mean ± SEM PDTs and CPDLs of 6 independent experiments. *P < .01 by Student paired t test. (D) DNA synthesis of cord (CB) and adult (AB) EPC-derived endothelial cells. Early-passage (1-2) cord blood EPC-derived endothelial cells demonstrate increased DNA synthesis in response to 10% FBS, rhVEGF, and rhbFGF compared with adult cells. Results represent the average of 4 independent experiments using endothelial cells derived from different donors. *P < .01 by Student paired t test.
We next compared the proliferation of cord blood and adult EPC-derived cells in response to stimulation by either of 2 endothelial cell mitogens, rhVEGF and rhbFGF. Early-passage cord blood and adult EPC-derived cells were serum starved and then cultured in the presence or absence of rhVEGF or rhbFGF. Cells were cultured for 16 hours and were pulsed with tritiated thymidine before harvest to measure DNA synthesis. Cord blood EPC-derived cells displayed greater DNA synthesis in response to stimulation with rhVEGF or rhbFGF compared with adult EPC-derived cells (Figure 4D). Collectively, these experiments demonstrate that the proliferative rate and the expandability of cord blood EPC-derived cells are greater than those of adult EPC-derived cells in short- and long-term assays and that EPC-derived endothelial cells form distinct cell colonies of various sizes and morphology when plated at limiting dilution.
Quantitation Of The Clonogenic And Proliferative Potential Of Single Cord Blood And Adult Endothelial Cells Derived From Epc Colonies
Our data argue that cord blood and adult EPC-derived ECCs are composed of cells with different proliferative and clonogenic potential. The most rigorous test for the clonogenic potential of a progenitor cell is to determine whether a single cell will divide and form a colony in the absence of other cells. Therefore, we developed an assay to quantitate the proliferative and clonogenic potential of single cord blood and adult endothelial cells derived from EPCs (Figure 5A).
Remarkably, the percentage of single cells undergoing at least 1 cell division was increased 5-fold for cord blood endothelial cells compared with adult cells (Figure 5B). In addition, the average number of cell progeny derived from a single plated cord blood cell was 100-fold greater than the number of cells derived from an individual adult cell (Figure 5C). More than 80% of the single adult endothelial cells that divided gave rise to small colonies or clusters ranging from 2 to 50 cells each (Figure 5D). A small population of single adult endothelial cells did form colonies containing more than 500 cells (Figure 5D). In contrast, at least 60% of the single-plated cord blood endothelial cells that divided formed well-circumscribed colonies containing from 2000 to 10 000 cells in the 14-day culture period (Figure 5D). Photomicrographs of the size and morphology of the various ECCs and clusters of cells derived from a single cord blood or adult cell are shown in Figure 5E. These single-cell studies demonstrate that there are different types of cord and adult EPC-derived ECCs that can be distinguished by their proliferative and clonogenic potential and that EPCs display a hierarchy of proliferative potentials similar to the hematopoietic progenitor cell hierarchy.
Cell progeny of single-cord blood endothelial cells can be serially replated and expanded exponentially in long-term cultures
In the hematopoietic cell system, the most proliferative progenitor cell type that can be cultured in vitro in the absence of a stromal cell monolayer is termed the high proliferative potential-colony-forming cell (HPP-CFC).11,24 We tested whether a cell with similar proliferative potential is present within adult and cord blood EPC-derived ECCs to further define and classify the endothelial progenitor cell hierarchy. The clonal progeny derived from a single plated cord blood or adult EPC-derived cell were trypsinized, replated, and cultured into 24-well tissue culture plates for 7 days. After replating the clonal progeny of more than 1000 single adult EPC-derived cells (primary wells containing more than 50 cells), we detected only 1 secondary colony in the wells (1 of 1000 plated) after 14 days of culture (Figure 6A). In contrast, approximately half (205 of 421 plated) clonal progeny of single-plated cord blood EPC-derived cells (primary wells containing more than 50 cells) formed secondary colonies or rapidly grew to confluence in 24-well plates (Figure 6A). A representative photomicrograph of the secondary endothelial cell colonies or confluent cell monolayers derived from the progeny of a cord blood endothelial cell is shown in Figure 6B. Because we could not detect secondary colonies in wells that had rapidly grown to confluence in 5 days, we performed limiting-dilution analysis on those confluent monolayers. At least 9% of the wells replated (from confluent secondary wells) formed tertiary ECCs of more than 100 cells (data not shown). This experiment verifies that individual cells derived from or representing cord blood EPCs are capable of forming secondary or tertiary colonies.
We next tested the long-term proliferative potential of the cells derived from a single plated cord blood EPC-derived endothelial cell. Secondary colonies or confluent cell monolayers derived from single cord blood endothelial cells were serially passaged into progressively larger tissue culture plates. We tested the cell progeny of 11 single endothelial cells, originally derived from 3 different cord blood donors. Strikingly, some single cord blood endothelial cells yielded at least 1012 cells in long-term culture, and all cells produced at least 10 million progeny (Figure 6C). The average CPDL of the 11 single cord blood endothelial cells tested was 30.8. Thus, we have identified a population of HPP-ECFC in cord blood that forms secondary and tertiary colonies.
EPC-derived cord blood endothelial cells contain high levels of telomerase activity
Our data demonstrate that endothelial cells derived from cord blood EPCs can be serially passaged for at least 100 PDs (Figure 4B) and that some single cord blood EPCs can be expanded for 30 PDs. The only other primary endothelial cells with similar growth kinetics are those genetically engineered to overexpress telomerase.25,26 Thus, we measured telomerase activity in cord blood- and adult EPC-derived cells as a potential molecular explanation for the differences in their growth kinetics. Remarkably, early- and late-passage cord blood EPC-derived progeny display significantly elevated levels of telomerase activity compared with adult EPC-derived cells, reminiscent of the previously described primary endothelial cell lines genetically engineered to overexpress telomerase (Figure 6D-E). Thus, consistent with extensive proliferative potential, cord blood EPC-derived cells retain high levels of telomerase activity with serial passage in culture.
Discussion
A hallmark of stem cells and progenitor cells is their ability to proliferate and give rise to functional progeny. Progenitor cells of various lineages are identified by their clonogenic and proliferative potential. Based on these well-established principles, the present study used a single-cell clonogenic assay to define a hierarchy of EPCs based on their proliferative potential. We also identified a unique population of HPP-ECFCs in human cord blood with extensive proliferative potential ex vivo.
Currently, there is no uniform clonogenic definition of EPCs, nor is there an experimental method to distinguish between different populations of EPCs. In fact, a recent expert panel of investigators suggested that the development of a single cell assay to distinguish between mature endothelial cells and their progenitors is an important research priority.27 Further, given that EPCs are intensely studied as potential cell therapies for cardiovascular diseases or biomarkers of disease progression, it is imperative to define EPCs based on reliable clonogenic assays.1-6 Numerous studies show that populations of cells termed EPCs can be isolated from human umbilical cord blood or adult peripheral blood by culturing sorted cells expressing the surface antigen CD34 or mononuclear cells in defined culture conditions (for a review, see Rafii and Lyden1 ). In these studies, EPCs were defined by their ability to ingest DiI-Ac-LDL or to express antigens shared by hematopoietic and endothelial progenitor cells (for a review, see Rafii and Lyden1 ). Several recent reports demonstrate that EPCs isolated from adult mononuclear cells using these culture techniques contain CD14+ monocytic cells that also express the hematopoietic cell surface antigen CD45.18,23 This cell population secretes angiogenic growth factors, which may explain why some studies have documented that in vivo infusion of these cells promotes angiogenesis in animal models of ischemia and that numbers of these cells correlate with cardiovascular complications in patients with diabetes and coronary artery disease.1,23 Although it is assumed that EPCs isolated and defined by these techniques function like stem and progenitor cells, no data exist to directly show that these cells are clonogenic at the single-cell level or that they can be serially passaged and expanded in culture up to or beyond the normal stage of replicative senescence. In fact, Rehman et al23 suggest that these cells should be renamed circulating angiogenic cells.
In contrast to these studies, Lin et al22 recently identified a population of endothelial progenitor cells termed endothelial outgrowth cells (EOCs). Using patients who had undergone sex-mismatched bone marrow transplantation, this study demonstrated that EOCs originate from human bone marrow and can be derived from long-term culture (28 days) of mononuclear cells isolated from adult peripheral blood.22 Since publication of this original study, several groups have successfully isolated EOCs from cord and adult peripheral blood.16-19 In contrast to other populations of EPCs or circulating angiogenic cells isolated in short-term culture of adult or cord blood mononuclear cells, EOCs uniformly express endothelial but not hematopoietic cell-specific surface antigens.22 In addition, EOCs can be expanded for at least 30 PDs in culture with serial passage, which are proliferative kinetics not associated with angiogenic cells isolated by different methodologies.16,19 Thus, EOCs are not contaminated with hematopoietic cells, and they demonstrate extensive proliferative potential, consistent with the definition of a progenitor cell. However, these important studies did not use limiting-dilution or single-cell assays to test whether EOCs harbored different populations of endothelial progenitor cells that could be distinguished by their proliferative or clonogenic potential. We speculate that the EOC-derived ECC may represent an EPC similar to the lower proliferative potential ECC we isolated from adult peripheral blood in the present studies. Whether the adult bone marrow harbors HPP-ECFC remains to be determined.
Hematopoietic cell progenitors have been defined by their ability to form colonies or small clusters of cells based on differences in proliferative or differentiation potential.11 HPPCFCs are the most primitive hematopoietic progenitors that can be assayed in vitro in the absence of a stromal cell monolayer.11,24 In contrast to the more differentiated low proliferative potential-colony forming cell (LPP-CFC), this cell is characterized by its ability to form secondary and tertiary colonies on replating.11,24 Given that murine genetic studies clearly show that the origin of endothelial cells is closely linked to hematopoietic cell development,7-10 it is not surprising that our data show that EPCs can be distinguished by similar clonogenic and growth characteristics. We propose that EPCs can be identified using terminology similar to that used for defining hematopoietic cell progenitors (Figure 7). HPP-ECFCs give rise to macroscopic colonies that form secondary and tertiary colonies on replating.11,24 We have provided evidence that HPP-ECFCs give rise to all subsequent stages of endothelial progenitors in addition to replating into secondary HPP-ECFCs. LPP-ECFCs form colonies that contain more than 50 cells, but they do not form secondary LPP-ECFC colonies on replating (represent the most proliferative population of EPCs that can be isolated from adult peripheral blood). They do give rise to endothelial cell clusters (fewer than 50 cells). Finally, endothelial cell clusters are composed of fewer than 50 cells, and they do not replate into colonies or clusters.
Hematopoietic stem and progenitor cells are enriched in umbilical cord compared with adult peripheral blood.28,29 One intriguing observation from our studies was that EPCs were also enriched in umbilical cord blood compared with adult peripheral blood. Further, EPC-derived ECCs contained relatively high levels of telomerase activity, which may account for the observation that these cells can be expanded for at least 100 PDs without obvious signs of cell senescence. At the single-cell level, some cord blood EPC-derived cells can be expanded 1010- to 1012-fold. To our knowledge, no other primary human endothelial cell has been identified with similar growth characteristics or clonogenic capacity. Whether this cell represents a true endothelial stem cell awaits more rigorous in vivo transplantation studies, which are currently under way. We are also examining whether a clonal relationship may exist between HSCs and HPP-ECFCs.
In summary, we provide data to support a hierarchy of EPCs that can be assayed in limiting dilution. Additional study of these subpopulations of EPCs should yield insight into endothelial cell development and may provide more specific biomarkers for monitoring the progression of cardiovascular disorders and other human diseases linked to endothelial cell dysfunction. This population of cells, with its vast proliferative potential, may represent a novel source of cells for applications of regenerative medicine.
Prepublished online as Blood First Edition Paper, June 29, 2004; DOI 10.1182/blood-2004-04-1396.
Supported by the Division of Neonatal and Perinatal Medicine, Indiana University School of Medicine, Indianapolis.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal