Abstract
Tumor/host-generated thrombin (endogenous thrombin) was investigated with tumor growth and metastasis experiments in mice by the use of hirudin, a highly potent specific inhibitor of thrombin. Pretreatment with hirudin inhibited tumor implantation in nude or syngeneic mice, following subcutaneous injection of 2 human and 2 murine tumors. Hirudin induced a considerable lag period in the appearance of tumor growth, compared with phosphate-buffered saline (PBS) treatment, but had no effect on established tumor nodule growth in vivo or on tumor growth in vitro. Hirudin treatment induced central necrosis of the tumor nodule compared with no effect with PBS treatment. Greater protection was noted with longer duration of treatment. Tumor seeding into blood was examined with green fluorescent protein (GFP)-labeled tumor cells. Hirudin inhibited seeding into the blood as well as systemic organs which varied from complete protection to 15- to 32-fold in the blood and 17- to 395-fold in the lung. Hirudin inhibited spontaneous metastases from subcutaneously implanted tumor by reducing the number of tumor nodules in the lungs. Mouse survival in animals injected subcutaneously with highly aggressive 4T1 cells revealed 5 of 5 deaths of PBS-treated animals on day 40 compared with no deaths with hirudin treatment, with prolongation of survival with hirudin treatment of 16 days to more than 31 days. Thus, endogenous thrombin contributes to tumor implantation, seeding, and spontaneous metastasis. A potent antithrombin agent should be of clinical benefit to patients with cancer. (Blood. 2004;104:2746-2751)
Introduction
The association of thrombosis and cancer (platelet and fibrin deposition) is well established,1-5 but the role that activation of the coagulation pathway plays in promoting neoplastic progression is not well defined. We and others have shown that exogenous thrombin (1 U/mL) acting through its PAR-1 receptor, is capable of enhancing tumor adhesion to platelets,6-8 endothelial cells,9 fibronectin, and von Willebrand factor7 in vitro. Studies have also revealed that exogenous thrombin promotes tumor growth in vitro10 and in vivo6,7,11 as well as experimental (tail-vein injection) metastasis.6-8,12 Exogenous thrombin is also capable of inducing angiogenesis in a chorioallantoic membrane model.13 However, the pathophysiologic relevance of thrombin in the host, that is, the end result of endogenous generation of host thrombin during tumor growth and spontaneous metastasis, has not been examined. Indeed, the concentration of thrombin generated by a developing tumor in the plasma at the interface is unknown and uncertain. In addition, the effect of thrombin on tumor growth in vitro is biplasic (activation at < 0.5 U/mL and inhibition at higher concentration).10 Most tumor cells have constitutively active tissue factor on their surface capable of generating thrombin in vitro. However, the host has antithrombin mechanisms capable of neutralizing this activity.
An approach was therefore designed to explore the mechanism of endogenously generated thrombin with its possible effect on tumor growth, seeding, and spontaneous metastasis by employing the highly potent and specific inhibitor of thrombin, hirudin. Hirudin is twice as potent as heparin in animal thrombosis models.14-16 Unlike heparin, it is capable of neutralizing thrombin bound to the tumor thrombus as well as in the plasma,17,18 with a Ki (inhibitory constant) of 0.05 pM.19 It has other antithrombotic properties. It is able to dissociate thrombin-platelet receptor complexes (greater affinity of hirudin for thrombin than for platelets).20 It can displace reversibly bound Factor Xa from vascular endothelium,20 which then becomes inactivated by plasma protease inhibitors, leading to reduced prothrombin activation. It has also been reported to reduce the adhesion of leukocytes to injured blood vessel walls.21 Experiments were therefore designed to test the effect of hirudin on subcutaneous inoculation of tumor cells into the mouse, with respect to tumor implantation, seeding, and spontaneous metastasis. In this report, we demonstrate that hirudin given at various dosing regimens before and after tumor inoculation inhibits tumor implantation, seeding, and spontaneous metastasis.
Materials and methods
Cell lines and culture media
Human MDAMB231 breast carcinoma, A549 non-small cell lung carcinoma, murine B16F10 melanoma, and 4T1 breast carcinoma were obtained from the American Tissue Culture Company (ATCC, Manassas, VA). 4T1 cells were grown in RPMI 1640 with 10% fetal bovine serum (FBS). All other cell lines were grown in Dulbecco modified Eagle medium (DMEM) plus 10% FBS. Cells were harvested at subconfluence with trypsin-EDTA (ethylenediaminetetraacetic acid) and washed in phosphate-buffered saline (PBS) prior to counting and inoculation into nude mice (Taconic Farms, Germantown, NY) or syngeneic C57BL/6 (Jackson Labs, Bar Harbor, ME) or Balb/c mice (Taconic).
Reagents
Refludan (recombinant hirudin) was obtained from Hoechst-Marion Roussel (Eugene, OR). Tissue media DMEM and RPMI, FBS, and trypsin-EDTA were obtained from Sigma (St Louis, MO). The green fluorescent protein (GFP) plasmid with SV40 promoter was obtained from Invitrogen (Carlsbad, CA). The in vitro proliferation MTT assay kit was obtained form Roche Applied Science (Indianapolis, IN). The KI-67 stain was performed by the NYU Medical Center routine Hemato-Pathology laboratory and kindly interpreted by its director, Dr Giorgio Inghirami.
Tumor inoculation and hirudin treatment
A quantity of 100 uL tumor suspension (1 × 105-1 × 106 cells) was injected subcutaneously into the flank of mice. Hirudin (10 mg/kg-20 mg/kg) was injected intraperitoneally 5 minutes before and 4 hours after injection of tumor suspension. Daily hirudin injections were continued for an additional 4 to 9 days or for an additional 10 injections given every other day.
Tumor photomicroscopy
A Leica Microsystems MML B 100S microscope (Wetzlar, Germany) was interfaced with a Boeckler Instruments Model 3-MR camera (Tucson, AZ) and RZM Biometrics BQ Nova Prime Software (Nashville, TN).
Transfection of B16F10 and 4T1 cells with GFP
A pcDNA3.1/CT-GFP-TOPO expression vector was purchased from Invitrogen (K4820-01). The plasmid is designed to produce stable expression GFP, driven by an SV40 promoter, and is neomycin resistant. The plasmid was transfected into tumor cells with the Lipofectamine 2000 reagent (Invitrogen) as recommended by the manufacturer. Highly fluorescent GFP cells were sorted by flow cytometry and regrown in culture media containing the antibiotic that is permissive for GFP-labeled tumor cells but toxic for other cells.
Detection of tumor in blood
After inoculation of GFP-labeled cells subcutaneously into the flanks of mice, blood was drawn by cardiac puncture at various time intervals for assay of GFP-labeled cells by flow cytometry. Whole blood was anticoagulated in 0.02% EDTA and treated with 0.15 M NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA, pH 7.4, to lyse red blood cells. The flow cytometer was gated with standard GFP cells prior to enumeration of cells in the blood. Approximately 80% of injected cells were GFP positive.
Detection of tumor in various organs
Animals were killed and various organs (lungs and draining lymph nodes, liver, heart, spleen) were removed under sterile conditions at different time intervals. The organs were minced with a scissors and grown in tissue culture for 7 days in the presence of geneticin. Viable cells were enumerated and employed as an index of cell seeding into organs.
Results
Hirudin inhibits tumor implantation and growth of human tumors (A549 non-small cell lung carcinoma and MDAMB231 breast carcinoma) in nude mice
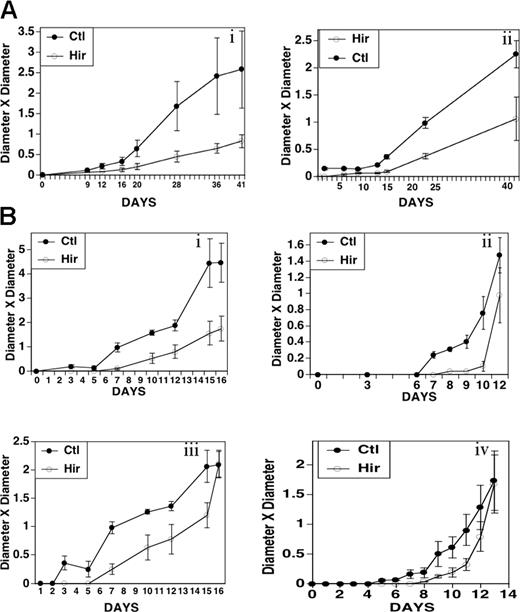
We employed the highly specific thrombin inhibitor hirudin to test whether endogenous thrombin generation within the host-tumor environment had any effect on tumor implantation and growth. Both A549 and MDAMB231 cells have PAR-1 and PAR-4 thrombin receptors (absence of PAR-3) and undergo Ca++ flux with ligand stimulation.22,23 Figure 1A demonstrates inhibition of human tumor growth of A549 cells with hirudin, following implantation of 1 × 106 cells into the flank of nude mice. Hirudin (20 mg/kg) or PBS control was given 5 minutes before and then 4 hours after tumor injection. Note a lag period of 10 to 12 days before the appearance of hirudin-treated animals, as demonstrated by 3- to 4-fold less growth at 12 to 15 days and 2- to 3-fold less growth at 41 days. Similar results were noted with 1.5 × 106 MDAMB231 cells (Figure 1B) with 4- to 11-fold less growth at 7 to 9 days and 1.5- to 3-fold less growth at 12 to 16 days. The difference in tumor volumes generally decreased with increasing time after hirudin injection.
Effect of hirudin on growth of A549 non-small cell lung carcinoma and MDAMB 231 breast carcinoma cells in nude mice. Nude mice were injected intraperitoneally with hirudin (20 mg/kg) 5 minutes before and 4 hours after the subcutaneous injection of 1 × 106 cells into the flank. Tumor size was measured on the designated days. Standard error of the mean (SEM) is given. (A) 1 × 106 A549 cells in 2 experiments (i, ii; n = 5). (B) 1.5 × 106 MDAMB 231 cells in 4 sets of experiments (i-iv; n = 4). Ordinate units are cm2.
Effect of hirudin on growth of A549 non-small cell lung carcinoma and MDAMB 231 breast carcinoma cells in nude mice. Nude mice were injected intraperitoneally with hirudin (20 mg/kg) 5 minutes before and 4 hours after the subcutaneous injection of 1 × 106 cells into the flank. Tumor size was measured on the designated days. Standard error of the mean (SEM) is given. (A) 1 × 106 A549 cells in 2 experiments (i, ii; n = 5). (B) 1.5 × 106 MDAMB 231 cells in 4 sets of experiments (i-iv; n = 4). Ordinate units are cm2.
Hirudin does not inhibit tumor implantation and growth 4 to 8 days after implantation of tumor
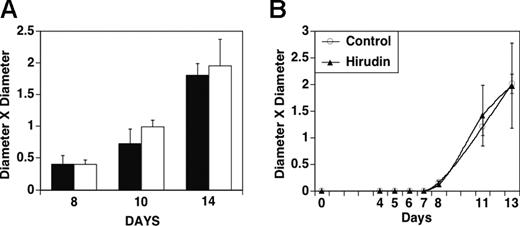
Figure 2A demonstrates no effect of hirudin after 5 days of daily treatment started at 8, 10, and 14 days after implantation. Figure 2B demonstrates that giving hirudin before detectable tumor at 4 days after implantation also does not protect against tumor growth. Similar results were obtained with the highly aggressive breast cell line 4T1 in which hirudin (10 mg/kg) was given on day 10 after tumor implantation and then for an additional 10 days to syngeneic Balb/c mice (data not shown, n = 5).
Effect of hirudin treatment on growth of MDAMB 231 cells in nude mice 4 to 8 days after subcutaneous implantation. (A) Tumors were grown for 8, 10, or 14 days prior to the initiation of hirudin treatment (20 mg/kgm) for 5 days. ▪ indicates hirudin injection; □, PBS injection (n = 4). (B) Initiation of treatment with hirudin (20 mg/kg × 5 days) 4 days after implantation of tumor when tumor nodule was neither visible nor palpable. Bars indicate SEM.
Effect of hirudin treatment on growth of MDAMB 231 cells in nude mice 4 to 8 days after subcutaneous implantation. (A) Tumors were grown for 8, 10, or 14 days prior to the initiation of hirudin treatment (20 mg/kgm) for 5 days. ▪ indicates hirudin injection; □, PBS injection (n = 4). (B) Initiation of treatment with hirudin (20 mg/kg × 5 days) 4 days after implantation of tumor when tumor nodule was neither visible nor palpable. Bars indicate SEM.
Hirudin prolongs survival of mice undergoing experimental pulmonary metastasis of A549 cells following tail-vein injection
Although tumor implantation is an early event, one can also consider experimental pulmonary metastasis as a late form of persistent implantation. The next experiment was designed to determine whether implantation of circulating tumor in a metastatic organ was also affected by hirudin treatment. Figure 3 demonstrates that hirudin given 5 minutes before implantation, 4 hours after implantation, and then every other day for 10 days (20 mg/kg) resulted in prolongation of survival of mice. At 40 days after tail-vein injection of 1 × 106 A549 cells, all hirudin-treated mice were alive, whereas all 5 control mice were dead by day 32 in one experiment (Figure 3A) and 5 were dead by day 38 in a second experiment (Figure 3B). Autopsied lungs of dead animals revealed multiple metastases. When animals implanted with 5 × 106 cells were followed for 121 days after hirudin, every other day for 10 treatments, 5 of 5 hirudin-treated animals were alive at 70 days, compared with 2 of 5 control animals with a hirudin-induced prolongation of life of 24 days on days 56 to 80 and 10 days on days 71 to 81 (Figure 3C). In a second identical experiment followed for 121 days, 5 of 5 hirudin-treated animals were alive at 60 days compared with 3 of 5 control animals. At 90 days, 2 of 5 animals from both groups were dead, however, with a hirudin-induced prolongation of survival of 20 days (Figure 3D). A statistical analysis of the combined experiments of Figure 3C-D was statistically significant by Breslow test, P = .046.
Survival of nude mice treated with A549 cells and hirudin. (A,B) Mice were treated with hirudin (20 mg/kg) 5 minutes before and 4 hours after intravenous injection of 1 × 106 A549 cells, and then every other day for 10 days. (C,D) Similar experiment with 5 × 106 cells.
Survival of nude mice treated with A549 cells and hirudin. (A,B) Mice were treated with hirudin (20 mg/kg) 5 minutes before and 4 hours after intravenous injection of 1 × 106 A549 cells, and then every other day for 10 days. (C,D) Similar experiment with 5 × 106 cells.
Hirudin inhibits tumor implantation and growth of murine tumors in syngeneic mice (B16F10 melanoma and 4T1 breast carcinoma)
To rule out the possibility that an immunocompromised mouse might be contributing to the effect of hirudin on tumor growth and experimental metastasis, experiments were also performed in syngeneic mice. B16F10 cells have the PAR-1 thrombin receptor (absence of PAR-311 and PAR-4 [Lu Hu and S.K., unpublished data, January 2004]) and undergo cell division in vitro10,11 and in vivo11 with ligand. 4T1 cells have the PAR-1 receptor (absence of PAR-3 and PAR-4 [Lu Hu and S.K., unpublished data, January 2004]). Figure 4 demonstrates the effect of hirudin (20 mg/kg per day) at various time intervals. Figure 4A demonstrates a lag period of 12 days prior to the appearance of B16F10 tumors in hirudin-treated animals (5 minutes before and every day for 5 days) with a difference in tumor volume of 4- to 1.3-fold on days 11 and 16, respectively. Figure 4B tests the effect of an additional course of hirudin given every other day for 10 days. These experiments demonstrate a lag period of 14 to 15 days (compared with 12 days for controls) before the appearance of B16F10 tumor in animals with a greater difference in the protection of tumor growth of 11- to 3-fold at days 12 and 18, respectively. The mean fold increase in protection for both hirudin-treated groups from 12 to 16 days was significant for both groups: 2.9 ± 1.2 for Figure 4A, P < .03 (Student t test); and 10.5 ± 0.05 for Figure 4B, P < .04. The 3.6-fold improved treatment response over animals shown in Figure 4A (5 days of treatment) compared with those shown in Figure 4B (5 days plus every other day for 10 days) was statistically significant at P < .04.
Effect of hirudin treatment on growth of tumors in syngeneic mice. B16F10 cells (1 × 106) or 4T1 cells (1 × 105) were injected subcutaneously into C57BL/6 or BALB/C mice, respectively, in similar experiments as in Figure 1. (A) Hirudin (10 mg/kgm) was given 5 minutes before B16F10 implantation, as well as 5 consecutive days afterward (n = 4) or (B) 5 days followed by every other day for 10 days (n = 4). (C) Hirudin (10 mg/kg) was given 5 minutes before 4T1 implantation followed by 10 days or (D) 10 days followed by every other day for 10 treatments (n = 5). Bars indicate SEM.
Effect of hirudin treatment on growth of tumors in syngeneic mice. B16F10 cells (1 × 106) or 4T1 cells (1 × 105) were injected subcutaneously into C57BL/6 or BALB/C mice, respectively, in similar experiments as in Figure 1. (A) Hirudin (10 mg/kgm) was given 5 minutes before B16F10 implantation, as well as 5 consecutive days afterward (n = 4) or (B) 5 days followed by every other day for 10 days (n = 4). (C) Hirudin (10 mg/kg) was given 5 minutes before 4T1 implantation followed by 10 days or (D) 10 days followed by every other day for 10 treatments (n = 5). Bars indicate SEM.
Similar results were noted with a highly malignant breast tumor cell line, 4T1, known to metastasize to the lung before the implanted nodule reaches a size requiring sacrifice of the animal, 1 cc to 2 cc. In animals treated 5 minutes before and 10 days thereafter, the inhibition of tumor growth by hirudin (Figure 4C) ranged from 3- to 7-fold on days 9 to 10 and 1.7- to 1.9-fold on days 17 to 20. In animals given an additional 10 treatments (one every other day), the inhibition of tumor growth by hirudin (Figure 4D) ranged from 3- to 7.5-fold on days 9 to 10 and 4.5- to 5.1-fold on days 17 to 20. The mean fold increase in protection of hirudin from 9 to 20 days was significant for both groups: 3.0 ± 0.39 for Figure 4C, P < .001; and 6.6 ± 2.2 for Figure 4D, P < .01. The 2.2-fold improved treatment response over animals shown in Figure 4C (10 days of treatment) compared with animals shown in Figure 4D (20 days treatment) was statistically significant at P = .01.
Hirudin induces tumor nodule necrosis of B16F10 and 4T1 cells
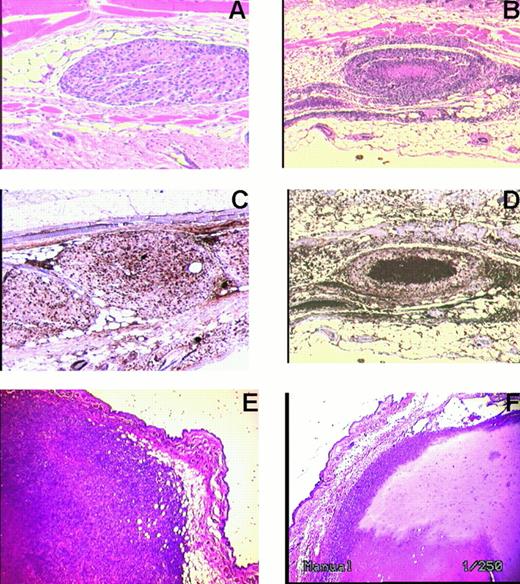
Of note was the observation that hirudin-treated animals developed B16F10 as well as 4T1 tumor nodules with central necrosis on day 15, following 10 days of hirudin treatment, whereas PBS-treated animals did not (Figure 5). The KI-67 stain measures dividing cells, as opposed to quiescent cells. It is strongly positive in the control sample. Necrotic cells nonspecifically take up stain.
Histochemical analysis of subcutaneous B16F10 and 4T1 tumor cells 10 days after treatment with hirudin, 10 mg/kg 5 minutes before and for 10 days thereafter. Tumor nodules remained fixed and stained with hematoxylin and eosin. (A) B16 F10 contral nodule. (B) B16 F10 nodule of hirudin-treated animal with central necrosis. (C) KI-67 stain of B16 F10 nodule of untreated control nodule. (D) KI-67 stain of B16 F10 nodule from hirudin-treated animal. (E) 4T1 control nodule. (F) 4T1 nodule following hirudin treatment. Representative of 3 different experiments for each tumor. Original magnification × 250 (A-D) and × 1000 (E, F).
Histochemical analysis of subcutaneous B16F10 and 4T1 tumor cells 10 days after treatment with hirudin, 10 mg/kg 5 minutes before and for 10 days thereafter. Tumor nodules remained fixed and stained with hematoxylin and eosin. (A) B16 F10 contral nodule. (B) B16 F10 nodule of hirudin-treated animal with central necrosis. (C) KI-67 stain of B16 F10 nodule of untreated control nodule. (D) KI-67 stain of B16 F10 nodule from hirudin-treated animal. (E) 4T1 control nodule. (F) 4T1 nodule following hirudin treatment. Representative of 3 different experiments for each tumor. Original magnification × 250 (A-D) and × 1000 (E, F).
Hirudin had no effect on growth of 5 × 103 tumor cells in vitro as measured by MTT cell proliferation assay. Cells treated with and without hirudin, 5 mg/mL for 24 hours, gave MTT optical density (OD) for B16F10 of 1.98 ± 0.025 and 1.96 ± 0.025, respectively. Similar measurements for 4TI cells were 2.70 ± 0.087 and 2.65 ± 0.035, respectively.
Hirudin impairs the release of GFP-labeled B16F10 cells from implanted tumor into the blood
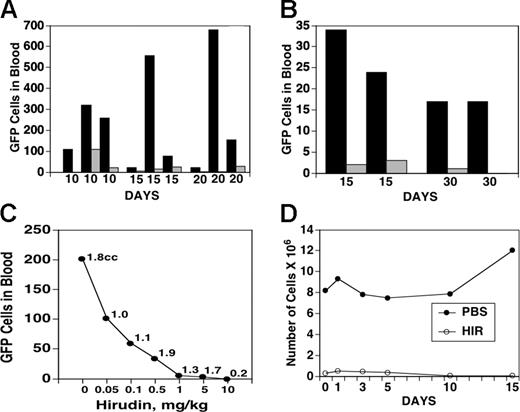
The previous data on impaired tumor implantation and experimental tail-vein metastasis strongly suggested that hirudin treatment might inhibit tumor seeding. GFP-transfected tumor cells were therefore implanted subcutaneously as in Figure 1 and cardiac puncture performed on varying days after implantation for monitoring of GFP fluorescence. Figure 6 demonstrates a dramatic hirudin-induced inhibition of tumor release into the circulation after tumor implantation in 5 sets of experiments monitored at 10 to 30 days, which could not be accounted for on the basis of differences in subcutaneous tumor volume in these experiments. Figure 6A demonstrates 3 sets of experiments with B16F10 implanted subcutaneous tumors, in which the differences varied from complete protection of seeding to a mean protection of 32 ± 16 fold, P < .04. Similar results were obtained in 2 sets of experiments with highly metastatic 4T1 cells which revealed results varying from complete protection to a mean protection of 15- to 2.3-fold, P < .01 (Figure 6B). The individual variance of released tumor cells with both sets of tumors should be noted. It is likely to reflect heterogeneity of the tumor population after growth in culture as well as variation in the host's local response at injected tumor sites. The lower number of released 4T1 cells compared with B16F10 cells could reflect the 10-fold lower number of cells injected. Despite this variation, hirudin had a dramatic protective effect on tumor release into the blood in all 13 pairs of animals examined.
Effect of hirudin on release of GFP-labeled B16F10 or 4T1 cells into the blood. Hirudin and GFP-labeled B16F10 cells (A; 1 × 106) or 4T1 cells (B; 1 × 105) were injected into mice, as described in Figure 5, except for 10 days of hirudin treatment after tumor implantation. Blood was removed on days 10, 15, 20, and 30 and assayed for GFP-labeled cells by flow cytometry. ▪ indicates PBS-treated mice; ▦, hirudin-treated mice. (C) Effect of hirudin concentration on release of GFP-labeled B16F10 cells into the blood. Tumor was injected and mice treated with varying concentrations of hirudin. Blood was collected on day 15 and assayed for GFP-labeled cells. Numbers on curve refer to volumes of the tumor at day 15 of each of the 7 animals studied. (D) Number of viable tumor cells in the lung following 7 days of ex vivo culture of mice.
Effect of hirudin on release of GFP-labeled B16F10 or 4T1 cells into the blood. Hirudin and GFP-labeled B16F10 cells (A; 1 × 106) or 4T1 cells (B; 1 × 105) were injected into mice, as described in Figure 5, except for 10 days of hirudin treatment after tumor implantation. Blood was removed on days 10, 15, 20, and 30 and assayed for GFP-labeled cells by flow cytometry. ▪ indicates PBS-treated mice; ▦, hirudin-treated mice. (C) Effect of hirudin concentration on release of GFP-labeled B16F10 cells into the blood. Tumor was injected and mice treated with varying concentrations of hirudin. Blood was collected on day 15 and assayed for GFP-labeled cells. Numbers on curve refer to volumes of the tumor at day 15 of each of the 7 animals studied. (D) Number of viable tumor cells in the lung following 7 days of ex vivo culture of mice.
Effect of hirudin concentration on release of GFP-labeled B16F10 cells into the circulation
The employment of GFP-labeled B16F10 cells to sensitively detect tumor seeding permitted an analysis of the concentration dependence of hirudin in these experiments. Figure 6C demonstrates hirudin inhibition of seeding of GFP-labeled B16F10 cells at an inhibitory concentration (IC50) of 0.05 mg/kg, well within the clinical range used in patients.24-26
Effect of hirudin on spontaneous seeding of tumor by harvesting of viable tumor cells from various organs
In order to sensitively and accurately assess the destination of viable seeded tumor cells into the circulation, we examined various organs for spontaneous seeding by incubation of minced organs for tumor culture recovery in the presence of the GFP-resistant antibiotic geneticin. This manipulation serves to kill nontumor cells that do not contain the antibiotic-resistant gene. Spontaneously released tumor cells were found in lungs, draining lymph nodes, and liver in control animals at day 15 after subcutaneous implantation of tumor. Hirudin treatment eliminated detectable viable tumor cells in lymph nodes and liver. Spontaneous pulmonary tumor seeding was 17- to 395-fold less in hirudin-treated animals compared with control animals (Figure 6D).
Effect of hirudin on spontaneous pulmonary metastasis of 4T1 breast carcinoma cells
Since spontaneous seeding of tumor cells into organs does not directly reflect implantation and growth of these cells, animals were tested for parenchymal tumor nodule development. This could be done with the highly metastatic 4T1 cells. Animals were killed on day 31 after implantation of 1 × 105 cells and the lungs examined microscopically for tumor nodules with a dissection microscope. Hirudin (10 mg/kg) was given 5 minutes before and every day thereafter for 10 days. The number of tumor nodules in the 3 of 5 surviving control mice was 2.2-fold greater than in the 5 surviving hirudin-treated mice (35.3 ± 3.4 vs 16.0 ± 2.3, respectively, P = .05).
Effect of hirudin on survival of mice injected subcutaneously with 4T1 cells
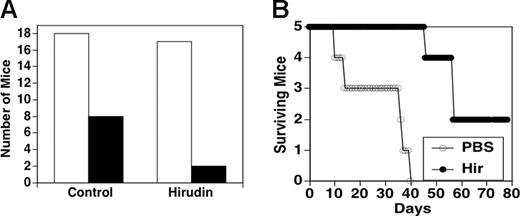
Animals were injected subcutaneously with spontaneously metastatic 1 × 105 4T1 cells with and without hirudin for 10 days followed by every other day for 10 days. Figure 7A depicts the survival at 30 days of 18 control mice and 17 hirudin-treated mice. Of 18 control mice, 8 (44%) died at various days within the 30-day observation period, whereas of 17 hirudin-treated mice, only 2 (12%) were dead following this time period (x2 with Yates correction, P < .05). Figure 7B provides a time course of death for 5 control animals versus 5 hirudin-treated animals. Note that 60% of control mice were dead at 14 days, with all dead at 40 days, whereas all hirudin-treated mice were alive at 45 days (χ2P < .05), with one dead at 46 days, 3 dead at 55 days, and 2 alive at more than 80 days.
Effect of hirudin on survival of syngeneic mice injected subcutaneously with 1 × 105 4T1 breast carcinoma cells. Animals were treated with either PBS (control group) or hirudin (10 mg/kg) 5 minutes before and 10 days thereafter plus every other day for 10 days, and observed. (A) Refers to number of dead mice in control and hirudin-treated groups. □ indicates number of mice at start of experiment; ▪, number of dead mice by day 30. (B) Kinetic analysis of animal survival.
Effect of hirudin on survival of syngeneic mice injected subcutaneously with 1 × 105 4T1 breast carcinoma cells. Animals were treated with either PBS (control group) or hirudin (10 mg/kg) 5 minutes before and 10 days thereafter plus every other day for 10 days, and observed. (A) Refers to number of dead mice in control and hirudin-treated groups. □ indicates number of mice at start of experiment; ▪, number of dead mice by day 30. (B) Kinetic analysis of animal survival.
Discussion
These data clearly demonstrate a role for endogenously generated thrombin in tumor implantation, seeding, growth, and spontaneous metastasis. The highly specific, potent, thrombin inhibitor hirudin inhibits tumor implantation and growth of 4 different tumors in nude as well as syngeneic mice, inhibits spontaneous seeding of tumor into the blood and systemic organs (lungs, liver, spleen, lymph nodes), and most importantly inhibits spontaneous tumor metastasis.
A major and novel observation from these studies is the requirement of endogenous tumor/host thrombin for tissue implantation. Indeed, thrombin or its downstream activation products are required to prevent central necrosis of 2 different tumor cell lines tested: B16F10 and 4T1. The role of thrombin in preventing tumor necrosis in an expanding tumor cell mass may conceivably relate to its ability to enhance tumor cell growth7,10-12 and host angiogenesis.13
A second major and novel observation is the requirement of endogenously generated thrombin for tumor seeding. The effect of hirudin on seeding of tumor into the blood and systemic organs was dramatic. These observations would suggest that tumor PAR receptor activation leads to the activation and/or up-regulation of invasive genes. For example, we have recently observed that thrombin up-regulates the tumor invasive proteins osteopontin and S100A4 (calcium-binding protein) in 2 different tumor cell lines (T. Tang, L.H., and S.K., unpublished data, January 2004). Osteopontin is markedly elevated in metastatic tumors and is cleaved by thrombin into N-terminal and C-terminal products that bind to their respective receptors, leading to tumor migration and haptotaxis.27-32 S100A4 is also highly elevated in metastatic tumor cell lines and S100A4 transfection enhances the invasiveness of tumor cell lines.33-36
A third major and novel observation is the requirement of endogenous tumor/host-generated thrombin for spontaneous metastasis. This observation is of particular significance because it demonstrates for the first time that spontaneous tumor metastasis, as opposed to highly artificial experimental tail-vein metastasis,37 requires thrombin generation for its development. Experiments with tail-vein metastasis measure entrapment and adhesion in the lungs of a huge number of cells (1 × 106) injected into the blood stream, whereas spontaneous metastasis in the host represents the release of approximately 10 000-fold fewer cells into circulation, with an as-yet-unknown fraction of these cells actually implanting and growing in host organs.
The theoretical clinical implications of these observations are apparent. Tumor cells are unique in that they have constitutively active tissue factor on their surface.38,39 This could induce thrombin generation, which in turn could stimulate tumor cell growth, adhesion, and invasion. Thus a “vicious” autocrine cycle is established. It is of interest that tissue factor expression correlates with hematogenous metastases in melanoma cells40-42 and is associated with the leading edge of invasive breast carcinomas,43 although tissue factor can also stimulate tumor growth independently of thrombin generation via the induction of vascular endothelial growth factor (VEGF) and angiogenesis.44,45 In addition, enzymatically active thrombin has been reported to be present on surgically removed tumor specimens, including malignant melanoma, by affinity-ligand histochemical analysis,46 and thrombin-receptor (PAR-1) overexpression has been reported in malignant invasive melanoma11 and breast tumor cell lines in vitro, as well as human breast metastatic tissue in vivo.47 In this regard, it is of interest that low-grade intravascular coagulation with generation of thrombin has been observed in most patients with solid tumors,3,5,48,49 and in 60% of cancer patients at the time of diagnosis. It progresses with greater tumor burden, and is associated with a poor prognosis.3
Of particular interest are the observations of Shulman and Lindmarker50 who studied 854 patients treated for 6 months versus 6 weeks with coumadin for deep vein thrombosis for 6 years. Surprisingly, those patients treated for 6 months developed significantly less cancer during the 6-year observation period than those exposed to anticoagulant for 6 weeks (odds ratio 1.6; 95% confidence interval [CI] 1.1-2.4; P = .02), and when confined to 40 patients with urogenital tumors, the odds ratio increased to 2.5 (95% CI 1.3-5.0). Although the coumadin effect could have been mediated by inhibition of factors Xa or VIIa rather than thrombin, it is intriguing to speculate that these data, as well as our present animal observations, strongly suggest that thrombin nourishes tumor dormancy and growth. Indeed, we further speculate from these observations that tumor cells lie dormant in the circulation and/or systemic organs of many cancer-prone individuals and that this dormancy is maintained by endogenous anticoagulants (antithrombin III, protein C, α2 macroglobulin, thrombomodulin, tissue factor pathway inhibitor [TFPI]), and other undescribed factors, as well as immune surveillance.
Thus, tumor cell-induced endogenous thrombin plays a significant role in tissue implantation, seeding, and spontaneous metastasis. These observations support a clinical trial of an effective thrombin inhibitor. We would predict that this would inhibit tumor implantation, seeding, and metastasis. The treatment should start immediately after diagnosis (before extensive tumor development) and in conjunction with chemotherapy.
Prepublished online as Blood First Edition Paper, July 20, 2004; DOI 10.1182/blood-2004-03-1047.
Supported by the National Institutes of Health grant HL-13336, a grant from the Hildegarde D. Becher Foundation, the Dorothy and Seymour Research Fund and the Helen Polonsky Research Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Dr Robert Holzman for help with the statistical analysis.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal