Abstract
Severe anemia is one of the major complications of malaria in Africa. We studied 2 populations, one in a village and the second in a periurban area in Mali, to understand the preventable factors in the disease. The 2 correlates of disease were parasitemia above 100 000 parasitized red blood cells per microliter (0.1 × 1012/L) and a low baseline hemoglobin level. All cases of moderate to severe anemia occurred in children under 3.2 years of age. Raising the baseline hemoglobin level and lowering peak parasitemia in infants and young children may reduce the incidence of severe anemia resulting from malarial infection. (Blood. 2004;104: 1198-1200)
Introduction
Severe anemia (hemoglobin level < 50 g/L [< 5 g/dL]) in malaria causes increased mortality in African children, especially when associated with dyspnea (respiratory distress), sepsis, and cerebral malaria.1 If severe anemia presents with respiratory distress, chemotherapy alone is not adequate; transfusion lessens mortality2 but has the problem of availability and exposure to blood-borne pathogens, including HIV and hepatitis B and C. Although the pathogenesis of severe malarial anemia is multifactorial, the major preventable factors have not been adequately identified. The clearest part of the equation is bone marrow suppression (dyserythropoiesis), which was carefully characterized in 19803 ; however, transient suppression of bone marrow without associated hemolytic anemia cannot explain the sudden drop in hemoglobin level that is characteristically seen in malarial infections. Thus, an important unanswered question is whether the hemolytic anemia that accompanies the bone marrow suppression in malarial infection is caused by high parasitemia or is related to another mechanism, such as immune-mediated red blood cell (RBC) destruction. In a study in Kenya, severe anemia was associated with a drop in RBC CR1 and CD55 and a rise in immune complexes on the RBC surface.4 No difference in parasitemia was observed between the children with a drop in hemoglobin level below 50 g/L (5 g/dL) and the control group with symptomatic malaria (fever and parasitemia) in the absence of severe anemia.4 In the present report, factors associated with moderate to severe anemia in children infected with malaria were determined in a longitudinal village-based study.
Study design
A longitudinal study of malaria in 397 (1999) and 398 (2000) subjects ages 6 months to 20 years in the village of Donéguébougou and the periurban setting of Sotuba in Mali presented the opportunity to study factors that may contribute to moderate to severe anemia (hemoglobin level < 60 g/L [< 6 g/dL]) in the setting of malarial infection. The transmission in both study sites is seasonal (from June to November). The intensity of transmission (number of infectious bites per person during the transmission season) determined by spray catch/human landing catch5 was 21.1/167 and 19.2/137.3 in Donéguébougou and 0.74/12.2 and 0.28/3.64 in Sotuba for 1999 and 2000, respectively. The hemoglobin level was determined by hemoglobin analyzer (hemacue, Labnet International, Buckley, WA) and the parasitemia (parasites/μL) was determined on a Giemsa-stained thick film (parasites per 300 leukocytes, assuming a leukocyte count of 7.5 × 109/L (7500/μL). The children were seen weekly from July to December 31 for evaluation of symptoms and axillary temperature. Any child who was sick either during the visit or between visits was evaluated clinically, including a malarial blood film. Baseline hemoglobin level and parasitemia were determined at the beginning of study and monthly. The study was approved by institutional review boards in Mali and at the National Institutes of Health, and informed consent was obtained for all subjects.
Results and discussion
Moderate to severe anemia (defined as hemoglobin level < 60 g/L [< 6 g/dL]) occurred in 6 children in Donéguébougou and in 2 children in Sotuba (Table 1). All cases occurred in children younger than 3.2 years of age from a total study population of 63 and 48 children in this age group in Donéguébougou and Sotuba, respectively. This is the same age distribution of anemia seen in cases from Gambia,6 where the intensity of transmission is similar to that of Donéguébougou. Of the 9 episodes of severe anemia (2 in the same child), 6 were associated with high parasitemia (> 0.1 × 1012/L infected RBCs [> 100 000 infected RBCs/μL]). The hemoglobin level in children with high parasitemia continued to fall after
Moderate to severe anemia in malaria
. | . | . | . | . | . | . | . | . | Hb, g/L . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Age, y . | Sex . | Site . | Hb type . | G-6-PD . | Infected RBCs × 106/L (%)* . | Treatment . | Treatment failure . | Baseline . | At treatment . | Lowest . | Time to lowest . | |||
| 1 | 0.7 | M | D | AA | Normal | 3 500 (—) | P/Su | No | 95 | 53 | 53 | 0 d | |||
| 2 | 1.0 | M | D | AA | Normal | 509 000 (30.6) | Q | No | 94 | 53 | 43 | 3 d | |||
| 3 | 1.0 | F | D | AS | Normal | 176 000 (6.9) | Q | ? | 104 | 82 | 59 | 3 d | |||
| 4A† | 2.0 | F | D | AA | Normal | 214 000 (8.9) | P/Su | No | 96 | 77 | 44 | 3 d | |||
| 4B† | 2.2 | F | D | AA | Normal | 145 000 (4.9) | Q | No | 96 | 95 | 54 | 7 d | |||
| 5 | 2.5 | F | D | AA | Normal | 264 000 (9.6) | P/Su | No | 104 | 88 | 48 | 7 d | |||
| 6 | 3.2 | F | D | AA | Normal | 15 025 (—) | None | — | 86 | — | 53 | — | |||
| 7 | 1.6 | M | S | AC | Normal | 170 000 (6.6) | C | Yes | 89 | 82 | 49 | 14 d | |||
| 8 | 2.0 | F | S | AA | Low | 9 600(—) | P/Su,C | No | 89 | 83 | 52 | 14 d | |||
. | . | . | . | . | . | . | . | . | Hb, g/L . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient . | Age, y . | Sex . | Site . | Hb type . | G-6-PD . | Infected RBCs × 106/L (%)* . | Treatment . | Treatment failure . | Baseline . | At treatment . | Lowest . | Time to lowest . | |||
| 1 | 0.7 | M | D | AA | Normal | 3 500 (—) | P/Su | No | 95 | 53 | 53 | 0 d | |||
| 2 | 1.0 | M | D | AA | Normal | 509 000 (30.6) | Q | No | 94 | 53 | 43 | 3 d | |||
| 3 | 1.0 | F | D | AS | Normal | 176 000 (6.9) | Q | ? | 104 | 82 | 59 | 3 d | |||
| 4A† | 2.0 | F | D | AA | Normal | 214 000 (8.9) | P/Su | No | 96 | 77 | 44 | 3 d | |||
| 4B† | 2.2 | F | D | AA | Normal | 145 000 (4.9) | Q | No | 96 | 95 | 54 | 7 d | |||
| 5 | 2.5 | F | D | AA | Normal | 264 000 (9.6) | P/Su | No | 104 | 88 | 48 | 7 d | |||
| 6 | 3.2 | F | D | AA | Normal | 15 025 (—) | None | — | 86 | — | 53 | — | |||
| 7 | 1.6 | M | S | AC | Normal | 170 000 (6.6) | C | Yes | 89 | 82 | 49 | 14 d | |||
| 8 | 2.0 | F | S | AA | Low | 9 600(—) | P/Su,C | No | 89 | 83 | 52 | 14 d | |||
Hb indicates hemoglobin; D, Donéguébougou; —, not done; P/Su, pyrimethamine/sulfadoxine (Fansidar); Q, quinine; ?, unknown; S, Sotuba; and C, chloroquine.
Infected RBCs at time of treatment, expressed as number/μL blood and as percent infected (estimated from hemoglobin and number per μL).
Same patient at 2 years (A) and 2 months later (B).
1199 treatment, reaching a nadir within 7 days in 5 of 6 episodes, similar to the observations in a study performed in Thailand.7 The relative risk of moderate to severe anemia (hemoglobin level < 60 g/L [< 6 g/dL]) in children younger than 3 years of age who had fever and parasitemia more than 0.1 × 1012/L (> 100 000/μL) compared with those who had fever and parasitemia between 0.0025 and 0.1 × 1012/L (2500-100 000/μL) was 9.5 (95% CI, 2.4-37.3; P = .001). In a multivariate analysis, hyperparasitemia in children younger than 3 years at the beginning of the study year, when adjusted for baseline hemoglobin level, remained significantly associated with hemoglobin level less than 60 g/L (6 g/dL) (RR = 6.3; 95% CI = 1.49-26.7; P = .012). A similar correlation between admission parasitemia and the level of anemia has been described in Thailand7 and Tanzania.8 Previous studies have demonstrated a reduction in the incidence of severe and moderate to severe anemia by 50% or more in the setting of intermittent treatment with antimalarial drugs,9,10 although rebound of high parasitemia occurred after discontinuation of therapy.11
Why did severe anemia not develop in the 14 other children younger than 3.2 years of age who had parasitemia equal to or more than 0.1 × 1012/L (≥ 100 000/μL) (Table 2)? The children without severe anemia had a higher baseline hemoglobin level (average of 3 highest hemoglobin determinations) than those who developed severe anemia (11.3 ± 0.19 [SE] and 9.7 ± 0.25 [SE], respectively; P < .001). These children also had a significantly higher mean hemoglobin levels at the time of treatment (10.2 ± 0.17 [SE]) than the children who had severe anemia (7.95 ± 0.59 [SE]; P < .001). Furthermore, the baseline hemoglobin level was signifi-cantly associated with a hemoglobin level less than 60 g/L (6 g/dL) (RR = 0.60; 95% CI, 0.46-0.80; P = < .001). As in the severely anemic group, hemoglobin levels fell in most children (11 of 14) following treatment and reached a nadir (between 76 and 97 g/L [7.6 and 9.7 g/dL]) within 7 days of the initiation of therapy. Thus, the major differences between the 2 groups of children with high parasitemia were the baseline hemoglobin level and the hemoglobin level at the time of treatment. The lower hemoglobin level at the time of treatment in the children who had severe anemia may reflect higher parasitemia during the time leading up to treatment. The lower baseline hemoglobin level that predisposes these children to severe anemia may result from repeated malarial infections and iron deficiency.
Children younger than 3 years who have parasitemia above 100 000 × 106/L without developing a hemoglobin level less than 60 g/L (6 g/dL)
. | . | . | . | . | . | . | . | . | Hb, g/L . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient identifier . | Age, y . | Sex . | Site . | Hb type . | G-6-PD . | Infected RBCs × 106/L (%) . | Treatment . | Treatment failure . | Baseline . | At treatment . | Lowest . | Time to lowest . | |||
| 111015 | 0.63 | M | D | AA | Normal | 119 000 (3.7) | P/Su | No | 109 | 104 | 92 | 3 d | |||
| 110997 | 1.1 | F | D | AC | Normal | 204 000 (6.3) | Q,C | Yes | 107 | 103 | 83 | 11 d | |||
| 110730 | 1.5 | M | D | AA | Normal | 110 000 (3.6) | Q | No | 114 | 98 | 84 | 14 d | |||
| 110991 | 1.4 | M | D | AA | Normal | 196 000 (5.6) | P/Su | No | 103 | 112 | 82 | 3 d | |||
| 110272 | 1.8 | F | D | AA | Unknown | 108 000 (3.3) | P/Su | Yes | 125 | 106 | 91 | 3 d | |||
| 110978 | 2.3 | M | D | AA | Normal | 110 000 (3.2) | Q | No | 116 | 110 | 95 | 7 d | |||
| 110251 | 2.5 | M | D | AA | Normal | 246 000 (8.4) | P/Su | No | 124 | 94 | 97 | 7 d | |||
| 110609 | 2.9 | M | D | AA | Normal | 205 000 (6.8) | P/Su | No | 112 | 96 | 88 | 3 d | |||
| 110070 | 2.9 | M | D | AA | Normal | 134 000 (4.6) | P/Su | Yes | 113 | 93 | 82 | 3 d | |||
| 110305 | 2.9 | M | D | AA | Normal | 123 100 (3.6) | P/Su | No | 105 | 107 | 96 | 3 d | |||
| 110968 | 2.4 | M | D | AA | Normal | 121 875 (3.9) | Q | No | 123 | 100 | 89 | 3 d | |||
| 110983 | 0.8 | M | D | AA | Normal | 103 050 (3.3) | P/Su | No | 106 | 101 | 76 | 7 d | |||
| 210029 | 2.3 | F | S | AS | Normal | 149 000 (5.1) | P/Su | Yes | 107 | 92 | 81 | 7 d | |||
| 210286 | 2.8 | M | S | AA | Normal | 127 000 (3.8) | P/Su | No | 113 | 108 | 80 | 14 d | |||
. | . | . | . | . | . | . | . | . | Hb, g/L . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient identifier . | Age, y . | Sex . | Site . | Hb type . | G-6-PD . | Infected RBCs × 106/L (%) . | Treatment . | Treatment failure . | Baseline . | At treatment . | Lowest . | Time to lowest . | |||
| 111015 | 0.63 | M | D | AA | Normal | 119 000 (3.7) | P/Su | No | 109 | 104 | 92 | 3 d | |||
| 110997 | 1.1 | F | D | AC | Normal | 204 000 (6.3) | Q,C | Yes | 107 | 103 | 83 | 11 d | |||
| 110730 | 1.5 | M | D | AA | Normal | 110 000 (3.6) | Q | No | 114 | 98 | 84 | 14 d | |||
| 110991 | 1.4 | M | D | AA | Normal | 196 000 (5.6) | P/Su | No | 103 | 112 | 82 | 3 d | |||
| 110272 | 1.8 | F | D | AA | Unknown | 108 000 (3.3) | P/Su | Yes | 125 | 106 | 91 | 3 d | |||
| 110978 | 2.3 | M | D | AA | Normal | 110 000 (3.2) | Q | No | 116 | 110 | 95 | 7 d | |||
| 110251 | 2.5 | M | D | AA | Normal | 246 000 (8.4) | P/Su | No | 124 | 94 | 97 | 7 d | |||
| 110609 | 2.9 | M | D | AA | Normal | 205 000 (6.8) | P/Su | No | 112 | 96 | 88 | 3 d | |||
| 110070 | 2.9 | M | D | AA | Normal | 134 000 (4.6) | P/Su | Yes | 113 | 93 | 82 | 3 d | |||
| 110305 | 2.9 | M | D | AA | Normal | 123 100 (3.6) | P/Su | No | 105 | 107 | 96 | 3 d | |||
| 110968 | 2.4 | M | D | AA | Normal | 121 875 (3.9) | Q | No | 123 | 100 | 89 | 3 d | |||
| 110983 | 0.8 | M | D | AA | Normal | 103 050 (3.3) | P/Su | No | 106 | 101 | 76 | 7 d | |||
| 210029 | 2.3 | F | S | AS | Normal | 149 000 (5.1) | P/Su | Yes | 107 | 92 | 81 | 7 d | |||
| 210286 | 2.8 | M | S | AA | Normal | 127 000 (3.8) | P/Su | No | 113 | 108 | 80 | 14 d | |||
Abbreviations and treatments are explained in Table 1.
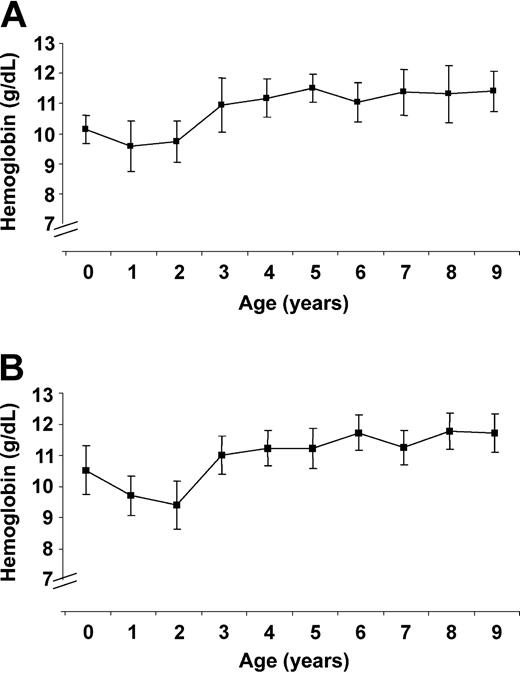
To further evaluate the factors in severe anemia, we looked at baseline hemoglobin levels in children younger than 10 years of age. The hemoglobin level dropped after infancy and then rose after age 3 to a plateau at age 5 years (Figure 1A-B). In a placebo-controlled study comparing the effect of chloroquine prophylaxis for malaria from birth to age 3 years, the hemoglobin level in the control group fell below that in the treatment group to hemoglobin levels similar to the levels in present study.12 This study12 and the present one indicate that baseline hemoglobin level is affected by malaria. The rise in baseline hemoglobin level at age 3 in the present study suggests that children younger than age 3 are affected differently by malaria than older children, because high parasitemia (> 0.1 × 1012/L [> 100 000/μL]) continued to occur in Sotuba and Denégué bougou children after the age of 3 years without severe anemia.
Baseline hemoglobin levels. (A) The baseline hemoglobin level (bars, 95% CI) in Donéguébougou during 1999 and 2000. (B) The baseline hemoglobin level (bars, 95% CI) in Sotuba during 1999 and 2000.
Baseline hemoglobin levels. (A) The baseline hemoglobin level (bars, 95% CI) in Donéguébougou during 1999 and 2000. (B) The baseline hemoglobin level (bars, 95% CI) in Sotuba during 1999 and 2000.
Three children developed severe anemia without high parasitemia. One child had no parasitemia above 0.006 × 1012/L (> 6000/μL) on 3 blood films during the 2 weeks before treatment; another had a parasitemia of 0.15 × 1011/L (15 000/μL). The lowest hemoglobin level in both of these children was 53 g/L (5.3 g/dL). One child's level rose immediately after treatment. The failure to evaluate and treat the other child was an oversight because the protocol dictated a complete evaluation for the presence of severe anemia even in an asymptomatic child. Fortunately, the child's hemoglobin level returned to baseline without treatment. Anemia in these children may fit into the category of other causes of anemia3 such as immune hemolysis.4 Its course may be similar to the severe anemia that developed during Plasmodium falciparum infections in partially immune Aotus monkeys,13,14 some of whom had no parasitemia on blood films but were positive for parasites on polymerase chain reaction of the blood. The third child had glucose-6-phosphate dehydrogenase (G-6-PD) deficiency and was treated with pyrimethamine/sulfadoxine, likely precipitating severe hemolytic anemia that reached its low point 14 days after treatment.
African children with severe anemia and malaria clearly fall into multiple categories,3 including those without high parasitemia. In our study, however, low baseline hemoglobin levels and high parasitemia were the most significant risk factors for severe anemia. Other factors such as dyserythropoiesis3 and pyrimethamine/sulfadoxine treatment in G-6-PD deficiency were probably also responsible for the anemia in a minority of cases. The difference between our data and those of Stoute et al,4 in which immune-mediated RBC destruction was thought to be the major cause of malaria-associated anemia, may be explained by differences in the intensity of transmission; the intensity in their study reached hundreds of infections per year and the infections occurred throughout the year, as compared with lower rates and seasonal transmission in our study.
The major implications of our study are 2-fold. First, raising the baseline hemoglobin level by intermittent antimalarial therapy may decrease the incidence of severe anemia, particularly in settings of seasonal malarial transmission. Second, effective vaccines against malaria that lower peak parasitemia may raise baseline hemoglobin levels and reduce the frequency of severe anemia.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, April 27, 2004; DOI 10.1182/blood-2003-11-3884.
We thank Dr Richard Sakai for logistical support and the populations of the 2 villages for their cooperation throughout the study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal