Abstract
Graft-versus-host disease (GVHD) is characterized by an impairment of mechanisms that underlie the development of immunologic tolerance. Although the cytokine storm associated with GVHD leads to expression of cell surface markers on both effector and regulatory T cells, regulatory CD4+ T cells that play an instrumental role in the maintenance of tolerance appear to uniquely express the Foxp3 transcriptional repressor. Foxp3 mRNA expression was significantly decreased in peripheral blood mononuclear cells from patients with either allogeneic GVHD or autologous GVHD compared with patients without GVHD. Expression of Foxp3 negatively correlated with the severity of GVHD but positively correlated with recent thymic emigrants. The results suggest that defective thymic function contributes to the impaired reconstitution of immune regulatory mechanisms following transplantation. The decrease in regulatory mechanisms after transplantation appears to provide an environment permissive to the development of GVHD. (Blood. 2004;104:2187-2193)
Introduction
Patients with hematologic malignancies can be treated by myeloablative conditioning and transplantation of hematopoietic stem cells (HSCs), which reconstitute the hematopoietic system, and results in a new immune system in the recipient. A major consequence of allogeneic hematopoietic stem cell transplantation (alloSCT) is allogeneic graft-versus-host disease (alloGVHD) in which donor lymphocytes recognize recipient histocompatibility antigens (Ags). Although associated with significant morbidity and mortality, alloGVHD can help to eradicate residual tumor cells by way of a graft-versus-tumor (GVT) effect.1,2 Despite the use of immunosuppressants, including steroids, cyclosporine A (CsA), and mycophenolate mofetil, severe GVHD is still a significant clinical problem. For autologous (auto) SCT patients, the absence of GVHD leads to higher rates of tumor relapse. Administration of CsA following autoSCT, however, can paradoxically elicit a systemic autoimmune syndrome resembling alloGVHD.3-6 This experimental autoaggression syndrome, termed autoGVHD, appears to have significant antitumor activity mediated by autoreactive T cells.4,5 Immunoregulatory mechanisms play a critical role in both alloGVHD and autoGVHD.7-11
Regulatory CD4+ T cells that express the interleukin 2 (IL-2) receptor α-chain (CD25) play a vital role in the maintenance of tolerance to self-Ag and are required for the induction of nonresponsiveness to allo-Ag.12-14 These regulatory cells prevent the activation and proliferation of auto and allo Ag-reactive T cells. Foxp3, which encodes a forkhead/winged helix transcription factor designated Scurfin, was identified as a key regulatory gene required for the development and functional activity of CD4+CD25+ regulatory T cells.15-17 Studies suggest that gene transfer of Foxp3 confers CD4+ T cells with a regulatory T-cell phenotype that ameliorate autoaggression.15,17
CD4+CD25+ regulatory T cells preferentially express Foxp3, cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), glucocorticoid-induced tumor necrosis factor receptor (GITR), and programmed death 1 (PD-1) and produce regulatory cytokines such as IL-4, IL-10, or transforming growth factor β (TGF-β).18 To examine the hypothesis that expression of these regulatory molecules correlates with the development and progression of GVHD, expression of these molecules was temporally examined in patients undergoing allogeneic (allo) bone marrow transplantation (BMT) or autoBMT (with autoGVHD induction by CsA treatment after transplantation). Real-time quantitative polymerase chain reaction (PCR) analysis revealed that Foxp3 and CTLA-4 mRNA expression was significantly decreased, whereas the levels of CD25 and TGF-β mRNA were increased in peripheral blood mononuclear cells (PBMCs) from patients with either alloGVHD or autoGVHD. Additional studies revealed that the severity of alloGVHD was inversely correlated with low Foxp3 expression. However, Foxp3 expression was positively correlated with de novo T-cell development as revealed by the assessment of T-cell receptor (TCR) gene rearrangement excisional circles (TRECs). In vitro studies indicated that specific stimulation of CD4+ T cells with self-Ag induced the up-regulation of Foxp3 expression, and these CD4+ T cells inhibit the proliferative response of CD8+ T cells. Taken together, although the regulatory mechanisms are reduced in GVHD, ex vivo stimulation of CD4+ T cells with self-Ag could provide a potential therapeutic approach to enhance immunoregulatory mechanisms.
Patients, materials, and methods
Patients
All patients gave written informed consent to participate in this study according to a protocol reviewed and approved by the institutional review board of the Johns Hopkins University School of Medicine and Rush University Medical Center. PBMC samples were obtained from 34 patients (18 men and 16 women, aged 19-68 years [median, 41 years]) undergoing alloSCT and from 39 patients (all women with metastatic breast cancer, aged 18-60 years [median, 47 years]) undergoing autoSCT with autoGVHD induction. Bone marrow (BM) was used as a source of stem cells for both alloSCT and autoSCT patients. For the alloSCT patients, the transplantation preparative regimens included either total body irradiation/cyclophosphamide (TBI/CY; n = 15), busulfan/cyclophosphamide (BU/CY; n = 9), TBI/CY/etoposide (n = 2), fludarabine/2 Gy TBI (n = 7), or fludarabine/melphalan/antithymocyte globulin (n = 1).19,20 Five patients received donor lymphocyte infusion (DLI) for relapse after myeloablative transplantation. Twenty-one alloSCT patients received related donor BM, 17 that were HLA-matched and 4 that received haploidentical BM. Thirteen alloSCT patients received HLA-matched unrelated donor BM. The GVHD prophylaxis regimen included CsA/methotrexate (n = 19), tacrolimus/methotrexate (n = 8), and tacrolimus/CY/mycophenolate mofetil (n = 7). The transplantation preparative regimen for the autoBMT group was CY/thiotepa.5,21 AutoGVHD was induced by the intravenous administration of CsA (2.5 μg/kg/d for 28 days; Novartis, Hanover, NJ) beginning on the day of transplantation. Recombinant interferon-γ (IFN-γ; 0.025 μg/m2; National Cancer Institute, Bethesda, MD) was administered subcutaneously every other day from days 7 through 28. One group of control patients (n = 6) underwent autoBMT using the same preparative regimens but without the administration of CsA and IFN-γ. Diagnosis of GVHD was made on the basis of both clinical and histologic criteria.19-21 Peripheral blood (PB) from the BMT patients developing alloGVHD was obtained within 24 to 48 hours of clinical presentation of GVHD and prior to initiation of active therapy. For the alloBMT patients not developing GVHD, PB was obtained in the same time frame after BMT as the patients developing GVHD. For the autoBMT patients, PB was drawn weekly starting from day 5 after transplantation with onset of clinical symptoms occurring within 5 to 26 days after BMT.21,22
Cell isolation
PBMCs were separated with use of density-gradient centrifugation. Monocytes were isolated by plastic adherence and harvested by gentle scraping. CD4+ and CD8+ cell populations were purified with immunomagnetic bead (Dynal Biotech, Oslo, Norway).5 CD4+CD25+ cells or CD4+CD25- cells were sorted by way of a FACSVantage (Becton Dickinson, San Jose, CA). Total RNA and genomic DNA were prepared from 5 × 106 cells with Trizol or DNAzol reagent (Life Technologies, Gaithersburg, MD).
Quantitative real-time PCR
Real-time PCR reactions were performed with use of TaqMan assay (Applied Biosystems Inc. [ABI], Foster City, CA) and PCR amplifications in ABI PRISM 7700 Sequence Detection System (Applied Biosystems) as described.22,23 Threshold cycle (CT) during the exponential phase of amplification was determined by real-time monitoring of fluorescent emission after cleavage of sequence-specific probes by nuclease activity of Taq polymerase. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or RNaseP was used as an internal control gene for mRNA expression or genomic DNA, respectively. Relative transcripts were determined by the formula: 1/2(CTtarget - CTcontrol). CTLA-4, Foxp3, GITR, PD-1, and TREC genes were synthesized with the specific primer sequences (Integrated DNA Technologies, Coralville, IA). PCR primer pairs and probe were as follows: CTLA-4, 5′-CCATGGACACGGGACTCTACAT-3′, 5′-GCACGGTTCTGGATCAATTACATA-3′ (junction between exons 2 and 3), and 5′-FAM-TGCAAGGTGGAGCTCATGTACCCACC-TAMRA-3′; GITR, 5′-TGCAAACCTTGGACAGACTGC-3′ (junction between exons 3 and 4), 5′-ACAGCGTTGTGGGTCTTGTTC-3′, and 5′-FAM-CCAGTTCGGGTTTCTCACTGTGTTCCCT-TAMRA-3′; Foxp3, 5′-GGCACTCCTCCAGGACAG-3′, 5′-GCTGATCATGGCTGGGCTCT-3′, and 5′-FAMATTTCATGCACCAGCTCTGAACGG-TAMRA-3′ (junction between exons 2 and 3); PD-1, 5′-ATCAAAGAGAGCCTGCGGG-3′, 5′-GGTGGGCTGTGGGCACT-3′, and 5′-FAM-AGCTCAGGGTGACAGAGAGAAGGGCATAMRA-3′ (junction between exons 2 and 3). Primers and probe were designed so that amplicons spanned intron/exon boundaries to minimize amplification of genomic DNA. Real-time PCR efficiencies of target (Foxp3, CTLA-4, GITR, and PD-1) and reference (GAPDH) were approximately equal over a concentration of 0.1 to 200 ng total cDNA. PCR primer pairs and probe for TREC24,25 were 5′-CACATCCCTTTCAACCATGCT-3′, 5′-GCCAGCTGCAGGGTTTAGG-3′, and 5′-FAM-ACACCTCTGGTTTTTGTAAAGGTGCCCACTTAMRA-3′. Primers and fluorogenic probes for other targets were from TaqMan kits (ABI).
Gene expression following in vitro stimulation
CD4+ or CD8+ subset (2.5 × 106 cells) was diluted with 1 mL RPMI-1640 culture medium supplemented with 10% auto serum and incubated for 3, 12, and 24 hours in 24-well cell culture plates at 37°C in 5% CO2. Concanavalin A (ConA; Sigma, St Louis, MO) or lipopolysaccharide (LPS; Sigma) were added to final concentration of 0.4 μg/mL or 0.1 μg/mL, respectively. ConA induced maximal induction of cytokine (IFN-γ or IL-4) expression and proliferation of lymphocytes. LPS also induced significant proliferative response of lymphocytes and is produced by intestinal damage associated with GVHD. Responder cells (1 × 104) were cocultured with irradiated 5 × 104 stimulator cells per well plates for mixed lymphocyte reactions (MLRs). T cells were stimulated with specific target peptide presented by dendritic cells (DCs) generated from blood monocyte precursors as described.26 Plastic adherent cells were cultured in RPMI supplemented with 1% auto plasma, 800 U/mL IL-4 (Sigma), and 100 IU/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; Immunex, Seattle, WA) for 4 days. DCs were pulsed with Ag on day 3 after initiation of culture. The cells were washed, and tumor necrosis factor α (TNF-α) was added to the culture medium on day 4 (10 ng/mL; Sigma). The peptide-specific Ags used in these studies included the major histocompatibility (MHC) class II-associated invariant chain peptide (CLIP) N-terminal variant (KPVSKMRMATPLLM; Quality Controlled Biochemicals, Hopkinton, MA), the C-terminal variant (MRMATPLLMQALPM), and the MHC class II binding domain variant (MRMATPLLM) added to the culture at a final concentration of 10 μM. CD4+ T cells (106/well) were stimulated with 5 × 104 Ag-pulsed auto DCs.
Assessment of regulatory T-cell activity
CD8+ cells (1 × 105 cell per well) were stimulated with 100 U/mL IL-2 (Chiron, Emeryville, CA) in the presence of irradiated (3000 rad) auto DCs (5 × 103).27 CD4+Foxp3+ cells (1 × 105) generated from CLIP-pulsed DCs were added to the assay. Cultures were incubated for 72 hours at 37°C and pulsed with [3H] thymidine at 1 μCi (0.037 MBq) per well for the last 8 hours of culture.
Statistical analysis
Data were analyzed by Fisher exact test, Pearson correlation (r), or Student t test with the use of StatView software (version 5; SAS Institute, Cary, NC), with P values less than .05 considered statistically significant.
Results
Immunoregulatory gene expression
Initial studies quantified gene expression in CD4+CD25+, CD4+CD25-, and CD8+ subsets isolated from healthy control individuals. Significant levels of Foxp3, CTLA-4, GITR, PD-1, and IL-4 mRNA transcripts were detected in CD4+CD25+ T cells compared with the CD4+CD25- subset, whereas GITR and IL-4 mRNA transcripts were comparable in CD4+CD25+ and CD8+ subsets (Figure 1A). TGF-β mRNA transcripts were comparable in these subsets. IL-10, IFN-γ, TNF-α, and Fas ligand mRNA transcripts were also comparable in these subsets (data not shown). Levels of IL-18 and perforin were lower in the CD4+CD25+ subset.23 Foxp3 mRNA levels in PBMCs from patients at the onset of alloGVHD and autoGVHD (within 24-48 hours of onset and before active treatment) were significantly decreased compared with patients who did not develop alloGVHD or autoGVHD (2.9-fold or 2.4-fold, P < .01, respectively; Figure 1B). Foxp3 mRNA levels in PBMCs from patients at the onset of alloGVHD and autoGVHD were significantly decreased compared with the healthy control group (n = 10, highlighted by gray zone in Figure 3A; 9.5-fold or 6.0-fold, P < .01, respectively). Foxp3 mRNA levels were not associated with absolute T-cell numbers comparing patients developing alloGVHD or autoGVHD with patients who did not develop alloGVHD or autoGVHD (data not shown). CTLA-4 mRNA levels in PBMCs from patients at the onset of alloGVHD and autoGVHD were significantly decreased compared with the healthy controls (3.1-fold or 2.3-fold, P < 0.01, respectively; data not shown). CTLA-4 expression in patients with autoGVHD was significantly decreased compared with patients without autoGVHD (2.1-fold, P < 0.01; Figure 1D) and correlated with Foxp3 expression in patients undergoing autoBMT (r = 0.71, P < .01). Assessment of CD25, GITR, PD-1, and TGF-β mRNA levels revealed a dichotomy between patient groups (Figure 1C,E-G). Compared with patients who did not develop alloGVHD, expression of CD25 and TGF-β mRNA was significantly increased in patients with alloGVHD (1.9-fold or 1.3-fold, respectively; P < .05). For patients with autoGVHD, CD25 and TGF-β mRNA levels were also significantly elevated than patients without autoGVHD (1.9-fold or 1.4-fold, respectively; P < .05). CD25, PD-1, and TGF-β mRNA levels in PBMCs from patients with alloGVHD were significantly elevated compared with healthy controls (5.2-fold, 6.7-fold, or 2.1-fold, respectively; P < .05).
Immunoregulatory gene expression. (A) Gene expression in CD4+CD25+ subpopulation. A representative experiment (1 of 3) is illustrated. Foxp3 (B), CD25 (C), CTLA-4 (D), GITR (E), PD-1 (F), and TGF-β (G) gene expression from peripheral blood mononuclear cells (PBMCs) of patients with alloGVHD (n = 28) and patients evaluated at the onset of autoGVHD (n = 16). The results include patients without clinical manifestations of alloGVHD (n = 8), patients without autoGVHD (n = 23), or non-CsA-treated autoBMT patients (n = 6). Expression of Foxp3 mRNA transcripts in CD8+ T cells was approximately 20-fold lower compared with the CD4+ T-cell subset. Expression of Foxp3 was not detected in the CD8+CD25- subset. Expression of other markers was similar both in the CD25- and CD25+ fractions of CD4+ T cells. BMT indicates bone marrow transplantation; CsA, cyclosporine A; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; GITR, glucocorticoid-induced tumor necrosis factor receptor; PD-1, programmed death-1; TGF, transforming growth factor. Error bars indicate SEM.
Immunoregulatory gene expression. (A) Gene expression in CD4+CD25+ subpopulation. A representative experiment (1 of 3) is illustrated. Foxp3 (B), CD25 (C), CTLA-4 (D), GITR (E), PD-1 (F), and TGF-β (G) gene expression from peripheral blood mononuclear cells (PBMCs) of patients with alloGVHD (n = 28) and patients evaluated at the onset of autoGVHD (n = 16). The results include patients without clinical manifestations of alloGVHD (n = 8), patients without autoGVHD (n = 23), or non-CsA-treated autoBMT patients (n = 6). Expression of Foxp3 mRNA transcripts in CD8+ T cells was approximately 20-fold lower compared with the CD4+ T-cell subset. Expression of Foxp3 was not detected in the CD8+CD25- subset. Expression of other markers was similar both in the CD25- and CD25+ fractions of CD4+ T cells. BMT indicates bone marrow transplantation; CsA, cyclosporine A; CTLA-4, cytotoxic T lymphocyte-associated antigen-4; GITR, glucocorticoid-induced tumor necrosis factor receptor; PD-1, programmed death-1; TGF, transforming growth factor. Error bars indicate SEM.
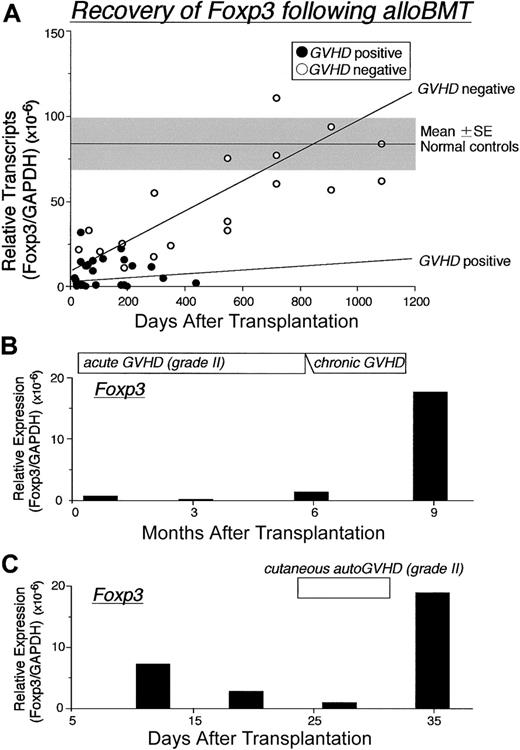
Recovery of Foxp3 expression following BMT. (A) Changes in the Foxp3 mRNA expression in PBMCs from 34 patients following alloSCT. Three of 8 patients without GVHD were serially evaluated at 18, 24, 30, and 36 months after BMT, and patients with GVHD were assessed at time of first diagnosis; (•) indicates GVHD positive; (○), GVHD negative. Changes in Foxp3 expression over time were assessed in PBMCs from 2 representative patients with GVHD following BMT. Gray zone indicates mean ± SE of 10 healthy controls. Regression lines indicate correlation with GVHD (top: negative, bottom: positive). (B) Patient received transplant from a partially HLA-mismatched related donor developed severe continuous alloGVHD. (C) Patient developing autoGVHD after autoBMT and autoGVHD induction treatment. Relative expression of Foxp3 mRNA levels normalized against GAPDH was determined by real-time PCR.
Recovery of Foxp3 expression following BMT. (A) Changes in the Foxp3 mRNA expression in PBMCs from 34 patients following alloSCT. Three of 8 patients without GVHD were serially evaluated at 18, 24, 30, and 36 months after BMT, and patients with GVHD were assessed at time of first diagnosis; (•) indicates GVHD positive; (○), GVHD negative. Changes in Foxp3 expression over time were assessed in PBMCs from 2 representative patients with GVHD following BMT. Gray zone indicates mean ± SE of 10 healthy controls. Regression lines indicate correlation with GVHD (top: negative, bottom: positive). (B) Patient received transplant from a partially HLA-mismatched related donor developed severe continuous alloGVHD. (C) Patient developing autoGVHD after autoBMT and autoGVHD induction treatment. Relative expression of Foxp3 mRNA levels normalized against GAPDH was determined by real-time PCR.
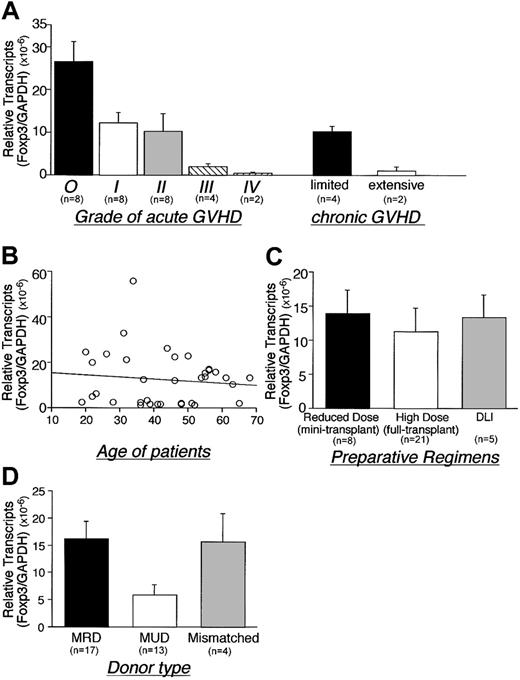
Foxp3 expression inversely correlated with the severity of acute GVHD and with the extent of chronic GVHD (P < .01 or P = .02, respectively; Figure 2A). Patient age and type of preparation had no significant effect on Foxp3 gene expression (Figure 2B-C). Foxp3 expression in patients who received transplants from matched unrelated donors (MUDs) was, however, lower than patients who received transplants from matched related donors (MRDs) (P = .01) (Figure 2D).
Role of GVHD severity, age, preparative regimen, and donor source on Foxp3 expression following alloBMT. Correlation between Foxp3 gene expression and (A) grade of GVHD, (B) age of recipients, (C) preparative regimens, and (D) donor type were for patients undergoing alloBMT (assessed < 500 days after transplantation). The results include 8 cases of grade I, 8 cases of grade II, 4 cases of grade III, and 2 cases of grade IV acute GVHD. Four patients with limited and 2 patients with extensive chronic GVHD were analyzed. GVHD developed in 11 of 17 MRD recipients, 11 of 13 MUD recipients, and 4 of 4 haploidentical donor recipients. Regression analysis demonstrates negative correlation that does not reach significance (B). *Two patients had both acute and chronic GVHD. DLI indicates donor lymphocyte infusion, MRD; matched related donor, MUD; matched unrelated donor. Error bars indicate SEM.
Role of GVHD severity, age, preparative regimen, and donor source on Foxp3 expression following alloBMT. Correlation between Foxp3 gene expression and (A) grade of GVHD, (B) age of recipients, (C) preparative regimens, and (D) donor type were for patients undergoing alloBMT (assessed < 500 days after transplantation). The results include 8 cases of grade I, 8 cases of grade II, 4 cases of grade III, and 2 cases of grade IV acute GVHD. Four patients with limited and 2 patients with extensive chronic GVHD were analyzed. GVHD developed in 11 of 17 MRD recipients, 11 of 13 MUD recipients, and 4 of 4 haploidentical donor recipients. Regression analysis demonstrates negative correlation that does not reach significance (B). *Two patients had both acute and chronic GVHD. DLI indicates donor lymphocyte infusion, MRD; matched related donor, MUD; matched unrelated donor. Error bars indicate SEM.
Temporal analysis revealed that following alloBMT, Foxp3 mRNA transcripts approach normal levels in patients who did not develop GVHD (Figure 3A). Levels of Foxp3 mRNA transcripts remained low for patients who developed GVHD. In accord are the results (Figure 3B), demonstrating barely detectable Foxp3 levels in a patient with a prolonged course of severe alloGVHD. The onset and progression of autoGVHD also paralleled decreasing expression levels of Foxp3 mRNA (Figure 3C). Importantly, a rise in Foxp3 mRNA expression was concurrent with the improvement of both alloGVHD and autoGVHD.
TREC assay
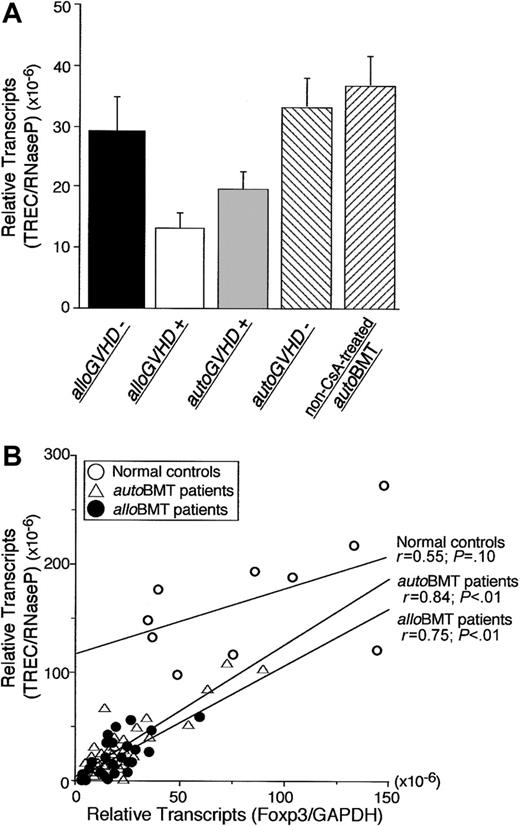
PBMCs from the patients were monitored for TREC levels by real-time PCR following transplantation to estimate recent thymic output of T lymphocytes.24,25 TREC levels in PBMCs from patients with alloGVHD or autoGVHD were significantly decreased compared with patients who did not develop GVHD (2.3-fold and 1.8-fold, P < .01; Figure 4A). TREC levels in PBMCs from patients with alloGVHD or autoGVHD were also significantly decreased compared with healthy controls (12.6-fold and 8.8-fold, P < .01). TRECs correlated with Foxp3 mRNA expression in patients undergoing alloBMT or autoBMT (r = 0.84 or r = 0.75, P < .01, respectively) but not healthy controls (Figure 4B).
Correlation between Foxp3 gene expression and de novo T-cell development. (A) T-cell receptor gene rearrangement excisional circle (TREC) levels from PBMCs of patients without clinical manifestations of alloGVHD (n = 8), patients with alloGVHD (n = 28), patients evaluated at the onset of autoGVHD (n = 16), patients without autoGVHD (n = 23), and non-CsA-treated autoBMT patients (n = 6). Error bars indicate SEM. (B) Correlation between TRECs and Foxp3 expression in PBMCs of healthy controls (n = 10) and patients undergoing alloBMT (assessed < 500 days after transplantation) or undergoing autoBMT with autoGVHD induction. Relative expression of Foxp3 or TRECs was normalized against GAPDH or RNaseP, respectively. Regression lines correspond to coefficient correlations (top: healthy individuals, middle: autoBMT patients, and bottom: alloBMT patients).
Correlation between Foxp3 gene expression and de novo T-cell development. (A) T-cell receptor gene rearrangement excisional circle (TREC) levels from PBMCs of patients without clinical manifestations of alloGVHD (n = 8), patients with alloGVHD (n = 28), patients evaluated at the onset of autoGVHD (n = 16), patients without autoGVHD (n = 23), and non-CsA-treated autoBMT patients (n = 6). Error bars indicate SEM. (B) Correlation between TRECs and Foxp3 expression in PBMCs of healthy controls (n = 10) and patients undergoing alloBMT (assessed < 500 days after transplantation) or undergoing autoBMT with autoGVHD induction. Relative expression of Foxp3 or TRECs was normalized against GAPDH or RNaseP, respectively. Regression lines correspond to coefficient correlations (top: healthy individuals, middle: autoBMT patients, and bottom: alloBMT patients).
Effect of in vitro stimulation on immunoregulatory gene expression
Immunoregulatory gene expression was assessed in CD4+ or CD8+ T cells 3, 12, and 24 hours after ConA or LPS stimulation. CD25, GITR, and PD-1 mRNA expression in CD4+ cells was increased after ConA stimulation, whereas LPS stimulation led to an increase in CD25, CTLA-4, GITR, PD-1, and TGF-β mRNA expression (Table 1). CD25, PD-1, and TGF-β expression was increased after MLR, whereas CTLA-4 expression was decreased. In contrast, Foxp3 expression, at best, was minimally increased after stimulation with either ConA or LPS and MLR. To assess whether Ag-specific stimulation is required for up-regulation of Foxp3 (Table 1, bottom panel), the response to the peptide identified as the target Ag in autoGVHD, CLIP, was used as a model system.5,6 Truncated variants of CLIP that express either the N- or C-terminal flanking regions differentially activate type 1 or type 2 T-cell responses, respectively.28,29 Stimulation of CD4+ T cells with DCs pulsed with C-terminal CLIP markedly induced up-regulation of Foxp3 mRNA. The levels of Foxp3 mRNA were higher than naturally occurring CD4+CD25+ T cells (8- to 16-fold). Additional studies revealed that FoxP3 expression in CD4+CD25+ subset after stimulation with DCs pulsed with C-terminal CLIP was 128-fold higher compared with the CD4+CD25- subset. In comparison, there was a slight increase in Foxp3 mRNA expression following stimulation with DCs pulsed with the N-terminal or MHC class II-binding domain CLIP variants. Levels of CD25, CTLA-4, and GITR mRNA were also enhanced following stimulation of CD4+ T cells with DCs loaded with N-terminal CLIP. Fewer effects on gene expression in CD8+ T cells were obtained after in vitro stimulation, except for CD25.
Effect of in vitro stimulation on immunoregulatory gene expression
. | Foxp3 . | CD25 . | CTLA-4 . | GITR . | PD-1 . | TRF-β . |
|---|---|---|---|---|---|---|
| Mitogen stimulation | ||||||
| ConA | ||||||
| CD4 | 2.7 ± 0.4 | 18.9 ± 2.9* | 6.7 ± 2.1 | 73.1 ± 11.5* | 27.7 ± 7.1* | 1.6 ± 0.6 |
| CD8 | 2.3 ± 0.9 | 23.1 ± 8.6 | 3.2 ± 1.3 | 5.8 ± 2.0 | 28.2 ± 12.0 | 1.6 ± 1.1 |
| LPS | ||||||
| CD4 | 2.9 ± 0.5 | 10.5 ± 1.7* | 97.5 ± 9.7* | 112.0 ± 17.0* | 86.2 ± 14.3* | 6.6 ± 0.9* |
| CD8 | 2.4 ± 1.1 | 60.6 ± 19.8 | 26.6 ± 9.5 | 29.9 ± 12.1 | 6.0 ± 1.9 | 1.3 ± 0.6 |
| Mixed lymphocyte reaction | ||||||
| PBMC | 1.8 ± 0.3 | 26.3 ± 6.7* | 0.5 ± 0.2 | 2.3 ± 0.9 | 3.2 ± 0.8* | 3.7 ± 0.7* |
| Ag stimulation | ||||||
| C-terminal CLIP | ||||||
| CD4 | 226.7 ± 14.8* | 14.8 ± 1.5* | 39.3 ± 5.4* | 880.0 ± 83.0* | 1.8 ± 0.9 | 1.4 ± 0.2 |
| CD8 | 14.8 ± 3.8 | 7.8 ± 2.0 | 1.2 ± 0.4 | 5.9 ± 2.8 | 1.5 ± 0.4 | 1.3 ± 0.3 |
| N-terminal CLIP | ||||||
| CD4 | 28.3 ± 2.6 | 12.0 ± 1.9 | 13.9 ± 2.0 | 223.0 ± 31.0 | 1.2 ± 0.2 | 1.2 ± 0.2 |
| CD8 | 5.2 ± 2.7 | 3.3 ± 1.5 | 0.5 ± 0.2 | 3.4 ± 1.8 | 1.0 ± 0.2 | 0.8 ± 0.2 |
| Class II-binding domain CLIP | ||||||
| CD4 | 23.7 ± 2.0 | 5.3 ± 1.6 | 8.5 ± 1.3 | 118.0 ± 27.0 | 1.1 ± 0.2 | 0.7 ± 0.1 |
| CD8 | 6.2 ± 2.8 | 2.3 ± 0.9 | 0.6 ± 0.3 | 2.2 ± 1.1 | 0.9 ± 0.3 | 0.5 ± 0.1 |
| No peptide | ||||||
| CD4 | 4.1 ± 1.2 | 4.7 ± 1.0 | 15.9 ± 2.5 | 73.1 ± 12.1 | 1.0 ± 0.2 | 0.6 ± 0.2 |
| CD8 | 2.6 ± 1.8 | 1.9 ± 0.9 | 1.0 ± 0.2 | 3.1 ± 1.4 | 1.2 ± 0.3 | 0.6 ± 0.3 |
. | Foxp3 . | CD25 . | CTLA-4 . | GITR . | PD-1 . | TRF-β . |
|---|---|---|---|---|---|---|
| Mitogen stimulation | ||||||
| ConA | ||||||
| CD4 | 2.7 ± 0.4 | 18.9 ± 2.9* | 6.7 ± 2.1 | 73.1 ± 11.5* | 27.7 ± 7.1* | 1.6 ± 0.6 |
| CD8 | 2.3 ± 0.9 | 23.1 ± 8.6 | 3.2 ± 1.3 | 5.8 ± 2.0 | 28.2 ± 12.0 | 1.6 ± 1.1 |
| LPS | ||||||
| CD4 | 2.9 ± 0.5 | 10.5 ± 1.7* | 97.5 ± 9.7* | 112.0 ± 17.0* | 86.2 ± 14.3* | 6.6 ± 0.9* |
| CD8 | 2.4 ± 1.1 | 60.6 ± 19.8 | 26.6 ± 9.5 | 29.9 ± 12.1 | 6.0 ± 1.9 | 1.3 ± 0.6 |
| Mixed lymphocyte reaction | ||||||
| PBMC | 1.8 ± 0.3 | 26.3 ± 6.7* | 0.5 ± 0.2 | 2.3 ± 0.9 | 3.2 ± 0.8* | 3.7 ± 0.7* |
| Ag stimulation | ||||||
| C-terminal CLIP | ||||||
| CD4 | 226.7 ± 14.8* | 14.8 ± 1.5* | 39.3 ± 5.4* | 880.0 ± 83.0* | 1.8 ± 0.9 | 1.4 ± 0.2 |
| CD8 | 14.8 ± 3.8 | 7.8 ± 2.0 | 1.2 ± 0.4 | 5.9 ± 2.8 | 1.5 ± 0.4 | 1.3 ± 0.3 |
| N-terminal CLIP | ||||||
| CD4 | 28.3 ± 2.6 | 12.0 ± 1.9 | 13.9 ± 2.0 | 223.0 ± 31.0 | 1.2 ± 0.2 | 1.2 ± 0.2 |
| CD8 | 5.2 ± 2.7 | 3.3 ± 1.5 | 0.5 ± 0.2 | 3.4 ± 1.8 | 1.0 ± 0.2 | 0.8 ± 0.2 |
| Class II-binding domain CLIP | ||||||
| CD4 | 23.7 ± 2.0 | 5.3 ± 1.6 | 8.5 ± 1.3 | 118.0 ± 27.0 | 1.1 ± 0.2 | 0.7 ± 0.1 |
| CD8 | 6.2 ± 2.8 | 2.3 ± 0.9 | 0.6 ± 0.3 | 2.2 ± 1.1 | 0.9 ± 0.3 | 0.5 ± 0.1 |
| No peptide | ||||||
| CD4 | 4.1 ± 1.2 | 4.7 ± 1.0 | 15.9 ± 2.5 | 73.1 ± 12.1 | 1.0 ± 0.2 | 0.6 ± 0.2 |
| CD8 | 2.6 ± 1.8 | 1.9 ± 0.9 | 1.0 ± 0.2 | 3.1 ± 1.4 | 1.2 ± 0.3 | 0.6 ± 0.3 |
Immunoregulatory gene expression was analyzed following in vitro stimulation of PBMCs from 3 different individuals. CD4+ or CD8+ T cells were incubated for 3, 12, and 24 hours with Concanavalin A (ConA) or lipopolysaccharide (LPS). Time to maximal expression of individual marker transcripts varied. Data presented the maximal transcript levels assessed over 24 hours. In addition, gene expression was evaluated after 5 days of stimulation with MHC unrelated lymphocytes (mixed lymphocyte reaction). CD4+ or CD8+ T cells were also stimulated with Ag-pulsed autologous dendritic cells. Ag peptides used for study included the CLIP N-terminal variant, C-terminal variant, and MHC class II-binding domain variant. Gene expression of Ag-specific T cells was evaluated after 3 days of culture. Fold increases were determined by comparison to unstimulated CD4+ or CD8+ T cells or PBMCs. Data are expressed as mean ± SE.
Significantly greater than control (P < .05)
Suppression assay
In vitro activation of Foxp3+CD4+ regulatory T cells by DCs pulsed with the C-terminal CLIP significantly inhibited the proliferation of CD8+ T cells (> 80%) in response to DCs that express parent CLIP5 (Figure 5). CD4+CD25+ T cells dominantly suppressed the proliferation of CD8+ T cells compared with the CD4+CD25- subset (91% ± 16% versus 12% ± 5%). Modest levels of regulatory activity were observed after activation by DCs pulsed with either the N-terminal or the class II binding domain variants of CLIP. Significant suppression, however, did not occur after stimulation of CD4+ T cells with nonpeptide-loaded DCs.
Suppression of the CD8+ T-cell response. CD8+ T cells (1 × 105) were stimulated with IL-2 (100 U/mL) in the presence of irradiated auto 5 × 103 DCs. CD4+Foxp3+ T cells generated from CLIP-pulsed DCs were added to the assay as suppressor cells at a ratio of 1:1. The cells were cultured 72 hours and pulsed with [3H] thymidine for the 8 hours of culture. Proliferation data are expressed as the mean ± SD from triplicate wells and represent 3 experiments.
Suppression of the CD8+ T-cell response. CD8+ T cells (1 × 105) were stimulated with IL-2 (100 U/mL) in the presence of irradiated auto 5 × 103 DCs. CD4+Foxp3+ T cells generated from CLIP-pulsed DCs were added to the assay as suppressor cells at a ratio of 1:1. The cells were cultured 72 hours and pulsed with [3H] thymidine for the 8 hours of culture. Proliferation data are expressed as the mean ± SD from triplicate wells and represent 3 experiments.
Discussion
Regulatory T cells can be assigned to different subsets according to the expression of cell surface makers and production of cytokines and to mechanisms of action.30-32 CD25 expression on a subset of naturally occurring CD4+ T cells demarcates a regulatory population.12,13 Substantial evidence indicates that donor CD4+CD25+ regulatory T cells can suppress alloGVHD lethality in animal models.7-10 In contrast, increased frequencies of CD25+ T cells in donor grafts are associated with development of GVHD.33 Moreover, the frequencies of CD4+ T cells expressing CD25 are markedly increased in patients who develop chronic alloGVHD.34 Consistent with these observations are the findings that CD25 mRNA expression reflecting active gene transcription is increased in PBMCs from patients who develop either autoGVHD or alloGVHD. The results certainly indicate that CD25 expression is not restricted to regulatory T cells. Expression of GITR and PD-1, molecules associated with regulatory T cells, is not also exclusive. These molecules are expressed in not only CD4+CD25+ T cells but also CD8+ T cells. Expression of GITR and PD-1 as well as TGF-β and IL-1022 is increased in both PBMCs from patients with GVHD and after in vitro stimulation with ConA or LPS. These molecules are likely to be expressed in activated T cells, including CD4+ regulatory T cells. However, CTLA-4 is preferentially expressed in naturally occurring CD4+CD25+ T cells. CTLA-4 expression in PBMCs from patients with either autoGVHD or alloGVHD is significantly lower than healthy controls. However, the levels were comparable in patients without alloGVHD. CTLA-4 expression was increased following LPS stimulation but reduced after allo-Ag stimulation. Hence, discrimination of regulatory and activated effector T cells cannot be based solely on expression of these molecules. Global up-regulation because of the cytokine storm associated with GVHD or infections further complicates discrimination between these T-cell subsets.2
In contrast, the results from the present study reveal that Foxp3 expression may correlate with the regulatory population. Foxp3 expression was significantly decreased in PBMCs from patients who developed both autoGVHD and alloGVHD with levels inversely correlating with the severity of GVHD. Foxp3 expression on regulatory T cells can only be elicited by Ag-specific stimulation. These data resolve an important problem regarding activated and regulatory T-cell subsets. Expression of Foxp3 appears to provide a unique marker to identify and monitor the development of the regulatory population following clinical SCT. Increased expression of Foxp3 appeared to be related to resolution of GVHD in a limited number of patients. Critical examination of larger number of alloSC transplant recipients, however, will be necessary to determine adequately whether increased Foxp3 expression and regulatory function after SCT correlates with the response to therapy. Despite GVHD observed in all 4 HLA-mismatched haploidentical recipients, there was no difference in Foxp3 expression between MRD and haploidentical recipient pairs. However, the number of patients analyzed was small. Studies of Foxp3 levels in larger cohorts of patients randomized among donor type, regimens, or stem cell sources will be also necessary to determine whether there are specific effects on regulatory function.
Of additional importance are the findings that the Foxp3 expression correlates with TREC levels in patients following SCT, indicating that Foxp3+ regulatory cells are most likely derived from a subset of recent thymic emigrants rather than the expansion of preexisting naive T cells that had been infused with the HSCs or HSC-derived thymic precursor cells generated from recipient BM. Alternatively, GVHD leads to destruction of the thymus, with the consequent reduction of TREC levels. Severity of GVHD in the present study was the critical factor associated with decreased Foxp3 levels. MUD recipients, in whom GVHD is more frequent and severe than in MRD recipients, was also associated with low Foxp3 expression. Moreover, increased Foxp3 expression correlated with the resolution of GVHD, implicating that reconstitution of the regulatory system results in the down-regulation of GVHD. In animal models, thymectomized mice are more susceptible to alloGVHD induction compared with euthymic mice.35 It seems likely that thymic dysfunction caused by GVHD compromises the reconstitution of the immune system, including immunoregulatory mechanisms. High-affinity TCR/self-peptide-MHC interactions in the thymus are required for selection of CD25+ regulatory T cells with immunosuppressive potential.36,37 Moreover, the expression of class II MHC on thymic cortical epithelial cells was found to be sufficient for the development of CD4+CD25+ regulatory T cells.38,39 Thus, recognition of self-peptides by the TCR is necessary for the ontogeny and development of regulatory T cells. The findings in the present study that Foxp3 mRNA expression is induced only after stimulation of CD4+ T cells with the self-Ag are consistent with this concept. Walker et al40 demonstrated that stimulation of CD4+CD25- T cells with plate-bound anti-CD3 and soluble anti-CD28 led to enhanced expression of Foxp3 gene. Additional ongoing studies attempted to enhance Foxp3 expression of CD4+ T cells along with soluble anti-CD3. However, stimulation with soluble anti-CD3 alone did not induce expression of Foxp3 (data not shown). Up-regulation of Foxp3 expression most likely requires costimulation in addition to engagement of the TCR. These data strongly suggest that antigen-specific stimulation by professional antigen-presenting cells (APCs), in fact, are required for expression of Foxp3. Furthermore, clonal expansion of T cells was observed as assessed by TCR spectratyping analysis after CLIP-specific stimulation (data not shown). This clonal expansion was also associated with enhanced regulatory T-cell function and enhanced Foxp3 expression, suggesting that a subset of CD4+CD25+ T cells may specifically recognize CLIP-presenting professional APCs. Interestingly, stimulation with allo-Ag did not induce significant up-regulation of Foxp3. This difference may be due to the marked expansion of alloreactive CD4+ T cells that do not have regulatory T-cell activity.
The present study also compares and contrasts autoGVHD and alloGVHD and reveals important insights into the regulation of alloimmune and autoimmune responses after BMT, effects that have the thymus as a common target element. In animal models of alloGVHD, effector T cells attack the thymic microenvironment, destroying the thymic architecture and leading to thymic involution.41-43 The net consequence of thymic damage is the failure of normal thymopoiesis, leading to a marked lymphopenia. Furthermore, negative selection of potentially autoreactive T cells is compromised, leading to the export of effector cells capable of recognizing self-Ag.44,45 Similar processes occur in autoGVHD. The effector T cells arise from HSCs with thymic “maturation” modified by CsA treatment. CsA disrupts the thymic architecture and microenvironment retarding T-cell development46,47 and directly inhibits the clonal deletion of autoreactive T cells.48 The autoreactive T cells further exacerbate the destruction of the thymus. In both settings, there is a failure to produce CD4+Foxp3+ regulatory T cells, providing a permissive environment for immune aggression.
Although the adverse effect of GVHD on regulatory functions in rodents is well established, accurate quantification of regulatory functions in humans is rudimentary. The evidence clearly suggests that the decrease in regulatory mechanisms allows for the development of GVHD. Defective thymic function contributes to the impaired reconstitution of immune regulatory mechanisms after SCT. Early successful treatment of GVHD may reduce thymic damage43 and improve regulatory functions. Alternatively, because Ag-specific stimulation in vitro can lead to the activation of CD4+ regulatory cells, ex vivo activation and expansion of these cells may provide a viable cell-based therapy to control GVHD. Furthermore, because regulatory T cells may specifically suppress GVHD, such an approach may help to limit GVHD but preserve a GVT effect.10
Prepublished online as Blood First Edition Paper, June 1, 2004; DOI 10.1182/blood-2004-03-1040.
Supported by grants from the National Institutes of Health (CA 15396, CA 82583, and AI 52213).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 5. Suppression of the CD8+ T-cell response. CD8+ T cells (1 × 105) were stimulated with IL-2 (100 U/mL) in the presence of irradiated auto 5 × 103 DCs. CD4+Foxp3+ T cells generated from CLIP-pulsed DCs were added to the assay as suppressor cells at a ratio of 1:1. The cells were cultured 72 hours and pulsed with [3H] thymidine for the 8 hours of culture. Proliferation data are expressed as the mean ± SD from triplicate wells and represent 3 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/7/10.1182_blood-2004-03-1040/6/m_zh80190467440005.jpeg?Expires=1769711118&Signature=eCnImCJiYUvyax6mZDVmvD75u9rB7g5BmEeT6DnPd4ESt8QY78Sszmy8EEdqDdjZGWdCOHX9IKMvf6-llMPaMd3czLAfQUd5KH--GYTdMBLGyyhuase~nJalM8bn~pWjoafdTNc5U62AKIOWGrBJnaZorSC8vRtr0HH90QeJEykozHG8oEgFpFj73p-O7h-SBPDzxryuXVKyNVdiMKNh9ciJQs689~nEbqspMsn2I-z95u01ffSKi74qIHcUNNUoHXC05uMI6Y4Faco3at4e2DYRbr4vqS9AtAQS65eu7H87h--c14FQv~MbNWpT7eOlA3LXpV7pA4Veg5jxcwXdQA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal