Abstract
Immunosuppression after organ transplantation is an acknowledged risk factor for skin cancer and lymphoma. We examined whether there was also an excess of leukemia in patients after transplantation and whether this might be related to a particular immunosuppressive treatment. Data from more than 170 000 patients indicated that organ transplantation is associated with a significantly increased risk for acute myeloid leukemia (AML). AML was more frequent after heart transplantation and lung transplantation than after kidney transplantation and was associated with immunosuppression by azathioprine, a thiopurine prodrug. Cellular resistance to thiopurines is associated with DNA mismatch repair (MMR) deficiency. We demonstrate that thiopurine treatment of human cells in vitro selects variants with defective MMR. Consistent with a similar selection in patient bone marrow, in 7 of 7 patients, transplant-related AML/myelodysplastic syndrome (MDS) exhibited the microsatellite instability (MSI) that is diagnostic for defective MMR. Because MSI occurs infrequently in de novo AML, we conclude that the selective proliferation of MMR-defective, azathioprine-resistant myeloid cells may contribute significantly to the development of AML/MDS in patients who have received organ transplants. Identifying azathioprine as a risk factor for AML/MDS suggests that discontinuing the use of azathioprine as an immunosuppressant might reduce the incidence of posttransplantation AML/MDS.
Introduction
The immunosuppression required to prevent the rejection of a transplanted organ is associated with an increased risk for cancer. Skin carcinoma, non-Hodgkin lymphoma, and other cancers are more frequent among transplant recipients.1-3 Part of the increased cancer risk reflects immunosuppression and is paralleled by a high incidence of malignancies in HIV-immunocompromised patients (for reviews see Penn1 and Mueller4 ). Long-term treatment with drugs may also be a risk factor for cancer. The ability of therapeutic drugs to inflict DNA damage may contribute to the increasing incidence of therapy-related malignancy, particularly therapy-related acute myeloid leukemia/myelodysplastic syndrome (tAML/MDS) (for a review, see Leone et al5 ).
Azathioprine is a thiopurine prodrug that is related to 6-mercaptopurine and 6-thioguanine (6-TG).6 Azathioprine is widely used as an immunosuppressant in recipients of organ transplant, often in conjunction with cyclosporin A and steroids. Although the mechanism of action of the thiopurines is not completely understood despite more than 3 decades of clinical use, the cell's DNA mismatch repair (MMR) system is known to be an important cofactor. It is widely accepted that the formation of thiopurine nucleotides and the eventual incorporation of 6-TG into DNA underlie the cytotoxicity and therapeutic effect of thiopurines (for a review, see McLeod et al7 ). Although substituting DNA with 6-TG is not, in itself, particularly detrimental, nonenzymatic methylation of DNA 6-TG generates DNA lesions.8 The rare DNA 6-thiomethylguanine (6-meTG) bases are then processed by MMR.9 MMR-dependent processing is linked to apoptosis, and inactivation of repair provides an escape from thiopurine-induced cell death. Thus, MMR-deficient cells can tolerate 6-meTG in their DNA, and their resistance to killing by 6-TG is well documented. MMR performs similar lethal processing on the structurally related DNA base, O6-methylguanine, that is produced when cells are treated with alkylating agents. Repair defective cells are also tolerant to N-methyl-N-nitrosourea (MNU), N-methyl-N′-nitro-N-nitrosoguanidine, and their clinical counterparts, such as temozolomide (for a review, see Karran10 ).
The principal function of MMR is to edit replication and to reverse DNA polymerase errors. Most correction events in human cells involve 2 heterodimers: hMutSα, comprising the hMSH2 and hMSH6 proteins, and hMutLα (hMLH1 and hPMS2). Both complexes participate in locating and replacing mismatched DNA tracts (for a review, see Buermeyer et al11 ), and both are required for the toxicity of DNA 6-meTG and O6-methylguanine. Inactivation of any of the 4 constituent proteins confers substantial resistance to 6-TG and to alkylating agents.
Inactivation of MMR greatly increases spontaneous mutation rates. This mutator effect is observed as microsatellite instability (MSI+), an accumulation of frameshiftlike mutations that change the lengths of DNA regions comprising extended tracts of mononucleotides or dinucleotides.12-14 MSI+ is diagnostic for defective MMR and is associated with familial and sporadic malignancy. It is widespread in certain types of cancer—for example, colorectal and endometrial carcinoma—but is rare in many others, including primary leukemia.15-17 Increasing evidence indicates, however, that MSI is common in AML/MDS that develops secondary to cancer therapy.18-20
In experimental model systems, methylating agents are used to select drug-resistant, MMR-defective variants from sensitive cell populations. Cells with defective hMLH1, hPMS2, hMSH6, or hMSH2 have been selected from human cell cultures using MNU or N-methyl-N′-nitro-N-nitrosoguanidine.21,22 These variants display the properties of cells derived from MMR-deficient tumors. In particular, they are MSI+, stably resistant to 6-TG, and cannot correct DNA mispairs in biochemical assays. In many cases, they do not express one of the key MMR proteins. Our hypothesis20 is that selection for drug resistance may be a factor in the development of tAML/MDS. Here, we adduce evidence that thiopurine treatment may contribute significantly to the development of tAML/MDS after organ transplantation by facilitating the selective expansion of MMR-deficient clones. First, we show that organ transplantation is a significant risk factor for AML and that this is associated with azathioprine treatment. Second, we show that chronic thiopurine treatment of repair-proficient cultured human cells selects drug-resistant clones that are MMR deficient. Third, we demonstrate that the characteristic MSI+ phenotype of MMR-defective cells occurs frequently in AML/MDS in recipients of organ transplants.
Patients, materials, and methods
Population studies
AML incidence. Since 1985, information on recipients of organ transplants, including details of treatment, has been collected from more than 300 transplantation centers participating in the Collaborative Transplant Study. Data from clinical follow-up, including cancer development, were obtained at 3, 6, and 12 months after transplantation and at annual intervals thereafter. All cancer incidence data were verified annually by means of a questionnaire. Only data on AML or MDS that arose during the retention of a functional graft have been included. Incidence data for AML were compared using Kaplan-Meier analysis. Data for expected AML incidence were obtained from a cohort of identical size, matched for age and sex, from the Cancer Incidence in Five Continents standard. This cohort was monitored for the same duration as the transplant cohort. The most appropriate regional reference registry was used for each transplant patient.
Data. Data collection and processing were approved by the Data Protection Agency in Germany, and all participating centers complied with local ethical and privacy legislation.
Cell culture studies
Cell culture and drug treatment. MNU and O6-benzylguanine (O6-bzGua) were gifts from Professor Peter Swann (University College London, London, United Kingdom) and Dr Jurgen Thomale (University of Essen, Essen, Germany). DLD-1, SW48, HCT116, A2780 (obtained from Professor Robert Brown; Cancer Research UK Beaston Research Institute, Glasgow, United Kingdom), and its hMLH1-defective variant, A2780MNU1, were cultured as described.23,24 A subclone (A2780SCA5) of A2780 was isolated and grown in medium containing 100 μM hypoxanthine (HPRT), 400 nM aminopterin, and 160 μM thymidine (HAT) for 10 days to remove preexisting 6-TG resistant HPRT- mutants. Then, 200 to 500 purged A2780SCA5 cells were seeded in normal medium into five 25-cm2 flasks and treated with 6-TG (100 ng/mL) for 7 days. The 6-TG concentration was then increased to 400 ng/mL and to 1 μg/mL 14 days later. After 1 month of continuous proliferation in 1 μg/mL 6-TG, the contents of each flask were cloned and screened for growth in HAT medium and MNU resistance. Throughout the culture, cells were maintained at subconfluence.
Screening for methylation tolerance. Clones were screened for HAT and MNU resistance. Each clone (200 cells) was plated in duplicate into 6-well plates. One well was supplemented with HAT. To the other, O6-bzGua (25 μM) was added to inactivate the O6-methylguanine–DNA methyltransferase that confers MNU resistance, followed 2 hours later by MNU (200 μM). Colonies were scored 10 days later. Under these conditions, survival of A2780SCA5 is less than 1% and survival of MMR-defective A2780MNU1 is greater than 95%.22 Wells containing more than 40 colonies (more than 50% survival) were designated MNU resistant. Appropriate clones were retrieved from frozen stocks for further investigation. Cloning efficiency and growth rates in normal and HAT medium were similar for parental cells and drug-resistant clones.
Cell survival. Sensitivity to 6-TG and MNU was determined by clonal assay.25 Briefly, exponentially growing cells were seeded into 6-well plates and were treated with MNU in the presence of O6-bzGua (25 μM). For 6-TG the cells were continuously exposed to drug. Colonies were scored after 7 days' growth. Fold resistance was determined from the ratio of D37 (the drug concentration required to reduce survival to 37%) doses.
Mismatch repair assays. RNA prepared from resistant clones was analyzed by reverse transcription–polymerase chain reaction (RT-PCR) for the expression of 5 MMR genes, and the levels of the hMLH1, hPMS2, hMSH2, and hMSH6 proteins were determined by Western blotting, as previously described.26
Cell extracts were assayed for MMR.27 A 4.47-kilobase (kb) nicked circular DNA substrate containing a single C/T mispair was incubated with cell extract, recovered, and digested with MluI. Digestion products were separated by agarose gel electrophoresis. Uncorrected DNA contains a single MluI site; restitution of a C/G base pair by MMR creates a second MluI site, and the appearance of a 3.9-kb digestion product is diagnostic for repair.
Posttransplantation AML/MDS studies
Patient material. Bone marrow aspirates were examined after staining with May-Grünwald-Giemsa and Perl stains. Diagnosis of AML/MDS was based on the French-American-British (FAB) criteria.28,29 Explanted organs, bone marrow clots, and trephine biopsy samples were fixed in 10% formol saline and decalcified in EDTA (ethylenediaminetetraacetic acid). Histologic specimens were embedded in paraffin wax. Sections were stained with hematoxylin-eosin for cell morphology. Reticulin content of trephine biopsy samples was assessed by the method of Gordon and Sweets.30 Marrow clots were stained for iron using Perl Prussian blue. Full local research ethics approval was obtained from Harefield Hospital, and patient material was kept anonymous. Written consent was obtained from the 2 surviving patients.
Microsatellite analysis. DNA was recovered from paraffin block sections using the DNeasy kit (Qiagen, Valencia, CA). The standard microsatellites BAT26, BAT25, D2S123, D17S250, D5S346, and BAT40 were amplified by PCR using fluorescent primers.31 Products were analyzed on an ABI Prism 377 Sequencer (Applied Biosystems, Warrington, United Kingdom).
Results
AML in recipients of transplants
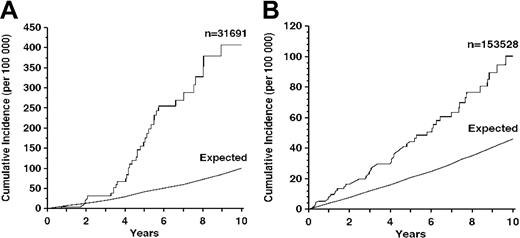
Organ transplantation was associated with an increased risk for AML (Figure 1). Thirty-seven of 31 691 recipients of heart or lung (or both) transplants and 56 of 153 528 recipients of renal transplants had AML. The relative risks for AML in transplant recipients compared with controls matched for age, sex, and geographic origin were 5.5 (95% confidence interval [CI], 4.0-7.7; P < .0001) for heart/lung patients (Figure 1A) and 2.1 (95% CI, 1.6-2.7; P < .0001) for kidney patients (Figure 1B). Transplant-related AML was more common in men. After heart/lung transplantation, 89.6% of patients in whom AML developed were male. For kidney transplantation, the figure was 63.7%. It seems likely that this bias simply reflects the predominance of men among transplant recipients (76.4% for heart/lung; 61.6% for kidney) (G.O. and Collaborative Transplant Study, unpublished data, January 2004). These figures refer only to AML and do not include patients with MDS. Because MDS generally precedes AML in this patient population,5 these are minimum values. (MDS data were excluded from this analysis because expected values cannot be computed from the Cancer Incidence in 5 Continents database. For all other analyses, we used the combined incidence of AML/MDS). It is notable that the excess of AML was less marked during the first 3 to 4 years after transplantation and that the observed incidence diverged sharply from the expected incidence thereafter. This apparent lag period distinguishes AML from the more common transplant-related non-Hodgkin lymphomas in which incidence is highest during the first posttransplantation year,3 suggesting that these secondary malignancies develop by different mechanisms.
AML in recipients of transplants. (A) Heart, lung, or both. Expected incidence in control subjects matched for age, sex, and geographic location is included for comparison. (B) Cadaver kidney. n indicates number of patients.
AML in recipients of transplants. (A) Heart, lung, or both. Expected incidence in control subjects matched for age, sex, and geographic location is included for comparison. (B) Cadaver kidney. n indicates number of patients.
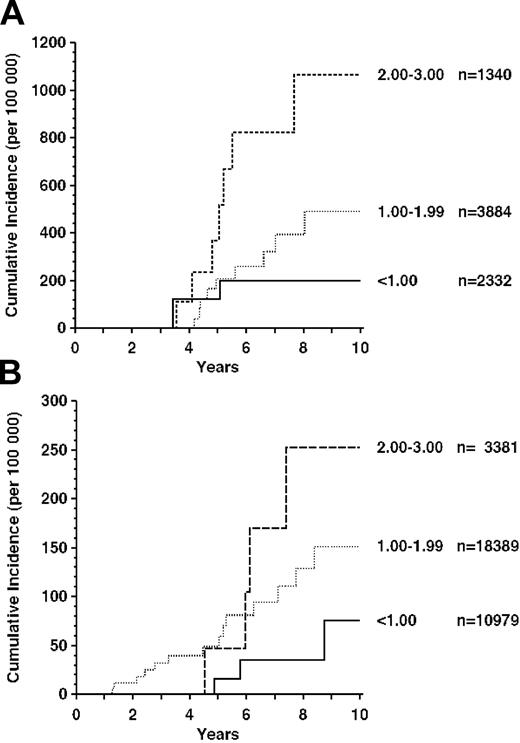
Most (90% of heart/lung and 60% of kidney transplant) patients had received the standard immunosuppression by azathioprine with or without cyclosporin A and steroids. The frequency of AML/MDS was approximately correlated with azathioprine dosage at 1 year after transplantation, when the dose is usually stable. The effect was particularly marked for recipients of heart/lung transplants (Figure 2A).
AML/MDS in recipients treated with azathioprine. (A) Heart, lung, or both. Azathioprine dosage (mg/kg/d) 1 year after transplantation is shown to the right of the curves. n indicates number of patients in each dosage category. (B) Cadaver kidney.
AML/MDS in recipients treated with azathioprine. (A) Heart, lung, or both. Azathioprine dosage (mg/kg/d) 1 year after transplantation is shown to the right of the curves. n indicates number of patients in each dosage category. (B) Cadaver kidney.
The incidence of AML/MDS was significantly higher in patients who received 2.0 to 3.0 mg/kg per day compared with the group who received less than 1.0 mg/kg per day (P = .031, weighted regression analysis). A similar trend was apparent for recipients of kidney transplants (Figure 2B), though this fell short of statistical significance (P = .136).
Mismatch repair defects in thiopurine-resistant cells
We examined the properties of human cells that had been selected for resistance to 6-TG treatment. Given that the myeloid precursor cells that probably represent the true target cell population for tAML/MDS are experimentally intractable, we used the A2780 cell line as a model. These cells are widely used in drug-resistance studies. They are HPRT+ and, therefore, are capable of salvaging 6-TG for incorporation into DNA. They are sensitive to methylating agents and to 6-TG. A subclone, A2780SCA5, was used to investigate the development of thiopurine resistance in vitro after exposure to escalating concentrations of 6-TG. After treatment, surviving A2780SCA5 cells grew at normal rates, even in the presence of 6-TG. Approximately 30 clones from each of 5 cultures were screened for HPRT deficiency and for resistance to MNU. All clones grew in HAT medium, indicating functional HPRT; most (140 of 154) were cross resistant to MNU.
MMR gene expression was analyzed in representative clones (Figure 3A). Most expressed detectable hMSH2, hMLH1, hPMS2, hMSH6, and hPMS1 mRNAs. However, hMLH1 mRNA was absent or diminished in several clones. Two examples of independent, nonexpressing clones, JA5 and JA8, are included in Figure 3A.
Characterization of 6-TG–resistant clones. (A) Expression of MMR genes. RNA from representative 6-TG– and MNU-resistant A2780SCA5 clones was amplified by RT-PCR. RNA was included from control cell lines Raji (MMR proficient), SW48 (hMLH1 deficient), LoVo (hMSH2 deficient), and DLD-1 (hMSH6 deficient). Products were separated using agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV light. *Clones JA5 and JA8 arose in different flasks. (B) MMR protein expression. Extracts from parental A2780SCA5, control MMR-defective cells, and JA5 and JA8 were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), blotted, and probed sequentially with antibody against hMLH1, hMSH2, hMSH6, and hPMS2. (C) Mismatch correction in vitro. Extracts (100 μg) of HeLa, A2780SCA5, JA5, or JA8 were incubated with a standard nicked circular DNA substrate containing a T/C mispair. DNA was recovered and digested with MluI, and products were separated using agarose gel electrophoresis. The arrow indicates a band that was diagnostic for correction. (D) 6-TG (left) and MNU (right) sensitivity of JA5 and JA8. Cells were treated as described in “Patients, materials, and methods,” and survival was determined by clonal assay. A2780SCA5 (♦); A2780MNU1 (▪); JA5 (▴); JA8 (•). Error bars represent standard deviations.
Characterization of 6-TG–resistant clones. (A) Expression of MMR genes. RNA from representative 6-TG– and MNU-resistant A2780SCA5 clones was amplified by RT-PCR. RNA was included from control cell lines Raji (MMR proficient), SW48 (hMLH1 deficient), LoVo (hMSH2 deficient), and DLD-1 (hMSH6 deficient). Products were separated using agarose gel electrophoresis, stained with ethidium bromide, and visualized under UV light. *Clones JA5 and JA8 arose in different flasks. (B) MMR protein expression. Extracts from parental A2780SCA5, control MMR-defective cells, and JA5 and JA8 were separated using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), blotted, and probed sequentially with antibody against hMLH1, hMSH2, hMSH6, and hPMS2. (C) Mismatch correction in vitro. Extracts (100 μg) of HeLa, A2780SCA5, JA5, or JA8 were incubated with a standard nicked circular DNA substrate containing a T/C mispair. DNA was recovered and digested with MluI, and products were separated using agarose gel electrophoresis. The arrow indicates a band that was diagnostic for correction. (D) 6-TG (left) and MNU (right) sensitivity of JA5 and JA8. Cells were treated as described in “Patients, materials, and methods,” and survival was determined by clonal assay. A2780SCA5 (♦); A2780MNU1 (▪); JA5 (▴); JA8 (•). Error bars represent standard deviations.
Independent clones that did not express measurable hMLH1 mRNA were analyzed further. The hMLH1 protein was not detectable in extracts of numerous clones, including JA5 and JA8 (Figure 3B and data not shown) but was present in extracts of parental A2780SCA5 cells. hPMS2, the heterodimer partner of hMLH1, was also undetectable in JA5 and JA8 extracts. hPMS2 is unstable in the absence of hMLH1,32 and this finding is consistent with a primary defect in hMLH1 gene expression in JA5 and JA8. The hMSH2 and hMSH6 proteins were present at similar levels in parental, JA5, and JA8 cells (Figure 3B).
Biochemical assays and microsatellite analysis confirmed that JA5 and JA8 were MMR defective. Unlike parental A2780SCA5 cell extracts, JA5 and JA8 cell extracts did not correct a C/T mispair in a standard substrate (Figure 3C). Analysis of the recommended panel of 5 mononucleotide and dinucleotide repeat microsatellites33 in JA5 and JA8 subclones, isolated after continuous growth for approximately 50 generations, confirmed an MSI+ phenotype. Two or more loci were altered in 14 (50%) of 28 JA5 subclones and in 20 (71%) of 28 JA8 subclones (data not shown). JA5 and JA8 exhibited stable resistance to methylating agents and thiopurines. They were, respectively, 39- and 34-fold more resistant to MNU and 6.7- and 5.4-fold more resistant to 6-TG than the parental A2780SCA5 cells (Figure 3D). In this regard, JA5 and JA8 resemble A2780MNU1, a previously characterized MMR-defective A2780 variant.34 Both clones also exhibited a 6-fold increased resistance to 6-mercaptopurine, but their sensitivity to cyclosporin A or prednisolone was unchanged (less than 2-fold difference; data not shown).
Thus, clones that survived chronic treatment with 6-TG had impaired hMLH1 gene expression that resulted in loss of the hMutLα repair complex. They were deficient in MMR in biochemical assays, MSI+, tolerant to methylating agents, and resistant to thiopurines. All (5 of 5) treated cultures yielded clones with this phenotype, and we conclude that thiopurine treatment permits the efficient clonal expansion of MMR-defective cells from an initially repair-competent population.
Microsatellite instability in transplant-related AML/MDS
MSI was analyzed in 7 recipients of organ transplants who had AML or MDS. Available treatment histories are presented in Table 1.
MSI analysis of patients with AML/MDS
. | Age at MDS, y . | Transplant type (year) . | Explant histology . | Clinical details . | Status . | Bone marrow findings . | Cytogenetic analysis . | Aza, mo . | Total Aza, mg/kg . | Other treatment . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | OCT (1995) | Coronary heart disease | Jan 2002 Pancytopenia | Alive | Jan 2002 Trilineage MDS (18% blasts) | del (6q), del (7q) | 14.7 | 275 | Steroids, FK506, ATG |
| 2 | 62 | SLT (1993) | Fibrosing alveolitis | 1999 Pancytopenia | Dead | Jul 1999 Trilineage MDS; Aug 1999 Trilineage MDS (7% blasts) | del (9q), –21 | 74 | 554 | CyA, steroids |
| 3 | 60 | OCT (1995) | Dilated cardiomyopathy | 1999 Acute gum swelling | Dead | Jun 1999 AML (FAB type M4Eo) | del (16q) | 43.4 | 1696 | CyA, steroids, ATG |
| 4 | 72 | OCT (1986) | Coronary heart disease | 1994 Pancytopenia | Dead | Aug 1994 Hypoplastic MDS (10% blasts); Apr 1995 MDS (26% blasts) | del (5q), –7, +8 | 91.6 | 3080 | CyA, steroids, TLI |
| 5 | 50 | HLT (1991) | Bronchiectasis | Unexplained anemia | Alive | Nov 1995 Acquired sideroblastic anemia (35% ringed sideroblasts); Jan 2003 (70% ringed sideroblasts) | del (17q) | 59.8 | 1325 | CyA, steroids, ATG |
| 6 | 59 | SLT (1989) | α-1 Antitrypsin deficiency | Pancytopenia | Dead | Hypoplastic trilineage dysplasia (18% blasts) | Complex karyotype with –5, –7 | 132 | 2571 | CyA, FK506, steroids, TLI |
| 7 | ND | CK | Glomerulonephritis | No details | Dead | AML | No details | No details | 50 mg/d | CyA, steroids |
. | Age at MDS, y . | Transplant type (year) . | Explant histology . | Clinical details . | Status . | Bone marrow findings . | Cytogenetic analysis . | Aza, mo . | Total Aza, mg/kg . | Other treatment . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | OCT (1995) | Coronary heart disease | Jan 2002 Pancytopenia | Alive | Jan 2002 Trilineage MDS (18% blasts) | del (6q), del (7q) | 14.7 | 275 | Steroids, FK506, ATG |
| 2 | 62 | SLT (1993) | Fibrosing alveolitis | 1999 Pancytopenia | Dead | Jul 1999 Trilineage MDS; Aug 1999 Trilineage MDS (7% blasts) | del (9q), –21 | 74 | 554 | CyA, steroids |
| 3 | 60 | OCT (1995) | Dilated cardiomyopathy | 1999 Acute gum swelling | Dead | Jun 1999 AML (FAB type M4Eo) | del (16q) | 43.4 | 1696 | CyA, steroids, ATG |
| 4 | 72 | OCT (1986) | Coronary heart disease | 1994 Pancytopenia | Dead | Aug 1994 Hypoplastic MDS (10% blasts); Apr 1995 MDS (26% blasts) | del (5q), –7, +8 | 91.6 | 3080 | CyA, steroids, TLI |
| 5 | 50 | HLT (1991) | Bronchiectasis | Unexplained anemia | Alive | Nov 1995 Acquired sideroblastic anemia (35% ringed sideroblasts); Jan 2003 (70% ringed sideroblasts) | del (17q) | 59.8 | 1325 | CyA, steroids, ATG |
| 6 | 59 | SLT (1989) | α-1 Antitrypsin deficiency | Pancytopenia | Dead | Hypoplastic trilineage dysplasia (18% blasts) | Complex karyotype with –5, –7 | 132 | 2571 | CyA, FK506, steroids, TLI |
| 7 | ND | CK | Glomerulonephritis | No details | Dead | AML | No details | No details | 50 mg/d | CyA, steroids |
Aza indicates azathioprine; OCT, cardiac transplant; SLT, single lung transplant; CK, cadaver kidney transplant; HLT, heart/lung transplant; ATG, antithymocyte globulin; CyA, cyclosporin A; TLI, total lymphoid irradiation; ND, not determined.
Six patients received thoracic organ (3 heart, 1 heart/lung, 2 lung) transplants. The seventh patient had received a cadaver kidney on 2 occasions. All had been treated with azathioprine (Table 1). Cytogenetic analysis at diagnosis revealed 3 examples of loss of all or part of chromosome 5, 7, or both. For patients 1 to 6, a biopsy sample of the explanted tissue was available as a source of matched nontumor DNA. The standard panel of microsatellites33 was analyzed, together with an additional mononucleotide repeat, BAT40. Examples are shown in Figure 4, and the results are summarized in Table 2. Two or more microsatellites were altered in each of the 6 bone marrow samples that were compared at all loci. These met the criteria for MSI positivity. There was also evidence of instability at BAT26 and BAT25, but not at BAT40, in the AML patient for whom there was no matched tissue. This was also designated MSI+ based on widely accepted criteria.35 The caspase 5 gene contains an A10 repeat that is frequently mutated in MMR-defective tumors.36 Two of the 7 AML patients had a heterozygous -1 base deletion in this sequence (A9/A10; Table 2), confirming their MSI+ status. Thus, 7 of 7 transplant-related AML/MDS patients were MSI+. This differs significantly from the frequency (0 of 28) of MSI+ we previously reported in patients with de novo leukemia20 (P < .0001; Fisher exact test). A precise estimate of the frequency of MSI+ in transplant-related AML/MDS must await the analysis of a larger number of cases. Our findings suggest, however, that the incidence is high and that often AML/MDS associated with organ transplantation is likely to be defective in MMR.
Microsatellite instability in AML/MDS from recipients of organ transplants. Examples of MSI in AML/MDS (patients 1, 5, and 6). DNA recovered from paraffin blocks of bone marrow (tumor) or explanted tissue (normal) was amplified at the indicated microsatellites. *Unstable loci.
Microsatellite instability in AML/MDS from recipients of organ transplants. Examples of MSI in AML/MDS (patients 1, 5, and 6). DNA recovered from paraffin blocks of bone marrow (tumor) or explanted tissue (normal) was amplified at the indicated microsatellites. *Unstable loci.
MSI in MDS/AML
Patient . | BAT26 . | BAT25 . | D2S123 . | D5S346 . | D17S250 . | Caspase 5 . |
|---|---|---|---|---|---|---|
| 1 | + | + | – | – | + | A10 |
| 2 | + | + | – | + | + | A9/A10 |
| 3 | + | + | + | + | + | A9/A10 |
| 4 | + | + | + | + | + | A10 |
| 5 | + | + | – | + | + | A10 |
| 6 | – | – | + | – | + | ND |
| 7 | + | + | — | — | — | A10 |
Patient . | BAT26 . | BAT25 . | D2S123 . | D5S346 . | D17S250 . | Caspase 5 . |
|---|---|---|---|---|---|---|
| 1 | + | + | – | – | + | A10 |
| 2 | + | + | – | + | + | A9/A10 |
| 3 | + | + | + | + | + | A9/A10 |
| 4 | + | + | + | + | + | A10 |
| 5 | + | + | – | + | + | A10 |
| 6 | – | – | + | – | + | ND |
| 7 | + | + | — | — | — | A10 |
+ indicates unstable; –, stable; ND, not determined; and —, not applicable.
Discussion
There are sporadic case reports of AML/MDS in recipients of organ transplants (see, for example, Ferry et al37 and Subar et al38 ), and Huebner et al39 have described 5 within a single institution. Our analysis of more than 170 000 patients provides unequivocal evidence that organ transplantation and long-term immunosuppression are associated with an increased risk for this disease. AML was less common in recipients of kidneys, though it was still significantly more frequent than in controls who did not undergo transplantation. This is consistent with previous findings of a generally lower incidence of cancer in recipients of kidney transplants. Possible links between immunosuppressive drug dosage and non-Hodgkin lymphoma in the 2 groups have been discussed.3 Diagnostic and therapeutic radiation is also a potential risk factor for tAML/MDS, and there is evidence that radiotherapy might increase the risk for chemotherapy-related AML/MDS (discussed in Pagano et al40 ). Although the Collaborative Transplant Study registry does not provide detailed information about posttransplantation radiation exposure, recipients of thoracic organ transplants generally undergo more diagnostic radiation procedures, such as coronary angiography. In addition, a small fraction of patients receive therapeutic total lymphoid irradiation (TLI) for refractory rejection. The finding that 2 of 5 recipients of thoracic organ transplants who acquired tAML/MDS had undergone TLI is consistent with the increased risk and suggests that a more detailed investigation of the role of radiation as a cofactor in chemotherapy-related tAML/MDS might be warranted.
Some evidence links thiopurine treatment to the development of AML/MDS. Patients with low thiopurine methyltransferase (TPMT) activity cannot catabolize thiopurines efficiently. When they are treated with thiopurines, they accumulate abnormally high levels of the thioguanine nucleotides that are the precursors of DNA damage. These patients have significantly increased risk for tAML/MDS after thiopurine treatment,41 suggesting that thiopurine-induced DNA damage may contribute to cancer development. tAML/MDS also occurs in patients treated with thiopurines for nonmalignant conditions (see, for example, Krishnan et al42 and Heizer and Peterson43 ; for a review, see Karran et al44 ). Immunosuppression by thiopurines might contribute to AML/MDS simply by providing a permissive environment for the clonal expansion of altered cells, but it does not provide a ready explanation for the high frequency of MSI in our transplant-related cases. To date, apart from de novo AML in elderly patients,45 reports of MSI+ AML/MDS have been largely confined to cases secondary to alkylating agent treatment. Thiopurines and alkylating agents differ markedly in their structures and mechanisms of action, but they share the ability to produce DNA damage that interacts with MMR. The cross-resistance of MMR-deficient cells to both types of drug is well established, and this provides a plausible link between transplant- and chemotherapy-related MSI+ AML/MDS. In addition to a high frequency of MSI, alkylation- and immunosuppression-related AML/MDS share other features that are consistent with a common cause. For example, loss of all or part of chromosome 5 or 7 is often apparent in AML/MDS secondary to alkylating agents.5,46 Deletions affecting the same chromosomes are frequently noted in case reports of solid organ transplant–related AML/MDS. Interestingly, in 3 of our 6 patients who underwent thoracic transplantation and acquired AML/MDS and for whom cytogenetic data were available, chromosome 5 or 7 was involved. None of these patients had been treated with alkylating agents.
Alkylating agents and thiopurines also share a dose-limiting myelotoxicity.47,48 Treatment with these drugs will, therefore, recapitulate in the bone marrow the conditions that we have shown favor the outgrowth of MMR-deficient cultured cells. Recent evidence indicates that this selection can be effected in situ in myeloid cells. Alkylating agent treatment enriches the fraction of repair-defective cells in mouse bone marrow reconstituted with mixtures of normal and msh2-/- cells.49 Similar experiments have not been carried out with thiopurines, though their attested myelotoxicity suggests that azathioprine treatment is also likely to provide a considerable selective pressure for the emergence of MMR-defective myeloid cell clones.
The expansion of an MMR-deficient clone represents an early step in the development of MSI+ AML/MDS. Progression to malignancy requires the acquisition of mutations in genes controlling cell proliferation, death, or both. The absence of MMR in the MSI+ myeloid clones would increase the probability of mutation in 2 ways. MSI+ cells have an intrinsic mutator phenotype and accumulate mutations because of uncorrected replication errors. MSI+ cells are also hypermutable by DNA-damaging drugs,50 including alkylating agents and thiopurines.51 The increased susceptibility to mutation by drug treatment may be particularly significant in the case of lifelong posttransplantation immunosuppressive treatment.
In summary, our findings demonstrate that organ transplantation/immunosuppression is a significant risk factor for leukemia and that the AML/MDS that develops in recipients of transplants is likely to be MSI+. The relationship between azathioprine dose and transplant-related AML/MDS and the clear association between thiopurine resistance and MSI+ in cultured cells suggest a plausible mechanism by which transplant-related MSI+ AML/MDS might develop. Our study supplies reasonable grounds to implicate azathioprine in the development of AML. This finding may have implications beyond organ transplantation. In addition to their use as immunosuppressants in patients who have undergone transplantation, thiopurines are widely used to treat common, non–life-threatening inflammatory disorders. Although the incidence of leukemia among these patients has not been examined systematically, there are case reports of AML/MDS, many of which mention chromosome 5 or 7 alterations.42,43,52,53 We suggest that the ability of azathioprine to promote the clonal expansion of rare MMR-defective myeloid cells is consistent with a role in the development of tAML/MDS. This implies that the incidence of immunosuppression-related AML/MDS might be reduced by replacing azathioprine with a nonthiopurine alternative, such as mycophenolate, sirolimus, or everolimus.54-56
Prepublished online as Blood First Edition Paper, April 15, 2004; DOI 10.1182/blood-2003-11-3938.
Supported in part by a B'nai B'rith Leo Baeck Lodge Scholarship (J.O.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all our colleagues in the Collaborative Transplant Study who provided data, and we particularly thank Prof G. Kirste for patient material. We thank Prof R. Brown, Prof P. Swann, and Dr J. Thomale for providing materials, Dr P. Maddox for help with processing the clinical samples, and Prof P. Swann for helpful discussions. We also thank the staff of the Cell Services, Oligonucleotide Synthesis, and the Equipment Park of the Cancer Research UK London Research Institute.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal