Abstract
The standard initial therapy for acute graft-versus-host disease (GVHD) is corticosteroids. Daclizumab is a humanized monoclonal antibody against the interleukin 2 (IL-2) receptor expressed on activated T lymphocytes. Because of daclizumab's favorable toxicity profile and response rate in steroid-resistant GVHD, a multicenter, double-blinded, randomized study of corticosteroids with or without daclizumab for initial treatment of acute GVHD was conducted. A total of 102 evaluable subjects of the targeted 166 were enrolled at 5 participating sites. Methylprednisolone at a dose of 2 mg/kg or daily equivalent was given in conjunction with daclizumab 1 mg/kg or placebo on study days 1, 4, 8, and weekly as long as clinically indicated. The groups were balanced for clinical characteristics. GVHD response rates by study day 42 were similar (53% vs 51%; P = .85). The study was halted after a planned interim analysis showed a significantly worse 100-day survival in the group receiving corticosteroids plus daclizumab (77% vs 94%; P = .02). Overall survival at 1 year was also inferior in the combination arm (29% vs 60%; P = .002). Both relapse- and GVHD-related mortality contributed to the increased mortality in the combination group. The combination of corticosteroids and daclizumab should not be used as initial therapy of acute GVHD.
Introduction
Acute graft-versus-host disease (GVHD) is one of the major complications of allogeneic hematopoietic stem cell transplantation (HSCT). Grades II to IV acute GVHD occur in 30% to 50% of matched related donor recipients1-3 and 50% to 70% of unrelated donor recipients.4 Standard first-line treatment consists of methylprednisolone at a dose of 2 mg/kg/d or equivalent. Response rates vary by severity of GVHD: the 1994 consensus conference on acute GVHD grading reported response rates of 63% to 95% for grade II, 17% to 39% for grade III, and 0% to 6% for grade IV.5 In aggregate, approximately 40% to 50% of patients with acute GVHD experience a complete or partial response with primary therapy, whereas 20% to 60% eventually receive salvage treatments.2,3,6
Despite several randomized studies seeking to improve first-line therapy for acute GVHD, standard care remains moderate-dose corticosteroids. Higher initial doses of corticosteroids,7 a more prolonged steroid tapering course,8 and addition of murine or equine anti–T-cell antibodies to standard GVHD therapy9-11 all failed to improve response rates. Despite corticosteroids, acute GVHD remains a detrimental complication of transplantation that is difficult to manage. The 1994 consensus conference on acute GVHD grading reported 100-day survival rates of 78% to 90% with grade I, 66% to 92% with grade II, 29% to 62% for grade III, and 23% to 25% for grade IV.5 The higher mortality rates were directly attributable to acute GVHD or the subsequent immunosuppression from high-dose corticosteroids and other medications required for treatment of GVHD.
Daclizumab (Zenapax) is a 144-kDa humanized immunoglobulin G1 (IgG1) monoclonal antibody that binds specifically to the alpha subunit (p55 alpha, CD25, or Tac subunit) of the human high-affinity interleukin-2 (IL-2) receptor, which is expressed on activated lymphocytes, and inhibits IL-2 binding. Most studies using daclizumab as a prophylactic agent have been conducted in solid organ transplantation where daclizumab appears to decrease the number and severity of rejection episodes without increasing adverse events, infectious complications, or late malignancies.12-15 No anaphylactic reactions have been reported. Low titers of anti-idiotype antibodies to daclizumab have been detected in less than 10% of treated patients but do not affect the efficacy, safety, or serum levels of daclizumab.16
In human HSCT, Przepiorka et al17 published an encouraging phase 2 study of daclizumab efficacy in steroid-refractory acute GVHD with a complete response rate of 47% after 43 days and a 120-day survival of 53%. Anasetti and colleagues18 reported a 40% response rate in 20 patients with steroid-refractory GVHD. Correlative laboratory studies show that circulating lymphocytes were saturated with antibody, but there was only a small decrease in the absolute number of circulating T cells. Since the high-affinity IL-2 receptor is only expressed on activated lymphocytes, it was hypothesized that daclizumab would delete activated alloreactive T cells while sparing resting T cells and allow greater subsequent immunologic recovery than a pan–T-cell antibody.
Based on daclizumab's apparent safety profile and encouraging results in treating steroid-refractory acute GVHD, a randomized, double-blinded, multicenter study was conducted to determine whether addition of humanized anti–IL-2 receptor antibody to initial corticosteroid therapy for GVHD improves outcome.
Patients, materials, and methods
Study design
The study was designed as a randomized, placebo-controlled, double-blinded study of humanized IL-2 receptor antibody (daclizumab) versus placebo added to corticosteroids as initial therapy for acute GVHD. The primary end point of the study was the proportion of patients in each treatment arm who experienced a decrease of acute GVHD overall severity by at least one grade on study day 42 without failing treatment (death, unblinding of study medication because of worsening acute GVHD, or initiation of any secondary treatment for acute GVHD before study day 42). Prespecified secondary end points included mortality by day 100 after transplantation, proportion of complete responses (return to grade 0 acute GVHD), total days of antibiotics and antifungal agents administered within the first 100 days, total steroid dose administered, and incidence of steroid-related complications such as hyperglycemia requiring insulin, steroid myopathy, and psychosis. Subjects were followed for at least one year to assess the incidence of chronic GVHD, overall survival, and relapse rates.
Eligibility requirements included allogeneic transplantation, acute GVHD (skin stage II or overall grades II-IV within the first 100 days of transplantation), and signed informed consent. The institutional review board (IRB) of the Dana-Farber Cancer Institute approved the study, as did the IRBs of the 4 other participating sites. Potential subjects were excluded if they had treatment with corticosteroids at a dose of greater than 1 mg/kg/d methylprednisolone or equivalent within the preceding 7 days, isolated upper gastrointestinal acute GVHD, hypersensitivity to daclizumab or prior therapy with daclizumab, GVHD from donor lymphocyte infusion, or treatment with investigational (non–Food and Drug Administration [FDA]–approved) therapeutics within 30 days of study entry. Randomization was stratified by type of GVHD prophylaxis (T-cell depletion vs other), organ involvement (skin only vs other), donor type (6/6 HLA-matched siblings vs all others), and study site to ensure balance between the groups. However, the analysis was not stratified.
Study methods
Subjects were required to start study medication (daclizumab or placebo) within 24 hours of initial corticosteroid therapy (2 mg/kg methylprednisolone or equivalent). After subjects provided informed consent they were registered and randomized centrally by the Investigational Drug Pharmacy of the Brigham and Women's Hospital. Five institutions enrolled 102 evaluable subjects between January 3, 2001 and September 11, 2003. Daclizumab was given at a dose of 1 mg/kg actual body weight as a 15-minute intravenous infusion on study days 1, 4, 8, and then weekly as indicated up to 100 days after transplantation. Placebo consisted of an identical-appearing saline solution. Discontinuation of study drug was at the discretion of the managing physician and was usually because of acute GVHD resolution or GVHD worsening with subsequent need for salvage therapy. If salvage therapy was indicated, treating physicians could request unblinding of the subject's assignment to allow optimal clinical decision making. The protocol suggested a tapering steroid regimen, but it was not mandated. Supportive care was according to standard institutional practices. Treating physicians assigned GVHD organ stages.
Roche Pharmaceuticals (Nutley, NJ) provided the daclizumab used in the study and financial support for data management but did not participate in study design, study conduct, or data analysis.
Case analysis
After the study was closed, a clinical abstract was prepared by each site on all subjects who either died or failed initial GVHD therapy, with particular attention to GVHD management and circumstances surrounding deaths. Information was requested on whether treatment failure was due to a GVHD flare after initial response, progression, or no response and subsequent medications used to treat GVHD. These abstracts were reviewed by the principal investigator (S.J.L.), blinded whenever possible to treatment assignment. Deaths were considered attributable to GVHD if either GVHD was listed as a primary, secondary, or tertiary cause of death on the case report forms or GVHD was determined to have contributed to the deaths based on interpretation of the abstract.
Biostatistical analysis
All analyses were performed on an intention-to-treat basis. We assumed that 30% of patients receiving corticosteroids and placebo would experience a reduction of at least one level in the grade of their GVHD on study day 42 without additional treatment, death, or unblinding and be classified a success. In addition, we hypothesized that 50% of patients receiving corticosteroids and daclizumab would be considered a success. At a .05 one-sided significance level, we would have 80% power to detect this difference if 166 evaluable subjects were randomized. Sequential monitoring of results and anticipated nonevaluable patients (eg, retraction of the GVHD diagnosis after study entry) caused us to inflate the desired enrollment to 190 subjects. Interim efficacy monitoring used the O'Brien-Fleming boundary.19 Lack of efficacy was assessed using the method of repeated confidence intervals of Jennison and Turnbull.20 The first planned interim analysis took place in October 2002 after 30% of evaluable subjects were enrolled. The second planned interim analysis took place in October 2003 after 50% of evaluable subjects were enrolled and had reached day 100 after transplantation. The study was permanently closed on November 11, 2003 based on the results of the second interim analysis, which included an overall survival analysis.
Results
Subject characteristics
A total of 105 subjects signed informed consent and were enrolled in the trial. Three patients were not evaluable according to prespecified criteria: one received high-dose steroids for diffuse alveolar hemorrhage within 7 days of enrollment; the second never received the study drug because of an administrative issue in one of the pharmacies; and treatment for the third was cancelled before any study medication was given. Subjects' treatment assignments were double-checked twice by the pharmacy prior to analysis.
Table 1 shows the subject characteristics for the 2 arms. The groups were well balanced for patient, donor, and transplant characteristics. In particular, the spectrum of disease risk groups and acute GVHD stages and grades at time of study entry, proportion of nonmyeloablative procedures, and time from graft infusion to study enrollment were similar between the 2 groups. Subjects in both groups received a median of 3 doses of study drug (range, 2-8 doses).
Subject characteristics
. | Randomized treatment arm . | . | . | ||
|---|---|---|---|---|---|
| Baseline characteristics . | Steroids + placebo; n = 49 . | Steroids + daclizumab; n = 53 . | P* . | ||
| Median age, y (range) | 42 (18-59) | 45 (8-65) | .65 | ||
| Institution, no. (%) | .69 | ||||
| Baylor | 14 (29) | 10 (19) | |||
| DFCI/BWH | 25 (51) | 32 (60) | |||
| MINN | 7 (14) | 8 (15) | |||
| OHSU | 3 (6) | 2 (4) | |||
| RPCI | 0 (0) | 1 (2) | |||
| Donor/patient sex, no. (%) | .82 | ||||
| Male/male | 14 (29) | 19 (36) | |||
| Male/female | 13 (27) | 11 (21) | |||
| Female/female | 12 (24) | 11 (21) | |||
| Female/male | 10 (20) | 12 (22) | |||
| Donor/patient CMV, no. (%) | .37 | ||||
| —/— | 17 (35) | 20 (38) | |||
| —/— | 8 (16) | 8 (15) | |||
| —/+ | 13 (27) | 11 (21) | |||
| —/+ | 5 (10) | 13 (24) | |||
| Unknown | 6 (12) | 1 (2) | |||
| Type of transplant, no. (%) | .66 | ||||
| Matched related | 19 (39) | 21 (40) | |||
| Matched unrelated | 20 (41) | 23 (43) | |||
| Mismatched related | 1 (2) | 3 (6) | |||
| Mismatched unrelated | 9 (18) | 6 (11) | |||
| Source of progenitor cells, no. (%) | .61 | ||||
| Bone marrow | 8 (16) | 13 (25) | |||
| PBSC | 35 (72) | 35 (66) | |||
| Cord | 6 (12) | 5 (9) | |||
| Risk group, no. (%)† | .56 | ||||
| Good | 12 (24) | 12 (22) | |||
| Intermediate | 21 (43) | 28 (53) | |||
| Poor | 16 (33) | 13 (25) | |||
| Nonmyeloablative transplant, no. (%) | .56 | ||||
| Yes | 5 (10) | 8 (15) | |||
| No | 44 (90) | 45 (85) | |||
| T-cell depletion, n (%) | 9 (18) | 10 (19) | .99 | ||
| Acute GVHD grade at time of study entry, no. (%) | .47 | ||||
| I | 10 (20) | 11 (21) | |||
| II | 27 (55) | 34 (64) | |||
| III-IV | 12 (25) | 8 (15) | |||
| Organ involvement at time of study entry, no. (%) | |||||
| Skin | 48 (98) | 59 (98) | .99 | ||
| Liver | 16 (33) | 20 (38) | .68 | ||
| Gastrointestinal | 19 (39) | 28 (53) | .17 | ||
| Median time from HSCT to study day 0, d (range) | 20 (8-63) | 23 (6-90) | .36 | ||
. | Randomized treatment arm . | . | . | ||
|---|---|---|---|---|---|
| Baseline characteristics . | Steroids + placebo; n = 49 . | Steroids + daclizumab; n = 53 . | P* . | ||
| Median age, y (range) | 42 (18-59) | 45 (8-65) | .65 | ||
| Institution, no. (%) | .69 | ||||
| Baylor | 14 (29) | 10 (19) | |||
| DFCI/BWH | 25 (51) | 32 (60) | |||
| MINN | 7 (14) | 8 (15) | |||
| OHSU | 3 (6) | 2 (4) | |||
| RPCI | 0 (0) | 1 (2) | |||
| Donor/patient sex, no. (%) | .82 | ||||
| Male/male | 14 (29) | 19 (36) | |||
| Male/female | 13 (27) | 11 (21) | |||
| Female/female | 12 (24) | 11 (21) | |||
| Female/male | 10 (20) | 12 (22) | |||
| Donor/patient CMV, no. (%) | .37 | ||||
| —/— | 17 (35) | 20 (38) | |||
| —/— | 8 (16) | 8 (15) | |||
| —/+ | 13 (27) | 11 (21) | |||
| —/+ | 5 (10) | 13 (24) | |||
| Unknown | 6 (12) | 1 (2) | |||
| Type of transplant, no. (%) | .66 | ||||
| Matched related | 19 (39) | 21 (40) | |||
| Matched unrelated | 20 (41) | 23 (43) | |||
| Mismatched related | 1 (2) | 3 (6) | |||
| Mismatched unrelated | 9 (18) | 6 (11) | |||
| Source of progenitor cells, no. (%) | .61 | ||||
| Bone marrow | 8 (16) | 13 (25) | |||
| PBSC | 35 (72) | 35 (66) | |||
| Cord | 6 (12) | 5 (9) | |||
| Risk group, no. (%)† | .56 | ||||
| Good | 12 (24) | 12 (22) | |||
| Intermediate | 21 (43) | 28 (53) | |||
| Poor | 16 (33) | 13 (25) | |||
| Nonmyeloablative transplant, no. (%) | .56 | ||||
| Yes | 5 (10) | 8 (15) | |||
| No | 44 (90) | 45 (85) | |||
| T-cell depletion, n (%) | 9 (18) | 10 (19) | .99 | ||
| Acute GVHD grade at time of study entry, no. (%) | .47 | ||||
| I | 10 (20) | 11 (21) | |||
| II | 27 (55) | 34 (64) | |||
| III-IV | 12 (25) | 8 (15) | |||
| Organ involvement at time of study entry, no. (%) | |||||
| Skin | 48 (98) | 59 (98) | .99 | ||
| Liver | 16 (33) | 20 (38) | .68 | ||
| Gastrointestinal | 19 (39) | 28 (53) | .17 | ||
| Median time from HSCT to study day 0, d (range) | 20 (8-63) | 23 (6-90) | .36 | ||
DFCI indicates Dana-Farber Cancer Institute; BWH, Brigham and Women's Hospital; MINN, University of Minnesota; OHSU, Oregon Health and Science University; and RPCI, Roswell Park Cancer Institute.
Unknown values were not used in the calculation of the P values
Good risk indicates CML in first chronic phase (CP1), acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) in complete response 1 (CR1), aplastic anemia. Intermediate risk indicates CML in accelerated phase (AP); AML and ALL in CR2 or above; non-Hodgkin lymphoma (NHL), multiple myeloma (MM), Hodgkin disease (HD) in partial response (PR); plus everyone else not good or poor. Poor risk indicates refractory or relapsed disease
GVHD response
The primary end point of the study was acute GVHD response, defined as a decrease of acute GVHD by at least one grade on study day 42 without failing therapy (death prior to study day 42, unblinding of study medication because of worsening acute GVHD, or initiation of any secondary treatment for acute GVHD). Complete data for all enrolled subjects were available. The GVHD response rate was 26 (53%) of 49 for corticosteroids alone compared with 27 (51%) of 53 for combination therapy (P = .85; Table 2); almost all responders had complete responses by day 42 (49% vs 43%, respectively; P = .77). The rate of unblinding in order to guide subsequent therapy was similar between the groups: 18 (37%) of 49 in the corticosteroids alone arm compared with 16 (30%) of 53 in the combination arm. Table 3 presents data for the subjects not responding to initial GVHD treatment. Subjects assigned to corticosteroids and placebo were more likely to fail because of stable GVHD compared with people who failed after study combination treatment, who tended to flare or progress with new organ involvement. Salvage treatments also differed between the groupsAjects randomized to corticosteroids plus placebo were much more likely to be treated with daclizumab for steroid-refractory disease compared with the combination arm that received other agents including denileukin diftitox, infliximab, and sirolimus.
Outcomes
Variable . | Steroids + placebo; n = 49 . | Steroids + daclizumab; n = 53 . | P . |
|---|---|---|---|
| Follow-up of surviving subjects, median mo (range) | 10 (3-30) | 11 (3-28) | NA |
| GVHD response 42 d after enrollment, no. (%) | 26 (53) | 27 (51) | .85 |
| Alive without GVHD on d 100, no. (%) | 32 (65) | 22 (42) | .02 |
| Within the first 100 transplant days | |||
| No. of hospital d (range) | 36 (15-89) | 44 (9-99) | .04 |
| Total days of antibiotics (range) | 13 (0-64) | 14 (0-58) | .42 |
| Total days of antifungal agents (range) | 10 (0-64) | 12 (0-61) | .96 |
| Total days of antiviral agents (range) | 20 (0-64) | 14 (0-49) | .63 |
| Total steroid dose, mg/kg prednisone (range) | 70.8 (16.2-191.5) | 67.7 (18.6-151.3) | .74 |
| Hyperglycemia requiring insulin, no. (%) | 17 (35) | 21 (43) | .53 |
| Steroid myopathy, no. (%) | 5 (10) | 2 (4) | .26 |
| Steroid psychosis, no. (%) | 1 (2) | 2 (4) | .99 |
| Overall survival at d 100 after transplantation, % ± SE | 94 ± 3 | 77 ± 6 | .02 |
| Overall survival at 1 y after transplantation, % ± SE | 60 ± 8 | 29 ± 7 | .002 |
| Disease-free survival at 1 y after transplantation, % ± SE | 56 ± 8 | 25 ± 7 | .005 |
| Cumulative incidence of chronic GVHD, % ± SE | 64 ± 8 | 49 ± 7 | .16 |
| Cumulative incidence of relapse at 1 y, % ± SE | 17 ± 6 | 29 ± 7 | .19 |
Variable . | Steroids + placebo; n = 49 . | Steroids + daclizumab; n = 53 . | P . |
|---|---|---|---|
| Follow-up of surviving subjects, median mo (range) | 10 (3-30) | 11 (3-28) | NA |
| GVHD response 42 d after enrollment, no. (%) | 26 (53) | 27 (51) | .85 |
| Alive without GVHD on d 100, no. (%) | 32 (65) | 22 (42) | .02 |
| Within the first 100 transplant days | |||
| No. of hospital d (range) | 36 (15-89) | 44 (9-99) | .04 |
| Total days of antibiotics (range) | 13 (0-64) | 14 (0-58) | .42 |
| Total days of antifungal agents (range) | 10 (0-64) | 12 (0-61) | .96 |
| Total days of antiviral agents (range) | 20 (0-64) | 14 (0-49) | .63 |
| Total steroid dose, mg/kg prednisone (range) | 70.8 (16.2-191.5) | 67.7 (18.6-151.3) | .74 |
| Hyperglycemia requiring insulin, no. (%) | 17 (35) | 21 (43) | .53 |
| Steroid myopathy, no. (%) | 5 (10) | 2 (4) | .26 |
| Steroid psychosis, no. (%) | 1 (2) | 2 (4) | .99 |
| Overall survival at d 100 after transplantation, % ± SE | 94 ± 3 | 77 ± 6 | .02 |
| Overall survival at 1 y after transplantation, % ± SE | 60 ± 8 | 29 ± 7 | .002 |
| Disease-free survival at 1 y after transplantation, % ± SE | 56 ± 8 | 25 ± 7 | .005 |
| Cumulative incidence of chronic GVHD, % ± SE | 64 ± 8 | 49 ± 7 | .16 |
| Cumulative incidence of relapse at 1 y, % ± SE | 17 ± 6 | 29 ± 7 | .19 |
NA indicates not applicable.
Subjects not responding to initial GVHD treatment
. | Steroids + placebo; n = 23 . | Steroids + daclizumab; n = 26 . |
|---|---|---|
| GVHD response to initial therapy, no. (%) | ||
| Flare, new organ | 0 (0) | 3 (12) |
| Flare, prior organ | 6 (26) | 6 (23) |
| Progression, new organ | 2 (9) | 5 (19) |
| Progression, prior organ | 6 (26) | 9 (35) |
| Stable, no response | 7 (30) | 3 (12) |
| Other reason for treatment failure* | 2 (9) | 0 (0) |
| Second-line GVHD therapies, no. | ||
| Daclizumab | 16 | 4 |
| Denileukin diftitox | 3 | 7 |
| Mycophenolate mofetil | 14 | 11 |
| Infliximab | 1 | 4 |
| Rapamycin | 1 | 5 |
| Antithymocyte globulin | 1 | 0 |
. | Steroids + placebo; n = 23 . | Steroids + daclizumab; n = 26 . |
|---|---|---|
| GVHD response to initial therapy, no. (%) | ||
| Flare, new organ | 0 (0) | 3 (12) |
| Flare, prior organ | 6 (26) | 6 (23) |
| Progression, new organ | 2 (9) | 5 (19) |
| Progression, prior organ | 6 (26) | 9 (35) |
| Stable, no response | 7 (30) | 3 (12) |
| Other reason for treatment failure* | 2 (9) | 0 (0) |
| Second-line GVHD therapies, no. | ||
| Daclizumab | 16 | 4 |
| Denileukin diftitox | 3 | 7 |
| Mycophenolate mofetil | 14 | 11 |
| Infliximab | 1 | 4 |
| Rapamycin | 1 | 5 |
| Antithymocyte globulin | 1 | 0 |
One subject had mycophenolate mofetil added as a steroid-sparing agent before study day 42; a second subject was unblinded, although GVHD was improving
Other treatment effects
The spectrum of grade 3, 4, and 5 toxicities of the renal/bladder, pulmonary, neurologic, and cardiac systems and infections were not different between the 2 arms (data not shown). Seventy-eight percent of subjects in the corticosteroids alone arm and 75% in the combination arm experienced at least one grade 3 toxicity. The rates of hyperglycemia requiring insulin, steroid myopathy, and steroid psychosis were similar. Total days of antibiotic, antifungal, and antiviral treatment were similar between the groups, and the rate of grade 4 or 5 infections was similar (16% with corticosteroids alone vs 25% with combination therapy; P = .34). Subjects on the combination arm spent more days in the hospital during the first 100 days after transplantation (median, 44 days versus 36 days; P = .04).
Overall survival and causes of death
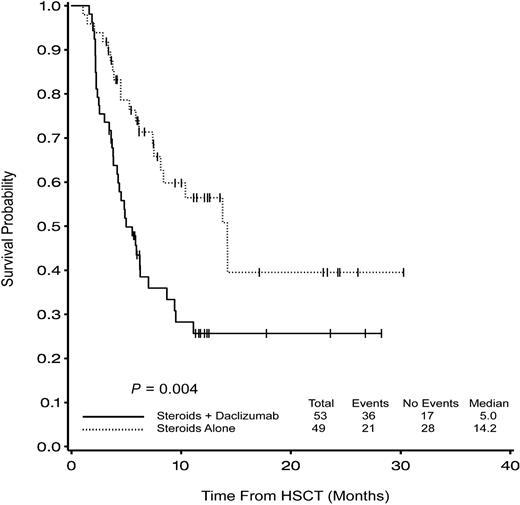
One hundred–day survival was a prespecified secondary end point and was statistically worse in the combination arm (77% vs 94%; P = .02) as was overall survival (log rank; P = .002). The hazard ratio (HR) comparing combination therapy to corticosteroids alone was 2.4 (95% confidence interval [CI], 1.3, 4.2). In other words, the risk of death for subjects randomized to combination therapy was more than 2 times higher than for subjects randomized to corticosteroids and placebo (Figure 1; Table 2). Disease-free survival showed a similar pattern (56% versus 25% at 1 year; P = .005; Figure 2). Nine subjects in the steroids alone group relapsed a median of 6.2 months after transplantation compared with 14 subjects in the combination arm at a median of 3.9 months (P = .42). The cumulative incidence of relapse at one year with death considered a competing risk was 17% for the corticosteroids alone arm and 29% for the combination arm (P = .19). The distribution of timing and causes of death in the 2 groups are listed in Table 4. Disease-related and GVHD-related deaths occurred in 8% and 10%, respectively, of patients randomized to corticosteroids alone compared with 23% and 28% in the combination arm. Deaths not related to relapse or GVHD occurred in 18% of people on the corticosteroids alone arm compared with 11% in the combination arm; no predominant other cause could be identified.
Probability of overall survival is better in the corticosteroids alone arm at 100 days (94% vs 77%; P = .02) and 1 year (60% vs 26%; P = .002).
Probability of overall survival is better in the corticosteroids alone arm at 100 days (94% vs 77%; P = .02) and 1 year (60% vs 26%; P = .002).
Timing and cause of death
Variable . | Steroids + placebo; n = 49 (%) . | Steroids + daclizumab; n = 53 (%) . |
|---|---|---|
| Currently alive, no. (%) | 31 (63) | 18 (34) |
| Timing of death, no. (%) | ||
| Within 100 d of transplantation | 3 (6) | 12 (23) |
| 100 d to 1 y after transplantation | 13 (27) | 22 (42) |
| More than 1 y after transplantation | 2 (4) | 1 (2) |
| Cause of death, no. (%) | ||
| Disease related | 4 (8) | 14 (26) |
| GVHD related | 5 (10) | 15 (28) |
| Not due to disease or GVHD | 9 (18) | 6 (11) |
Variable . | Steroids + placebo; n = 49 (%) . | Steroids + daclizumab; n = 53 (%) . |
|---|---|---|
| Currently alive, no. (%) | 31 (63) | 18 (34) |
| Timing of death, no. (%) | ||
| Within 100 d of transplantation | 3 (6) | 12 (23) |
| 100 d to 1 y after transplantation | 13 (27) | 22 (42) |
| More than 1 y after transplantation | 2 (4) | 1 (2) |
| Cause of death, no. (%) | ||
| Disease related | 4 (8) | 14 (26) |
| GVHD related | 5 (10) | 15 (28) |
| Not due to disease or GVHD | 9 (18) | 6 (11) |
The observed treatment effect on survival was isolated to subjects who entered the study with overall GVHD grades II-IV (HR, 3.1; 95% CI, 1.6, 6.0) and the effect was similar in grade II versus grades III-IV. There was no treatment effect among subjects entering with grade I (skin stage II) overall (HR, 1.0; 95% CI, 0.3, 3.4), and exclusion of these subjects did not change conclusions. Only one subject entered with grade IV GVHD. Univariate and multivariate modeling was performed to investigate possible associations between patient characteristics and group effects. The candidate variables were female donor/male patient versus other sex combinations, patient age, cytomegalovirus (CMV)–negative donor and patient status, related versus unrelated donor, good/intermediate disease risk versus poor risk, and myeloablative versus nonmyeloablative preparative therapy. No characteristics changed the significance of treatment group on overall survival. There was no evidence of center effects either in GVHD response rates or overall survival, as assessed by interaction terms for the 2 centers enrolling the largest number of subjects.
Exploratory subset analyses showed that results were consistent with the findings in the overall population. Within the group of GVHD responders (n = 53), the HR comparing combination therapy versus corticosteroids alone was 3.1 (95% CI, 1.2, 8.2; P = .02), whereas it was 2.1 (95% CI, 1.0, 4.3; P = .05) within the group of 49 nonresponders. Eliminating the GVHD-related deaths (n = 20), the HR for death with combination therapy was 2.3 (95% CI, 1.1, 4.5; P = .02).
Chronic GVHD
We evaluated the incidence of chronic GVHD via 2 methods. In neither analysis was there a statistically significant difference. If we censored patients at time of death and calculated a Kaplan-Meier statistic for comparison with prior reports, the rates would be similar in each group and within the range of historic reports (77% vs 76%; P = .4) The cumulative incidence of chronic GVHD at one year was 64% in the corticosteroids alone arm and 49% in the combination arm (P = .16; Figure 3).
Cumulative incidence of chronic GVHD considering death as a competing event.
Discussion
In this randomized, placebo-controlled, multicenter study of 102 evaluable subjects, we found that the addition of daclizumab to corticosteroids did not improve response rates compared with corticosteroids alone for initial treatment of acute GVHD. Moreover, subjects randomized to combination therapy had a statistically inferior overall survival. The higher mortality rate in the combination arm was due to both GVHD- and relapse-related deaths. Based on these results, the combination of corticosteroids and daclizumab should not be used as initial therapy for acute GVHD.
The finding that daclizumab therapy was associated with an adverse outcome in patients with previously untreated acute GVHD was surprising as there is little in the HSCT literature to suggest any effect on relapse or nonrelapse mortality. A single randomized study of 101 patients evaluating prophylactic use of a rat monoclonal IL-2 receptor antibody did show a higher rate of relapse in patients receiving antibody, although overall survival was the same.21 Other studies, including one using the same antibody22 and another using daclizumab,23 did not find a difference in relapse rates.24,25 A randomized study, reported in abstract form, of daclizumab added as GVHD prophylaxis in unrelated donor HSCT did not detect any difference in GVHD rates, GVHD severity, or overall and disease-free survival.23 Nevertheless, our findings strongly suggest daclizumab had a profound negative impact on survival contributed to in part by increases in disease relapse and GVHD-related mortality. The rate of relapse in the combination therapy arm was approximately twice that seen in the corticosteroids-alone arm, raising the concern that blockade of the high-affinity IL-2 receptor at the time of evident alloimmunity may interfere with the graft-versus-tumor response.
It is difficult to propose a single unifying hypothesis that can explain the increased rates of both relapse and nonrelapse mortality while producing equivalent GVHD control rates. However, the IL-2 receptor targeted by daclizumab is expressed both on antigen-activated conventional T cells (thought responsible for tumor control, graft rejection,26,27 GVHD, and protection from infectious agents28,29 ) and on so-called CD4+CD25+ T regulatory cells (thought to be important in down-regulating the immune system and providing protection from GVHD).30,31 Despite undetectable changes in T-cell number and cell surface markers, in vitro studies suggest that IL-2 receptor antibodies inhibit IL-12–independent interferon-γ production.32 The observed clinical effects could occur with daclizumab treatment if CD25+ blockade crippled conventional T cells enough to interfere with graft-versus-tumor effects while inhibition of CD4+CD25+ activity allowed residual conventional T cells to perpetuate GVHD. Some evidence from murine studies suggests that CD4+CD25+ cells are involved in protection from GVHD but leave tumor immunity intact.31 Unfortunately, corollary biologic studies were not incorporated into our trial to address some of these hypotheses.
The similarity in GVHD control rates with approximately 50% of subjects responding in both arms suggests that there is no apparent benefit for increased immunosuppression in this particular form in the treatment of new onset acute GVHD. The GVHD response rate is consistent with prior literature, suggesting that the study population did not differ significantly from those of other studies or between the 2 randomized arms of this trial.6
Our findings have significance beyond the recognition that daclizumab should not be added to corticosteroids for the initial treatment of acute GVHD due to an unexplained deleterious effect compared with corticosteroids alone. Our results should send a cautionary message to practitioners considering use of powerful immune-suppressing combinations without proper evaluation with randomized controlled trials.
Prepublished online as Blood First Edition Paper, May 11, 2004; DOI 10.1182/blood-2004-03-0854.
Supported in part by grant P01 HL070149 from the National Heart, Lung, and Blood Institute and by Roche Pharmaceuticals.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank their colleagues who facilitated this study. Qiheng Yang helped with database management.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal