Abstract
The lymphoid follicle is a specialized microenvironment for the differentiation of antigen (Ag)–activated B cells; the major stromal cell components in lymphoid follicle are the follicular dendritic cells (FDCs). At the same time, most of the B-cell lymphomas originate from the germinal center, and the generation and blast transformation of B-cell lymphoma occurs in close association with FDCs in the early stage of tumorigenesis. To study the functional roles of FDCs in lymphomagenesis, we established an inducible tumor model. The human B-cell lymphoma cell line, L3055, formed solid tumors only when inoculated with an FDC line, HK. In addition, 2 FDC-signaling molecules (FDC-SMs), a novel protein 8D6 and 4G10/CD44, are required for tumor formation in vivo, because monoclonal antibodies (mAbs) specific to these 2 proteins inhibited lymphomagenesis completely when they were inoculated with L3055 and HK cells. However, these 2 FDC-SMs have distinct functional roles in tumor formation. FDC-SM-8D6 enhances L3055 cell proliferation, whereas FDC-SM-4G10/CD44 inhibits its apoptosis. Identification of the functional roles of these critical FDC-SMs may lead to the discovery of therapeutic drugs that suppress the survival and growth of lymphoma cells.
Introduction
B-cell lymphomas emerge in the germinal center (GC) by selection for additional genetic changes or through adaptation to the protumorigenic environment provided by the microenvironment. The stromal cells in the adjacent tissue provide a supporting microenvironment for the survival and proliferation of the malignant transformed cells in the early stage of tumorigenesis. However, the molecular events that endow the lymphoma cells with tumorigenic competence and the function of the stromal cells in lymphomagenesis are not clearly understood.
The GC of lymphoid follicles is a microenvironment where antigen (Ag)–activated B cells undergo clonal expansion and selection to differentiate into plasma cells and memory B cells.1,2 Follicular dendritic cells (FDCs), derived from non–bone marrow origin, are stromal cells localized in the GC. Reticular networks formed by FDCs play critical roles in the organization of local microenvironments. GC formation requires the cellular interaction among FDCs, activated B and T cells in the milieu of appropriate cytokines.
We have described the distinct roles of T cells and FDCs in the proliferation and differentiation of human GC B cells.3 Activated T cells induce differentiation of centroblasts by providing CD40L and lymphokines. FDCs rescue GC B cells from apoptosis and stimulate their proliferation.4,5 We have identified a novel growth factor, FDC-signaling molecule (SM)–8D6, for GC B cells.6 This factor stimulates GC B-cell proliferation in collaboration with CD44.7 However it is not known whether and how these factors mediate lymphoma formation in vivo.
We previously showed that a Burkitt lymphoma cell line, L3055, recapitulates typical features of centroblasts, and the subclone generated from the original L3055 cell line proliferates in vitro continuously in the presence of FDC or an FDC line, HK, whereas they undergo apoptosis in the absence of FDC/HK cells.8
In this paper, we report that in an inducible tumor model a novel FDC-SM-8D6 collaborates with FDC-SM-4G10/CD44 in supporting tumor formation of a centroblast-like cell line, L3055. FDC-SM-8D6 enhances L3055 cell proliferation, whereas FDC-SM-4G10/CD44 inhibits its apoptosis.
Materials and methods
Antibodies and reagents
Antibodies (Abs) used in this study were as follows: purified mouse immunoglobulin G1 (IgG1; clone MOPC-21) and biotin-conjugated mouse anti-Flag were purchased from Sigma Chemical (St Louis, MO); biotin-conjugated mouse IgG1 and purified mouse anti–human FDC monoclonal Ab (mAb) DRC-1 (IgM, clone R4/23) from DAKO (Carpinteria, CA); fluorescein isothiocyanate (FITC)–or phycoerythrin (PE)–conjugated mouse IgG1 (clone MOPC-21), FITC- or PE-conjugated goat anti–mouse immunoglobulin, FITC- or PE-conjugated mouse anti–human CD20 (IgG1, clone 2H7 or L27), purified mouse anti–human CD44 (IgG1, clone L178), and FITC-conjugated mouse anti–human CD38 (IgG1, clone HIT2) from BD Pharmingen (San Diego, CA); biotin-conjugated mouse anti–human CD38 (IgG1, clone LMHCD3815) from CALTAG Laboratories (Burlingame, CA); purified mouse anti–human CD44 (IgG1, clone NK1-P1) from Dr C. G. Figdor (University of Nijmegen, The Netherlands); and FITC-conjugated mouse anti–human CD44 mAb (IgG1, F10-44-2) from Biosource International (Camarillo, CA). FDC-specific murine mAbs 8D6, 4G10, and 3C8 (all IgG1) used in this study were generated as described previously.6 PE-conjugated streptavidin was purchased from BD Pharmingen. SNARF-1 (SNARF-1 carboxylic acid, acetate, succinimidyl ester), 3, 3′-dihexyloxacarbocyanine iodide (DiOC6(3)), Alexa-Fluor 488 labeling kit, Pro-Long AntiFade mounting medium, and 4′, 6-diamidino-2-phenylindole, dilactate (DAPI) were purchased from Molecular Probes (Eugene, OR).
FDC-SM-8D6–transfected NIH 3T3 (8D6-3T3) cell preparation
The FDC-SM-8D6 was previously expression cloned from an HK cell cDNA library using mAb 8D6 and identified as a novel protein of 282 amino acids.6 Flag tag fused FDC-SM-8D6 cDNA was cloned into the modified pEF/myc/cyto vector (Invitrogen, Carlsbad, CA). NIH 3T3 (3T3) cells were transfected with this vector by using Lipofectamine (Invitrogen), and stable clones were established by selection with antibiotic G418 sulfate (Calbiochem, San Diego, CA).
Cell lines
HK cells were established and maintained as described previously.9 The L3055 cell line, originated from a patient with Burkitt lymphoma, was obtained from Dr Chris Gregory (Institute of Cell Signaling and School of Biomedical Sciences, University of Nottingham Medical School, Queen's Medical Centre, Nottingham, United Kingdom). L3055 cells were subcloned for the property of HK cell dependency and maintained on a layer of HK cells in Iscoves modified Dulbecco medium (Irvine Science, Santa Ana, CA) supplemented with 10% fetal calf serum (FCS; Sigma Chemical), 3 mM glutamine, 100 U/mL penicillin G, and 100 μg/mL streptomycin (Irvine Science). 3T3 and 8D6-3T3 cells were maintained in Dulbecco modified Eagle medium (Irvine Science) supplemented with 10% FCS, 3 mM glutamine, 100 U/mL penicillin G, and 100 μg/mL streptomycin. Antibiotic G418 sulfate (800 μg/mL) was added in the 8D6-3T3 cell cultures.
In vitro lymphoma cell proliferation assay
For proliferation experiments, HK, 3T3, and 8D6-3T3 cells were irradiated with 5000 rad and seeded (2 × 104 cells/well, if not otherwise indicated) 1 day before the addition of L3055 cells in 24-multiwell plates. For inhibition experiments, HK cells were pretreated with mAbs 3C8, 8D6, and/or 4G10 (20 μg/mL each, optimal dose) for 30 minutes at 37°C and then cocultured with L3055 cells (2 × 104 cells/well) in complete RPMI media. At the time of harvest, L3055 cells were collected, and viable cells were enumerated by counting the cells with intact morphology after staining with trypan blue. Contaminating HK cells were excluded in cell counting by their larger cell size.
Experimental animals and in vivo lymphomagenesis
Female, 8-week-old, BALB/c background nude mice (Jackson Laboratory, Bar Harbor, ME) were subcutaneously injected with lymphoma cells for tumor formation. For this purpose, L3055 and HK cells grown in a log phase were obtained from the in vitro culture. L3055 cells that had been maintained in the culture with HK cells were collected and divided into equal aliquots (2 × 106 cells) and injected with or without HK cells (1 × l06 cells) into mice subcutaneously. The viability of the cells was more than 95% immediately before inoculation, preventing the possible false-negative result. For Ab-blocking experiments, HK cells were pretreated with mAbs 8D6 and/or 4G10 (1 mg/mL) at 4°C for 30 minutes before mixing with L3055 cells. All cell suspensions (0.1 mL/injection site) were injected with a 25-gauge needle in the posterior flank of nude mice. At least 4 animals were used in each group. Tumorigenesis procedure was observed by measuring solid tumors in 3 dimensions twice a week with a caliper for 60 days. Tumor size was expressed in volume (volume = length × width × height, in mm3). A Student t test was used for statistical analysis (P values).
Fluorescence-activated cell sorting (FACS) analysis
The expression of cell-surface antigens was detected by FACS analysis. For staining, HK cells or 8D6-3T3 cells were released by using trypsin and EDTA (ethylenediaminetetraacetic acid), tumor cells were harvested from tumor biopsy 60 days after the inoculation, and L3055 cells were collected from in vitro culture. Single cell suspensions were stained with murine IgG1; mAbs 8D6, 4G10, and 3C8; anti-Flag Ab followed by FITC-conjugated goat anti–mouse immunoglobulin, and FITC-conjugated mouse IgG1, mAbs against CD44, CD20, and CD38. Flow cytometric analysis was carried out on a FACSCalibur (Becton Dickinson, San Jose, CA) with use of CELLQuest Pro software (Becton Dickinson). Dead cells were excluded by appropriate forward- and side-scatter gating.
Immunofluorescent microscopy
To evaluate tumor formation, a series of dermal biopsies were performed with a 4-mm diameter Dermal Biopsy Punch (Miltex Instrument, Bethpage, NY) at the injection sites on day 3, 7, 10, and 14 after inoculations. Biopsy tissues were prepared into cryosections or fixed in formalin and embedded with paraffin for tissue sections. Tissue slides were subjected to Hematoxylin and Eosin staining (formalin fixed) as well as immunohistochemistry staining (cryosections). For immunofluorescent staining, Alexa-Fluor 488–conjugated mAb 3C8 was prepared according to the manufacturer's instructions to detect HK cells. Biotin-conjugated CD38 followed by PE-conjugated streptavidin was used to locate L3055 cells, and DAPI was used for nuclei counter-staining. A Leica fluorescent microscope (Leica Microsystems Wetzlar GmbH, Watzlar, Germany) equipped with a digital camera and SPOT software (Diagnostic Instruments, Sterling Heights, MI) was used to evaluate tumor formation and specific cell location.
Soluble FDC-SM-8D6 (GST-8D6) preparation
To make recombinant GST-8D6 protein, the extracellular portion of 8D6 cDNA was cloned into a pGEX-3T vector. Purification of GST-8D6 protein was carried out according to the manufacturer's instructions (Amersham Biosciences AB, Uppsala, Sweden) after being produced in Escherichia coli. The purity of GST-8D6 protein was confirmed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Purified glutathione-S-transferase (GST) was used as control.
DiOC6(3) staining
DiOC6(3) is a fluorochrome, which incorporates into cells depending on their mitochondrial transmembrane potential. Viable cells are DiOC6(3) positive, showing yellow fluorescence, whereas apoptotic cells show a reduced uptake of DiOC6(3). To detect apoptotic cells, L3055 cells (1 × 104 cells/well) were cultured on a layer of HK cells (2 × 104 cells/well) in complete RPMI medium for 72 hours. DiOC6(3) (final concentration of 80 nM) was pulse added into cell culture 15 minutes before harvest. L3055 cells were then collected and resuspended in RPMI medium for cytometric analysis.
CD44-GFP–transfected HK cell and L3055 cell interaction
HK cells (1.5 × 105 cells per dish) were seeded 1 day before transfection on glass-bottom culture dishes (35 mm, MatTek, Ashland, MA) to obtain 70% to 80% confluence. Cells were transfected with CD44-GFP construct using Effectene Transfection Reagent (Qiagen, Valencia, CA). L3055 cells were labeled with 1 μM SNARF-1 and incubated on a layer of HK cells overnight. After collection, labeled L3055 cells were transferred onto CD44-GFP–transfected HK cells. Cells were then spun down at 327g for 5 minutes to obtain a better cell-cell contact. The supernatant was then removed, and the dishes were mounted with ProLong AntiFade mounting medium. Images were captured using a 63× oil objective lens at room temperature on a deconvolution microscope (Axiover 200; Zeiss, Göttinggen, Germany) and SlideBook software (Intelligent Imaging Innovations, Denver, CO).
Results
FDC-specific mAbs inhibit HK cell–mediated lymphoma cell growth in vitro
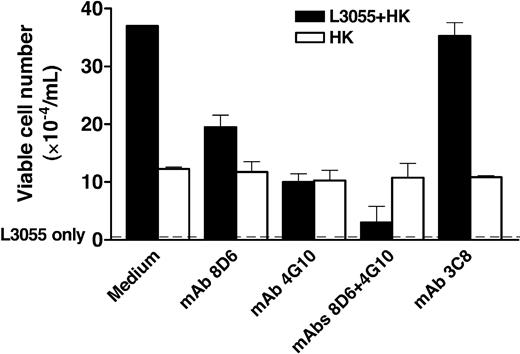
A Burkitt lymphoma cell line, L3055, has the phenotype of the GC centroblast. A subclone that requires FDC/HK cells for its growth was isolated from the original L3055 cell line.8 This subclone does not grow in the culture without FDCs or HK cells. We investigated whether FDC-SM-8D6 and FDC-SM-4G10/CD44 affect in vitro proliferation of L3055 cells by using mAbs 8D6 and 4G10. When added separately in the beginning of L3055 and HK cell coculture, both mAbs partially inhibited the growth of L3055 cells (Figure 1). However, when these 2 mAbs were added together to the culture, there was an additive effect in the cell growth inhibition. The reduction of L3055 cell recovery reflects the blocking of FDC-SMs by mAbs, because mAbs 8D6 and 4G10 had no cytotoxicity to HK cells. The addition of mAbs did not reduce the viable cell recovery of HK cells (Figure 1). An isotype-matched control mAb 3C8 (mouse IgG1)5 that binds FDCs and HK cells (Figure 2E) did not have any influence on L3055 or HK cell proliferation (Figure 1), confirming that specificity of mAbs 8D6 and 4G10 are directed to FDC-SMs. Inhibition did not appear to be limited to mAb 4G10 because other anti-CD44 mAbs (clones NK1-P1 and L178) showed similar results (data not shown).
FDC-specific mAbs 8D6 and 4G10 inhibit L3055 cell but not HK cell proliferation in vitro. Irradiated HK cells were pretreated with medium (control) and mAbs 3C8, 8D6, and/or 4G10 (20 μg/mL) as indicated and were then cultured with L3055 cells. Viable L3055 cell numbers were obtained at the end of a 4-day culture, and the results are shown as mean of triplicate ± SD (▪). No viable cells were recovered from the culture with L3055 cells alone (dashed line). In a separate experiment, HK cells (4 × 104 cells/well in 6-well plates) were cultured in the presence of indicated mAbs for 4 days before cell count (□). Data from 1 of 4 reproducible experiments is shown.
FDC-specific mAbs 8D6 and 4G10 inhibit L3055 cell but not HK cell proliferation in vitro. Irradiated HK cells were pretreated with medium (control) and mAbs 3C8, 8D6, and/or 4G10 (20 μg/mL) as indicated and were then cultured with L3055 cells. Viable L3055 cell numbers were obtained at the end of a 4-day culture, and the results are shown as mean of triplicate ± SD (▪). No viable cells were recovered from the culture with L3055 cells alone (dashed line). In a separate experiment, HK cells (4 × 104 cells/well in 6-well plates) were cultured in the presence of indicated mAbs for 4 days before cell count (□). Data from 1 of 4 reproducible experiments is shown.
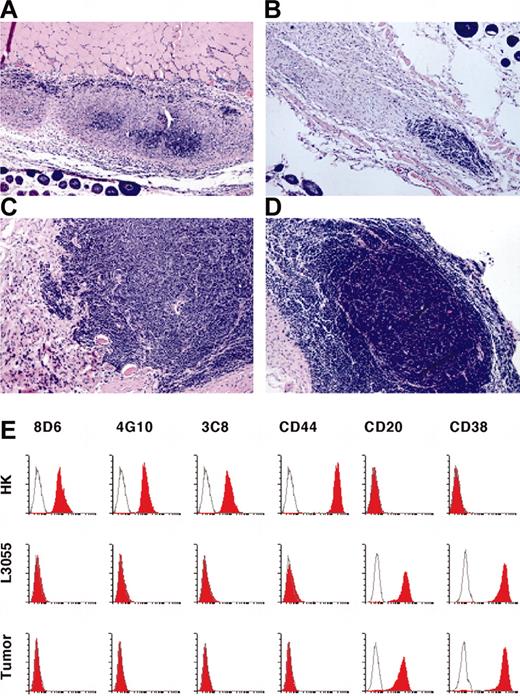
In vivo tumor formation. (A-D) Kinetics of tumor formation. L3055 and HK cells were inoculated in nude mice for tumor formation. Paraffin sections of tumor tissue were stained with Hematoxylin and Eosin on day 3 (A), 7 (B), 10 (C), and 14 (D) after the inoculation. Original magnification, × 200. (E) Cell-surface phenotype indicates that the tumor cells are derived from L3055 cells. HK, L3055, and tumor cells (60 days after the inoculation) were stained with murine IgG1 as a negative control (white histograms); mAbs 8D6, 4G10, 3C8, CD44, CD20, and CD38 (red histograms) are as indicated. Flow cytometric analysis was carried out on a FACSCalibur by using CELLQuest Pro software.
In vivo tumor formation. (A-D) Kinetics of tumor formation. L3055 and HK cells were inoculated in nude mice for tumor formation. Paraffin sections of tumor tissue were stained with Hematoxylin and Eosin on day 3 (A), 7 (B), 10 (C), and 14 (D) after the inoculation. Original magnification, × 200. (E) Cell-surface phenotype indicates that the tumor cells are derived from L3055 cells. HK, L3055, and tumor cells (60 days after the inoculation) were stained with murine IgG1 as a negative control (white histograms); mAbs 8D6, 4G10, 3C8, CD44, CD20, and CD38 (red histograms) are as indicated. Flow cytometric analysis was carried out on a FACSCalibur by using CELLQuest Pro software.
FDCs/HK cells are essential for lymphoma cell growth in vivo
To test whether FDCs support lymphomagenesis, we investigated the effect of HK cells on the growth of L3055 cells in vivo by adopting a tumor generation model in nude mice.10 When L3055 cells (2 × 106 cells/site) and HK cells (1 × 106 cells/site) were inoculated subcutaneously into nude mice, a palpable tumor mass grew only at the injection site within 3 weeks with no evidence of dissemination7 (Figure 3). In contrast, there was no tumor formation when either L3055 cells or HK cells alone were injected. The lack of tumor formation by L3055 cells alone did not result from poor cell viability or an insufficient number of injected cells, because more than 95% of L3055 cells were alive. No tumor was formed when an increasing number of L3055 cells (up to 4 × 106 cells) was injected. In addition, the failure of tumorigenesis was not attributed to the inoculation period of 60 days, because no tumor emerged even after 4 months. HK cell titration experiments showed that tumors could form, although delayed, when a lower dose of HK cells (5 × 105 cells/site) was inoculated with L3055 cells. When an increasing number of L3055 cells were injected with a fixed number of HK cells (1 × 106 cells/site), the time period required for tumor formation was shortened (data not shown). The effect of FDCs/HK cells on tumor formation in vivo was specific because fibroblast cells (ie, CD32-transfected L cells) did not support L3055 cell growth in vitro.6
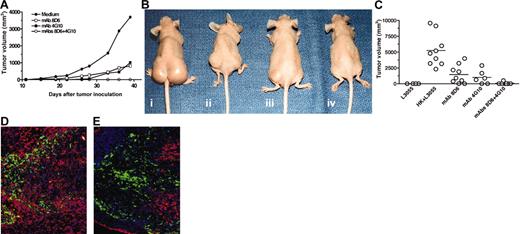
The combination of FDC-specific mAbs 8D6 and 4G10 inhibits FDC-mediated lymphomagenesis. (A-B) Kinetics of tumor formation. L3055 cells (2 × 106 cells) and HK cells (1 × 106 cells) were injected into nude mice in the presence of medium (i), mAb 8D6 (ii), mAb 4G10 (iii), and mAbs 8D6 plus 4G10 (iv) as indicated. A tumor growth chart (A) and tumor-bearing mice images taken 38 days after tumor cell inoculation (B) are shown. (C) A summary of in vivo tumor formation experiments shows tumor size and incidence. Horizontal bars indicate average tumor volume in each group. (D-E) Seven days after the inoculation of L3055 and HK cells with (E) or without (D) mAbs 8D6 and 4G10, cryosections of tissue from the injection site were prepared for immunofluorescent staining. Alexa-Fluor 488–conjugated mAb 3C8 (green) was used to locate HK cells in tumor tissue. Biotin-conjugated CD38 was used to detect L3055 cells followed by PE-conjugated streptavidin (red). Blue fluorescence dye DAPI was used for nuclei counterstaining. Original magnification, ×200.
The combination of FDC-specific mAbs 8D6 and 4G10 inhibits FDC-mediated lymphomagenesis. (A-B) Kinetics of tumor formation. L3055 cells (2 × 106 cells) and HK cells (1 × 106 cells) were injected into nude mice in the presence of medium (i), mAb 8D6 (ii), mAb 4G10 (iii), and mAbs 8D6 plus 4G10 (iv) as indicated. A tumor growth chart (A) and tumor-bearing mice images taken 38 days after tumor cell inoculation (B) are shown. (C) A summary of in vivo tumor formation experiments shows tumor size and incidence. Horizontal bars indicate average tumor volume in each group. (D-E) Seven days after the inoculation of L3055 and HK cells with (E) or without (D) mAbs 8D6 and 4G10, cryosections of tissue from the injection site were prepared for immunofluorescent staining. Alexa-Fluor 488–conjugated mAb 3C8 (green) was used to locate HK cells in tumor tissue. Biotin-conjugated CD38 was used to detect L3055 cells followed by PE-conjugated streptavidin (red). Blue fluorescence dye DAPI was used for nuclei counterstaining. Original magnification, ×200.
To examine tumor formation kinetics in the early stage, the tissues of the injection sites were examined at day 3, 7, 10, and 14 (Figure 2A-D). At day 7, a small viable tumor mass was observed. At day 10, tumors expanded in size. This process continued to day 14. Some growing tumors were surrounded by fibroblast-like cells forming a GC-like structure. No significant tumor cells were found at the site injected with L3055 cells alone after 7 days (data not shown), confirming that HK cells were required for tumor formation.
Flow cytometric analysis of the tumor cells showed that they originated from CD20+CD38+CD44-L3055 cells8 (Figure 2E). There is no difference in CD20 and CD38 expression between tumor cells and L3055 cells, indicating no change in cell differentiation status. These results suggest that FDCs/HK cells provide an essential microenvironment for tumor formation in vivo.
FDC-specific mAbs 8D6 and 4G10 inhibit HK cell–mediated lymphoma cell growth in vivo
To investigate the functional roles of FDC-SMs in tumor formation, mAbs 8D6 and/or 4G10 were coinoculated subcutaneously along with L3055 and HK cells. Figure 3A shows a growth chart of solid tumors by volume measured at different time points after the animals received different mixtures of cells and mAbs. Figure 3B shows the tumor-bearing mice at day 38. These results illustrate that solid tumors were formed within 3 weeks after the injection of L3055 and HK cells, and they grew in an exponential phase thereafter. Consistent with the in vitro results, injection of either mAb 8D6 (Figure 3A,Bii) or 4G10 (Figure 3A,Biii) (200 μg/site, optimal dose) inhibited or delayed tumor formation and decreased tumor size in comparison to that observed in control animals receiving no mAb (Figure 3A,Bi). Neither mAb completely inhibited tumor formation, suggesting that blocking either FDC-SM-8D6 or FDC-SM-4G10/CD44 alone may not be sufficient to block the FDC/HK signals for lymphomagenesis in vivo. No tumor was observed when both mAbs were added (Figure 3A,Biv). These results were reproducible because multiple experiments (Figure 3C) showed that there were significant differences in both tumor incidence and size between the control group (tumor formation 9 of 9) and the group injected with mAb 8D6 (tumor formation 7 of 9, P = .0014) or mAb 4G10 (tumor formation 4 of 6, P = .0023). The combination of mAbs 8D6 and 4G10 had a more remarkable inhibitory effect (tumor formation 1 of 7, P = .0001) on tumorigenicity of L3055 cells. These results are consistent with the additive inhibitory effect of mAbs 8D6 and 4G10 on in vitro growth of L3055 cells (Figure 1).
To analyze the cell populations in tumor tissue, in situ immunohistochemical examination of the tissues was performed. Cryosections of tumor tissues 7 days after injection were examined to investigate the effect of FDC-specific mAbs on tumor formation. The specific surface markers for HK cells (3C8+CD38-) and L3055 cells (3C8-CD38+) were used to distinguish these 2 cell populations. Although 3C8+ HK cell numbers were comparable between the injection sites with or without mAbs 8D6 and 4G10, there was a significant reduction of CD38+ L3055 cells at the injection site with mAbs (Figure 3D-E), where HK and L3055 cells were not in as close contact compared with the control tissue (Figure 3D). They tended to be segregated. In the area where HK cells were absent, L3055 cells underwent apoptosis. This result confirms the essential role of FDC-SMs for lymphoma formation by L3055 cells.
FDC-SM-8D6 is a lymphoma cell growth factor
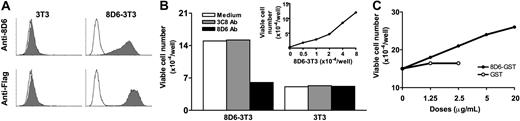
To demonstrate the function of FDC-SM-8D6 in lymphoma cell growth, FDC-SM-8D6 was expressed on 3T3 cells (8D6-3T3, Figure 4A). When 8D6-3T3 cells were used to coculture with L3055 cells, they supported L3055 cell growth in a dose-dependent manner. The supporting capacity of 8D6-3T3 cells was much less than that of HK cells (Figure 4B, insert, and Figure 1), suggesting that other factors on HK cells may be needed for lymphoma cell growth. This stimulatory effect is FDC-SM-8D6 specific because mAb 8D6 blocked L3055 cell growth induced by 8D6-3T3 cell, whereas a control mAb 3C8 showed no blocking effect. The wild-type 3T3 cell supported a minimum growth of L3055 cell. This background level of cell growth was not interfered by either mAbs.
Recombinant FDC-SM-8D6 supports L3055 cell growth. 8D6-3T3 cells were prepared as described in “Materials and methods.” (A) Transfected 3T3 cells express Flag tag-FDC-SM-D6. 8D6-3T3 cells were trypsinized and subjected to FACS analysis by using mAb 8D6 and anti-Flag Ab followed by FITC-conjugated goat anti–mouse immunoglobulin (solid histograms). 3T3 cells were used as a negative control. (B and insert) 8D6-3T3 cells stimulate L3055 cell growth. Different numbers of irradiated 8D6-3T3 and 3T3 cells were seeded in 24-multiwell plates 1 day before the addition of L3055 cells. Monoclonal Ab 8D6 (20 μg/mL) was used to block stimulatory effect of 8D6-3T3 cells, and mAb 3C8 was used as an isotype control (empty histograms). Viable L3055 cell number at day 4 from 1 of 3 reproducible experiments is shown. (C) Soluble FDC-SM-8D6 further enhanced HK cell–supported L3055 cell growth. L3055 cells (2 × 104 cells/well) were cocultured with HK cells (1 × 104 cells/well) in the presence of different doses of GST-8D6 or GST as indicated. Viable L3055 cells were counted at day 4. Data from 1 of 3 experiments are shown.
Recombinant FDC-SM-8D6 supports L3055 cell growth. 8D6-3T3 cells were prepared as described in “Materials and methods.” (A) Transfected 3T3 cells express Flag tag-FDC-SM-D6. 8D6-3T3 cells were trypsinized and subjected to FACS analysis by using mAb 8D6 and anti-Flag Ab followed by FITC-conjugated goat anti–mouse immunoglobulin (solid histograms). 3T3 cells were used as a negative control. (B and insert) 8D6-3T3 cells stimulate L3055 cell growth. Different numbers of irradiated 8D6-3T3 and 3T3 cells were seeded in 24-multiwell plates 1 day before the addition of L3055 cells. Monoclonal Ab 8D6 (20 μg/mL) was used to block stimulatory effect of 8D6-3T3 cells, and mAb 3C8 was used as an isotype control (empty histograms). Viable L3055 cell number at day 4 from 1 of 3 reproducible experiments is shown. (C) Soluble FDC-SM-8D6 further enhanced HK cell–supported L3055 cell growth. L3055 cells (2 × 104 cells/well) were cocultured with HK cells (1 × 104 cells/well) in the presence of different doses of GST-8D6 or GST as indicated. Viable L3055 cells were counted at day 4. Data from 1 of 3 experiments are shown.
Because 3T3 cell is a mouse fibroblast cell line that causes some background growth of L3055 cells through the feeder effect, the soluble form of FDC-SM-8D6, GST-8D6, was prepared as described in “Materials and methods.” GST-8D6 but not GST alone enhanced L3055 cell proliferation in a dose-dependent manner in the presence of a suboptimal dose of HK cells (Figure 4C). This soluble factor alone did not stimulate L3055 cell growth (data not shown). These results strongly suggest that FDC-SM-8D6 is indeed a growth factor for L3055 cells.
FDC-SM-4G10/CD44 is an antiapoptosis factor for lymphoma cell
CD44 is known to be involved in B-cell proliferation and antiapoptosis.11,12 DiOC6(3) is often used to detect mitochondria membrane potential, an indicator for apoptosis.13 When cells undergo apoptosis, DiOC6(3) staining becomes negative. Figure 5A shows that HK cells rescue L3055 cells from apoptosis in a dose-dependent manner. In the culture without HK cells, about 90% of L3055 cells underwent apoptosis in 72 hours, whereas in the presence of HK cells (2 × 104 cells/well), only 48% of cells showed apoptotic phenotype. The presence of mAb 4G10 increased the apoptotic cell percentage (Figure 5B), suggesting FDC-SM-4G10/CD44 could rescue L3055 cells from apoptosis. The addition of mAb 8D6 did not alter this ratio, confirming the previous results that mAb 8D6 did not interfere with the capacity of HK cells to prevent apoptosis of L3055 cells.6
FDC-SM-4G10/CD44 protects L3055 cells from spontaneous apoptosis. (A) HK cells rescue L3055 cells from spontaneous apoptosis. L3055 cells (1 × 104 cells/well) were cultured in the presence of different concentrations of HK cells for 72 hours as indicated. L3055 cells were pulse stained with DiOC6(3) 15 minutes before harvesting. DiOC6(3) staining was analyzed by FACS (▪), and viable L3055 cells were counted by a trypan blue exclusive assay (□). (B) Blocking of CD44 molecule augments L3055 cell apoptosis. L3055 cells (1 × 104 cells/well) were cultured with mAb-pretreated HK cells (2 × 104 cells/well) as indicated. DiOC6(3) staining was performed as described earlier. DiOC6(3)-negative cell percentages from 1 of 3 similar experiments are shown. (C) Localization of GFP-CD44 on HK cells where L3055 cells make contact (arrows). HK cells were transfected with GFP-CD44, and L3055 cells were labeled with SNARF-1 as described in “Materials and methods.” After centrifugation, cell-cell contact was investigated under a deconvolution microscope using SlidBook software. Original magnification, × 630.
FDC-SM-4G10/CD44 protects L3055 cells from spontaneous apoptosis. (A) HK cells rescue L3055 cells from spontaneous apoptosis. L3055 cells (1 × 104 cells/well) were cultured in the presence of different concentrations of HK cells for 72 hours as indicated. L3055 cells were pulse stained with DiOC6(3) 15 minutes before harvesting. DiOC6(3) staining was analyzed by FACS (▪), and viable L3055 cells were counted by a trypan blue exclusive assay (□). (B) Blocking of CD44 molecule augments L3055 cell apoptosis. L3055 cells (1 × 104 cells/well) were cultured with mAb-pretreated HK cells (2 × 104 cells/well) as indicated. DiOC6(3) staining was performed as described earlier. DiOC6(3)-negative cell percentages from 1 of 3 similar experiments are shown. (C) Localization of GFP-CD44 on HK cells where L3055 cells make contact (arrows). HK cells were transfected with GFP-CD44, and L3055 cells were labeled with SNARF-1 as described in “Materials and methods.” After centrifugation, cell-cell contact was investigated under a deconvolution microscope using SlidBook software. Original magnification, × 630.
To further analyze the antiapoptosis effect of CD44, a GFP-fused CD44 plasmid was constructed (GFP-CD44), and HK cells were transfected with GFP-CD44 to visualize CD44 expression by deconvolution microscopy (Figure 5C). GFP-CD44 localized on the surface of HK cells as expected. When SNARF-tracked L3055 cells were cocultured with GFP-CD44–transfected HK cells, GFP-CD44 clustered at the contact zone with L3055 cells. CD44 clustering was not observed on HK cell surface without L3055 cell contact. These data suggested an important functional role of CD44 in the cellular contact area.
Discussion
In this report, we demonstrate that FDCs are required for lymphoma formation in vivo by L3055 cells. L3055 cells alone are not tumorigenic in nude mice, and the addition of FDCs/HK cells adequately reconstitutes the microenvironment for tumor formation. Furthermore, 2 of the FDC-specific mAbs, 8D6 and 4G10, completely inhibited tumor formation. Seven days after inoculation, tumor cells staying in close contact with HK cells survived and proliferated, whereas L3055 cells underwent apoptosis in the presence of the blocking mAbs.
Monoclonal Abs 8D6 and 4G10 inhibit L3055 cell growth in vitro and tumor formation in vivo by blocking signaling molecules from HK cells. The inhibition is specific to blocking FDC-SMs rather than affecting HK cells, because (1) mAb 8D6 and 4G10 do not have any inhibition of HK cell growth in vitro; (2) the injection of the control mouse IgG1 or HK cell binding, non–blocking mAb 3C8 has no effect on tumor formation in vivo; and (3) it is not likely that HK cells are killed by Ab-dependent cytotoxic cells in nude mice because both mAbs are mouse IgG1 Abs (not IgG2a). These results suggest that FDC-SM-8D6 and FCD-SM-4G10/CD44 are required for L3055 cell growth in vitro and in vivo. However, the mode of action of these 2 FDC-SMs appears to be distinct.
FDC-SM-8D6 is a growth factor but does not have an antiapoptotic effect because mAb 8D6 did not stimulate apoptosis of L3055 cells in vitro. Furthermore, the growth-stimulating activity of FDC-SM-8D6 was shown by expressing FDC-SM-8D6 in 3T3 cells. 8D6-3T3 cells enhance L3055 cell growth compared with 3T3 cells. The supportive effect is specific because the growth stimulation is completely blocked by mAb 8D6 but not by mAb 3C8.
The FDC-SM-8D6 is expressed on the HK cell membrane surface as well as in the intracellular compartments.6 To distinguish the activity of soluble and membrane-bound FDC-SM-8D6s in L3055 cell growth, a recombinant GST-8D6 fusion protein was prepared. In both soluble and membrane form, FDC-SM-8D6 supported L3055 cell proliferation. The membrane form of FDC-SM-8D6 expressed on 3T3 cells seems more effective compared with its soluble form GST-8D6 fusion protein. In the absence of HK cells, GST-8D6 fusion protein did not sustain L3055 cell survival. It is possible that HK cells or 3T3 cells provide some feeder cell components in addition to the FDC-SMs, because 3T3 cells support a minimum level of L3055 cell growth. As reported previously, coinjection of fibroblast cells with tumor cells can enhance their tumorigenicity.10
CD44 is a class I transmembrane glycoprotein known to be expressed on most mammalian cells.14 The N-terminal domain outside the cell binds multiple components of the extracellular matrix, including hyaluronic acid (HA), collagen, laminin, and fibronectin. The cytoplasmic domain binding to cytoskeleton serves as a signal transduction site by associating with other proteins. CD44 has been implicated in a number of cellular functions such as adhesion, growth, differentiation, and apoptosis. Many of these functions are important in tumor progression. Disruption of CD44-HA interaction suppresses the in vitro growth of myeloma cells in the presence of bone marrow stromal cells.15,16 HA-binding peptides inhibit tumor progress in vivo.17,18 It is not clear how CD44-HA interaction stimulates tumor growth. Although CD44 is a signal transduction molecule for tumor cells in these studies, our experimental model is quite different in that L3055 cells are CD44- and interact with CD44+ FDCs/HK cells. It is possible that CD44+ FDCs/HK cells facilitate the growth of CD44- lymphoma cells through other cytokines.
The adherence of B cells to FDCs appears to be crucial for B-cell survival, because apoptosis of GC B cells is prevented by binding to FDCs.19 Although intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) are reported to mediate B-cell binding to FDCs,20 these surface molecules do not stimulate GC B-cell proliferation. Antiapoptosis function of CD44 expressed by FDCs is critical for lymphomagenesis, because (1) 90% of L3055 cells undergo apoptosis after 3 days of culture without HK cells and (2) blocking of CD44 signaling reduces HK cell capacity of rescuing L3055 cell from apoptosis.
The interaction, stimulation, and benefit between tumor cells and the tumor microenvironment are often mutual. A recent report indicates that tumor necrosis factor (TNF) functions in the maintenance of FDC networks by regulating its survival or death.21 Clustering of CD44 molecules on HK cell surface during tumorstromal interaction is one of the examples that the tumor cell not only gains help from its counterpart, it also sends signals to the stromal cell, modulating CD44 expression on HK cells. More investigations are under way to reveal the effects of L3055 cells on FDCs/HK cells. Although the cells in the microenvironment may not be genetically altered, their behavior can change through interactions with tumor cells. Both the tumor cells and their surrounding environment need to be fully characterized to understand how cancer grows in the body. Recently, it has been reported that a high incidence of glycosylation sites in the variable regions of the surface immunoglobulin was observed in follicular lymphoma and Burkitt lymphoma.22,23 It is intriguing to find the glycosylation site in these tumors, suggesting the selection of the tumors with this somatic mutation site in lymphomagenesis. Such a glycosylation site may interact with lectin on the stromal cells, protecting tumor cells from apoptosis. It would be interesting to investigate whether FDC-SMs such as CD44 and 8D6 interact with the glycosylation site of L3055 cells.
In summary, our data show that stromal cells prevent CD44-L3055 cells from apoptosis by providing a CD44 signal and stimulating L3055 cell growth via the growth factor FDC-SM-8D6. These results underscore the important collaboration between CD44 and growth factors of the stromal cells in tumor growth in vivo. Consistent with this notion, it has been reported recently that inhibition of apoptosis alone is insufficient to support tumor formation, whereas overexpression of ErbB2 oncogene in tumor cells promotes proliferation while suppressing apoptosis, which leads to tumor formation.24-26 The interaction between CD44R of B cells and CD44 of FDCs cooperates with FDC-SM-8D6 to augment the survival and proliferation of lymphoma cells. Indeed, it has been reported that integrins can collaborate with growth factors in phosphorylation of receptor kinase and subsequent activation of mitogen-activated protein (MAP) kinase.27
B-cell lymphomas often need stimuli supplied by other cells in the tumor microenvironment for survival and growth.28 Thus, identifying the functional roles of these critical FDC-SMs may lead to the discovery of therapeutic drugs that suppress the survival and growth of lymphoma cells. In addition, our inducible tumor model can be used to test the efficacy of the putative therapeutic reagents.
Prepublished online as Blood First Edition Paper, April 15, 2004; DOI 10.1182/blood-2004-01-0292.
Supported in part by grants from the National Institutes of Health (CA89057 [to L.L.] and CA092126 [to Y.S.C.]).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Leander Taylor, Russell G. Hendrick Jr, and Farheen Khan for excellent technical assistance and critical reading of the manuscript; Dr Chris Gregory for providing the L3055 cell line; Dr Clive Wood for helping clone FDC-signaling molecules; Dr Jawed Gill for pathologic advices; and Drs Chan-Sik Park and Chung-Gi Lee for comments.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal