Abstract
Bortezomib (PS-341), a selective inhibitor of proteasomes, induces apoptosis in multiple myeloma (MM) cells; however, prolonged drug exposure may result in cumulative toxicity and the development of chemoresistance. Here we show that combining PK-11195 (PK), an antagonist to mitochondrial peripheral benzodiazepine receptors (PBRs), with bortezomib triggers synergistic anti-MM activity even in doxorubicin-, melphalan-, thalidomide-, dexamethasone-, and bortezomib-resistant MM cells. No significant cytotoxicity was noted in normal lymphocytes. Low-dose combined PK and bortezomib treatment overcomes the growth, survival, and drug resistance conferred by interleukin-6 or insulin growth factor within the MM bone marrow milieu. The mechanism of PK + bortezomib–induced apoptosis includes: loss of mitochondrial membrane potential; superoxide generation; release of mitochondrial proteins cytochrome-c (cyto-c) and Smac; and activation of caspases-8/-9/-3. Furthermore, PK + bortezomib activates c-Jun NH2 terminal kinase (JNK), which translocates to mitochondria, thereby facilitating release of cyto-c and Smac from mitochondria to cytosol. Blocking JNK, by either dominant-negative mutant (DN-JNK) or cotreatment with a specific JNK inhibitor SP600125, abrogates both PK + bortezomib–induced release of cyto-c/Smac and induction of apoptosis. Together, these preclinical studies suggest that combining bortezomib with PK may enhance its clinical efficacy, reduce attendant toxicity, and overcome conventional and bortezomib resistance in patients with relapsed refractory MM.
Introduction
Multiple myeloma (MM) remains fatal despite all available therapies.1 Recent studies show that bortezomib/proteasome inhibitor PS-341 induces apoptosis even in MM cells refractory to multiple prior therapies including dexamethasone (Dex), melphalan, and thalidomide.2,3 Based on our preclinical and clinical studies, the FDA recently approved bortezomib for the treatment of relapsed refractory MM.2,4 Although initial treatment with bortezomib triggers apoptosis in MM cells, de novo PS-341 resistance ultimately develops in some cases. Chemoresistance is associated with defects in apoptotic signaling in response to drugs; overexpression of antiapoptotic proteins, such as B-cell leukemia/lymphoma-2 protein (Bcl2) or inhibitors of apoptosis protein (IAPs),5 and expression of multidrug resistance (MDR) and other resistance genes6,7 ; and tumor cell binding to bone marrow (BM) extracellular matrix proteins and BM stromal cells, as well as cytokines within the BM microenvironment including interleukin-68 and insulin growth factor-I (IGF-I).9-11 The mechanisms mediating bortezomib resistance and strategies to overcome it are now being delineated.
Conversely, recent reports have linked mitochondria to cell death; and alterations in expression and/or function of mitochondrial signaling proteins lead to chemoresistance and treatment failure.12-14 For example, modulation of peripheral benzodiazepine receptors (PBRs) alters the sensitivity of acute myeloid leukemic cells to chemotherapy.15 PBRs are predominantly localized in mitochondrial pores and function as a prosurvival/antiapoptotic protein.16-18 Higher levels of PBR density have been noted in rapidly proliferating breast cancer cells and correlate with tumor malignancy and patient survival.16 PK-11195 (PK) blocks the cytoprotective effects of Bcl2 against multiple chemotherapeutic agents without any cytotoxic effects of its own.16 Inhibition of PBR function by its antagonist PK-11195 (PK) sensitizes AML (MDR+) cells to daunorubicin and cytarabine, without associated toxicity to normal myeloid cells.15 Importantly, PK facilitates apoptosis by opening mitochondrial permeability transition pores (PTPCs).16,19 Moreover, PK also inhibits p-glycoprotein–mediated drug efflux thereby enhancing daunomycin cytotoxicity.15 In the context of MM, we and others have established a role of mitochondria during both conventional and novel agent–triggered cell death.5,12-19 Whether targeting PBRs in MM cells can sensitize these cells to bortezomib and overcome bortezomib resistance remains undefined.
In the present study, we asked (1) whether inhibition of PBRs using PK affects MM cell viability, and (2) whether combination of minimally toxic doses of PK and bortezomib induces apoptosis in MM cells, overcomes bortezomib resistance, and enhances anti-MM activity of bortezomib. We show that PK and bortezomib triggers synergistic apoptosis, even in MM cells resistant to bortezomib, via both mitochondria-dependent and -independent apoptotic signaling pathways. Moreover, activation of c-Jun NH2 terminal kinase (JNK) is an obligatory event during PK + bortezomib–induced apoptosis in MM cells. These preclinical studies provide the framework for clinical evaluation of PK in combination with less toxic doses of bortezomib to inhibit MM cell growth and overcome drug resistance.
Patients, materials, and methods
Cell culture and reagents
Dex-sensitive MM.1S and Dex-resistant MM.1R human MM cell lines20,21 were kindly provided by Dr Steven Rosen (Northwestern University, Chicago, IL). Doxorubicin (Dox)–resistant (Dox-40) and melphalan-resistant (LR-5) RPMI-8226 cells were kindly provided by Dr William Dalton (Moffit Cancer Center, Tampa, FL). The U266 MM cell line was obtained from the American Type Culture Collection (Rockville, MD). SUDHL-4 (DHL-4) lymphoma cells were kindly provided by Dr Margaret Shipp, Dana Farber Cancer Institute, Boston. Human B-cell lymphoma cell line RC-K8 was kindly provided by Dr Thomas Gilmore (Boston University, MA). All cell lines were grown in RPMI-1640 media supplemented with 10% heat inactivated fetal bovine serum (FBS), 100 units/mL penicillin, 100 μg/mL streptomycin, and 2 mM l-glutamine. Drug-resistant cell lines were cultured with low doses of drugs to confirm their lack of drug sensitivity. MM cells were freshly isolated from patients relapsing after multiple prior therapies including dexamethasone, melphalan, thalidomide, or bortezomib. An informed consent was obtained from all patients in accordance with the Helsinki protocol. Mononuclear cells were prepared from MM patient BM samples by Ficoll-Hypaque density gradient centrifugation. Tumor cells (97 ± 2.0% CD138+) were isolated by CD138+ selection21 using CD138 (Syndecan-1) Micro Beads and the Auto magnetic-activated cell separation (MACS), according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). Cells were treated with various concentrations of PK-11195 (25, 50, 75, 100, 200 micromolar; Sigma Chemical, St Louis, MO) and/or bortezomib (Millennium Pharmaceuticals, Cambridge, MA).
Cell viability assays
Cell viability was assessed by 3-(4,5-dimethylthiozol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Chemicon International, Temecula, CA) assay according to the manufacturer's instructions (Roche Molecular Biochemical, Indianapolis, IN), with some modifications. Cells were seeded in 96-well plates in RPMI-1640 medium containing 10% FBS. PK or bortezomib was added 24 hours later and incubated for 24 hours. Cells were also treated with PK + bortezomib in the presence or absence of IL-6 (10 ng/mL)/IGF-I (50 ng/mL) and analyzed for cell viability, as previously described.21,22
Quantification of apoptosis
Cell Death Detection ELISAplus was used to quantitate cell death, as per the manufacturer's instructions (Roche Applied Sciences, Indianapolis, IN). Apoptosis was also assessed by dual fluorescence staining with DNA-binding fluorochrome Hoechst 33342 (HO) and propidium iodide (PI) to quantitate the percentage of apoptotic (PI–HO+) cells using flow cytometry (The Vantage; Becton Dickinson, San Jose, CA), as previously described.23 Cell death was quantified by Annexin V staining, as previously described.24
Mitochondrial membrane potential (Δψm) and generation of superoxide (O–2) anions
Serum-starved MM.1S cells were treated with bortezomib (2 nM) alone, PK (50 μM) alone, or bortezomib + PK for 12 hours, with CMXRos added for the last 20 minutes; stained with lipophilic cationic dye CMXRos (Mitotracker Red; Molecular Probes, Eugene, OR) in phosphate-buffered saline (PBS) for 20 minutes at 37° C; and analyzed by flow cytometry to assay for alterations in Δψm.25 Superoxide production was measured by staining cells with membrane permeable dye dihydroethidium (HE), as previously described.26 Superoxide anions oxidize HE to fluorescent ethidium, permitting analysis by flow cytometry (The Vantage, FACScan; Becton Dickinson) using excitation at 480 nm and emission at 630 nm.
In vitro immune complex kinase assays
In vitro immune complex c-Jun kinase assays were performed as a measure of JNK activity, as previously described.27
Preparation of cytosolic and mitochondrial extracts from MM.1S MM cells
MM.1S cells were washed twice with PBS, and the pellet was suspended in 3 volumes of ice cold buffer A (20 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, pH 7.5], 1.5 mM MgCl2, 10 mM KCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM EGTA [ethylene glycol tetraacetic acid], 1 mM dithiothreitol [DTT], 0.1 mM phenylmethylsulfonyl fluoride [PMSF], and 10 μg/mL leupeptin, aprotinin, and pepstatin A) containing 250 mM sucrose. The cells were homogenized using a Dounce homogenizer, and cytosolic or mitochondrial extracts were isolated as previously described.27
Western blotting
Protein lysates were prepared and Western blot analysis was performed as previously described.28 Briefly, equal amounts of proteins were resolved by 10% or 12.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto nitrocellulose membranes. Filters were blocked by incubation in 5% dry milk in PBST (0.05% Tween-20 in PBS) and probed with anti–cytochrome-c (cyto-c); anti-Smac (kindly provided by Dr Xiaodong Wang, University of Texas Southwestern Medical Center at Dallas); antitubulin (Sigma, St Louis, MO); and anti–caspases-8, -9, or -3 (Cell Signaling, Beverly, MA) antibodies (Abs). Blots were then developed by enhanced chemiluminescence (ECL; Amersham, Arlington Heights, IL). The immunoblots were scanned using an LKB produkter (Bromma, Sweden) Ultrascan XL laser densitometer and analyzed with the Gelscan software package (Bromma, Sweden). Signal intensity was determined in a linear range and normalized to that for tubulin. Preparation of cell lysates for poly adenosine diphosphate (ADP) (ribose) polymerase (PARP) immunoblot analysis was performed as described using C-2-10 anti-PARP monoclonal antibody.29
Transient transfections
MM.1S cells were transiently transfected using the Cell line Nucleofector kit V, according to the manufacturer's instructions (Amaxa Biosystems, Cologne, Germany), with vector alone or DN-JNK (kindly provided by Dr Donald Kufe, Dana Farber Cancer Institute, Boston, MA), and cotransfected with vector containing green fluorescence protein (GFP) alone. Following transfections, GFP-positive cells were selected by flow cytometry, treated with PK + bortezomib, and analyzed for cytotoxicity.
Results
PBR antagonist PK-11195 (PK) and bortezomib decreases viability of MM cells
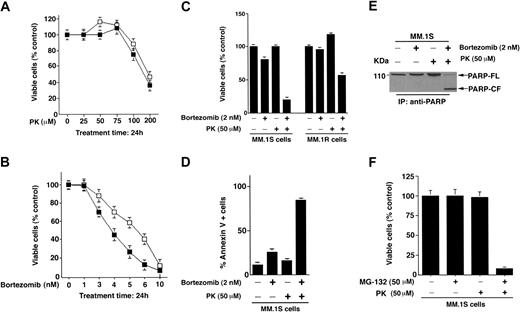
We first determined whether PK affects the viability of Dexsensitive (MM.1S) or Dex-resistant (MM.1R) MM cells using an MTT assay. As seen in Figure 1A, treatment of MM cells with PK for 24 hours induces a significant (P < .005) decrease in cell viability in a dose-dependent manner in both cell lines. A 50% decrease in viable cells was noted at 200 μM. The median inhibitory concentration (IC50) of bortezomib for both cells lines was 4 to 6 nM(P < .005; Figure 1B).
PK-11195 (PK) and bortezomib treatment triggers synergistic anti-MM activity in MM cell lines and patient MM cells. (A) Dex-sensitive (MM.1S, ▪) and Dex-resistant (MM.1R, □) cells were treated with various concentrations of bortezomib (1-10 nM) for 24 hours and assessed for viability using MTT assays. Results are means ± SDs of 3 independent experiments (P < .005). (B) MM.1S (▪) and MM.1R (□) cells were treated with various concentrations of PK (25-200 μM) for 24 hours and assessed for viability using MTT assays. Results are means ± SDs of 3 independent experiments (P < .005). (C) MM.1S and MM.1R cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours and assessed for viability using MTT assays (P = .05 for MM.1S cells and P = .04 for MM.1R cells, one-sided Wilcoxon rank-rank sum test). Error bars indicate standard error. (D) MM.1S cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours and assessed for apoptosis by Annexin V staining assays. Results are means ± SDs of 3 independent experiments (P < .003). (E) MM.1S cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours and assessed for apoptosis by PARP cleavage assays. Total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP Abs. FL indicates full length; CF, cleaved fragment. (F) MM.1S cells were treated with PK (50 μM), MG-132 (50 μM), or PK + MG-132 for 24 hours and assessed for viability using MTT assays. Results are means ± SDs of 3 independent experiments (P < .005).
PK-11195 (PK) and bortezomib treatment triggers synergistic anti-MM activity in MM cell lines and patient MM cells. (A) Dex-sensitive (MM.1S, ▪) and Dex-resistant (MM.1R, □) cells were treated with various concentrations of bortezomib (1-10 nM) for 24 hours and assessed for viability using MTT assays. Results are means ± SDs of 3 independent experiments (P < .005). (B) MM.1S (▪) and MM.1R (□) cells were treated with various concentrations of PK (25-200 μM) for 24 hours and assessed for viability using MTT assays. Results are means ± SDs of 3 independent experiments (P < .005). (C) MM.1S and MM.1R cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours and assessed for viability using MTT assays (P = .05 for MM.1S cells and P = .04 for MM.1R cells, one-sided Wilcoxon rank-rank sum test). Error bars indicate standard error. (D) MM.1S cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours and assessed for apoptosis by Annexin V staining assays. Results are means ± SDs of 3 independent experiments (P < .003). (E) MM.1S cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours and assessed for apoptosis by PARP cleavage assays. Total protein lysates were subjected to SDS-PAGE analysis. Immunoblot analysis of the lysates was performed with anti-PARP Abs. FL indicates full length; CF, cleaved fragment. (F) MM.1S cells were treated with PK (50 μM), MG-132 (50 μM), or PK + MG-132 for 24 hours and assessed for viability using MTT assays. Results are means ± SDs of 3 independent experiments (P < .005).
PK + bortezomib triggers synergistic anti-MM activity
Based on our viability data, we selected minimally toxic concentrations of PK (50 μM) and bortezomib (2 nM) to determine their combined effect on MM cell viability. As seen in Figure 1C, treatment of MM.1S or MM.1R MM cells with PK + bortezomib for 24 hours induces synergistic growth inhibition. Median viability after each treatment was as follows: bortezomib + PK = 12 ± 1.2%; bortezomib alone = 78 ± 3.1%; and PK alone = 98% (P = .05, one-sided Wilcoxon rank-sum test). Similarly, median viability of MM.1R cells was as follows: bortezomib + PK = 50 ± 0.8%; bortezomib alone = 95 ± 2.4%; and PK alone = 115 ± 6.1% (P = .04, one-sided Wilcoxon rank-sum test). Isobologram analysis confirmed a synergistic anti-MM activity of PK with bortezomib (combination index [CI] < 1.0). Moreover, maximal anti-MM activity was noted when PK and bortezomib were given concomitantly than in other drug treatment schedules (data not shown).
To confirm whether decreases in MM cell viability in response to PK + bortezomib are due to apoptosis, we performed both Annexin V staining and poly ADP (ribose) polymerase (PARP) cleavage assays. As seen in Figure 1D, treatment of MM.1S with low doses of PK (50 μM) and bortezomib (2 nM) triggered a significant (P < .003) increase in Annexin V–positive cells, whereas neither agent alone induces apoptosis at these concentrations. Examination of proteolytic cleavage of PARP, a hallmark of apoptosis,30 demonstrated similar results (Figure 1E).
To exclude the possibility that synergistic anti-MM activity of PK is specific to bortezomib, we performed experiments using another proteasome inhibitor MG-132. MM.1S cells were treated with PK (50 μM), MG-132 (50 μM), or PK + MG-132 for 24 hours, and then analyzed for viability by MTT assays. As seen in Figure 1F, treatment of MM.1S cells with either PK or MG-132 alone triggered minimal decrease in viability, whereas PK + MG-132 induced significant cytotoxicity in these cells. Median viability of cells after each treatment was as follows: PK = 98%; MG-132 = 99.1%; and PK + MG-132 = 13 ± 1.2%; P < .005. These findings suggest that PK has synergistic anti-MM activity when combined with proteasome inhibitors other than bortezomib. These data are consistent with prior studies demonstrating similar effects of PK combined with other anticancer drugs against leukemic cells.15
Combined PK and bortezomib treatment induces apoptosis in drug-resistant MM cell lines and patient cells
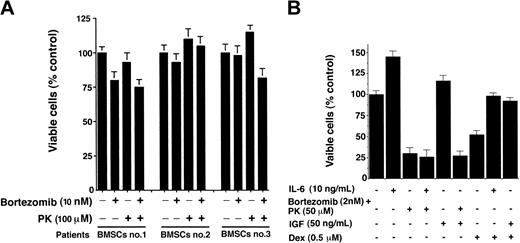
Since PK + bortezomib triggered synergistic apoptosis in Dexresistant (MM.1R) MM cells, we asked whether similar effects were also induced in other drug-resistant MM cells, including doxorubicin- and melphalan-resistant RPMI-8226 MM cells. As seen in Figure 2A, combined treatment with low doses of PK and bortezomib induced marked decreases in viability of all these cell types (synergy: CI < 1.0). We next directly examined the effects of PK + bortezomib on MM cells freshly isolated from patients relapsing after multiple prior therapies including bortezomib (Figure 2B, patient nos. 1-2), dexamethasone (Figure 2B, patient nos. 3-4), and thalidomide (Figure 2B, patient no. 5). As seen in Figure 2B, treatment of patient MM cells with PK (50 μM) + bortezomib (2 nM) for 24 hours significantly (P = .05; n = 2) triggered apoptosis in all 5 patient MM cells, as measured by DNA fragmentation assays. Together, these findings show that synergy between PK and bortezomib overcomes conventional and bortezomib drug resistance in MM cells.
Combined PK and bortezomib treatment triggers synergistic anti-MM activity in human MM cell lines and patient MM cells resistant to conventional drugs, without affecting the viability of normal PBMNCs. (A) MTT assays were performed after incubation of RPMI-8226, doxorubicin-resistant Dox-40, or melphalan-resistant (LR-5) MM cell lines with indicated doses of PK + bortezomib for 24 hours. Results are means ± SDs from 3 independent experiments (P < .0002 for all cells lines). (B) CD138+ MM patient cells (patient nos. 1-5) were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours and assessed for apoptosis using DNA fragmentation assays. Values are means ± SDs of triplicate samples (P = .05); experiments were repeated 2 times with similar results. (Ci) bortezomib-resistant SUDHL4 (DHL-4) lymphoma cells were treated with indicated concentrations of PK, bortezomib, or PK + bortezomib for 24 hours, and assessed for viability by MTT assays. Results are means ± SDs of 4 independent experiments (P < .005). (Cii) MM.1S cells were stably transfected with Bcl2 construct; treated with indicated concentrations of PK, bortezomib, or PK + bortezomib for 24 hours; and assessed for viability using MTT assays. Results are means ± SDs of 3 independent experiments (P < .005). (D) Normal lymphocytes from 5 healthy donors were treated with PK (50 μM) + bortezomib (2 nM) for 24 hours, and viability was assessed by an MTT assay. Each donor is indicated by a distinct symbol. Results are the mean ± SD of 3 independent experiments (P = .21 from Jonchkeere-Terpstra [J-T] trend test).
Combined PK and bortezomib treatment triggers synergistic anti-MM activity in human MM cell lines and patient MM cells resistant to conventional drugs, without affecting the viability of normal PBMNCs. (A) MTT assays were performed after incubation of RPMI-8226, doxorubicin-resistant Dox-40, or melphalan-resistant (LR-5) MM cell lines with indicated doses of PK + bortezomib for 24 hours. Results are means ± SDs from 3 independent experiments (P < .0002 for all cells lines). (B) CD138+ MM patient cells (patient nos. 1-5) were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours and assessed for apoptosis using DNA fragmentation assays. Values are means ± SDs of triplicate samples (P = .05); experiments were repeated 2 times with similar results. (Ci) bortezomib-resistant SUDHL4 (DHL-4) lymphoma cells were treated with indicated concentrations of PK, bortezomib, or PK + bortezomib for 24 hours, and assessed for viability by MTT assays. Results are means ± SDs of 4 independent experiments (P < .005). (Cii) MM.1S cells were stably transfected with Bcl2 construct; treated with indicated concentrations of PK, bortezomib, or PK + bortezomib for 24 hours; and assessed for viability using MTT assays. Results are means ± SDs of 3 independent experiments (P < .005). (D) Normal lymphocytes from 5 healthy donors were treated with PK (50 μM) + bortezomib (2 nM) for 24 hours, and viability was assessed by an MTT assay. Each donor is indicated by a distinct symbol. Results are the mean ± SD of 3 independent experiments (P = .21 from Jonchkeere-Terpstra [J-T] trend test).
PK + bortezomib decreases viability in bortezomib-resistant lymphoma cells
Having shown that PK + bortezomib overcomes bortezomib resistance in patient MM cells, we next examined whether it can also trigger cell death in other bortezomib-resistant cell types. Our recent study demonstrated SUDHL4 lymphoma cells are resistant to bortezomib therapy,31 and we therefore next asked whether PK + bortezomib affects the viability of these cells. SUDHL4 lymphoma cells were treated with PK (50 μM), bortezomib (10 nM), or PK + bortezomib for 24 hours, and then analyzed for viability by MTT assays. As seen in Figure 2Ci, PK + bortezomib markedly (P < .005) decreased viability in these cells, whereas either agent alone did not. These findings suggest that the treatment with PK + bortezomib overcomes bortezomib resistance even in other cell types besides MM. Furthermore, since our previous study showed that bortezomib resistance in SUDHL4 is conferred by high expression of heat shock protein 27 (Hsp-27),31-33 we next determined whether Hsp-27 expression is altered in response to either PK or PK + bortezomib in these cells. No significant changes in Hsp27 expression levels were noted in PK-treated cells; however, treatment with PK + bortezomib markedly decreased Hsp27 protein levels (data not shown). Together, these data indicate that PK + bortezomib overcomes the major growth/survival and chemoresistance mechanism mediated by Hsp-27 in SUDHL4 cells. Our data support the use of combining PK and bortezomib to induce synergistic antitumor activity in bortezomib refractory cancers besides MM.
Combined PK + bortezomib treatment overcomes Bcl2-mediated protective effects
Our recent study showed that Bcl2 can modestly attenuate bortezomib-triggered cell death in MM cells.3 We therefore examined whether PK + bortezomib affects the viability of MM.1S MM cell line stably transfected with the wild-type Bcl2 gene. As seen in Figure 2Cii, PK (75 μM) + bortezomib (5 nM) significantly (P < .05) decreased viability in Bcl2-overexpressing MM.1S cells after 24 hours of treatment, whereas neither agent alone at these concentrations reduced viability of these cells (Figure 2Cii). Moreover, PK + bortezomib induces 23 ± 1.5% less cell death in Bcl2-transfected MM.1S cells than in vector alone–transfected MM.1S cells (data not shown). These data suggest that Bcl2 confers residual protective effects despite combined treatment of MM cells with PK + bortezomib.
Combined treatment with PK + bortezomib does not affect viability of normal lymphocytes
We next examined whether PK + bortezomib affects the viability of normal cells. Normal lymphocytes from 5 healthy donors were treated with PK (50 μM) + bortezomib (10 nM) for 24 hours, and then analyzed for viability using MTT assays. In contrast to MM cells, survival of normal lymphocytes from 5 healthy donors was not significantly altered (P = .21 from J-T trend test), even after combined therapy with high-dose bortezomib (10 nM) and PK (50 μM) (Figure 2D). Treatment of MM cells with high doses of PK alone for 24 hours did not affect normal cell viability, whereas bortezomib (20 nM) decreased cell viability by 20% to 30% at 24 hours (data not shown). These findings are consistent with another study demonstrating the lack of cytotoxicity of PK at these doses on normal lymphocyte viability.34 Furthermore, no significant apoptosis in normal lymphocytes was observed after treatment with PK (50 μM) + bortezomib (10 nM) for 24 hours (data not shown). Taken together, these findings indicate that low-dose combined treatment with PK + bortezomib does not significantly affect viability of normal cells.
Effect of PK + bortezomib treatment on the viability of patient MM-derived bone marrow stromal cells (BMSCs)
MM cells are predominantly localized in the BM microenvironment due to their adherence both to extracellular matrix proteins and to BMSCs.35 This interaction between MM cells and BMSCs triggers production of cytokines mediating autocrine and paracrine growth and survival of MM cells, as well as protection against drug-induced apoptosis.11 We therefore next examined whether PK (100 μM) + bortezomib (20 nM) affects viability of patient MM-derived BMSCs. As seen in Figure 3A, treatment of BMSCs (patient nos. 1-3) with PK + bortezomib for 48 hours did not decrease their viability. DNA fragmentation assays confirmed that PK + bortezomib does not trigger cell death in BMSCs (data not shown). Together, these findings suggest that PK + bortezomib directly affects the viability of MM cells, but not BMSCs.
PK + bortezomib does not affect the viability of patient MM-derived bone marrow stroma cells (BMSCs) and overcome the antiapoptotic effects of interleukin-6 (IL-6) or insulin growth factor-1 (IGF-1). (A) Patient MM BMSCs (patient nos. 1-3) were treated with indicated concentrations of PK + bortezomib for 24 hours and analyzed for viability by MTT assays. Results are the mean ± SD of 3 independent experiments; P < .005. (B) MM.1S cells were treated with indicated concentrations of PK + bortezomib or Dex (0.5 μM) in the presence or absence of either IL-6 (10 ng/mL) or IGF (50 ng/mL). At 24 hours, cells were harvested and viability was analyzed by MTT assays. Results are means ± SDs of 3 independent experiments.
PK + bortezomib does not affect the viability of patient MM-derived bone marrow stroma cells (BMSCs) and overcome the antiapoptotic effects of interleukin-6 (IL-6) or insulin growth factor-1 (IGF-1). (A) Patient MM BMSCs (patient nos. 1-3) were treated with indicated concentrations of PK + bortezomib for 24 hours and analyzed for viability by MTT assays. Results are the mean ± SD of 3 independent experiments; P < .005. (B) MM.1S cells were treated with indicated concentrations of PK + bortezomib or Dex (0.5 μM) in the presence or absence of either IL-6 (10 ng/mL) or IGF (50 ng/mL). At 24 hours, cells were harvested and viability was analyzed by MTT assays. Results are means ± SDs of 3 independent experiments.
Treatment with PK + bortezomib overcomes the antiapoptotic effects of human recombinant IL-6 (rIL-6) and recombinant insulin growth factor-I (rIGF-I)
We next examined the effect of combined PK + bortezomib treatment on the 2 major growth and survival cytokines present within the BM microenvironment that protect against Dex-induced apoptosis.36-39 MM.1S cells were treated with PK (50 μM) + bortezomib (2 nM) or Dex (0.5 μM), in the presence and absence of IL-6 (10 ng/mL) or IGF (50 ng/mL). As seen in Figure 3B, the median cell viability was 28 ± 2.1% after treatment with PK + bortezomib and 24 ± 2.2% with PK + bortezomib + IL-6 (P = .23, Wilcoxon test), whereas median viability was 51 ± 4.1% after Dex therapy and 92 ± 5.4% due to combined Dex + IL-6 treatment (P = .05, as determined by one-sided Wilcoxon ranksum test). Similar results were obtained using IGF-I: median viability was 28 ± 2.1% after therapy with PK + bortezomib and 24.7% after treatment with PK + bortezomib + IGF-I (P = .27) (Figure 3B). Thus, neither IL-6 nor IGF-I block PK + bortezomib–induced cytotoxicity in MM.1S cells. In contrast and as in our prior studies,9,40 both IL-6 and IGF-I protect against Dex-induced decreased MM.1S cell viability. Taken together, these data demonstrate that PK + bortezomib overcomes the growth/survival effects of cytokines present within the MM BM milieu.
Combined treatment with PK + bortezomib induces alterations in mitochondrial membrane potential (Δψm), generation of superoxide ( \(\mathrm{O}_{2}^{-}\) ), release of mitochondrial proteins cytochrome-c and Smac, and activation of caspases
We next examined the molecular mechanisms whereby low-dose combined treatment with PK and bortezomib triggers apoptosis in MM cells. Stress-induced apoptosis correlates with loss of Δψm and generation of superoxide
Disruption of mitochondrial integrity is critical during stress-induced cell death.12 We therefore first determined whether treatment with PK + bortezomib induces a loss in Δψm. MM.1S cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 12 hours; stained with CMXRos; and analyzed by flow cytometry, as previously described.44 As seen in Figure 4A, neither PK nor bortezomib treatment alone at these doses triggered significant decreases in Δψm, whereas PK + bortezomib therapy induced a marked decrease in Δψm in MM.1S cells, evidenced by an increase in number of CMXRos-negative cells (P < .005). Since loss of Δψm is associated with
PK + bortezomib changes mitochondrial membrane potential (Δψm), generation of superoxide (
PK + bortezomib changes mitochondrial membrane potential (Δψm), generation of superoxide (
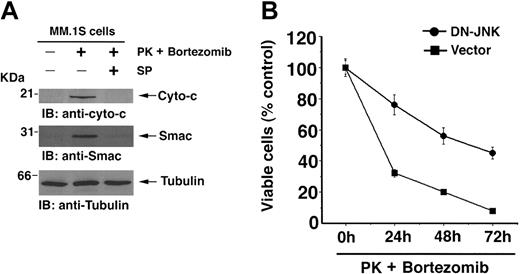
Loss of Δψm is associated with release of mitochondrial proteins Cyto-c and Smac to the cytosol.46 We therefore next examined whether Cyto-c or Smac release is similarly affected by synergistic doses of PK + bortezomib. MM.1S cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib; cytosolic extracts were then prepared and subjected to immunoblot analysis with anti–Cyto-c or anti-Smac Abs. As seen in Figure 4C (upper and middle panels), treatment of MM.1S cells with PK + bortezomib induces the release of both Cyto-c and Smac. In contrast, neither agent alone at these concentrations induced significant release of cyto-c and Smac. Importantly, PK + bortezomib–induced Cyto-c or Smac release is 2- to 3-fold higher than that induced by higher concentrations of bortezomib (10 nM) (data not shown). Reprobing the immunoblots with antitubulin Abs confirms equal protein loading (Figure 4C, lower panel). These findings show that
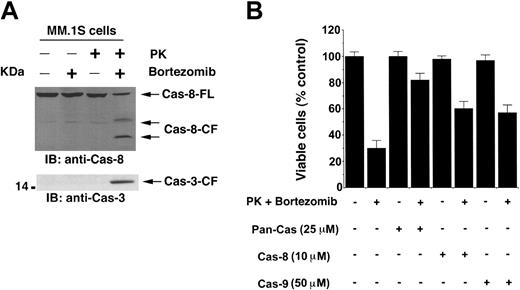
Stress-induced alterations in mitochondria trigger the release of Cyto-c/Smac from mitochondria to cytosol, which activates caspase-9.46,47 We next therefore determined whether PK + bortezomib–induced apoptosis is associated with caspase-9 activation by a colorimetric assay. MM.1S cells were treated with PK, bortezomib, or PK + bortezomib for 24 hours, and cytosolic extracts were assayed for protease activity using LEHD-pNA as substrate. As seen in Figure 4D, treatment of MM.1S cells with PK + bortezomib, but not with PK or bortezomib alone, activates caspase-9, as determined by LEHD-pNA cleavage. Since prior studies have shown that a high dose of bortezomib induces extrinsic apoptotic signaling via caspase-8 activation,3,48 we further examined whether a nontoxic dose of bortezomib (2 nM) in combination with PK can also trigger caspase-8 activation. As seen in Figure 5A (upper panel), PK + bortezomib, but not either agent alone, triggered marked caspase-8 cleavage. Both caspase-9 (mitochondria-dependent/intrinsic pathway) and caspase-8 (mitochondria-independent/extrinsic pathway) are known to activate a common downstream effector procapsase-3,49 and our data further showed that PK + bortezomib triggered caspase activation, as evidenced by caspase-3 cleavage (Figure 5A, lower panel). These data demonstrate that combined treatment with subtoxic concentrations of PK and bortezomib triggers mitochondrial perturbations sufficient to initiate apoptotic signaling, and suggest that this strategy may avoid dose-related toxic effects of therapy with bortezomib alone at higher concentrations.
PK + bortezomib–induced apoptosis is associated with activation of caspase-8 and caspase-3. (A) MM.1S cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours; cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti–caspase-8 (top panel) and anti–caspase-3 (bottom panel) Abs. Blots are representative of 3 independent experiments. FL indicates full length; CF, cleaved fragment. (B) PK + bortezomib–induced apoptosis is mediated by both caspase-8, caspase-9 >> caspase-3 pathway. MM.1S cells were treated with PK + bortezomib alone or in the presence of caspase-8 inhibitor, caspase-9 inhibitor, or pan–caspase-3 inhibitor for 24 hours; harvested; and assessed for viability using MTT assays. Results are means ± SDs of 3 independent experiments (P < .005).
PK + bortezomib–induced apoptosis is associated with activation of caspase-8 and caspase-3. (A) MM.1S cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours; cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti–caspase-8 (top panel) and anti–caspase-3 (bottom panel) Abs. Blots are representative of 3 independent experiments. FL indicates full length; CF, cleaved fragment. (B) PK + bortezomib–induced apoptosis is mediated by both caspase-8, caspase-9 >> caspase-3 pathway. MM.1S cells were treated with PK + bortezomib alone or in the presence of caspase-8 inhibitor, caspase-9 inhibitor, or pan–caspase-3 inhibitor for 24 hours; harvested; and assessed for viability using MTT assays. Results are means ± SDs of 3 independent experiments (P < .005).
To determine whether caspase activation is required for PK + bortezomib–triggered apoptosis, we treated MM.1S cells with caspase-8 inhibitor (IETD-fmk), caspase-9 inhibitor (LEHD-fmk), or pan-caspase inhibitor (z-VAD-fmk). As seen in Figure 5B, pan-caspase inhibitor, but not either caspase-8 or caspase-9 inhibitor, abrogates PK + bortezomib–induced apoptosis. We also determined whether caspase-9 activation triggers caspase-8 activity. The results demonstrate that inhibition of caspase-9 using LEHD-fmk does not affect caspase-8 activity triggered by combined PS + PK treatment of MM.1S cells (data not shown). These data suggest that PS + PK–induced caspase-8 activity is not secondary to activation of caspase-9. We next determined whether PK + bortezomib–induced apoptosis requires caspase-3 activity. Inhibition of caspase-3 using DEVD-fmk markedly abrogates PK + bortezomib–triggered apoptosis (data not shown), as observed using pan-caspase inhibitor. Together, these findings suggest that (1) activation of both caspase-8 and caspase-9 is required, and neither alone is sufficient for PK + bortezomib–induced MM cell death, and (2) caspase-3 activation is the predominant mechanism whereby PK + bortezomib triggers MM cell apoptosis.
Treatment with PK + bortezomib induces activation of cytosolic c-Jun NH2-terminal kinase (JNK) and its translocation to mitochondria
Having shown that PK + bortezomib induces mitochondria-dependent apoptotic signaling, we next examined an upstream activator of this pathway. JNK has been linked to apoptosis: 2 serine residues (Ser63 and Ser73) in the amino-terminal transactivation domain of c-Jun are substrates for JNK,50,51 and previous studies have shown that stress stimuli (eg, irradiation, tumor necrosis factor, sphingomyelinase, and ultraviolet [UV] light) activate JNK.50-52 Our prior studies showed that bortezomib induces JNK activation in MM cells, albeit at higher doses (8-10 nM).27,48 In the present study, we therefore examined whether low-dose bortezomib (2 nM) in combination with PK (50 μM) trigger JNK activation. MM.1S cells were exposed to PK + bortezomib for various time intervals, and JNK activity was analyzed by in vitro immune complex kinase assays using GST-Jun as a substrate. As seen in Figure 6A (upper panel), analysis of anti-JNK immunoprecipitates demonstrated significant (7- to 8-fold) increases in GST-Jun phosphorylation, indicating activation of JNK. Moreover, cotreatment of MM.1S cells with SP600125, a specific inhibitor of JNK,53 blocks PK + bortezomib–induced JNK activity (Figure 6A, upper panel). Importantly, synergistic doses of PK and bortezomib induce greater JNK activation compared with the high-dose bortezomib (10 nM) alone (Figure 6A, upper panel). Activation of JNK in response to PK + bortezomib is not associated with changes in JNK protein levels (Figure 6A, lower panel). Our findings are in concert with studies of Verheij et al, which demonstrate a role of JNK in ceramide- and TNF-α–induced apoptosis.52
PK and bortezomib treatment triggers activation of c-Jun NH3-terminal kinase (JNK) and translocation of JNK from cytosol to mitochondria in MM.1S MM cells. (A) Cells were treated with PK (50 μM), bortezomib (2 nM), PK + bortezomib, PK + bortezomib + JNK inhibitor SP600125 (SP), or bortezomib (10 nM) alone for 24 hours. Protein lysates were immunoprecipitated with anti-JNK Ab. Immune complex kinase assays were performed by addition of 5 μg GST-Jun (2-100), (γ32P) adenosine triphosphate (ATP), and incubation for 15 minutes at 30° C. The phosphorylated proteins were resolved by 10% SDS-PAGE and analyzed by autoradiography (top panel). Anti-JNK immunoprecipitates were also immunoblotted with anti-JNK Ab (bottom panel). Blots are representative of 3 independent experiments with similar results. (B) MM.1S cells were treated with indicated concentrations of PK + bortezomib for 24 hours. Cytosolic (Cyto) and mitochondrial (Mito) fractions were isolated and subjected to immunoblotting with anti-JNK (upper panel) or anti-Hsp60 (lower panel) Abs. Blots are representative of 2 independent experiments with similar results.
PK and bortezomib treatment triggers activation of c-Jun NH3-terminal kinase (JNK) and translocation of JNK from cytosol to mitochondria in MM.1S MM cells. (A) Cells were treated with PK (50 μM), bortezomib (2 nM), PK + bortezomib, PK + bortezomib + JNK inhibitor SP600125 (SP), or bortezomib (10 nM) alone for 24 hours. Protein lysates were immunoprecipitated with anti-JNK Ab. Immune complex kinase assays were performed by addition of 5 μg GST-Jun (2-100), (γ32P) adenosine triphosphate (ATP), and incubation for 15 minutes at 30° C. The phosphorylated proteins were resolved by 10% SDS-PAGE and analyzed by autoradiography (top panel). Anti-JNK immunoprecipitates were also immunoblotted with anti-JNK Ab (bottom panel). Blots are representative of 3 independent experiments with similar results. (B) MM.1S cells were treated with indicated concentrations of PK + bortezomib for 24 hours. Cytosolic (Cyto) and mitochondrial (Mito) fractions were isolated and subjected to immunoblotting with anti-JNK (upper panel) or anti-Hsp60 (lower panel) Abs. Blots are representative of 2 independent experiments with similar results.
Genotoxic stress triggers translocation of JNK from the cytosol to mitochondria,13 thereby facilitating the release of mitochondrial apoptogenic proteins to cytosol. To determine whether PK + bortezomib–activated JNK translocates to mitochondria, we assayed for JNK protein levels in cytosolic and mitochondrial fractions from untreated versus PK + bortezomib–treated MM.1S cells. As seen in Figure 6B (upper panel), treatment with PK + bortezomib significantly increases JNK protein level in the mitochondrial fraction (3- to 4-fold, measured by densitometric analysis of immunoblots). Blots were reprobed with mitochondrial-specific Hsp60 protein to confirm purity of these fractions (Figure 6B, lower panel). Together, these data demonstrate that PK + bortezomib–induced apoptosis is associated with JNK activation and its translocation to mitochondria.
Blocking JNK abrogates PK + bortezomib–induced Cyto-c/Smac release and apoptosis
Given that activated JNK translocates to mitochondria and facilitates Cyto-c/Smac release in response to PK + bortezomib, we next examined whether blocking JNK inhibits PK + bortezomib–induced Cyto-c/Smac release and cell death. MM.1S cells were treated with low-dose combination of PK (50 μM) + bortezomib (2 nM), in the presence or absence of JNK inhibitor SP600125 (10 μM); cytosolic extracts were prepared and subjected to immunoblot analyses with anti–Cyto-c or anti-Smac Abs. As seen in Figure 7A (upper and middle panel), PK + bortezomib induces the release of Cyto-c and Smac. Importantly, cotreatment with SP600125 significantly inhibits release of both Cyto-c and Smac (Figure 7A, upper and middle panel). Reprobing the immunoblots with antitubulin confirmed equal protein loading (Figure 7A, lower panel). These findings demonstrate that PK + bortezomib–induced apoptosis is mitochondria-mediated (intrinsic) signaling, since it requires JNK activation to allow for its translocation and subsequent release of Cyto-c/Smac.
JNK is involved during PK + bortezomib-induced apoptosis in MM cells. (A) SP6000125 (SP), inhibitor of JNK, abrogates PK + bortezomib–induced release of Smac or cyto-c. MM.1S cells were treated with PK (50 μM) + bortezomib (2 nM) in the presence or absence of SP600125 (SP) and harvested at 24 hours. Cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti–Cyto-c or anti-Smac (top and middle panels) Abs. As a control for equal loading of proteins, filters were also reprobed with antitubulin Ab (bottom panel). Blots are representative of 3 independent experiments with similar results. (B) Overexpression of DN-JNK enhances resistance to PK + bortezomib. Cells were transiently transfected with cDNA expression construct containing GFP with either DN-JNK (•) or empty vector (▪). Following transfections, GFP-positive cells were selected by flow cytometry; treated with PK (50 μM) + bortezomib (2 nM) for 24 hours, 48 hours, or 72 hours; and analyzed for cell viability by MTT assay (P = .05, as determined by one-sided Wilcoxon rank-sum test). Error bars indicate standard error.
JNK is involved during PK + bortezomib-induced apoptosis in MM cells. (A) SP6000125 (SP), inhibitor of JNK, abrogates PK + bortezomib–induced release of Smac or cyto-c. MM.1S cells were treated with PK (50 μM) + bortezomib (2 nM) in the presence or absence of SP600125 (SP) and harvested at 24 hours. Cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti–Cyto-c or anti-Smac (top and middle panels) Abs. As a control for equal loading of proteins, filters were also reprobed with antitubulin Ab (bottom panel). Blots are representative of 3 independent experiments with similar results. (B) Overexpression of DN-JNK enhances resistance to PK + bortezomib. Cells were transiently transfected with cDNA expression construct containing GFP with either DN-JNK (•) or empty vector (▪). Following transfections, GFP-positive cells were selected by flow cytometry; treated with PK (50 μM) + bortezomib (2 nM) for 24 hours, 48 hours, or 72 hours; and analyzed for cell viability by MTT assay (P = .05, as determined by one-sided Wilcoxon rank-sum test). Error bars indicate standard error.
We next directly examined the functional significance of PK + bortezomib–induced JNK activation. MM.1S cells were transiently transfected with either DN-JNK or empty vector, and then treated with low doses of PK (50 μM) and bortezomib (2 nM); survival was analyzed using MTT assay. As seen in Figure 7B, MM.1S DN-JNK transfectants survived significantly longer after treatment with PK + bortezomib than cells transfected with vector alone: median cell viability was 76% (24 hours), 56% (48 hours), and 45% (72 hours) after PK + bortezomib treatment of DN-JNK transfected cells versus 32% (24 hours), 20% (48 hours), and 8% (72 hours) after PK + bortezomib treatment of empty vector transfected cells (P = .05, as determined by one-sided Wilcoxon rank-sum test).
Discussion
Our study shows the following: (1) the combination of low doses of PK and bortezomib triggers synergistic antitumor activity in MM cells resistant to conventional and bortezomib therapy; (2) PK + bortezomib does not affect the viability of normal cells; (3) exogenous hIL-6 or hIGF-1 do not protect against PK + bortezomib–induced apoptosis; (4) PK + bortezomib–induced apoptosis is associated with caspase activation and occurs via both mitochondria-dependent (loss of Δψm, increase in
The finding that PK enhances the anti-MM activity of bortezomib is consistent with previous studies showing similar effects of PK combined with other anticancer drugs in leukemic cells. For example, PK sensitizes acute myeloid leukemic (AML) cells to cytarabine.15 PK blocks p-glycoprotein efflux in AML cells, thereby increasing daunomycin toxicity even in efflux competent AML cells.15 A recent study also showed that PK overcomes multiple drug-resistance mechanisms to increase gentuzumab ozogamicin (GO) sensitivity in AML cells.54 The mechanism whereby PK sensitizes AML cells to chemotherapy activity includes both inhibition of p-glycoprotein–mediated efflux and initiation of mitochondrial apoptosis.54 Our data using MM cells show that PK enhances bortezomib-triggered cytotoxicity in these cells via triggering both intrinsic and extrinsic apoptotic pathways. It is likely that PK increases the intracellular uptake of bortezomib, thereby enhancing its anti-MM activity; however, whether bortezomib is an efflux pump substrate remains under evaluation. Our data further show that JNK activation is an obligatory event during PK + bortezomib–induced apoptosis in MM cells, and these findings are consistent with a known role of JNK during stress response in other cell systems.52 Activated JNK translocates to mitochondria and facilitates the release of cyto-c and Smac; conversely, absence of JNK results in defect in the mitochondrial death signaling cascades, including failure to release Smac and cyto-c. These data indeed confirm the functional consequence of JNK activation during PK + bortezomib–induced MM cell apoptosis and establish that mitochondria are affected by proapoptotic signaling pathways via JNK.
Besides triggering apoptotic signaling pathways, PK + bortezomib also overcomes the major chemoresistance mechanisms in MM cells. For example, PK + bortezomib overcomes Hsp27-mediated bortezomib resistance. Additionally, PK + bortezomib triggers MM cell apoptosis even in the presence of growth and survival factors for MM cells, such as IL-6 or IGF-1, without affecting the viability of normals or BMSCs.
Overall, our data exemplify the role of PBR as an endogenous modulator of mitochondrial apoptosis in MM cells and as a potential therapeutic target. Importantly, a combination of subtoxic doses of PBR antagonist PK-11195 and bortezomib triggers significant apoptosis even in MM cells resistant to bortezomib. The present findings suggest that combination therapy with PK and bortezomib may avoid dose-related toxic effects of bortezomib therapy. Other studies have already shown that PK is nontoxic in nonobese diabetic–severe combined immunodeficiency (NOD-SCID) mice,54 and is well tolerated in humans.55,56 Together, these findings provide a framework for clinical trials of bortezomib with PK to enhance clinical efficacy, reduce toxicity, and overcome resistance to conventional and bortezomib therapy in patients with relapsed refractory MM.
Prepublished online as Blood First Edition Paper, June 24, 2004; DOI 10.1182/blood-2004-02-0547.
Supported by National Institutes of Health grants 50947, CA 78373, IP50 CA100707-01, and P01 CA078378-06; a Doris Duke Distinguished Clinical Research Scientist Award (K.C.A.); a Multiple Myeloma Research Foundation Senior Research Award (D.C.); and The Cure Myeloma Fund.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. Combined PK and bortezomib treatment triggers synergistic anti-MM activity in human MM cell lines and patient MM cells resistant to conventional drugs, without affecting the viability of normal PBMNCs. (A) MTT assays were performed after incubation of RPMI-8226, doxorubicin-resistant Dox-40, or melphalan-resistant (LR-5) MM cell lines with indicated doses of PK + bortezomib for 24 hours. Results are means ± SDs from 3 independent experiments (P < .0002 for all cells lines). (B) CD138+ MM patient cells (patient nos. 1-5) were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours and assessed for apoptosis using DNA fragmentation assays. Values are means ± SDs of triplicate samples (P = .05); experiments were repeated 2 times with similar results. (Ci) bortezomib-resistant SUDHL4 (DHL-4) lymphoma cells were treated with indicated concentrations of PK, bortezomib, or PK + bortezomib for 24 hours, and assessed for viability by MTT assays. Results are means ± SDs of 4 independent experiments (P < .005). (Cii) MM.1S cells were stably transfected with Bcl2 construct; treated with indicated concentrations of PK, bortezomib, or PK + bortezomib for 24 hours; and assessed for viability using MTT assays. Results are means ± SDs of 3 independent experiments (P < .005). (D) Normal lymphocytes from 5 healthy donors were treated with PK (50 μM) + bortezomib (2 nM) for 24 hours, and viability was assessed by an MTT assay. Each donor is indicated by a distinct symbol. Results are the mean ± SD of 3 independent experiments (P = .21 from Jonchkeere-Terpstra [J-T] trend test).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/8/10.1182_blood-2004-02-0547/6/m_zh80200467710002.jpeg?Expires=1769102454&Signature=jLxO4ZO1jtVZBEDkgtFTOYFLAHVSeOLMy-myELLD7IRPgOLBbSxRYuOPvbXPLay9AkQkUlxUHrWVOpGlkV~LV7binG4qzBWWQy74wzvU10pp5QNaQWBxrH9oIlMeVgAt8eFMfokoS~MF9p4AfTYY0cqk39QnxeLbhLJtCUt-Ves1W1IuuPxlDhSYXLrGGdFf0InjBmbpfhCbAjFD2eh5sn3ytarZ4ecKaZ1e-VPCifgcAa3eO8iJbwuwvuzZOr6TNMjTg1Qq2Xq4xMQem1-kfvRbrly5-GNnVwgsZw3IBvATw-NrUBNgkUh2Y03eKbF4LHCpBD9wQe1ZkLquS66A5w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. PK + bortezomib changes mitochondrial membrane potential (Δψm), generation of superoxide (\batchmode \documentclass[fleqn,10pt,legalpaper]{article} \usepackage{amssymb} \usepackage{amsfonts} \usepackage{amsmath} \pagestyle{empty} \begin{document} \(\mathrm{O}_{2}^{-}\) \end{document}), release of mitochondrial proteins Cyto-c and Smac, and activation of caspase-9. (A) MM.1S cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 12 hours; incubated with CMXRos for the last 20 minutes; and analyzed by flow cytometry to assay for alterations in Δψm. Increase in the number of CMXRos-negative cells indicates loss in Δψm. Results are means ± SDs of 3 independent experiments (P < .005). (B) MM.1S cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 12 hours; harvested; stained with membrane permeable dye dihydroethidium (HE) for the last 15 minutes; and analyzed by flow cytometry. Results are means ± SDs of 3 independent experiments (P < .005). Superoxide anions oxidize HE to fluorescent ethidium, permitting analysis by flow cytometry. (C) MM.1S cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours and harvested; cytosolic proteins were separated by 12.5% SDS-PAGE and analyzed by immunoblotting with anti–cyto-c (top panel) or anti-Smac (middle panel) Abs. As a control for equal loading of proteins, filters were also reprobed with antitubulin Ab (bottom panel). Blots are representative of 3 independent experiments. IB indicates immunoblot. (D) MM.1S cells were treated with PK (50 μM), bortezomib (2 nM), or PK + bortezomib for 24 hours. Cytosolic extracts were assayed for protease activity using LEHD-pNA as substrate as per the manufacturer's instructions (colorimetric assay kit; Biovision, Palo Alto, CA). Results are representative of 3 independent experiments (mean ± SD, P < .005).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/8/10.1182_blood-2004-02-0547/6/m_zh80200467710004.jpeg?Expires=1769102454&Signature=iuYVTmgJucZS2AINgGjp1-Zp7-N9gpo1jOwkRoW9qoFGmfWY9SVZol~xXXioysUax3RwJsRrBqu7ae0ZVf07ESyKGplaWKTd07WFJZyW5sE7GWja~pnCsAX5Y1m4rRZeYKFXImMF7epn3JvoXrtyAnQKNth~tEmEtEVoawcoNntWHx0xteWgi2bW0V0z6LDg~1xxDvso2pNw22ntnjksPrS5Q2pKwWVzD1UfMRNtV5BPFn24hNRF34fvNCglQA4MaOuUU8XF0FiAL7NGFMbXL1AzkvF8mrnfQ6txjOAHbaIjwi53D5ZK42n6WUwlcz~Q8Ydv-fuLLwOszDj58o7FyQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal