Abstract
Immunotherapeutic approaches to limit cytomegalovirus (CMV) morbidity and mortality after hematopoietic stem cell transplants (HSCTs) are currently under investigation as alternatives to antiviral drugs. In this context, we have inserted full-length and ubiquitin-modified CMV phosphoprotein 65 (pp65), phosphoprotein 150 (pp150), and immediate early protein 1 (IE1) immunodominant antigens into the virulent Western Reserve strain of vaccinia virus (VV) and the highly attenuated strain, modified vaccinia Ankara (MVA). Recombinant (r) VV or rMVA stimulated vigorous expansion of CMV-specific CD8+ T cells in CMV-positive donor peripheral blood mononuclear cells (PBMCs), which showed minimal alloreactivity and high levels of HLA tetramer binding, cytokine production, and cytotoxicity. Ubiquitinated antigens had a profound effect when expressed in VV. Single antigen rMVA expressing pp65 or IE1, either ubiquitin-modified or native, stimulated both cytotoxic T lymphocyte (CTL) populations to be expanded up to 500-fold in a 60-mL blood draw from the same donor. This result demonstrates the clinical feasibility of simultaneously amplifying multiple CMV-CTL populations. Transgenic HLA A2.1 (HHD II) mice, immunized with the same rMVA as used with human PBMCs, produced a robust cytotoxic response to both CMV pp65 and IE1. The specificity of the vigorous immunologic response to rMVA, both in vitro and in vivo, makes them candidates for clinical evaluation in the context of adoptive immunotherapy for hematopoietic stem cell transplant (HSCT) recipients or donor vaccination.
Introduction
Cytomegalovirus (CMV) is a major cause of morbidity in individuals whose immune system is immature or compromised. Primary CMV infection is among the leading causes of congenital birth defects in developed countries.1 It induces serious pathology in AIDS patients2 and in recipients of solid organ3 or hematopoietic stem cell transplants (HSCTs).4 Although the majority of patients at risk for CMV disease undergo prophylaxis with acyclovir or preemptive treatment with ganciclovir,5 the efficacy of these antiviral agents is often inadequate. Currently licensed anti-CMV drugs suffer from a number of shortcomings, including toxicity, graft failure, stimulation of drug resistance, and interference with immune reconstitution.6 The development of alternative strategies for prophylaxis and treatment of CMV disease is desirable.7 As the kinetics of the immune response to CMV become better defined,8 immunotherapeutic approaches are acquiring more relevance as a means to complement pharmacologic strategies.9-12
In the healthy immunocompetent child or adult, cell-mediated immunity plays a pivotal role in the control of persistent infection and the recovery from CMV disease.13,14 During natural infection, several immunodominant CMV antigens trigger the immune system.15 CMV target antigens include the matrix phosphoprotein 65 (pp65),16 the 72-kDa immediate early protein 1 (IE1),17 and the structural phosphoprotein 150 (pp150).18,19 Pioneering studies of Riddell et al20 showed the feasibility of using adoptive transfer of cytotoxic T lymphocytes (CTLs) from an HSCT donor to reconstitute protective immunity that limits CMV disease, and gave strong experimental evidence for cell-mediated immune control of CMV infection.10,21 Although adoptive immunotherapy holds promise as an alternative to antivirals for CMV treatment, the development of such a strategy, to be applicable on a clinical scale, has been impeded by the difficulty in finding robust methods for generating therapeutic numbers of CMV CTLs, without using CMV itself.
Viruses have been explored as recombinant vaccine vectors for both infectious disease22 and cancer.23 The feasibility of using pp65 expressed in adenovirus,24,25 retrovirus,26 or poxvirus27 to induce robust CTL response has been demonstrated. In a recent phase 1 trial, CMV-seronegative volunteers were vaccinated with an attenuated strain of canarypox virus (ALVAC) expressing CMV pp65, and they developed robust cellular immunity, equivalent to levels measured in CMV-positive individuals.27 A safe vehicle able to efficiently present CMV-CTL target proteins to the immune system would constitute an attractive tool to expand CMV memory T cells for adoptive immunotherapy, or, as a vaccine, to prime or boost CMV immunity.
Toward this goal we have explored the in vitro and in vivo vaccine properties of the virulent Western Reserve (WR) strain of vaccinia virus (VV) and highly attenuated modified vaccinia Ankara (MVA)28 engineered with CMV antigens,14 using the ubiquitination strategy.29,30 MVA is a live attenuated virus that easily accommodates multiple foreign genes and has shown attractive properties in clinical studies.31,32 Due to the lack of viral assembly and avirulence in mammals, MVA has proven safe for clinical use under conditions of immunosuppression,33,34 and is capable of inducing potent immunity in nonpermissive hosts, even if previously vaccinated with VV or MVA.22,35-37 Either recombinant (r) VV or rMVA used in vitro rapidly stimulated CMV-specific CD8+ T cells in peripheral blood mononuclear cells (PBMCs) from seropositive volunteers. Antigen ubiquitination significantly enhanced the immunogenicity of rVV, while it had a minor impact on rMVA. Large-scale simultaneous expansions of both pp65- and IE1-specific CTLs (∼ 0.5 × 109) were obtained when a mixture of rMVA expressing pp65 or IE1 was used as stimulator. Using the HHD II transgenic (Tg) mouse model,38 rMVA viruses were also evaluated in vivo. HHD II mice immunized with rMVA consistently showed vigorous and specific primary immunity to CMV antigens. These findings indicate that rMVA could have a beneficial role both for adoptive immunotherapy and a CMV vaccine candidate in HSCT.
Materials and methods
Study population
The City of Hope (COH) institutional review board approved the study protocol (IRB 93140). Blood specimens, acquired prospectively after obtaining informed consent (according to United States Public Health Service [USPHS] guidelines and the Helsinki doctrine) from the enrollees, were HLA typed by polymerase chain reaction (PCR)39 and tested for CMV status by immunofluorescence assay (IFA).40 Multiple blood samples were collected at the General Clinic Research Center at COH, during a 2-year period from 7 HLA A*0201 and 4 HLA B*0702 CMV-positive healthy donors. Ficoll-Paque (Amersham-Pharmacia, Piscataway, NJ) isolated PBMCs were used either fresh or after cryopreservation.
Synthetic peptides
Construction of recombinant (r) VV constructs
The WR strain of rVV expressing full-length pp65 protein (pp65-VV) was derived by homologous recombination.40 The ubiquitinated form of recombinant pp65-VV containing an Arg (R) at the NH2 terminus (UbRpp65-VV), instead of the native Met (M), was constructed according to the N-end rule model.30,44 The ubiquitinated rVV construct containing M at the pp65 amino terminus (UbMpp65-VV) was constructed using analogous procedures detailed for UbRpp65-VV.30 Briefly, the human ubiquitin gene29 was fused with the CMV pp65 gene45 to create a fusion gene that was inserted into pSC11-MCS30 to obtain UbMpp65-pSC11. UbMpp65-VV was generated by transfecting wild-type VV (wtVV)–infected HuTK-143B cells (American Type Culture Collection [ATCC], Manassas, VA; CRL 8303) with the UbMpp65-pSC11 plasmid, then screened30 and analyzed by Western blot (WB).45 All rVVs were pelleted by ultracentrifugation through a sucrose-density cushion and stored at -80°C.46
Pulse-chase metabolic labeling and immunoprecipitation
Pulse-chase and immunoprecipitation were performed using described methods.29 Briefly, HuTK-143B cells (ATCC; CRL 8303) were infected with pp65-VV, UbRpp65-VV, or UbMpp65-VV at a multiplicity of infection (MOI) of 20 for 2 hours, then washed and incubated for 1 hour with Met-free, Cys-free RPMI (ICN Biomedicals, Irvine, CA) containing 5% dialyzed fetal calf serum (FCS; HyClone, Logan, UT) and 50 U/mL penicillin–50 μg/mL streptomycin (1x Penn-Strep; Gibco-BRL Life Technologies, Rockville, MD). 35S-Met and 35S-Cys (300 μCi [11.1 MBq] of each) were added for 30 minutes. Cells were subsequently washed and incubated with RPMI 1640 medium containing 10× Met (1 mM) and Cys (5 mM), 1× Penn-Strep (Gibco-BRL Life Technologies), and 10% FCS (HyClone) for increasing time. After each time point, cells were immediately pelleted and lysed in 1 mL cell lysis buffer (10 mM Tris [tris(hydroxymethyl)aminomethane]–HCl, pH 7.4; 150 mM NaCl; 0.1% sodium dodecyl sulfate [SDS]; 1% Triton X-100; 1% sodium deoxycholate; 100 μg/mL phenylmethylsulfonyl fluoride [PMSF]; and 1 μg/mL aprotinin). Supernatants were precleared once with protein G–Sepharose beads (Amersham Bioscience, Uppsala, Sweden) and stored at -80°C. For pp65 immunoprecipitation, 2.4 μg purified anti-pp65 monoclonal antibody (mAb 28-10347 ) and 50 μL protein G–Sepharose beads were mixed with 250 μL cell lysate for 2 hours. Beads were washed and boiled in 50 μL SDS–polyacrylamide gel electrophoresis (PAGE) loading buffer, proteins were separated by SDS-PAGE, and dried gels were quantitated using a Typhoon 9410 Scanner (Amersham, Piscataway, NJ).
Derivation of recombination plasmids for construction of rMVA
The CMV pp65 gene was subcloned into pLW2248 to create pp65-pLW22.49 The UbRpp65 gene was excised from the UbRpp65-pSC11 plasmid30 and subcloned into pLW22. CMV pp150 was cloned into pMCO350 to create pp150-pMCO3. CMV-IE4 was amplified from CMV-IE149 with primers carrying both PmeI (underlined in primer A) and AscI sites (underlined in primer B), and cloned into the PmeI and AscI sites of pLW22. Primers A and B are as follows: A, (sense, 5′-AGCTTTGTTTAAACGCCACCACCATGGTCAAACAGATTAAGGTTCG-3′); and B, (antisense, 5′-TTGGCGCGCCTTTATTTGACGTGGGATCCATAACAGTAACTG-3′).
Primers C (ApaI site underlined) and D were used to amplify the human ubiquitin gene (Ub). CMV IE4 was amplified using primers E and F (StuI site underlined). The Ub and IE4 genes were fused together by PCR using the C and F primers as described previously.30 The resulting UbRIE4 fusion gene was cloned into the ApaI and StuI sites of pSC11MCS. In analogy to the procedure for obtaining UbRpp65-pLW22,30 the UbRIE4 fusion gene was transferred into pLW22 to generate UbRIE4-pLW22. All plasmids were confirmed by restriction enzyme digestion and DNA sequencing using IRD-800 labeled primers (Li-cor, Lincoln, NE). Primers C to F are as follows: C (sense, 5′-TTGATCGGGCCCATGCAGATCTTCGTGAAGACC-3′); D (antisense, 5′-CTCGAACCTTAATCTGTTTGACCCTACCCCCCCTCAAGCGCAGGAC-3′); E (sense, 5′-GTCCTGCGCTTGAGGGGGGGTATGGTCAAACAGATTAAGGTTCGAG-3′); and F (antisense, 5′-AAGAAGGCCTGGCGCGCCTTACTGGTCAGCCTTGCTTCTAG-3′).
Generation of rMVA
pp65/pp150-MVA, UbRpp65-MVA, IE4-MVA, and UbRIE4-MVA were generated according to published protocols.35,46,49 rMVAs were screened for β-galactosidase (β-gal) in the presence of Bluo-gal substrate (Sigma-Aldrich, St Louis, MO),51 except for pp65/pp150-MVA that was first screened in the presence of X-glcA (5-bromo-4-chloro-3-indolyl-b-d-glucuronide; Sigma-Aldrich), followed by Bluo-gal. Viruses were screened and purified by sucrose density ultracentrifugation, and high-titer stocks were stored at -80°C.46 The protein expression levels of pp65, pp150, and IE in rMVA-infected baby hamster kidney (BHK-21) cells (ATCC; CCL10) were evaluated by WB using an enhanced chemiluminescence (ECL) kit (Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom).35 Membranes were incubated first with purified mAb 28-103 against pp65 or mAb p63-27 against IE52 or mAb 36-14 against pp150,53 followed with a peroxidase-labeled goat anti–mouse Ab.
In vitro stimulation assay (IVS) of human PBMCs with rVV or rMVA
Autologous Epstein-Barr virus (EBV)–transformed B-cell lines (lympho-blastoid cell lines [LCLs])40 were used as antigen-presenting cells (APCs) for IVS and maintained in LCL medium.43 Autologous LCLs were infected with rVV or rMVA at an MOI of 5 for 2 hours in LCL medium containing 2% FCS, then irradiated (50 gray) using the Isomedix Model 19 Gammator (Nuclear Canada, Parsippany, NJ). When rVVs were used, LCLs were also ultraviolet (UV) irradiated for 66 seconds using a Stratalinker 1800 (Stratagene, Cedar Creek, TX).54 Loss of infectivity was confirmed by a plaque assay, using CV-1 cells (ATCC; CCL 70).46 Fresh Ficoll-separated PBMCs (20 million) from 60 mL whole blood were depleted of CD4+, CD16+, and CD56+ T cells by magnetic separation.54 Depleted PBMCs (0.5 × 106) used as effectors, together with 4 × 105 rVV or rMVA infected/irradiated LCLs, and 2.5 × 106 autologous γ-irradiated (2400 rads) PBMCs as feeder cells were plated in T-cell culture medium (TCM).43 Fresh or thawed aliquots from IVS cultures were analyzed between days 7 and 12 for CMV-specific cytotoxicity, interferon γ (IFN-γ) production, and tetramer binding (see the next 3 paragraphs).
Chromium release assay (CRA)
IVS cultures were evaluated in a 4-hour CRA to detect CMV-specific cytotoxic T lymphocytes (CTLs).43 Background lysis to rVV/rMVA-infected EBV-LCL targets was reduced using cold target inhibition.54 For some donors, CMV AD169–infected or peptide-pulsed fibroblasts were also used as targets.43 Specific cytotoxicity to autologous and allogeneic fibroblasts peptide pulsed with pp65 or IE1 peptides was detected in all post-IVS cultures (data not shown).
Tetramer binding analysis
The HLA A*0201 CMVpp65495-503, the HLA B*0702 CMV pp65417-426, the HLA A*0201 CMV IE1316-324, and HIV pol464-472 tetramers were refolded and purified in our laboratory.43,54 Cryopreserved IVS cells or PBMCs were labeled54 and analyzed on the FACSCalibur (BD, San Jose, CA) using propidium iodide (SIGMA, St Louis, MO) to exclude nonviable cells.46 Quadrants were set based on negative controls, stained with irrelevant HIV pol464-472 tetramer, and a minimum of 30 000 gated events were captured. Tetramer-positive cells are expressed as a percentage of the CD8+ T-cell population.
Intracellular cytokine staining (ICS)
Intracellular staining for IFN-γ production was performed on thawed aliquots of IVS cells or PBMCs using the Cytofix/Cytoperm Plus (with GolgiPlug) Kit from BD Biosciences (San Diego, CA). Briefly, 10 μM of relevant CMV or irrelevant peptide was used as stimulator. Labeling was performed with fluorescein isothiocyanate (FITC)–conjugated CD8 antibody (BD Biosciences) and APC-conjugated antibody to IFN-γ (BD Biosciences). The number of double-positive cells is expressed as a percentage of the CD8+ T-cell population.
Mouse immunization and generation of CMV-specific CTL response
HHD II Tg mice38 were bred and maintained under pathogen-free conditions in the Assessment and Accreditation of Laboratory Animal Care (AAALAC)–approved animal care facility at COH. Groups of HHD II mice (9- to 12-week-old) were immunized intraperitoneally with various amounts and combinations of rMVA (as described in figure legends). At 2 weeks after immunization, spleens were aseptically removed and splenocytes from individual mice were stimulated once in vitro (IVS) with syngeneic lipopolysaccharide (LPS) blasts as APCs, and loaded with CMV-CTL epitope,43 and cytotoxic activity was determined by a 4-hour CRA following IVS.43
Statistical analysis methods
Comparison of paired data before and after IVS, or with and without ubiquitination, was performed using the signed rank test. For CRA results where a P value is quoted, it is computed from the Welch 2-sided t test, after a logit transformation to improve variance homogeneity.
Results
Stability properties of ubiquitinated and unmodified CMV-pp65 expressed in rVV
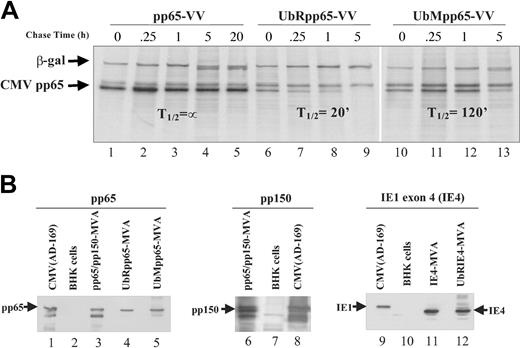
A study was carried out to examine whether modification of CMV-pp65 by ubiquitination would alter its stability. Chimerizing ubiquitin (Ub) to pp65 was hypothesized to enhance antigen degradation, resulting in more efficient antigen presentation and immune recognition.30,54,55 Since the substitution of the initiator amino acid (AA) of a protein with one that destabilizes it has been shown to further enhance instability, ubiquitinated pp65 was made with an N-terminal Arg (UbRpp65-VV).30 Ub fused to unmodified pp65 (UbMpp65-VV) was generated to compare the effect of the amino terminal substitution of M to R. UbRpp65-VV, UbMpp65-VV, and native pp65-VV were evaluated for changes in protein stability by conducting pulse-chase studies. A significant change in half-life (T½) was detected from either ubiquitinated protein, in contrast to the unmodified form, which remained stable for the 20-hour evaluation period (Figure 1A). These results substantiate that modification of CMV-pp65 alters the kinetics of its degradation, with the most radical change associated with the N-terminal R-substitution (T½, ∼ 20 minutes). Similar studies were conducted with modified forms of both IE and pp150 antigens, and substantially similar results were found concerning the impact of R-substitution (data not shown).
Pulse-chase experiments and characterization of poxvirus recombinants. (A) Pulse-chase and immunoprecipitation analysis were used to determine T½ of CMV-pp65 protein expressed by pp65-VV, UbRpp65-VV, and UbMpp65-VV. Hu TK- cells infected with VV expressing CMV pp65, UbRpp65, and UbMpp65 were pulsed with 35S-Met and 35S-Cys for 30 minutes, and chased with 10x unlabeled Met and Cys for 0, 0.25, 1, 5, and 20 hours. Cell lysates were immunoprecipitated by anti-pp65 mAb (103-28) and anti–β-gal mAb and analyzed by SDS-PAGE. Lanes 1 to 5 represent VV-pp65. Lanes 6 to 9 represent UbRpp65-VV. Lanes 10 to 13 represent UbMpp65-VV. (B) Western blot detection of pp65, pp150, and IE4 expressed by pp65/pp150-MVA, UbRpp65-MVA, UbMpp65-MVA, IE4-MVA, and UbRIE4-MVA. In lanes 1, 8, and 9 are AD169 CMV cell lysates (Microbix Biosystems, Toronto, ON, Canada) for detection of pp65, pp150, and IE4 and uninfected BHK cell lysates in lanes 2, 7, and 10. Cell lysates from pp65/pp150-MVA–infected BHK-21 cells (lanes 3, 6), UbRpp65-MVA–infected BHK-21 cells (lane 4), UbMpp65-MVA–infected BHK-21 cells (lane 5), IE4-MVA–infected BHK-21 cells (lane 11), and UbRIE4-MVA–infected BHK-21 cells (lane 12) are shown. All lanes were loaded with the same amount of protein as determined by Braford method. pp65 protein expression was detected using mAb (103-28), pp150 expression was detected using mAb (1.XPA 36), and IE1 exon 4 expression was detected using mAb (p63-27).
Pulse-chase experiments and characterization of poxvirus recombinants. (A) Pulse-chase and immunoprecipitation analysis were used to determine T½ of CMV-pp65 protein expressed by pp65-VV, UbRpp65-VV, and UbMpp65-VV. Hu TK- cells infected with VV expressing CMV pp65, UbRpp65, and UbMpp65 were pulsed with 35S-Met and 35S-Cys for 30 minutes, and chased with 10x unlabeled Met and Cys for 0, 0.25, 1, 5, and 20 hours. Cell lysates were immunoprecipitated by anti-pp65 mAb (103-28) and anti–β-gal mAb and analyzed by SDS-PAGE. Lanes 1 to 5 represent VV-pp65. Lanes 6 to 9 represent UbRpp65-VV. Lanes 10 to 13 represent UbMpp65-VV. (B) Western blot detection of pp65, pp150, and IE4 expressed by pp65/pp150-MVA, UbRpp65-MVA, UbMpp65-MVA, IE4-MVA, and UbRIE4-MVA. In lanes 1, 8, and 9 are AD169 CMV cell lysates (Microbix Biosystems, Toronto, ON, Canada) for detection of pp65, pp150, and IE4 and uninfected BHK cell lysates in lanes 2, 7, and 10. Cell lysates from pp65/pp150-MVA–infected BHK-21 cells (lanes 3, 6), UbRpp65-MVA–infected BHK-21 cells (lane 4), UbMpp65-MVA–infected BHK-21 cells (lane 5), IE4-MVA–infected BHK-21 cells (lane 11), and UbRIE4-MVA–infected BHK-21 cells (lane 12) are shown. All lanes were loaded with the same amount of protein as determined by Braford method. pp65 protein expression was detected using mAb (103-28), pp150 expression was detected using mAb (1.XPA 36), and IE1 exon 4 expression was detected using mAb (p63-27).
UbRpp65-VV is superior to pp65-VV in stimulating expansion of CMV-specific T cells
In order to test the hypothesis that stability of pp65 can influence efficiency of immunologic recognition, we compared pp65-VV and UbRpp65-VV to expand CMV-specific memory T cells in 4 HLA A*0201 and 4 B*0702 CMV-positive donors. HLA A*0201 and B*0702 were selected since they are among the most common HLA haplotypes for which CMV minimal cytotoxic epitopes (MCEs) have been identified and extensively studied.18 After 7 to 12 days of IVS, initiated with either the pp65-VV or UbRpp65-VV viruses, strong cell proliferation was observed in cultures from 3 donors. Maximum cell expansions exceeded 150-fold, with an average of 107-fold (Table 1). Amplified T cells from all donor PBMC cultures stimulated with either ubiquitinated or unmodified pp65-VV constructs were able to consistently elicit specific lytic activity against autologous LCL targets sensitized with HLA-matched MCE peptides (Table 1). IVS performed in all donor cultures (Table 1) stimulated with UbRpp65-VV (41%-91%) produced higher lytic activity than unmodified pp65-VV (28%-74%; P < .05).
CRA and cell expansion results after IVS with unmodified and ubiquitinated pp65-VV
. | % Cytotoxicity to pp65 MCE . | . | . | |
|---|---|---|---|---|
| HLA, donor UPN, and IVS VV-pp65 . | A2495-503 . | B7417-426 . | Expansion, fold . | |
| A*0201 | ||||
| 001 | 67 | |||
| Unmodified | 66 | — | ||
| Ubiquitinated | 91 | — | ||
| 002 | 7 | |||
| Unmodified | 28 | — | ||
| Ubiquitinated | 41 | — | ||
| 003 | 190 | |||
| Unmodified | 40 | — | ||
| Ubiquitinated | 70 | — | ||
| 004 | 151 | |||
| Unmodified | 66 | — | ||
| Ubiquitinated | 76 | — | ||
| B*0702 | ||||
| 005 | 23 | |||
| Unmodified | — | 46 | ||
| Ubiquitinated | — | 75 | ||
| 006 | 10 | |||
| Unmodified | — | 74 | ||
| Ubiquitinated | — | 79 | ||
| 007 | 65 | |||
| Unmodified | — | 42 | ||
| Ubiquitinated | — | 72 | ||
| 008 | 340 | |||
| Unmodified | — | 41 | ||
| Ubiquitinated | — | 55 | ||
. | % Cytotoxicity to pp65 MCE . | . | . | |
|---|---|---|---|---|
| HLA, donor UPN, and IVS VV-pp65 . | A2495-503 . | B7417-426 . | Expansion, fold . | |
| A*0201 | ||||
| 001 | 67 | |||
| Unmodified | 66 | — | ||
| Ubiquitinated | 91 | — | ||
| 002 | 7 | |||
| Unmodified | 28 | — | ||
| Ubiquitinated | 41 | — | ||
| 003 | 190 | |||
| Unmodified | 40 | — | ||
| Ubiquitinated | 70 | — | ||
| 004 | 151 | |||
| Unmodified | 66 | — | ||
| Ubiquitinated | 76 | — | ||
| B*0702 | ||||
| 005 | 23 | |||
| Unmodified | — | 46 | ||
| Ubiquitinated | — | 75 | ||
| 006 | 10 | |||
| Unmodified | — | 74 | ||
| Ubiquitinated | — | 79 | ||
| 007 | 65 | |||
| Unmodified | — | 42 | ||
| Ubiquitinated | — | 72 | ||
| 008 | 340 | |||
| Unmodified | — | 41 | ||
| Ubiquitinated | — | 55 | ||
CRA, described in “Materials and methods,” was performed after 7 to 12 days IVS with pp65-VV (unmodified) or UbRpp65-VV (ubiquitinated) for each CMV-positive donor. The lysis percentages to autologous LCL targets, pulsed with pp65495-503 for HLA A*0201 donors or with pp65417-426 for HLA A*0702 donors, are shown at E/T 20, except for donor 004 reported at E/T 5. In the last column, the highest cell expansion obtained after unmodified or ubiquitinated VV-pp65 IVS is shown for each donor. — indicates not applicable.
UbRpp65-VV is superior to pp65-VV in stimulating functional CMV-specific T cells
Using intracellular cytokine staining (ICC), IFN-γ production between 21% to 31% was detected in cultures stimulated with UbRpp65-VV, a much higher level than with pp65-VV (3%-8%, P < .05). Nonetheless, the difference in IFN-γ levels before and after pp65-VV IVS was still significant (P < .05, Figure 2A). Binding to CMV-pp65–specific tetramers was significantly enhanced (P < .05) following IVS with both ubiquitinated and unmodified pp65-VV (Figure 2B). Of 8 donors, 6 had higher tetramer binding following IVS with UbRpp65-VV than with pp65-VV (P < .05 for all donors). In IVS cultures performed with UbRpp65-VV, the CMV-specific tetramer frequency was higher (average, 22.4-fold) versus pp65-VV (average, 9.9-fold) compared with the respective fresh PBMCs (Figure 2B). In one representative example, a marked tetramer frequency difference (10-fold) was found between UbR and native forms of pp65 (Figure 2B-C). These results may reflect an advantage of UbRpp65-VV for rapid amplification of CMV-specific T cells to be used in adoptive transfer.
IFN-γ release and tetramer binding in unmodified and ubiquitinated pp65-VV IVS cultures. (A) Donors listed in Table 1 were tested for specific CD8+ IFN-γ production using ICC before (○) and after (▪) IVS with pp65-VV or UbRpp65-VV (▴). The following peptides were used during ICC: p53149-157, pp65495-503 with HLA A*0201 donors, and pp65417-426 with HLA B*0702 donors. Donor 002 was not tested, while for donor 005, ICC was performed only after UbRpp65-VV IVS. For each donor, percent CD8+ IFN-γ release to irrelevant peptide was subtracted from specific release. (B) CMV pp65495-503 tetramer was used with HLA A*0201 donors, CMV pp65417-426 with HLA B*0702 donors, and the nonrelated HIV pol464-472 as control tetramer for each donor. CD8+ binding percentages to control tetramer (0.07%-0.1%) were subtracted in each case. ○ denotes CD8+ tetramer binding percentages in donor PBMC before IVS; ▪, after pp65-VV IVS; and ▴, after UbRpp65-VV IVS. (C) Donor 001 tetramer binding fluorescence-activated cell-sorter (FACS) plots are shown. A 2-color FACS was used using anti-CD8 FITC-labeled mAb and tetramer conjugated with phycoerythrin (PE). Numbers on the upper-right quadrant indicate CD8+ T-cell percentages to (i) pp65495-503 tetramer, (ii) pol464-472 control tetramer after UbRpp65-VV IVS, (iii) pp65495-503 tetramer, and (iv) pol464-472 control tetramer after pp65-VV IVS.
IFN-γ release and tetramer binding in unmodified and ubiquitinated pp65-VV IVS cultures. (A) Donors listed in Table 1 were tested for specific CD8+ IFN-γ production using ICC before (○) and after (▪) IVS with pp65-VV or UbRpp65-VV (▴). The following peptides were used during ICC: p53149-157, pp65495-503 with HLA A*0201 donors, and pp65417-426 with HLA B*0702 donors. Donor 002 was not tested, while for donor 005, ICC was performed only after UbRpp65-VV IVS. For each donor, percent CD8+ IFN-γ release to irrelevant peptide was subtracted from specific release. (B) CMV pp65495-503 tetramer was used with HLA A*0201 donors, CMV pp65417-426 with HLA B*0702 donors, and the nonrelated HIV pol464-472 as control tetramer for each donor. CD8+ binding percentages to control tetramer (0.07%-0.1%) were subtracted in each case. ○ denotes CD8+ tetramer binding percentages in donor PBMC before IVS; ▪, after pp65-VV IVS; and ▴, after UbRpp65-VV IVS. (C) Donor 001 tetramer binding fluorescence-activated cell-sorter (FACS) plots are shown. A 2-color FACS was used using anti-CD8 FITC-labeled mAb and tetramer conjugated with phycoerythrin (PE). Numbers on the upper-right quadrant indicate CD8+ T-cell percentages to (i) pp65495-503 tetramer, (ii) pol464-472 control tetramer after UbRpp65-VV IVS, (iii) pp65495-503 tetramer, and (iv) pol464-472 control tetramer after pp65-VV IVS.
Immunologic characterization of pp65/pp150-MVA and UbRpp65-MVA
Since encouraging results were obtained with pp65 in VV, analogous viruses were produced in MVA.49 rMVAs were first investigated for expression in WB analysis (Figure 1B). Immunologic recognition of both pp65 and pp150 inserted in pp65/pp150-MVA and pp65 expressed in UbRpp65-MVA was verified using CMV-specific T-cell clones previously obtained in our laboratory.18,40,43 HLAA*0201 pp65495-503 and HLA B*0702 pp65417-426 T-cell clones derived from different CMV-seropositive donors were able to efficiently lyse (> 70% at effector-target [E/T] 10) LCL targets infected with rMVA at an MOI of 5 (data not shown).18 The cytotoxic activity was similar to that obtained when pp65-VVs were used to infect LCLs in an analogous experimental setting (data not shown). pp65/pp150-MVA–infected LCLs were also able to induce potent HLA-restricted cytotoxicity (> 60%, at E/T 10) in HLA A*0301 pp150945-955 and HLA A*6801/2 pp150792-802 specific T-cell clones (data not shown).
rMVAs promote robust and specific CMV cellular immune responses in PBMCs
Evaluation of rMVA viruses as tools for in vitro expansion of CMV memory CTLs (mCTLs) was performed with 3 HLA A*0201 donors (unique patient numbers [UPNs] 001, 009, and 011) and an HLA B*0702 donor (007), previously tested with pp65-VV (Table 1). Similar to findings with pp65-VV, cell expansions ranging between 30- to 270-fold were obtained at the end of the IVS with both ubiquitinated and unmodified pp65/pp150-MVA. Robust cytotoxicity to autologous LCL targets loaded with the relevant immunodominant HLA epitopes peptides was found in all donors after IVS (Figure 3A). pp65-tetramers detected amplified population of CMV-specific T cells (19- to 39-fold) after IVS (Figures 3B and 4C). In addition, IFN-γ production by CD8+ T cells measured by ICC substantially rose (9- to 13-fold) in the tested subjects (Figure 3C). Among 3 HLAA*0201 donors, UPN 011 was the only one to have HLA allele A*0301, for which a CTL epitope (pp150945-955) has been described.18 Following IVS, lytic activity against targets pulsed with this peptide was remarkable (Figure 3A), and 2.4% of the CD8+ T cells were IFN-γ+ by ICC (> 8-fold higher than fresh PBMCs, data not shown), which confirmed that pp150 expressed in MVA was promoting specific recognition. In contrast to the differences in IVS activity between pp65-VV and UbRpp65-V, UbRpp65-MVA did not increase cytotoxicity, IFN-γ production, or tetramer binding versus pp65/pp150-MVA in both HLA A*0201 and HLA B*0702 donors (data not shown).
Lytic activity, IFN-γ release, and tetramer binding in pp65/pp150-MVA IVS cultures. IVS using pp65/pp150-MVA was performed with HLA A*0201 donors 001, 009, 011, and 007, and for HLA B*0702. (A) Cytotoxic activity detected after IVS is shown for each donor. ▦ indicates background lysis to autologous LCLs loaded with p53149-157; ♦ indicates lysis of autologous LCLs pulsed with pp65495-503 (donors 001, 009, and 011) or pp65417-426 (donor 007); • indicates lysis of pp150945-955-pulsed autologous LCLs of donor 011. (B) Tetramer binding levels in CD8+ cells from fresh PBMCs (○) and pp65/pp150-MVA IVS cultures (•). pp65495-503 tetramers were used for donors 001, 009, and 011, while donor 007 was tested using pp65417-426 tetramers. CD8+ T-cell binding to HIV pol464-472 tetramers was subtracted. (C) Percentage IFN-γ release from CD8+ cells of fresh PBMCs (○) and pp65/pp150-MVA IVS cultures (•) detected in ICC. Peptides used during ICC incubation were p53149-157 and pp65495-503 for HLA A*0201 donors and pp65417-426 for HLA A*0702 donor 007. For each donor, percentages of IFN-γ CD8+ cells to irrelevant p53149-157 peptide were subtracted from the corresponding specific values.
Lytic activity, IFN-γ release, and tetramer binding in pp65/pp150-MVA IVS cultures. IVS using pp65/pp150-MVA was performed with HLA A*0201 donors 001, 009, 011, and 007, and for HLA B*0702. (A) Cytotoxic activity detected after IVS is shown for each donor. ▦ indicates background lysis to autologous LCLs loaded with p53149-157; ♦ indicates lysis of autologous LCLs pulsed with pp65495-503 (donors 001, 009, and 011) or pp65417-426 (donor 007); • indicates lysis of pp150945-955-pulsed autologous LCLs of donor 011. (B) Tetramer binding levels in CD8+ cells from fresh PBMCs (○) and pp65/pp150-MVA IVS cultures (•). pp65495-503 tetramers were used for donors 001, 009, and 011, while donor 007 was tested using pp65417-426 tetramers. CD8+ T-cell binding to HIV pol464-472 tetramers was subtracted. (C) Percentage IFN-γ release from CD8+ cells of fresh PBMCs (○) and pp65/pp150-MVA IVS cultures (•) detected in ICC. Peptides used during ICC incubation were p53149-157 and pp65495-503 for HLA A*0201 donors and pp65417-426 for HLA A*0702 donor 007. For each donor, percentages of IFN-γ CD8+ cells to irrelevant p53149-157 peptide were subtracted from the corresponding specific values.
Lytic activity, IFN-γ release, and tetramer binding in IE4-MVA IVS cultures. IVS using IE4-MVA was performed with HLA A*0201 donors 009, 010, and 011. (A) Cytotoxic activity detected after IVS is shown for each donor. ▦ indicates background lysis to autologous LCLs loaded with p53149-157; ♦ indicates lysis of autologous LCLs pulsed with HLA A*0201 IE1316-324 peptide. (B, left panel) IE1316-324 tetramer binding frequencies in CD8+ T cells from donor PBMCs (□) and IE4-MVA IVS cultures (▪). CD8+ T-cell binding to HIV pol464-472, used as control, was subtracted. (B, right panel) Percentages of CD8+ with IFN-γ release after incubation with IE1316-324 peptide in fresh PBMCs (□) or IE4-MVA IVS cultures (▪) were detected using ICC. Percentage of CD8+ T cells with IFN-γ release to p53149-157 was subtracted. (C) Donor 009 tetramer binding FACS plots are shown. Tetramers used were conjugated with APCs, and with PE for plot ii. Numbers on the upper-right quadrant indicate CD8+ T-cell tetramer binding percentages to (i) pp65495-503 tetramer after pp65/pp150-MVA IVS, (ii) IE1316-324 tetramer after IE4-MVA IVS, (iii) pp65495-503 tetramer, (iv) IE1316-324 tetramer, and (v) HIV pol464-472 control tetramer after combined pp65/pp150-MVA and IE4-MVA IVS.
Lytic activity, IFN-γ release, and tetramer binding in IE4-MVA IVS cultures. IVS using IE4-MVA was performed with HLA A*0201 donors 009, 010, and 011. (A) Cytotoxic activity detected after IVS is shown for each donor. ▦ indicates background lysis to autologous LCLs loaded with p53149-157; ♦ indicates lysis of autologous LCLs pulsed with HLA A*0201 IE1316-324 peptide. (B, left panel) IE1316-324 tetramer binding frequencies in CD8+ T cells from donor PBMCs (□) and IE4-MVA IVS cultures (▪). CD8+ T-cell binding to HIV pol464-472, used as control, was subtracted. (B, right panel) Percentages of CD8+ with IFN-γ release after incubation with IE1316-324 peptide in fresh PBMCs (□) or IE4-MVA IVS cultures (▪) were detected using ICC. Percentage of CD8+ T cells with IFN-γ release to p53149-157 was subtracted. (C) Donor 009 tetramer binding FACS plots are shown. Tetramers used were conjugated with APCs, and with PE for plot ii. Numbers on the upper-right quadrant indicate CD8+ T-cell tetramer binding percentages to (i) pp65495-503 tetramer after pp65/pp150-MVA IVS, (ii) IE1316-324 tetramer after IE4-MVA IVS, (iii) pp65495-503 tetramer, (iv) IE1316-324 tetramer, and (v) HIV pol464-472 control tetramer after combined pp65/pp150-MVA and IE4-MVA IVS.
IE4-MVA stimulates recall cellular immunity to CMV
IE4-MVA and UbRIE4-MVA were constructed to measure levels of recall cellular immunity. IE1 exons 1 to 3 were deleted because they contain potent transcription stimulation sites that might be a risk for clinical use.56 Exon 4 is the largest domain of IE1, and it contains most of the defined CTL epitopes.15,17,57 The expression of IE4 protein from both IE4-MVA and UbRIE4-MVA was found to be abundant by WB analysis (Figure 1B). Presentation of the IE4 proteins expressed in MVA was tested using an IE1316-324-specific T-cell clone (C.L.R. et al, unpublished data, May 2002) derived from an HLA A*0201 CMV-positive donor.17 The IE1316-324 T-cell clone lysed HLA A*0201 LCL targets infected at an MOI of 10 with IE4-MVA (45% at E/T 3) or with UbRIE4-MVA (78% at E/T 3) (data not shown). rMVAs were subsequently evaluated in IVS with PBMCs from 3 CMV-positive HLA A*0201 donors (UPNs 009, 010, and 011; Figure 4A-C). PBMCs from UPNs 009 and 011 were previously analyzed using pp65/pp150-MVA and UbRpp65-MVA (Figure 3A-C). IE4-MVA used in a 7- to 12-day IVS promoted substantial T-cell expansion (≤ 68-fold). In all 3 donors, IE4-MVA was able to elicit strong specific cytotoxicity against autologous LCL targets pulsed with IE1316-324 peptide (Figure 4A). An increase (12.2-fold average) in the percentages of specific IE1316-324 tetramer binding and IFN-γ production (13.8-fold average) was observed (Figure 4B-C). Very similar results were found using UbRIE4-MVA in IVS cultures from all 3 donors (data not shown).
IVS with pp65/pp150-MVA and IE4-MVA amplifies pp65 and IE1 CTLs in CMV-positives
We evaluated whether CMV-CTL could be expanded from PBMCs simultaneously using pp65/pp150-MVA and IE4-MVA or their ubiquitinated versions (UbRpp65-MVA and UbRIE4-MVA) in the same subject. Both rMVAs were used at an MOI of 2.5, in order to duplicate the same MOI of 5 used for the IVS performed with single viruses. The objective of eliciting an amplified mCTL response simultaneously to CMV-IE1 and pp65 was achieved in all 3 HLA A*0201 donors, following IVS with a mixture of both pp65/pp150-MVA and IE4-MVA (Table 2). As expected, donor 011 also amplified pp150-specific CTLs (data not shown) to comparable levels using pp65/pp150-MVA (Figure 3A). After 12 days of IVS, massive cell proliferation, producing between 3 to 5 × 108 cells, was obtained for donors 009 and 010, while 3 × 107 cells were recovered for donor 011. For UPNs 009 and 010, the degree of cell expansion after the combination IVS was about 1 log higher than after the IVS with IE4-MVA only, while for 011 it was similar. After combination IVS, cytotoxicity was robust against IE1316-324 and pp65495-503 in all donors (Table 2). Tetramer binding of PBMCs from donor 009 stimulated with either pp65-MVA or IE4-MVA or with the combination of 2 rMVAs (Figure 4C) showed similar high levels of expansion. UbRpp65-MVA and UbRIE4-MVA combined in the same IVS for all 3 donors gave comparable tetramer and IFN-γ+ T-cell percentages to those obtained with unmodified rMVA used in combination (data not shown). These data demonstrate the feasibility of simultaneously expanding separate CMV mCTL populations in the same individual.
Tetramer, IFN-γ T-cell frequency, and CRA results of PBMCs before and after IVS
. | % Tetramer binding . | . | . | % IFN-γ . | . | . | % CRA . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | IE1 . | pp65 . | Nonrelated . | IE1 . | pp65 . | Nonrelated . | IE1 . | pp65 . | Nonrelated . | ||||||
| 009 | |||||||||||||||
| PBMC* | 6.89 | 2.90 | 0.38 | 4.60 | 2.97 | 0.19 | 34.89 | 19.03 | 11.56 | ||||||
| After IVS† | 72.26 | 32.30 | 0.13 | 16.70 | 7.40 | 0.22 | 99.19 | 69.05 | 9.91 | ||||||
| 010 | |||||||||||||||
| PBMC | 2.30 | 8.45 | 0.50 | 1.60 | 1.10 | 0.02 | 8.73 | 26.51 | 5.32 | ||||||
| After IVS | 9.60 | 30.19 | 0.17 | 11.34 | 21.47 | 0.93 | 71.25 | 88.34 | 28.1 | ||||||
| 011 | |||||||||||||||
| PBMC | 0.37 | 3.00 | 0.08 | 0.45 | 2.00 | 0.05 | 3.64 | 9.68 | 0.01 | ||||||
| After IVS | 3.91 | 57.44 | 0.41 | 1.73 | 17.17 | 0.21 | 61.58 | 97.71 | 22.37 | ||||||
. | % Tetramer binding . | . | . | % IFN-γ . | . | . | % CRA . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN . | IE1 . | pp65 . | Nonrelated . | IE1 . | pp65 . | Nonrelated . | IE1 . | pp65 . | Nonrelated . | ||||||
| 009 | |||||||||||||||
| PBMC* | 6.89 | 2.90 | 0.38 | 4.60 | 2.97 | 0.19 | 34.89 | 19.03 | 11.56 | ||||||
| After IVS† | 72.26 | 32.30 | 0.13 | 16.70 | 7.40 | 0.22 | 99.19 | 69.05 | 9.91 | ||||||
| 010 | |||||||||||||||
| PBMC | 2.30 | 8.45 | 0.50 | 1.60 | 1.10 | 0.02 | 8.73 | 26.51 | 5.32 | ||||||
| After IVS | 9.60 | 30.19 | 0.17 | 11.34 | 21.47 | 0.93 | 71.25 | 88.34 | 28.1 | ||||||
| 011 | |||||||||||||||
| PBMC | 0.37 | 3.00 | 0.08 | 0.45 | 2.00 | 0.05 | 3.64 | 9.68 | 0.01 | ||||||
| After IVS | 3.91 | 57.44 | 0.41 | 1.73 | 17.17 | 0.21 | 61.58 | 97.71 | 22.37 | ||||||
Combined exposure of PBMCs to pp65/pp150-MVA and IE4-MVA was carried out as described in “Materials and methods.” Immunity to IE1316-324, pp65495-503, and nonrelated peptide p53149-157 was determined for HLA A*0201 donors 009, 010, and 011, as specified in “Materials and methods,” and listed as percentages. CRA killing is reported at E/T 100 for PBMCs and at E/T 20 for post-IVS cultures.
PBMC = fresh uncultured PBMC
After IVS = cultured cells after IVS
Recognition of CMV-infected fibroblasts
To address whether rMVA-stimulated CMV-specific effectors recognized CMV-infected cells, IVS cell cultures were assayed on dermal fibroblasts infected with the AD169 CMV strain.43 Fibroblasts were either autologous or mismatched at 2 or more HLA loci.12 Specific killing of CMV-infected autologous fibroblasts was detected in all specimens tested (Table 3). Polyclonal IVS cultures, without undergoing a purification step,12 also displayed strong HLA-restricted recognition against allogeneic HLA A*0201 (or HLA B*0702 for UPNs 005 and 008) CMV-infected fibroblasts, with a low percentage of nonspecific killing (average 6%; Table 3, “Allogeneic”). Substantial cytotoxicity against infected fibroblasts was found in cultures raised from UPNs 009, 010, and 011 stimulated by IE4-MVA and UbRIE4-MVA. This was surprising, since CMV-IE1 CTL clones were reported to inefficiently recognize AD169-infected fibroblasts.17,58,59
Recognition of CMV-infected fibroblasts after IVS with CMV-poxvirus constructs
. | . | Autologous, % lysis . | . | Allogeneic, % lysis . | . | ||
|---|---|---|---|---|---|---|---|
| UPN . | IVS . | CMV infected . | Untreated . | CMV infected . | Untreated . | ||
| 001 | pp65/pp150-MVA | 40.0 | 3.5 | 41.3 | 2.0 | ||
| 004 | pp65-VV | 20.7 | 0.9 | 22.4 | 3.1 | ||
| 005 | UbRpp65-VV | 48.4 | 10.1 | NA | NA | ||
| 008 | UbRpp65-VV | 31.0 | 2.9 | 27.0 | 6.0 | ||
| 009 | pp65/pp150-MVA | 43.3 | 7.3 | 43.8 | 9.9 | ||
| 009 | IE4-MVA | 28.9 | 0.5 | 32.8 | 2.0 | ||
| 009 | pp65/pp150-MVA + IE4-MVA | 34.5 | 7.2 | 27.4 | 6.0 | ||
| 010 | UbRpp65/pp150-MVA + IE4-MVA | NA | NA | 43.4 | 15.7 | ||
| 010 | UbRIE4-MVA | NA | NA | 30.5 | 3.3 | ||
| 011 | pp65/pp150-MVA + IE4-MVA | 43.8 | 0.9 | 46.0 | 6.4 | ||
| 011 | IE4-MVA | 30.4 | 3.4 | NA | NA | ||
. | . | Autologous, % lysis . | . | Allogeneic, % lysis . | . | ||
|---|---|---|---|---|---|---|---|
| UPN . | IVS . | CMV infected . | Untreated . | CMV infected . | Untreated . | ||
| 001 | pp65/pp150-MVA | 40.0 | 3.5 | 41.3 | 2.0 | ||
| 004 | pp65-VV | 20.7 | 0.9 | 22.4 | 3.1 | ||
| 005 | UbRpp65-VV | 48.4 | 10.1 | NA | NA | ||
| 008 | UbRpp65-VV | 31.0 | 2.9 | 27.0 | 6.0 | ||
| 009 | pp65/pp150-MVA | 43.3 | 7.3 | 43.8 | 9.9 | ||
| 009 | IE4-MVA | 28.9 | 0.5 | 32.8 | 2.0 | ||
| 009 | pp65/pp150-MVA + IE4-MVA | 34.5 | 7.2 | 27.4 | 6.0 | ||
| 010 | UbRpp65/pp150-MVA + IE4-MVA | NA | NA | 43.4 | 15.7 | ||
| 010 | UbRIE4-MVA | NA | NA | 30.5 | 3.3 | ||
| 011 | pp65/pp150-MVA + IE4-MVA | 43.8 | 0.9 | 46.0 | 6.4 | ||
| 011 | IE4-MVA | 30.4 | 3.4 | NA | NA | ||
Fibroblasts were infected with CMV AD169 and used as targets in CRA as described in “Materials and methods.”
Effectors were PBMC stimulated 7 to 12 days with various CMV-poxvirus constructs, as detailed in “Materials and methods.” The percentage killing of autologous (“Autologous”) and HLA A*0201 (or HLA A*0702 for UPNs 005 and 008) allogeneic (“Allogeneic”) fibroblasts, mismatched at least at 2 other HLA loci are shown at E/T 20 for each donor.
NA indicates that either fibroblasts or effector cells were not available for the assay.
Immunization using rMVA
We evaluated the immunogenicity and priming ability of rMVA in HHD II mice. In the Tg HLA A2/Kb model30,60 with a complete functional murine H-2 haplotype,41 we observed erratic recognition of full-length CMV antigens expressed in MVA or VV (data not shown). In contrast, pp65/pp150-MVA and UbRpp65-MVA were able to stimulate robust cytotoxicity after a single immunization in HHD II mice, which have no endogenous murine class I presentation38 (Figure 5A-B). Introduction of 107 IU pp65-MVA was sufficient for immune recognition, and responses reached plateau levels when 2 × 107 IU was administered (Figure 5B). IE4-MVA and UbRIE4-MVA also consistently elicited high levels of specific cytotoxicity after one IVS (Figure 5C). Reproducing the in vitro findings with the human subjects, ubiquitinated antigens did not increase immunogenicity of rMVA in CRA and HLA A2 tetramer binding studies (data not shown). pp65/pp150-MVA or UbRpp65-MVA with IE4-MVA or UbRIE4-MVA administered in combination to HHD II mice were able to elicit specific pp65 and IE1 cytotoxic response in the same mouse (Figure 5 D-E).
HHD II mice immunizations with rMVA. Splenocytes from HHD II mice immunized with rMVA were subjected to IVS and then tested for lytic function in a standard CRA. Filled symbols show killing of T2 cell targets loaded with a pp65 or IE peptides; open symbols indicate killing of the same target cells loaded with p53149-157. Each set of experiments was repeated at least twice. (A) Lytic activity of splenocytes from 2 mice immunized with 107 IU or (B) 2 × 107 IU of pp65/pp150-MVA, against targets loaded with (closed symbols) pp65495-503 or (open symbols) p53149-157. (C) UbRIE4-MVA (5 × 107 IU) was used to immunize 2 mice. Filled symbols represent individual mouse recognition of IE1297-306–loaded targets, and open symbols indicate background lysis of targets loaded with p53149-157. In panels D and E, 3 mice were immunized with a mixture of 2.5 × 107 IU pp65/pp150-MVA and 2.5 × 107 IU UbRIE4-MVA. Splenocytes from each spleen were stimulated separately with (D) pp65495-503 (filled symbols) and (E) IE1297-306 (filled symbols). In panels D and E, open symbols indicate background lysis to p53149-157. At E/T, 20 significant differences (P < .05) were detected between the activity against p53149-157 and pp65495-503 (D) and between p53149-157 and IE1297-306 (E), according to the Welch 2-sided t test.
HHD II mice immunizations with rMVA. Splenocytes from HHD II mice immunized with rMVA were subjected to IVS and then tested for lytic function in a standard CRA. Filled symbols show killing of T2 cell targets loaded with a pp65 or IE peptides; open symbols indicate killing of the same target cells loaded with p53149-157. Each set of experiments was repeated at least twice. (A) Lytic activity of splenocytes from 2 mice immunized with 107 IU or (B) 2 × 107 IU of pp65/pp150-MVA, against targets loaded with (closed symbols) pp65495-503 or (open symbols) p53149-157. (C) UbRIE4-MVA (5 × 107 IU) was used to immunize 2 mice. Filled symbols represent individual mouse recognition of IE1297-306–loaded targets, and open symbols indicate background lysis of targets loaded with p53149-157. In panels D and E, 3 mice were immunized with a mixture of 2.5 × 107 IU pp65/pp150-MVA and 2.5 × 107 IU UbRIE4-MVA. Splenocytes from each spleen were stimulated separately with (D) pp65495-503 (filled symbols) and (E) IE1297-306 (filled symbols). In panels D and E, open symbols indicate background lysis to p53149-157. At E/T, 20 significant differences (P < .05) were detected between the activity against p53149-157 and pp65495-503 (D) and between p53149-157 and IE1297-306 (E), according to the Welch 2-sided t test.
Discussion
We describe poxviruses (VV and MVA) engineered with CMV genes,30,35,49,54 which are attractive tools for expansion of CMV-specific T cells for clinical scale applications. We focused on immunodominant antigens pp65, pp150, and IE1, previously found to have a key role in CMV immunity in healthy CMV-positive adults, children, and infants.14,15,19 A cohort of healthy CMV-positive donors carrying representative haplotypes, among the highest frequencies in humans (HLA A*0201, A*0301, and B*0702),18 was selected to assess the immunogenicity of the viruses. An advantage to using full-length proteins expressed in poxviruses is their applicability regardless of an individual's HLA type, while for other methods, in which HLA-restricted peptide epitopes are used for T-cell induction, such limitations exist.10,11,26 Moreover, the use of synthetic peptides, although powerful to identify minimal epitopes, cannot stimulate the whole spectrum of potential epitopes generated from an antigen in bulk culture.61
We developed a reproducible IVS in which rVV and rMVA induced vigorous CMV-specific T-cell expansions in PBMCs from every donor (≤ 0.5 × 109), reaching levels suitable for clinical use. An infusion of 107 CMV-specific T cells/m2 is sufficient to suppress CMV infection in some HSCT recipients not responding to ganciclovir treatment.9 Our method of rapid expansion of T cells from PBMCs is both convenient and practical, since a single blood draw of 60 mL and one IVS is minimally required to reach clinically relevant numbers of CMV CTLs. In others approaches, not only is a larger amount of blood used, but repetitive stimulation cycles are performed to get acceptable amounts of infusible cells and to reduce alloreactivity.26,62 The rapid CMV-CTL expansion method was developed exclusively for seropositives, and was never intended for generating an in vitro priming response, since a single stimulation is performed and LCLs are used as APCs. While the possibility of generating an in vitro CMV response from seronegative donors remains debatable, it has been previously attempted with equivocal results.63,64 Dendritic cells (DCs) appear to be as essential a requirement as APCs, together with several restimulations (up to 664 ) and additional maturation stimuli, such as tumor necrosis α (TNFα63 ). However, in vivo results in the HHD II model prove that rMVAs are capable of priming, and may promote de novo CMV response in seronegative donors, as already shown for pp65-ALVAC.27 The rapid expansion technique we developed relies exclusively on autologous components for both APCs and feeder cells, in order to minimize the growth of alloreactive cells. Autologous LCLs used in this study as professional APCs have been previously used for adoptive immunotherapy with success.65 Other cell types (reviewed in van Rhee and Barrett66 ) could also be evaluated as APCs in a clinical setting, such as autologous DCs,10,64 artificial APCs expressing a single HLA class I molecule,12 CD40-activated B cells,26 or even PBMCs, which are infectable with MVA (C.L.R., Z.W., S.M., S.F.L., and D.J.D., manuscript in preparation).
pp65-VV and UbRpp65-VV30,54 both generated a remarkable level of cell proliferation, with some differences in specificity. The extent of specific IFN-γ secretion, after IVS with UbRpp65-VV, resulted in higher expression levels in all donors than with unmodified pp65-VV. UbRpp65-VV also promoted higher killing activity, and tetramer assays showed 1 log greater T-cell frequencies than was found with pp65-VV. Our findings are consistent with studies by Townsend et al,67 who observed that the vaccinia-induced defect in presentation of a foreign antigen was overcome by fusing ubiquitin to the foreign gene. Subsequently, Tobery and Siliciano29 demonstrated that the ubiquitination strategy enhances degradation and induces greater immunogenicity, especially when it is coupled to N-terminal Arg residue.30,44,67 These data indicate that Ub fusion can confer enhanced immunogenicity to pp65, which is advantageous in a clinical design in which high frequency of CMV-CTL amplification is sought.
Methods for isolating and selecting CMV CTLs has been described and could be adopted to further purify the enriched CMV-CTL post-IVS population.12,66 However, HLA class I tetramer binding and functional assays used in this study, such as CRA and ICC for IFN-γ, revealed that T-cell lines obtained after one IVS were largely CMV-epitope specific. They were able to kill both autologous and allogeneic (HLA A*0201 or HLA B*0702, mismatched at other loci12 ) CMV-infected fibroblasts with low alloreactivity (Table 3, “Allogeneic”). This result critically augments the feasibility of our approach for clinical use. Regardless of the subject's previous immune status to EBV or poxvirus, we were always able to detect substantial CMV cellular immunity. In particular IFN-γ production to immunodominant EBV (BMLF168 ) and poxvirus (VP35#169 ; 74A and 16570 ) epitopes never exceeded 2% among various post-IVS CD8+ population tested (data not shown). Our IVS method, in which PBMCs are magnetically depleted of T-helper and natural killer (NK) cells, can also be performed to generate CD4+ enriched T cells by depleting CD8+ and NK cells (C.L.R. et al, unpublished data). Since adoptively transferred CD8+ CMV-specific T-cell clones do not persist long term without endogenous recovery of CD4+ T cells,71 immunotherapy protocols would be more effective if CD4+ T cells were provided, especially for HSCT recipients who lack CMV-specific CD4+ T-help responses.9 We are investigating whether this system would support simultaneous induction of CD4+ T-help and CD8+ CTL populations.
Divergence between tetramer staining and IFN-γ levels found in some study subjects (Figures 2, 3, 4; Table 2) may result from individual variation of immune response kinetics and subject heterogeneity.72 Consistent with previous reports, levels of CMV-specific HLA tetramer staining that were associated with limited or no immune function were found in several examples of unstimulated donor PBMCs73,74 (Figures 2, 3, 4; Table 2). Nonetheless, simultaneous and proportionate increases in HLA tetramer–positive cells, cytotoxicity, and cytokine production were elicited in every donor following poxvirus stimulation (Figures 2, 3, 4; Table 2).
The choice of MVA for in vivo clinical applications is based on its avirulence and benign toxicity, which make this poxvirus particularly suitable for use in immunocompromised persons.33,36 In animal disease models, rMVA-based vaccines have elicited systemic immunity or protection against different infectious pathogens.75-78 rMVAs targeting pp65 and IE were effective tools to measure CMV-specific responses in infants and children ranging from birth to 2 years of age.49 In this work, rMVAs expressing pp65 or IE4 alone, and pp65 and pp150 simultaneously had excellent immunogenic properties (Figure 5). In our HHD II mouse pilot study, only the rMVA constructs were analyzed in vivo, since virulent rVV has safety concerns79 and could be ineffective for a large portion of the human population with pre-existing immunity to the VV vector.80 In contrast, rMVA expressing CMV soluble glycoprotein B caused durable immunity and neutralizing antibodies against multiple strains of human CMV, when used to immunize naive mice and even those previously vaccinated with VV or MVA.35
Using the same assays used for evaluating the pp65-VV vectors, we found that all rMVAs efficiently promoted rapid expansion of specific human CMV CTLs similarly to replication-competent VV. However, in contrast to rVV, we found that ubiquitination of CMV antigens in rMVA did not increase immunogenicity during IVS of human PBMCs or as a vaccine in the HHD II model. Our findings are consistent with the observed favorable biologic characteristics of MVA.37,81 Humoral and cellular immune response to the virus was considerably lower after MVA-versus VV-immunized mice.81 The different profile of proinflammatory cytokines induced by VV and MVA, including higher levels of IFN-γ and interleukin-12 (IL-12) in response to VV, may also contribute to greater insert immunogenicity expressed in MVA.81 Previous reports found that cellular and humoral immune responses to a foreign antigen expressed from MVA are higher than comparable rVV.77,82 pp65-VV stimulated IFN-γ levels (Figure 2A) to a lower extent than the corresponding ubiquitinated form (UbRpp65-VV), while no difference was found with either form of pp65 expressed in MVA (data not shown). The strong immune response against viral antigens of VV is associated with depressed recognition of VV-expressed foreign antigens.
Unexpectedly, good cytotoxicity to infected fibroblasts was found using IE4-MVA– and UbRIE4-MVA–stimulated PBMC cultures, comparable with that obtained with pp65-poxvirus–stimulated cultures (Table 3). Newly synthesized viral proteins, such as CMV-IE1, are not efficiently presented by CMV AD169–infected fibroblasts.58 Based on our findings, we conclude that IE1 epitopes are presented on CMV-infected fibroblasts, despite viral interference. Use of a bulk culture specific for full-length IE1 protein, rather than IE1 epitope-specific clones, may reflect the in vivo milieu in which there is evidence of abundant IE1-specific CTLs, and explain why we easily detected IE1-specific CTLs in mice and humans, in contrast to others.17,59,83
pp65/pp150-MVA elicited simultaneous responses to both antigens, prompting us to investigate performing IVS with MVA constructs expressing multiple CMV antigens. Thus, for some donors (Figure 3; Table 2) we not only assessed the response to both IE1 and pp65, but we also evaluated T-cell expansion to 3 different CMV-specific target antigens in the same individual. When APCs coinfected with both pp65/pp150-MVA and IE4-MVA were used in IVS with the PBMCs of the same subject, we were able to generate up to 0.5 × 109 CTLs for some donors. This is one log higher expansion than obtained with a single antigen MVA IVS. Concurrent specificity to IE1, pp150, and pp65 was robust in all donors tested, as assessed by CRA, IFN-γ, and HLA tetramer binding. Analogous results were found when simultaneous immunizations with a mixture of rMVA expressing pp65 and IE4 were performed in HHD mice (Figure 5D-E). Significantly, both in our in vivo and in vitro studies, no interference or selective inhibition of IE1 CTLs due to pp65 coexpression was found, as previously reported by others.84
Immunity to CMV antigens is likely dependent on the donor's pre-existing CMV precursor frequency. If the pp65 response already predominated over IE (Table 2, donors 010 and 011), the post-IVS expansion will generate a proportionally greater frequency of pp65-specific T cells than IE. The converse was also found (Table 2, donor 009). These represent the first published examples of simultaneous stimulation by a recombinant vaccine of CD8+ T-cell response to different CMV antigens. A recombinant chimeric IE1-pp65 protein succeeded in stimulating CD8+ T cells against pp65, however, the protein was unable to expand IE1-specific CD8+ T cells.61 The use of both pp65/pp150-MVA and IE4-MVA is feasible, since frequencies of CD8+ T cells that are stimulated by the viruses and directed against IE1 and pp65 are of similar magnitude. rMVA as a mixture is preferable for both immunotherapeutic and vaccination approaches, since some individuals recognize either IE1 or pp65.85,86 Adoptive cell therapy infusions of CMV-specific rMVA-expanded polyclonal T cells or rMVA immunization strategies are a promising path to reveal whether host response to IE1, pp65, and pp150 are protective against infection. In summary, our results suggest that unmodified CMV-pp65, IE4, and pp150 antigens expressed from MVA are the preferred virus for in vivo immunizations, and in any situation where VV is a risk to patients because of its virulence.
Prepublished online as Blood First Edition Paper, April 15, 2004; DOI 10.1182/blood-2003-10-3469.
Partially supported by grants from the National Cancer Institute (NCI; RO1-CA77544 and PO1-CA30206, Project III) to D.J.D. and a subcontract from AI52065 (M.C.V. and D.J.D.). The City of Hope Comprehensive Cancer Center is supported by the NCI (CA33572). D.J.D. and S.F.L. are partially supported by Translational Research Awards from the Leukemia and Lymphoma Society (LLS). The Laboratory of Vaccine Research (LVR) is partially supported by The Edwin and Bea Wolfe Charitable Foundation. W.J.B. is partially supported by R01 AI49537. Support for the City of Hope General Clinical Research Center (GCRC) (satellite of the University of Southern California GCRC) is from the National Institutes of Health (NIH; MO1-RR0043-38).
Z.W. and C.L.R. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We appreciate the kind gift of plasmid vectors from L. S. Wyatt and B. Moss of the Laboratory of Viral Diseases (NIH, Bethesda, MD). Advice on construction of rMVA from L. S. Wyatt is gratefully acknowledged. Plasmids containing modified HLA A2 genes and β2 microglobulin (β2M) were generously provided under MTA by Beckman-Coulter Immunomics. IL-2 was the gift of the NIH AIDS Research and Reference Reagent Program. HHD II mice have been kindly provided by F. Lemonnier under the Material Transfer Agreement (MTA) with the Institut Pasteur (Paris, France). The expert technical assistance of John Brewer and Rebecca Maas is gratefully acknowledged. We received assistance in volunteer recruiting and scheduling by GCRC personnel including Brenda Williams, RN, Joanne Shifflet, and Maria Madrigal. Maintenance and husbandry of HHD II mice were provided by the City of Hope Parvin Vivarium under the direction of Dr Richard Ermel. Dr John A. Zaia (COH), whose advice on clinical matters and collegial interactions has improved our understanding of CMV-host interactions, is gratefully acknowledged. The assistance of Julia Santos in the administration of the laboratory has greatly aided the completion of this work. Donna Packer is gratefully acknowledged for preparation of the manuscript.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal