Abstract
Endothelial progenitor cells (EPCs) play a role in the repair of ischemic or injured tissue. Because endothelial injury can be associated with apoptosis, we have investigated whether apoptotic bodies from mature endothelial cells (ECs) may affect growth and differentiation of EPCs in vitro. A 24-hour incubation of isolated human EPCs with apoptotic bodies-rich medium (ABRM) from ECs led to a significant increase in the number of spindle-shaped attached cells. EPCs were characterized by DiI-Ac-LDL/lectin staining and measurement of CD34 and kinase insert domain receptor (KDR) expression. The treatment with ABRM resulted in a 2-fold increase of DiI-Ac-LDL/lectin-positive cells and up-regulation of CD34 (22% ± 2% versus 13% ± 3%, P < .05 and KDR (49% ± 12% versus 19% ± 7%, P < .05). Fluorescence and confocal laser microscopy demonstrated the uptake of apoptotic bodies by the EPCs. Apoptotic bodies-depleted medium had no effect, whereas the incubation with suspension of apoptotic bodies induced effects similar to those of ABRM. Our results suggest that apoptotic bodies from ECs are taken up by EPCs, increasing their number and differentiation state. Such a mechanism may facilitate the repair of injured endothelium and may represent a new signaling pathway between progenitor and damaged somatic cells. (Blood. 2004;104:2761-2766)
Introduction
The vascular endothelium is a dynamic single-cell layer, producing important regulatory factors, such as prostaglandins and nitric oxide.1 Injury of the endothelial cells (ECs), which is often followed by programmed cell death (apoptosis), is a critical event in the pathogenesis of atherosclerosis.2 Therefore, the maintenance of an intact endothelium is of particular importance. Cells undergoing apoptosis partially transform into phosphatidylserine-containing apoptotic bodies, which are rapidly engulfed by phagocytes or neighboring cells to inhibit an inflammatory response or prevent the unwanted activation of the coagulation system.3
Evidence from the recent past suggests that human peripheral blood contains bone marrow-derived progenitor cells, which have the capacity to differentiate into mature ECs and are therefore termed endothelial progenitor cells (EPCs).4 Under culture conditions favoring endothelial growth, EPCs develop an elongated spindle-shaped morphology and are characterized in general by specific cell-surface markers, including CD133, CD34, and vascular endothelial growth factor receptor-2 (VEGFR-2).5,6 Furthermore, EPCs incorporate acetylated low-density lipoproteins (Ac-LDLs), bind lectin, and express with different intensity markers typical for the endothelial lineage.4-7 Myocardial infarction, vascular trauma, and application of certain drugs, growth factors, or cytokines induce a rapid mobilization of EPCs from the bone marrow and an increase of their number in the systemic circulation.7
However, possible interactions between apoptotic mature ECs and EPCs in the context of endothelial pathophysiology have not been studied. Because such an interaction may represent an important signaling mechanism for the regeneration of injured endothelium, we have investigated the possible effects of EC-derived apoptotic bodies on the number and differentiation of isolated human EPCs.
Materials and methods
ABRM from HUVECs
Isolated confluent human umbilical vein endothelial cells (HUVECs) between passage 1 and 4 were incubated for 24 hours in endothelial basal medium without serum and growth factors to induce apoptosis. Apoptosis was characterized by double staining with annexin V/fluorescein isothiocyanate (FITC; BD PharMingen, Hamburg, Germany) and propidium iodide (PI). Flow cytometry was performed using a single-cell gate. The data were analyzed in a fluorescence-1/fluorescence-2 dot plot to quantify the percentage of annexin V+/PI- cells, representing the apoptotic population. In addition, human promyelocytic leukemic HL-60 cells were incubated for 72 hours in serum-free RPMI 1640 medium to induce apoptosis.
Medium from apoptotic HUVECs was collected and clarified from dead cells and cell debris by centrifugation (800g, 10 minutes). This HUVEC-derived apoptotic bodies-rich medium (ABRM) was further centrifuged (16 000g, 20 minutes) to isolate the apoptotic bodies and to obtain apoptotic bodies-depleted medium (ABDM). Medium from apoptotic HL-60 cells was treated equally. Apoptotic bodies were characterized by flow cytometry after staining with annexin V/FITC or PI. The amounts of apoptotic bodies used were quantified by measuring their protein content as described in the manufacturer's instructions (Bio-Rad DC Protein Assay Kit; Bio-Rad, Munich, Germany).
Characterization of HUVEC-derived apoptotic bodies
Apoptotic bodies were analyzed in a fluorescence-activated cell sorter (FACScan; Becton Dickinson, Heidelberg, Germany) and in a Leica DMRBE microscope (Leica, Wetzlar, Germany). For flow cytometry, apoptotic bodies were stained for 30 minutes with annexin V/FITC (1:400). Platelets were used as a size marker (1-4 μm). For fluorescence microscopy, the suspension of apoptotic bodies was plated on coverslips coated with poly-l-lysine (10 mg/mL; Sigma, Taufkirchen, Germany), fixed with 2% paraformaldehyde (PFA), and stained with 1 μg/mL DAPI (4′,6-diamidino-2-phenylindole dihydrochloride; Sigma). In addition, together with serum and growth factor starvation, HUVECs were incubated with 1 μg/mL PI and after 24 hours apoptotic bodies were analyzed by flow cytometry or were plated on poly-l-lysine-coated coverslips and characterized by fluorescence microscopy. Images from the fluorescence microscope were taken using a Diagnostic Instruments SPOT cooled color digital camera and SPOT acquisition software (Visitron Systems, Puchheim, Germany).
Isolation and culture of EPCs
Peripheral blood mononuclear cells (PBMCs) were isolated by density gradient centrifugation with Biocoll separating solution (Biochrom, Berlin, Germany) from 50 to 150 mL anticoagulated peripheral blood from healthy donors. PBMCs were seeded (2 × 106/cm2) on 24-well plates or 4-well chamber slides (Nunc, Roskilde, Denmark) coated with 10 μg/mL human fibronectin (Sigma) in 0.5 mL microvascular endothelial growth medium with 5% FBS and supplements (EGM-MV BulletKit, Clonetics-Cambrex, Verviers, Belgium). After 24 hours, nonadherent cells were removed and transferred to new fibronectin-coated plates. After 7 days in culture, the number of spindle-shaped attached cells was determined in 3 different fields, 1 mm2 each.
Identification and characterization of EPCs
Uptake of Ac-LDL. For uptake of 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine-labeled Ac-LDL (DiI-Ac-LDL; Biomedical Technologies, Stoughton, MA) cells were incubated with 4.0 μg/mL DiI-Ac-LDL for 4 hours. Then the cells were fixed with 2% PFA and incubated with 10 μg/mL FITC-labeled Ulex europaeus agglutinin-I (lectin; Sigma) for 1 hour. DiI-Ac-LDL/lectin double-positive cells were judged as EPCs and counted in 3 different fields at a magnification of × 200 in a fluorescence microscope.
Flow cytometry. Adherent spindle-shaped cells were detached with Accutase (PAA Laboratories, Pasching, Austria) for 5 minutes at 37°C and incubated with antibodies to CD34 (Zymed Laboratories, Berlin, Germany), kinase insert domain receptor (KDR [VEGFR-2]; Sigma), von Willebrand factor (VWF; Dako, Hamburg, Germany), or CD68 (Dako) for 1 hour on ice. Staining for VWF was performed in permeabilized cells (0.1% saponin, 10 minutes). After washing, the cells were incubated with FITC-conjugated goat antimouse F(ab)2 antibody (Dako) for 45 minutes. The cells were washed again and fixed with 2% PFA. FITC-conjugated anti-IgG antibody (Dako) was used as a control. Analysis was performed on at least 5000 cells/sample in a FACScan using CellQuest software and manual gating. Additionally, to characterize the population of floating cells, staining with phycoerythrin (PE)-conjugated anti-CD4 antibody (Sigma) was performed. Isotype-specific PE-conjugated anti-IgG (Sigma) was used as a control.
Measurement of cell proliferation
Adherent cells were collected after 7 days in culture using trypsin-EDTA (ethylenediaminetetraacetic acid) solution (Sigma) and were plated on fibronectin-coated 48-well plates at a density of 40 000 cells/well. After 24 hours, cells were incubated with different dilutions of ABRM for 24 hours. Proliferation was measured using the CyQANT Cell Proliferation Assay Kit (Molecular Probes, Leiden, The Netherlands) according to the manufacturer's protocol. Briefly, frozen cells were thawed and lysed by addition of a buffer containing the CyQANT GR dye protected from light. Sample fluorescence was measured after 5 minutes of incubation in an enzyme-linked immunosorbent assay (ELISA) reader at 500 nm excitation and 540 nm emission wavelength. The proliferation rate was calculated as the percentage of untreated control (= 100%).
Uptake of HUVEC-derived apoptotic bodies by EPCs
The uptake of apoptotic bodies was visualized in a fluorescence microscope and in a confocal laser microscope (TCS-NT, Leica). For fluorescence microscopy, apoptotic bodies from HUVECs were stained with annexin V/PE (BD PharMingen; 1:400) for 30 minutes and incubated with EPCs for 0.5, 1.5, or 6 hours. The cells were fixed with 2% PFA and stained with lectin-FITC to identify EPCs. For confocal laser scanning microscopy, apoptotic bodies from HUVECs were stained with annexin V/FITC (BD PharMingen; 1:200) for 30 minutes and incubated for 0.5 or 1 hour with the adherent mononuclear cells, containing EPCs. Filamentous actin (F-actin) of the adherent cells was visualized using Alexa-568 phalloidin (1:200; Molecular Probes). The uptake of apoptotic bodies was investigated in the spindle-shaped adherent cells. Images from the confocal laser scanning microscope were taken using Leica TCS-NT software. Subsequent software for image processing included ImageJ 1.29 (National Institutes of Health, Bethesda, MD) and Adobe Photoshop 6.0 (San Diego, CA).
Statistical analysis
Data are expressed as mean ± SD and were analyzed using unpaired Student t test. Differences with a P value less than .05 were considered significant.
Results
Quantification of apoptosis in HUVECs after serum and growth factor deprivation
To grow in culture, HUVECs need a permanent supplementation with serum and growth factors.8,9 Under normal culture conditions only 5% ± 0.8% of the cells were positive for annexin V and negative for PI, whereas 2% ± 0.8% were double positive for both annexin V and PI. After a 24-hour period of serum and growth factor depletion, we found 23% ± 6% annexin V+/PI- cells, indicating a pronounced induction of apoptosis in HUVECs by these conditions. In addition, we found 13% ± 2% annexin V/PI double-positive cells, indicating necrotic or secondary necrotic cells.
Characterization of apoptotic bodies by flow cytometry and fluorescence microscopy
Apoptotic bodies were first analyzed by flow cytometry using forward/side scatter (FSC/SSC) dot plot and FL-1 or FL-2 histogram plots. We analyzed and compared conditioned medium from apoptotic HUVECs without centrifugation, after centrifugation at 800g for 10 minutes, and after centrifugation at 16 000g for 20 minutes. Platelets were used for size comparison (1-4 μm; Figure 1A). Conditioned medium contained dead cells, large cell debris, apoptotic bodies, and microparticles (Figure 1A). After the first centrifugation (800g, 10 minutes), dead cells and large cell debris were spun down and remained in the sediment, whereas the supernatant (ABRM) contained mainly apoptotic bodies and microparticles (Figure 1A). After centrifugation (16 000g, 20 minutes) of the ABRM, most of the apoptotic bodies were spun down and the supernatant (ABDM) contained mainly microparticles and some apoptotic bodies (Figure 1A). Apoptotic bodies are approximately in the size range of platelets (1-4 μm), whereas microparticles are much smaller (< 1 μm). Annexin V/FITC staining showed that both apoptotic bodies and microparticles are positive for annexin V (Figure 1B). In contrast, staining with PI showed that apoptotic bodies, but not microparticles, are positive for PI (Figure 1B). Furthermore, apoptotic bodies stained positive for DAPI, PI, and lectin, as demonstrated by fluorescence microscopy (Figure 1C). These findings demonstrate that EC-derived apoptotic bodies exist as small membrane vesicles, which contain DNA.
Characterization of HUVEC-derived apoptotic particles by flow cytometry and fluorescence microscopy. (A) FSC/SSC dot plot analysis of particles from apoptotic HUVECs. Platelets (blue) were used as a size marker (1-4 μm, gate R2). Conditioned medium from apoptotic HUVECs contains dead cells and large cell debris (gate R1), apoptotic bodies (orange, gate R2), and microparticles (green, gate R3). Apoptotic bodies-rich medium (ABRM), obtained after centrifugation (800g, 10 minutes), contains mainly apoptotic bodies and microparticles. Apoptotic bodies-depleted medium (ABDM), obtained after centrifugation (16 000g, 20 minutes) of the ABRM, contains mainly microparticles and some apoptotic bodies. The percentage of events is given in the upper left corner of each region gate. (B) Annexin V/FITC (FL-1) and PI (FL-2) dot plot analysis of live cells, dead cells, and cell-derived particles. Live cells (red) showed an extremely low binding of annexin V and PI. Dead cells, apoptotic bodies, and microparticles (as gated in panel A, black) stain positively with annexin V. Furthermore, dead cells and apoptotic bodies, but not microparticles, stain positive with PI. The quadrant gates were set on the respective unstained control population. The percentage of events is given in the upper right corner of the respective region. (C) Fluorescence microscopy of DAPI+, PI+, and lectin-FITC+ apoptotic bodies (scale bars represent 2.5 μm). Representative plots and images from 3 to 4 independent experiments.
Characterization of HUVEC-derived apoptotic particles by flow cytometry and fluorescence microscopy. (A) FSC/SSC dot plot analysis of particles from apoptotic HUVECs. Platelets (blue) were used as a size marker (1-4 μm, gate R2). Conditioned medium from apoptotic HUVECs contains dead cells and large cell debris (gate R1), apoptotic bodies (orange, gate R2), and microparticles (green, gate R3). Apoptotic bodies-rich medium (ABRM), obtained after centrifugation (800g, 10 minutes), contains mainly apoptotic bodies and microparticles. Apoptotic bodies-depleted medium (ABDM), obtained after centrifugation (16 000g, 20 minutes) of the ABRM, contains mainly microparticles and some apoptotic bodies. The percentage of events is given in the upper left corner of each region gate. (B) Annexin V/FITC (FL-1) and PI (FL-2) dot plot analysis of live cells, dead cells, and cell-derived particles. Live cells (red) showed an extremely low binding of annexin V and PI. Dead cells, apoptotic bodies, and microparticles (as gated in panel A, black) stain positively with annexin V. Furthermore, dead cells and apoptotic bodies, but not microparticles, stain positive with PI. The quadrant gates were set on the respective unstained control population. The percentage of events is given in the upper right corner of the respective region. (C) Fluorescence microscopy of DAPI+, PI+, and lectin-FITC+ apoptotic bodies (scale bars represent 2.5 μm). Representative plots and images from 3 to 4 independent experiments.
Culture and characterization of endothelial-like cells from the mononuclear fraction of human peripheral blood
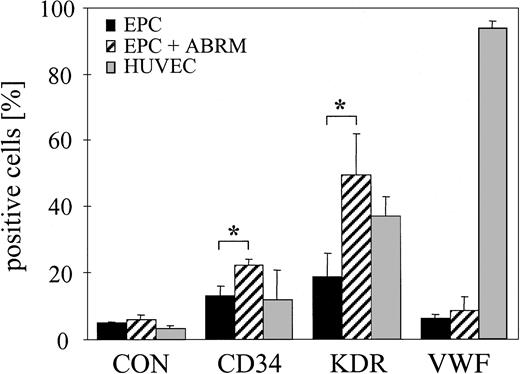
PBMCs were cultured in fibronectin-coated wells using microvascular endothelial growth medium with low serum concentration. This culture resulted in the adherence of a subset of cells. Analysis by flow cytometry of the remaining floating PBMCs demonstrated that 45% ± 17% of these cells express CD4, and therefore they were characterized as lymphocytes. Furthermore, 11% ± 3% of these lymphocytes are positive for annexin V after 5 days in culture. After 7 days in culture, some of the attached PBMCs developed a spindle-shaped appearance (Figure 2A) and these cells were counted (41 ± 16 cells/mm2,n = 4). To verify an endothelial-like subpopulation of these spindle-shaped attached cells, we used double staining with DiI-Ac-LDL/lectin. We found 40 ± 8 DiI-Ac-LDL/lectin double-positive cells/mm2 (n = 3) and these cells were judged as EPCs (Figure 2B). In contrast, monocyte/macrophage-like cells showed a larger size and were not double positive for DiI-Ac-LDL/lectin (Figure 2B). Next, we performed flow cytometry for EPC surface markers, including CD34 and KDR. EPCs are characterized by the expression of CD34 (13% ± 3%) and KDR (19% ± 7%), but lack the endothelial cell marker VWF (Figure 3). HUVECs also show expression of CD34 (12% ± 9%), KDR (37% ± 6%), and, in contrast to EPCs, VWF (94% ± 2%; Figure 3). Staining for the macrophage marker CD68 demonstrated that 17% ± 9% of the adherent cells were positive for CD68. In summary, our data indicate that adherent PBMCs after 7 days under these culture conditions contained spindle-shaped cells with characteristics of EPCs, some macrophages, and floating lymphocytes.
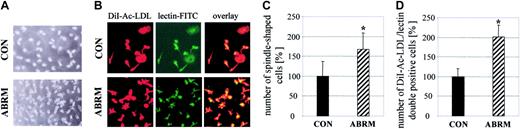
Effect of ABRM on attached cells. Increase in the number of spindle-shaped attached (A; light microscopy, original magnification × 100) and DiI-Ac-LDL/lectin-FITC double-positive cells (B; fluorescence microscopy, original magnification × 200) before (CON) and after treatment with ABRM. Bar graphs show cell numbers of spindle-shaped (C) or DiI-Ac-LDL/lectin double-positive cells (D) before (CON) and after treatment with ABRM, expressed as percent of untreated controls (mean ± SD, n = 3, *P < .05).
Effect of ABRM on attached cells. Increase in the number of spindle-shaped attached (A; light microscopy, original magnification × 100) and DiI-Ac-LDL/lectin-FITC double-positive cells (B; fluorescence microscopy, original magnification × 200) before (CON) and after treatment with ABRM. Bar graphs show cell numbers of spindle-shaped (C) or DiI-Ac-LDL/lectin double-positive cells (D) before (CON) and after treatment with ABRM, expressed as percent of untreated controls (mean ± SD, n = 3, *P < .05).
Flow cytometry of progenitor and endothelial markers. Percentage of CD34+, KDR+, and VWF+ HUVECs (▦) and EPCs (▪) from the mononuclear cell fraction of human peripheral blood. EPCs were treated for 24 hours with ABRM (▨) from HUVECs. IgG-FITC antibody was used as a control (CON). Mean ± SD, n = 4, *P < .05.
Flow cytometry of progenitor and endothelial markers. Percentage of CD34+, KDR+, and VWF+ HUVECs (▦) and EPCs (▪) from the mononuclear cell fraction of human peripheral blood. EPCs were treated for 24 hours with ABRM (▨) from HUVECs. IgG-FITC antibody was used as a control (CON). Mean ± SD, n = 4, *P < .05.
ABRM from HUVECs increases the number of adherent spindle-shaped cells
The protein content of the ABRM used was 16 ± 8 μg/mL (n = 4). A 24-hour treatment of adherent PBMCs with freshly prepared ABRM significantly increased their number from 41 ± 16 to 70 ± 17 cells/mm2 (n = 4, P = .01), corresponding to approximately a 65% increase (Figure 2C). Moreover, a change of the cell phenotype was observed. Before incubation with ABRM, most of the attached cells appear rounded, whereas after treatment they change their appearance and most of the cells are spindle-shaped (Figure 2A).
ABRM increases the number and initiates a differentiation of EPCs
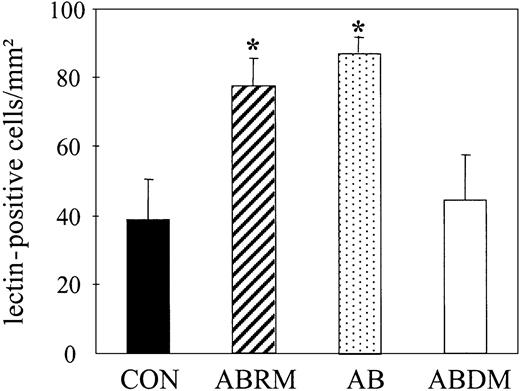
Treatment with ABRM increased the number of DiI-Ac-LDL/lectin double-positive cells from 40 ± 8 to 80 ± 11 cells/mm2 (n = 3, P = .01), corresponding to a 2-fold increase (Figure 2D). This confirmed the effects observed by light microscopy and suggests that ABRM induces an increase in the number of EPCs. After incubation with ABRM, EPCs showed a significantly increased expression of CD34 (22% ± 2% versus 13% ± 3%; n = 3, P < .05) and KDR (49% ± 12% versus 19% ± 7%; n = 3, P = .02; Figure 3). Freezing and thawing of the ABRM abolished the described effects and incubation with medium from nonapoptotic HUVECs had no effect (data not shown). Furthermore, ABDM from HUVECs did not affect the number of EPCs, whereas treatment with resuspended apoptotic bodies induced effects similar to those of ABRM (88 ± 5 versus 39 ± 12 cells/mm2; n = 3, P = .01; Figure 4). The expression of CD68, as determined by flow cytometry, was not changed by incubation of adherent cells with ABRM (17% ± 9% before and 18% ± 15% after treatment, n = 4) indicating that the number of macrophages was not changed after the treatment.
Effect of apoptotic bodies on the number of lectin-positive EPCs. Bar graphs represent the number of untreated lectin-FITC+ spindle-shaped EPCs (CON) and their number after treatment with ABRM, apoptotic bodies (AB), or ABDM. Mean ± SD, n = 3, *P < .05.
Effect of apoptotic bodies on the number of lectin-positive EPCs. Bar graphs represent the number of untreated lectin-FITC+ spindle-shaped EPCs (CON) and their number after treatment with ABRM, apoptotic bodies (AB), or ABDM. Mean ± SD, n = 3, *P < .05.
ABRM induces a dose-dependent proliferation of EPCs
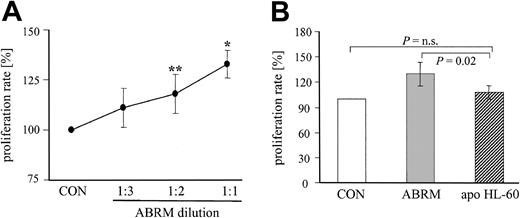
To test a dose-dependent effect, ABRM (protein content approximately 16 μg/mL) was diluted with endothelial basal medium in different ratios (1:2 and 1:3) and EPC proliferation was measured using a specific cell proliferation assay kit. Treatment with these different dilutions of ABRM for 24 hours dose dependently increased the proliferation rate of adherent EPCs, as compared with untreated control cells (Figure 5A). Undiluted ABRM enhanced the proliferation rate to 133% ± 7%, whereas serial dilutions of 1:2 and 1:3 increased the proliferation to 118% ± 10% and 111% ± 10%, respectively (Figure 5A). In contrast, a parallel incubation of the same batch of EPCs with supernatant from apoptotic HL-60 cells did not increase significantly the proliferation rate of EPCs (108% ± 8%, n = 5; Figure 5B).
Effect of different dilutions of ABRM and of apoptotic bodies derived from HL-60 cells on proliferation of EPCs. (A) Line graph depicts the proliferation rate of EPCs after treatment with different dilutions of ABRM. The protein content of the undiluted ABRM (1:1) was approximately 16 μg/mL. Incubation with endothelial basal medium, which was used to obtain and to dilute the ABRM, is shown as a control (CON). Proliferation rate is expressed as the percentage of control (= 100%; mean ± SD, n = 3-7, **P = .04, *P = .01). (B) Bar graph represents the proliferation rate of EPCs after a parallel incubation with ABRM (▦) or apoptotic bodies-rich supernatant from HL-60 cells (apo HL-60, ▨). Proliferation rate is expressed as the percentage of control (= 100%, □). Mean ± SD, n = 5-9.
Effect of different dilutions of ABRM and of apoptotic bodies derived from HL-60 cells on proliferation of EPCs. (A) Line graph depicts the proliferation rate of EPCs after treatment with different dilutions of ABRM. The protein content of the undiluted ABRM (1:1) was approximately 16 μg/mL. Incubation with endothelial basal medium, which was used to obtain and to dilute the ABRM, is shown as a control (CON). Proliferation rate is expressed as the percentage of control (= 100%; mean ± SD, n = 3-7, **P = .04, *P = .01). (B) Bar graph represents the proliferation rate of EPCs after a parallel incubation with ABRM (▦) or apoptotic bodies-rich supernatant from HL-60 cells (apo HL-60, ▨). Proliferation rate is expressed as the percentage of control (= 100%, □). Mean ± SD, n = 5-9.
Apoptotic bodies from HUVECs are phagocytosed by the EPCs
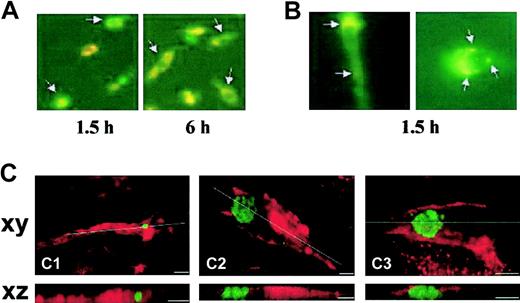
Finally, we characterized the interaction of EPCs with HUVEC-derived apoptotic bodies at different incubation times using fluorescence and confocal laser microscopy. After 0.5 hour of incubation, annexin V/PE-labeled apoptotic bodies were localized at and probably bound to the cell surface of lectin-FITC+ EPCs (data not shown). Between 0.5 and 6 hours we found intracellular localization of these structures (Figure 6A-B). Thereafter, the apoptotic bodies faint, start to disappear, and are no longer detectable as clear intracellular structures. Furthermore, confocal laser microscopy demonstrated the intracellular localization of annexin V/FITC-labeled apoptotic bodies in Alexa-568 phalloidin-labeled spindle-shaped attached cells (Figure 6C). These data indicate that HUVEC-derived apoptotic bodies are taken up by EPCs in culture.
Phagocytosis of HUVEC-derived apoptotic bodies by EPCs. (A) Uptake of annexin V/PE-labeled apoptotic bodies by lectin-FITC+ EPCs (arrows) at different time points. Overlay of both signals (orange), fluorescence microscopy, original magnification × 200. (B) Localization of apoptotic bodies (arrows) in EPCs after 1.5 hours of incubation. Overlay of both signals (orange), fluorescence microscopy, original magnification × 1000. (A-B) Representative images from 3 independent experiments. (C) Confocal laser scanning micrographs of spindle-shaped adherent cells (F-actin staining with Alexa-568-labeled phalloidin, red) containing apoptotic bodies (staining with FITC-labeled annexin V, green). Upper panels: xy-axis; lower panels: xz-axis. The xz sections were gained by sectioning 16 xy sections each time. Virtual cutting axes are indicated as white lines. Scale bars represent 10 μm; 3 images (Ci-iii) from 4 independent experiments.
Phagocytosis of HUVEC-derived apoptotic bodies by EPCs. (A) Uptake of annexin V/PE-labeled apoptotic bodies by lectin-FITC+ EPCs (arrows) at different time points. Overlay of both signals (orange), fluorescence microscopy, original magnification × 200. (B) Localization of apoptotic bodies (arrows) in EPCs after 1.5 hours of incubation. Overlay of both signals (orange), fluorescence microscopy, original magnification × 1000. (A-B) Representative images from 3 independent experiments. (C) Confocal laser scanning micrographs of spindle-shaped adherent cells (F-actin staining with Alexa-568-labeled phalloidin, red) containing apoptotic bodies (staining with FITC-labeled annexin V, green). Upper panels: xy-axis; lower panels: xz-axis. The xz sections were gained by sectioning 16 xy sections each time. Virtual cutting axes are indicated as white lines. Scale bars represent 10 μm; 3 images (Ci-iii) from 4 independent experiments.
Discussion
Circulating EPCs play a role in the repair of injured vessels and ischemic or damaged tissue.10 Because endothelial injury is often associated with apoptosis, we have investigated whether apoptotic bodies from mature ECs could affect the behavior of adult EPCs in vitro. We demonstrated here a stimulatory effect of HUVEC-derived apoptotic bodies on the number and differentiation state of progenitor cells with endothelial properties, isolated from human PBMCs and known in the literature as EPCs.4-7 Our PBMC culture system favored the growth of EPCs under control conditions. The amounts of macrophages did not increase and the lymphocytes gradually died by apoptosis during the culture period of 7 days. The incubation of the EPCs under these culture conditions with ABRM from HUVECs resulted in a significant increase of DiI-Ac-LDL/lectin double-positive cells, characteristic for endothelial-like cells, and in an enhancement of the expression of endothelial-like markers. Our experiments also demonstrated that apoptotic bodies, but not ABDM or medium from nonapoptotic HUVECs, are responsible for the observed effects and that apoptotic bodies are phagocytosed by the EPCs. Taken together, these data suggest that EPCs may represent a unique subpopulation of circulating, bone marrow-derived progenitor cells, which can respond to apoptotic material from mature ECs.
The idea of apoptotic bodies as transporters of cell-derived compounds (eg, DNA, peptides, or oxidized phospholipids) contained in these membrane vesicles to induce the maturation of progenitor cells in our experiments, is appealing. In vivo, such an intercellular communication would circumvent a “signal dilution” effect of soluble products released from apoptotic cells into the extracellular space or into the circulation. It should be emphasized that apoptotic bodies and microparticles appear to be different. Apoptotic bodies are formed as small membrane vesicles from cells during apoptosis only and have a larger size (1-4 μm) as compared to microparticles that have a size of less than 1 μm and occur as simple membrane fragments, which do not contain cell-specific material.11 Microparticles are released also from viable cells at low amounts.11,12
We found that serum and growth factor deprivation induced apoptosis in HUVECs as also previously described.8 Furthermore, in our study we have characterized and used the supernatant of serum- and growth factor-depleted HUVECs. Flow cytometry analysis demonstrated that this supernatant contained a mixture of dead cells, apoptotic bodies, and microparticles. A low-speed centrifugation of this mixture resulted in the removal of dead cells and an enrichment of apoptotic bodies. High-speed centrifugation of this preparation further yielded a supernatant free of apoptotic bodies and a sediment enriched in apoptotic bodies. The analysis of platelets with identical settings of the FACScan showed that apoptotic bodies are of similar size as platelets (1-4 μm). Apoptotic bodies, as well as microparticles, stained positively with annexin V, demonstrating their origin from apoptotic cells. Furthermore, apoptotic bodies but not microparticles contained DNA, as demonstrated by staining with DAPI or PI.
Most interestingly, incubation of EPCs with ABRM resulted in a dose-dependent increase of the proliferation rate of EPCs and induced an apparent morphologic change in the adherent cells, possibly indicating differentiation. Furthermore, the analysis of endothelial surface markers clearly suggested that EC-derived apoptotic bodies gave rise to a higher EPC differentiation state toward endothelial-like cells. In contrast to the effects observed with ABRM, treatment with ABDM had no effect, whereas the incubation with isolated and resuspended apoptotic bodies induced effects similar to those of ABRM. Therefore, it seems that EC-derived compounds contained in the apoptotic bodies induce the observed effects on EPCs. Freezing and thawing of ABRM abolished the described effects, inferring that the damage of the apoptotic bodies themselves or of sensitive compounds within the membrane vesicles (or both) may have resulted in a loss of biologic activity. Because apoptotic bodies stained positive for DAPI and PI, one possible active compound within the apoptotic blebs requiring phagocytotic uptake for inducing biologic effects could be DNA. Recently, an intercellular DNA transfer by uptake of apoptotic bodies has been demonstrated in embryonic fibroblasts, monocytes, and ECs.13,14 Such an intercellular transfer mediated by apoptotic bodies could potentially be exploited for gene and drug delivery.
Our experiments further demonstrate a phagocytotic activity of EPCs. Fluorescence microscopy suggested phagocytosis of HUVEC-derived apoptotic bodies by lectin-positive EPCs. For a more specific resolution of this uptake, analysis by confocal laser microscopy was used. An unequivocal intracellular localization of annexin V/FITC+ apoptotic bodies in spindle-shaped cells was found. Therefore, according to these findings the transfer of apoptotic bodies containing intracellular material from HUVECs, such as DNA, into EPCs seems feasible. In an additional set of experiments we have investigated the specificity of the uptake process. We found that EPCs also phagocytosed apoptotic bodies from HL-60 cells (data not shown). Thus, the uptake process is not specific for EC-derived apoptotic bodies. However, the apoptotic bodies derived from HL-60 did not increase significantly the proliferation rate of EPCs, suggesting that the magnitude of the effects is due to apoptotic bodies derived from ECs. These phagocytotic properties of isolated human EPCs may be explained by their recently described close relationship to professional phagocytes such as monocyte/macrophages.15 In this context, the phagocytotic capacity of EPCs has been therapeutically exploited for the first time by Nagaya et al, who demonstrated the uptake of plasmid DNA-gelatin complexes by EPCs.16
The number of circulating CD133+/CD34+/VEGFR-2+ EPCs in healthy subjects is rather low (about 0.002% of PBMCs) and very low amounts of circulating ECs (1-3/mL blood) are present.5,17 In addition, recent data have demonstrated that acute myocardial infarction or vascular trauma was followed by a rapid mobilization of EPCs.18,19 Furthermore, in patients with acute coronary syndromes elevated numbers of both desquamated ECs and circulating endothelial membrane microparticles have been found, indicating a considerable damage of the vascular endothelium.20,21 Vascular trauma in acute coronary syndromes is followed by apoptosis in vascular cells,22 and an increased formation of circulating apoptotic bodies from ECs may be required to induce differentiation of circulating progenitor cells. Our experiments infer, indeed, a possible link between injured vascular ECs, that is, increased formation of circulating apoptotic bodies and increased numbers of circulating bone marrow-derived EPCs in vivo.
In conclusion, our data indicate that isolated adult human EPCs react to apoptotic bodies from mature ECs by increasing their number and differentiation state. Such a mechanism, if occurring in vivo, could facilitate the repair of injured endothelium and may represent a new intercellular signaling pathway on the border between bone marrow-derived progenitor cells and damaged somatic cells.
Prepublished online as Blood First Edition Paper, July 8, 2004; DOI 10.1182/blood-2003-10-3614.
Supported in part by the Deutsche Forschungsgemeinschaft (GRK 438 “Vascular biology in medicine”) and by the August-Lenz-Stiftung.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Gabriele Berberich and Nicole Wilke for technical support.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal