Abstract
The high frequency of Kaposi sarcoma (KS) in immunodeficiency states, particularly in patients with AIDS, has been attributed to increased replication of KS-associated herpesvirus (KSHV), a necessary cofactor for KS development. However, experimental KSHV infection of endothelial lineage cells that compose KS lesions has been difficult even in the absence of immune cells. Here we show that HIV-1 Tat protein can directly promote KSHV transmission. Full-length HIV-1 Tat and a 13–amino-acid peptide corresponding to the basic region of Tat specifically enhances the entry of KSHV into endothelial and other cells, presenting evidence for an active role of HIV-1 in the development of KSHV-associated diseases. These results can explain why AIDS-KS is more frequent and clinically more aggressive than KS in other immunodeficiency states.
Introduction
Kaposi sarcoma (KS) is the most common neoplasm associated with HIV infection.1 KS-associated herpesvirus (KSHV) is the etiologic agent of KS,2 and it has been proposed that constant reactivation and reinfection by KSHV under immune deregulation may be necessary to sustaining the malignant state of this tumor.3 KS is more frequent and presents a more aggressive clinical course in AIDS than in other immunodeficiency states, suggesting that factors other than increased KSHV replication due to immunodeficiency are involved.4 KS lesions are characterized by neoangiogenesis and proliferating spindle cells of endothelial origin,5 including cells of lymphatic lineage.6 KSHV is constantly detectable in KS spindle cells,6 but experimental KSHV infection of endothelial cells has been difficult even in the absence of immune cells and using high multiplicities of infectious particles.7,8 The HIV-1 Tat protein is an 86– to 104–amino acid (aa) polypeptide, which is released by infected cells and regulates many viral and host functions.9,10 Recently, a 13-aa motif from the Tat basic region (BR) promoted entry of replication-deficient adenovirus into murine endothelial cells.11 Compared with adenovirus, KSHV is much larger in size and has a limited range of cell target infectivity.2 We tested whether HIV-1 Tat could contribute to overcome limitations in KSHV infectivity.
Materials and methods
Recombinant protein, peptides, and virus
An 86-aa recombinant protein of HIV-1 Tat was obtained from the National Institutes of Health (NIH) AIDS Research & Reference Reagent Program. HIV-1 Tat peptides corresponding to aa 48-60, GRKKRRQRRRPPQ, within the BR11 and aa 37-49, FITKALGISYGRK, within the core region (CR)9 were synthesized by Sigma Genosys (Woodlands, TX). Peptides were dissolved in phosphate-buffered saline (PBS; stock solution, 25 mM) and sterile filtered before use. A recombinant KSHV (rKSHV) expressing green fluorescent protein (GFP; a kind gift from Dr Jeffrey Vieira, University of Washington, Seattle) was prepared using tetradecanoyl phorbol acetate (TPA) as described previously.12 To harvest virus, the culture supernatant of BCBL-1 cells producing rKSHV-GFP was centrifuged at 15 000g for 4 hours; the pellet was resuspended in one-thirtieth of the original volume using serum-free OptiMEM (Invitrogen, Carlsbad, CA). The 30× concentrated virus stocks were stored at -80°C. rKSHV-GFP titer was estimated using HEK293 cells as described previously.12
KSHV infection
Human embryonic kidney (HEK) 293 (ATCC, Manassas, VA) and murine angiosarcoma ISOS-1 (the Institute for Fermentation, Osaka, Japan) were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM l-glutamine, and antibiotics. Primary human dermal microvascular endothelial cells (HDMECs; Emory University, Atlanta, GA) and human umbilical vein endothelial cells (HUVECs) were cultured as described previously.13,14 For infectivity assays, 1 mL rKSHV-GFP stock suspension (30x concentrated) was diluted with 4 mL OptiMEM, and this 5× virus suspension was incubated with the peptides or full-length Tat protein for 30 minutes at room temperature. The virus/peptide complex (300 μL/well) was added onto the cells in 12-well culture plates for 3 hours, followed by addition of the original growth media (1.2 mL) to the culture. To establish latent KSHV infection, 250 μg/mL G418 (Invitrogen) was added to the growth media. For immunofluorescent staining, infection was carried out in the same manner in gelatin-coated, 35-mm, glass-bottom plates (MatTek, Ashland, MA). Cell-to-cell KSHV transmission was induced as described previously8 by direct coculture of rKSHV-GFP–producing BCBL-1 cells on ISOS-1 cells. BCBL-1 cells were pretreated with 20 ng/mL TPA for 24 hours. After a 3-day coculture, suspension cells were removed by repeated vigorous washing with PBS.
Western blotting
Western blotting was performed as described previously.15 Antibodies for GFP and α-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibody for viral interleukin-6 (vIL-6) was prepared in our laboratory.15 Antibody detection was performed by standard enhanced chemiluminescence (ECL) techniques (Perkin-Elmer, Boston, MA).
Fluorescent microscopy, flow cytometry, and immunofluorescent staining
Cells infected with rKSHV-GFP were examined using a Zeiss Axiophot microscope (Oberkochen, Germany) equipped with a Roper Scientific CCD camera (Tucson, AZ). The image acquisition and processing were performed using IPLab Mac version 3.5 (Scanalytics, Fairfax, VA) and Adobe Photoshop version 6 (Adobe, San Jose, CA). Flow cytometry analysis for GFP and propidium iodide (PI; Sigma Chemical, St Louis, MO) was performed as described previously.16 Immunofluorescent staining for latency associated nuclear antigen (LANA) was carried out as described previously,12 using a rat monoclonal antibody against LANA6 (LN53; Advanced Biotechnologies, Columbia, MD) and Alexa Fluor 594–conjugated chicken antibody against rat immunoglobulin G (IgG; Molecular Probes, Eugene, OR).
Statistical analysis
Data were compared using Student t test; P values less than .05 were considered significant.
Results
HIV-1 Tat enhances KSHV infectivity
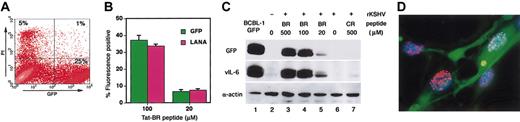
To monitor KSHV infection, we used replication-competent rKSHV encoding GFP.12 After a 24-hour incubation with the virus, only a small proportion of HEK293 were GFP-positive, reflecting the occurrence of KSHV infection. Of note, KSHV infectivity was markedly enhanced when the virus was incubated with Tat-BR peptide prior to addition to the cells (Figure 1A). Increased KSHV infectivity by Tat-BR peptide was also significant (P < .05) in primary human endothelial cells, HUVECs and HDMECs (Figure 1A–C). The GFP-positive cells expressed KSHV-encoded LANA,6 confirming the presence of KSHV in the infected cells (Figure 1B). In contrast to Tat-BR, addition of Tat-CR peptide did not increase KSHV infectivity (Figure 1C). A subset of GFP-positive cells displayed morphologic changes compatible with herpesvirus-induced cytopathic effects, including rounding and ballooning.13
Effects of HIV-1 Tat peptides on KSHV infectivity. (A) Fluorescence and phase-contrast microscopy of HEK293, HDMECs, and HUVECs 24 hours after infection with rKSHV-GFP in the absence (left) or presence (right) of Tat-BR peptide (100 μM). Original magnification, × 100. (B) Expression of KSHV LANA, as visualized with Alexa 594 (red) localized to the blue nucleus (DAPI [4′,6-diamidino-2-phenylindole]) in HUVECs infected by KSHV, as revealed by GFP. Merged 3-color image demonstrates the typical speckled pattern of nuclear LANA expression. Original magnification, × 320. (C) GFP-positive population in HEK293, HDMECs, and HUVECs analyzed by flow cytometry. Mean ± standard deviation (SD; triplicate samples) in a representative experiment. *P < .05 compared with rKSHV alone.
Effects of HIV-1 Tat peptides on KSHV infectivity. (A) Fluorescence and phase-contrast microscopy of HEK293, HDMECs, and HUVECs 24 hours after infection with rKSHV-GFP in the absence (left) or presence (right) of Tat-BR peptide (100 μM). Original magnification, × 100. (B) Expression of KSHV LANA, as visualized with Alexa 594 (red) localized to the blue nucleus (DAPI [4′,6-diamidino-2-phenylindole]) in HUVECs infected by KSHV, as revealed by GFP. Merged 3-color image demonstrates the typical speckled pattern of nuclear LANA expression. Original magnification, × 320. (C) GFP-positive population in HEK293, HDMECs, and HUVECs analyzed by flow cytometry. Mean ± standard deviation (SD; triplicate samples) in a representative experiment. *P < .05 compared with rKSHV alone.
HIV-1 Tat is known to be toxic for neuronal cells and has been implicated as a causative factor of AIDS-associated dementia.17 Among 3 cell lines we tested, morphologic changes were most evident in HEK293 cells when high concentrations (≥ 100 μM) of Tat-BR peptide were used in the presence (Figure 1A) or absence of KSHV (not shown). These changes could be due, at least in part, to Tat-induced nuclear factor κ B (NF-κB) activation, which is known to variously affect cell morphology.18 Since damaged cells often produce autofluorescence, we examined cell viability after KSHV infection in the presence of 100 μM Tat-BR peptide. As shown in Figure 2A, the vast majority of GFP-positive HEK293 cells did not stain with PI 24 hours after infection, providing evidence that most of the cells are alive and that GFP-positive status is not attributable to autofluorescence associated with cell death but rather to the occurrence of KSHV infection. In addition, the proportion of GPF-positive cells corresponded well with the proportion of LANA-positive cells (Figure 2B). Furthermore, by Western blotting, GFP- and KSHV-encoded vIL-6 were both detectable in HEK293 cells 24 hours after infection with the Tat-BR peptide/rKSHV-GFP complex (Figure 2C). KSHV infection was enhanced when Tat-BR peptide was present at concentrations ranging between 20 μM and 500 μM (Figure 2C) but was not evident at lower concentrations (data not shown). After longer film exposure, both GFP and vIL-6 were detected in cells infected with KSHV alone or together with Tat-CR peptide (data not shown). Although a large number of cells died in the first week after infection, latent KSHV infection was established in the remaining cells, as evidenced by their expression of both GFP and LANA for more than 2 months (Figure 2D).
GFP expression reflects KSHV infectivity. (A) Flow cytometry analysis of HEK293 cell viability 24 hours after infection with rKSHV-GFP. Most GFP-positive cells are PI-negative. Numbers in quadrants represent percentages of total cells in each cell fraction. Representative experiment. (B) GFP and LANA expression in HUVECs 24 hours after infection with rKSHV-GFP. Fluorescence-positive (GFP and LANA/Alexa 594) populations were counted under fluorescence microscopy after LANA staining. Mean ± SD (triplicate samples) in a representative experiment. (C) KSHV vIL-6 and GFP expression in HEK293 cells 24 hours after infection with rKSHV-GFP. Western blots show expression of GFP, KSHV vIL-6, and α-actin (loading control) in BCBL-1–GFP (positive control) and HEK293 cells. Representative experiment. (D) Latent KSHV infection in HUVECs. Two months after infection with rKSHV-GFP, expression of KSHV LANA was visualized with Alexa 594 (red) staining localized to the blue nucleus (DAPI). Original magnification, × 320.
GFP expression reflects KSHV infectivity. (A) Flow cytometry analysis of HEK293 cell viability 24 hours after infection with rKSHV-GFP. Most GFP-positive cells are PI-negative. Numbers in quadrants represent percentages of total cells in each cell fraction. Representative experiment. (B) GFP and LANA expression in HUVECs 24 hours after infection with rKSHV-GFP. Fluorescence-positive (GFP and LANA/Alexa 594) populations were counted under fluorescence microscopy after LANA staining. Mean ± SD (triplicate samples) in a representative experiment. (C) KSHV vIL-6 and GFP expression in HEK293 cells 24 hours after infection with rKSHV-GFP. Western blots show expression of GFP, KSHV vIL-6, and α-actin (loading control) in BCBL-1–GFP (positive control) and HEK293 cells. Representative experiment. (D) Latent KSHV infection in HUVECs. Two months after infection with rKSHV-GFP, expression of KSHV LANA was visualized with Alexa 594 (red) staining localized to the blue nucleus (DAPI). Original magnification, × 320.
Selectivity of Tat-BR–facilitated KSHV cell entry
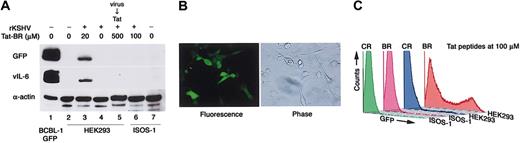
A previous report has shown that full-length Tat protein can induce KSHV replication in lymphoma cells that are infected with KSHV.19 We therefore examined whether the increased GFP expression induced by Tat-BR was attributable to stimulation of KSHV replication after virus entry. We and other investigators have shown that lytic KSHV replication is accompanied by increased vIL-6 expression.15,20 When Tat-BR peptide was added after KSHV infection, vIL-6 expression was not induced (Figure 3A), suggesting that Tat-BR peptide did not promote post-entry KSHV replication in these experiments.
Tat-BR peptide enhances receptor-dependent KSHV transmission. (A) Western blot analysis of GFP, KSHV vIL-6, and α-actin (loading control) expression in BCBL-1–GFP (positive control), HEK293, and murine angiosarcoma ISOS-1 cells. HEK293 cells were also treated with Tat-BR after removal of rKSHV-GFP from the supernatant (KSHV → Tat; lane 5). Representative experiment. (B) Stable KSHV infection in ISOS-1 cells after direct coculture of BCBL-1 cells producing rKSHV-GFP with ISOS-1 cells. Original magnification, × 100. (C) Flow cytometry profile of GFP fluorescence in HEK293 and ISOS-1 cells exposed to rKSHV-GFP in the presence of 100 μM Tat-BR and Tat-CR peptides.
Tat-BR peptide enhances receptor-dependent KSHV transmission. (A) Western blot analysis of GFP, KSHV vIL-6, and α-actin (loading control) expression in BCBL-1–GFP (positive control), HEK293, and murine angiosarcoma ISOS-1 cells. HEK293 cells were also treated with Tat-BR after removal of rKSHV-GFP from the supernatant (KSHV → Tat; lane 5). Representative experiment. (B) Stable KSHV infection in ISOS-1 cells after direct coculture of BCBL-1 cells producing rKSHV-GFP with ISOS-1 cells. Original magnification, × 100. (C) Flow cytometry profile of GFP fluorescence in HEK293 and ISOS-1 cells exposed to rKSHV-GFP in the presence of 100 μM Tat-BR and Tat-CR peptides.
The full-length Tat and Tat-BR have been shown to cross the cell membrane targeting the lipid bilayer.21 In addition, Tat can carry a variety of 15-kDa to 120-kDa proteins through the plasma membrane in a receptor- and transporter-independent manner.21 Although KSHV is much larger in size and the presence of a virus-specific receptor is usually required for herpesvirus transmission,22 it was unclear whether this requirement persisted in the presence of Tat-BR peptide. To address this question, we used the previously described murine ISOS-1 cell line, which displays endothelial cell properties and expresses H-2Dd.23 We found this cell line to be negative for KSHV and resistant to cell-free KSHV transmission (data not shown). However, we could establish KSHV infection in ISOS-1 cells by direct coculture with the BCBL-1 cells producing rKSHV-GFP (Figure 3B), suggesting that KSHV could be transmitted to these cells through cell-mediated entry.8 We tested if cell-free KSHV transmission could be achieved in the presence of Tat-BR peptide. However, Tat-BR peptide did not facilitate cell-free KSHV entry to ISOS-1 cells (Figure 3A,C), suggesting that Tat-facilitated transmission of KSHV is not universal, and thus differs from Tat-mediated intracellular delivery of protein or DNA, which is believed to occur independently of specific receptors.21
Full-length Tat protein is more potent than Tat-BR peptide at facilitating KSHV cell entry
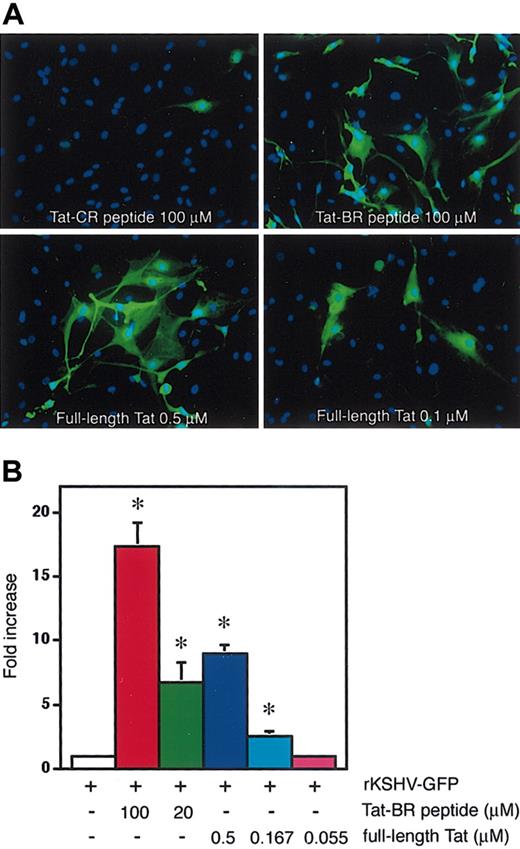
An early study using dot-blot analysis demonstrated that HIV-1 Tat is detectable at levels up to 10 nM in sera from HIV-positive individuals.24 Compared with serum Tat levels reported in this study, much higher concentrations of synthetic Tat-BR peptide were required to enhance KSHV transmission. In general, synthetic peptides do not reproduce the entire capacity of full-length proteins. We therefore tested if the full-length Tat protein could enhance KSHV transmission more efficiently than Tat-BR peptide. By fluorescence microscopy, enhanced KSHV infectivity was observed when the 86-aa Tat protein was present at nanomolar concentrations (Figure 4A). By fluorescence-activated cell sorting (FACS) analysis, increased infectivity was evident when the full-length Tat protein was used at concentrations as low as 0.167 μM (Figure 4B). This concentration of Tat protein is expected to be comparable with Tat levels in selected tissues such as lymph nodes where HIV-1 loads are increased by orders of magnitude compared with peripheral blood.24-26
Full-length Tat protein is more potent than Tat-BR peptide at promoting KSHV cell entry. (A) Fluorescence microscopy of HDMECs 24 hours after infection with rKSHV-GFP in the presence of Tat-CR (100 μM) and Tat-BR (100 μM) peptides or recombinant Tat protein (0.5 μM or 0.1 μM). The nucleus of endothelial cells is blue, as revealed by DAPI staining in the KSHV-infected cells (green). Original magnification, × 100. (B) Flow cytometry profile of GFP fluorescence in HEK293 cells exposed to rKSHV-GFP in the presence of Tat-BR peptide (100 μMor20 μM) or full-length Tat protein (0.5 μM, 0.167 μM, or 0.055 μM). The fold increase of the GFP-positive proportion compared with cells infected with rKSHV alone is shown. Mean ± SD (triplicate samples) in a representative of 4 independent experiments. *P < .05 compared with rKSHV alone.
Full-length Tat protein is more potent than Tat-BR peptide at promoting KSHV cell entry. (A) Fluorescence microscopy of HDMECs 24 hours after infection with rKSHV-GFP in the presence of Tat-CR (100 μM) and Tat-BR (100 μM) peptides or recombinant Tat protein (0.5 μM or 0.1 μM). The nucleus of endothelial cells is blue, as revealed by DAPI staining in the KSHV-infected cells (green). Original magnification, × 100. (B) Flow cytometry profile of GFP fluorescence in HEK293 cells exposed to rKSHV-GFP in the presence of Tat-BR peptide (100 μMor20 μM) or full-length Tat protein (0.5 μM, 0.167 μM, or 0.055 μM). The fold increase of the GFP-positive proportion compared with cells infected with rKSHV alone is shown. Mean ± SD (triplicate samples) in a representative of 4 independent experiments. *P < .05 compared with rKSHV alone.
Discussion
KS is rare and usually not aggressive except in association with HIV-1 infection. Previous studies have demonstrated that the HIV-1 Tat protein contributes to the highly aggressive nature of AIDS-KS by inducing inflammatory cytokines and angiogenesis.4,9,27 In this study, we show another active role of Tat in AIDS-KS pathogenesis. Although the mechanism is still unknown, HIV-1 Tat greatly enhances KSHV infectivity for cultured endothelial cells.
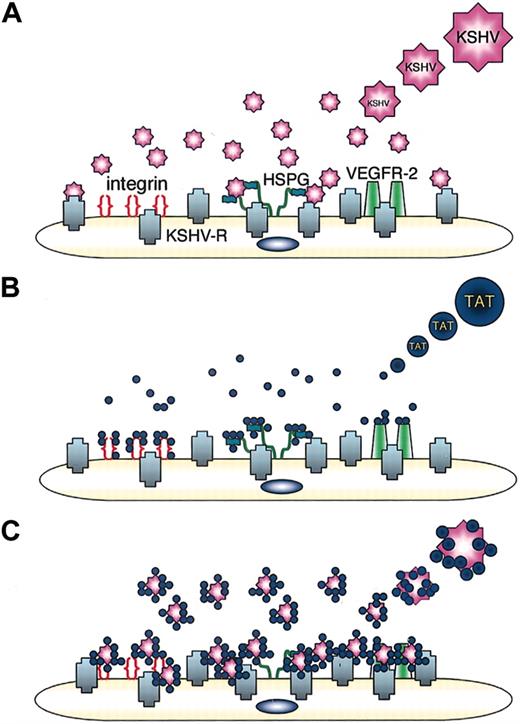
The transducing function of Tat for protein and DNA occurs in a receptor- and transporter-independent fashion, which appears to target directly the lipid bilayer.21 A number of studies suggested that Tat-enhanced entry of biologic materials occurs by membrane destabilization, temperature-dependent adsorptive endocytosis, or through caveolae.28-30 The lack of cell selectivity of Tat-mediated intracellular delivery of protein and DNA appears to differ from Tat-induced KSHV entry. KSHV virion is composed of a large DNA genome encased in a capsid, which is in turn coated with an envelope composed of a dozen viral proteins and glycoproteins in a lipid bilayer.22 The Tat-BR peptide has a net positive charge, which has been proposed to facilitate low-affinity binding of Tat protein to the lipid bilayer.31 Owing to its strong electrostatic interaction with negatively charged molecules, Tat could bind to the viral coat proteins thereby enhancing the effective cell-surface concentration of viral particles, as suggested for Tat-induced adenovirus entry.11 KSHV entry into target cells is believed to involve interactions of multiple viral glycoproteins with a variety of cell-surface determinants.22 Cell-surface heparan sulfate proteoglycans (HSPGs) can bind KSHV and may serve to concentrate the virus at the cell surface, and α3β1 integrin has been reported to serve as a coreceptor for KSHV.32 It is interesting that Tat can bind to cell-surface HSPG and to integrins,33 which were indicated as surface binding receptors for KSHV.32 Tat also binds to cell-associated thrombospondin-1, immunoglobulin-like domains of vascular endothelial growth factor receptor-2 (VEGFR-2) and the chemokine receptors CCR2 and CCR3.34 By binding to these molecules, Tat may serve to greatly increase the initial KSHV contact to cells.11,30,31 Thus, Tat could facilitate virus uptake by increasing virion concentration on the cell surface (Figure 5).
A schematic representation of how HIV-1 Tat could facilitate KSHV entry into cells. (A) KSHV entry into target cells is initiated by the interaction of viral glycoproteins with binding molecules such as HSPG, followed by viral membrane fusion with KSHV-entry receptor(s) (KSHV-R). (B) HIV-1 Tat protein binds to cell-surface integrins, HSPG, and VEGFR-2. (C) Tat binds to KSHV and to cell-surface integrins HSPG and VEGFR-2, thereby concentrating KSHV on the cell surface. By bridging KSHV to the cell surface, Tat could facilitate the interaction of the virus with its entry receptor.
A schematic representation of how HIV-1 Tat could facilitate KSHV entry into cells. (A) KSHV entry into target cells is initiated by the interaction of viral glycoproteins with binding molecules such as HSPG, followed by viral membrane fusion with KSHV-entry receptor(s) (KSHV-R). (B) HIV-1 Tat protein binds to cell-surface integrins, HSPG, and VEGFR-2. (C) Tat binds to KSHV and to cell-surface integrins HSPG and VEGFR-2, thereby concentrating KSHV on the cell surface. By bridging KSHV to the cell surface, Tat could facilitate the interaction of the virus with its entry receptor.
Although the synthetic Tat peptides mimic many functions of the full-length Tat protein, it is often difficult to reproduce the activities of the full-length protein using short synthetic peptides. For example, the carboxyl terminal region of Tat contains an Arg-Gly-Asp (RGD) motif responsible for Tat binding to integrin receptors.34 The RGD motif but not the BR peptide would facilitate concentrating Tat/KSHV on cell-surface integrins. Structural studies of the Tat peptides have revealed that much of the peptide is disordered, and that interaction with other proteins would be required to acquire a stable structure.35 Accordingly, the synthetic Tat-BR peptide may not have a proper conformation when used in vitro. In addition, the Tat-BR peptide used here is extremely hydrophilic (hydrophobicity = 0% at pH 2.0 and pH 6.8), compared with the full-length protein. Hydrophobicity is recognized as an important contributor to protein stability and structure,36 and thus, the Tat-BR peptide may be less stable than the full-length protein. In fact, full-length Tat could enhance KSHV transmission at significantly lower concentrations than those required by the synthetic Tat-BR peptide.
Nonetheless, increased KSHV infectivity by full-length Tat required approximately 10- to 15-fold-higher protein concentrations than those reported present in the circulation of HIV-1–infected individuals. It is important to note that serum levels of HIV-1 Tat are unlikely to accurately reflect Tat levels in tissues.24 It is to be expected that Tat concentrations measured in serum are significantly lower than those in lymph nodes of HIV-1–infected individuals, where productively infected cells are most frequent.25 In lymphoid tissues, HIV-1 load exceeds by orders of magnitude the quantity of free and cell-associated HIV-1 found in the bloodstream.26 Additionally, serum levels of Tat are unlikely to reflect Tat concentrations in tissues because HIV-1 Tat has heparin-binding capacity.33 As such, HIV-1 Tat would be expected to be locally trapped by HSPGs, which are expressed on the surface of most cells and in the extracellular matrix resulting in Tat being concentrated and sequestered away from serum.37
It is likely that HIV-1 Tat concentrations higher than those present in serum are required to facilitate KSHV entry into cells. In fact, only a minority of cells, particularly blood cells, are infected with KSHV in individuals who are dually infected with HIV-1 and KSHV.2,6 KS is thought to originate from KSHV infection of lymphatic endothelial or precursor cells, and arises most frequently in the skin and lymph nodes.6 The occurrence of most intense HIV-1 replication in lymphoid tissues and the association of primary KSHV infection with the development of lymph nodal KS in an HIV-infected patient38 suggest that lymphoid tissues are a likely preferential site of KSHV spread. Indeed, recent studies on the pathogenesis of KS propose models in which the KS precursor or seed cells are first infected with KSHV in transplanted grafts39 or other tissues40 and then migrate to the sites of KS development.
Based on the results presented here, we suggest that the sites of most intense HIV-1 replication are also those where KSHV infection of target cells takes place due to the facilitating effects of HIV-1 Tat. It is therefore no surprise that successful control of HIV replication by antiretroviral therapies have resulted in a dramatic decrease of KS incidence.1
Prepublished online as Blood First Edition Paper, April 13, 2004; DOI 10.1182/blood-2003-07-2533.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Jeffrey Vieira for providing rKSHV-GFP; Dr Lei Yao for maintaining HDMECs and HUVECs; and Drs Masashi Narazaki and Robert Yarchoan for helpful suggestions.

![Figure 1. Effects of HIV-1 Tat peptides on KSHV infectivity. (A) Fluorescence and phase-contrast microscopy of HEK293, HDMECs, and HUVECs 24 hours after infection with rKSHV-GFP in the absence (left) or presence (right) of Tat-BR peptide (100 μM). Original magnification, × 100. (B) Expression of KSHV LANA, as visualized with Alexa 594 (red) localized to the blue nucleus (DAPI [4′,6-diamidino-2-phenylindole]) in HUVECs infected by KSHV, as revealed by GFP. Merged 3-color image demonstrates the typical speckled pattern of nuclear LANA expression. Original magnification, × 320. (C) GFP-positive population in HEK293, HDMECs, and HUVECs analyzed by flow cytometry. Mean ± standard deviation (SD; triplicate samples) in a representative experiment. *P < .05 compared with rKSHV alone.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/3/10.1182_blood-2003-07-2533/6/m_zh80150464340001.jpeg?Expires=1769091908&Signature=MLG2o9El6LobN8LVO8Nz9Om1EE0GYzivTjb-wezmClcgaT7BWY49mGoA0kapSu~cjz3cH3NLwmNloG0b3dvir189niWiO7TLE--31Euj2GyDU8vROlTyPLgh4P309KseKVJu-1TTo9tbXH9ploh3rErro3GHCnc-WqpENBlxtTG~AzLuPmuYgXqFI0cz9GYQFLH9nkkzsuRumz4JsHBOQUTCLddQmYhFs76cSd1zobtKy72t-0phu6smyT28ZrHkp9F7wExKXEVXzQmfoDhzFIXlcujKix2q3pD89jgE-eq8DiSREX3f0qUiCou9RqW4kEYK820SwAlU5-RQZg5lrA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal