Abstract
Plasminogen (Pg) has been implicated in many biologic processes involving extracellular proteolysis. We investigated whether Pg, by virtue of its capacity to be deposited within the extracellular matrix, can serve as a ligand for cell surface integrins. We report here that Pg supports cell adhesion by engaging integrins αMβ2 and α5β1. The immobilized Glu-Pg, but not its derivatives with the N-terminal peptide lacking, plasmin and Lys-Pg, supported efficient adhesion that was abolished by anti-αMβ2 and anti-α5β1 integrin-specific monoclonal antibodies (mAbs). In addition, lysine binding sites of Glu-Pg contributed to cell adhesion inasmuch as tranexamic acid and ϵ-aminocaproic acid inhibited cell adhesion. The involvement of αMβ2 and α5β1 in adhesion to Glu-Pg was demonstrable with blood neutrophils, U937 monocytoid cells, and genetically engineered αMβ2-transfected human embryonic kidney (HEK) 293 cells. In αMβ2, the αMI-domain is the binding site for Glu-Pg because the “I-less” form of αMβ2 did not support cell adhesion and the recombinant αMI-domain bound Glu-Pg directly. In comparison with cell adhesion, the binding of soluble Glu-Pg to cells and the concomitant generation of plasmin activity was inhibited by anti-α5β1 but not by anti-αMβ2. These findings identify Glu-Pg as an adhesive ligand for integrins αMβ2 and α5β1 and suggest that α5β1 may participate in the binding of soluble Glu-Pg and assist in its activation.
Introduction
Plasminogen (Pg), the zymogen form of the serine proteinase plasmin (Pm), its activators, and inhibitors compose the Pg system, which has been implicated in numerous physiological and pathological processes involving extracellular proteolysis. Broad substrate recognition exhibited by Pm allows it to perform multiple tasks. Pm is the major enzyme responsible for degradation of fibrin clots. In addition, the interaction of Pg and its activators, tissue plasminogen activator (t-PA) and urokinase plasminogen activator (u-PA), with cell surfaces has been proposed to play a pivotal role in assisting cell migration by degrading the extracellular matrix directly or indirectly through activation of metalloproteinases. Numerous processes that involve cell migration and tissue remodeling, such as wound healing, angiogenesis, ovulation, trophoblast invasion, neurite outgrowth, and tumor cell invasion, depend on generation of Pm activity (reviewed by Irigoyen et al1 and Herren et al2 ). Direct evidence for the accessory role of Pg in cell migration was obtained in studies with mice genetically deficient in Pg; leukocyte migration in response to the inflammatory stimulus was significantly diminished.3,4
Binding of Pg to fibrin clots or cell surfaces enhances t-PA–induced Pg activation many fold (reviewed by Nieuwenhuizen5 and Plow et al6 ). The association of Pg with several extracellular matrix (ECM) proteins, including fibronectin, collagen IV, laminin, and fibrin(ogen), also dramatically stimulates its activation,7,8 suggesting that the ECM proteins may provide alternative surfaces for assembly and regulation of Pg conversion to Pm. The interactions of Pg with the ECM proteins in vitro point to the Pg potential to be deposited within the extravascular interstitial tissues. Pg is not synthesized by other tissues except liver and, therefore, should traverse the endothelial barrier in blood vessels to become associated with the underlying ECM. This might occur during the inflammatory response when plasma proteins leak through the vessels. Deposition of plasma proteins—for example, fibrin(ogen)—is well documented within inflamed tissues.9,10 Indeed, recent immunohistochemical studies localized Pg in human atherosclerotic coronary arteries in cellular and acellular portions of the plaque.11 The binding of Glu-Pg, the native form of the molecule, and its Lys-Pg derivative, which does not contain the N-terminal 77/78 residues, to cells and the ECM proteins is mediated primarily by low-affinity lysine binding sites (LBSs) present in kringle domains 1, 2, 4, and 5 of Pg.12-15 The occupation of LBSs induces a large conformational change in Pg and facilitates its activation.16 LBSs of Pg are responsible for multiple interactions of this protein and are of high capacity and ubiquitous in their nature. A growing list of candidate receptors on the cell surface for soluble Pg and their structural and functional heterogeneity supports the LBS-mediated recognition mechanism. Several proteins with carboxy-terminal lysines (α-enolase, annexin II) have been identified as Pg receptors on the cell surface,17,18 while other Pg-binding molecules (amphoterin, integrin glycoprotein IIbIIIa [GPIIbIIIa], cytokeratin-8, dipeptidyl peptidase, tissue factor) appear to utilize internal lysines.11,19-22 The interactions of surface-bound Pg with several proteins involves, in addition to LBSs, some other binding regions,23,24 suggesting that the alteration of Pg conformation may control recognition.
Because Pg is deposited within the ECM at the sites of vascular injury and can bind multiple cell surface proteins, we have examined whether integrins can engage Pg as an adhesive ligand. Our previous studies with model cells expressing integrin αMβ2 demonstrated that Pg is capable of supporting cell adhesion.25 However, the relationships between Pg/Pm and αMβ2 and/or other integrins on leukocytes that abundantly express this integrin have not been defined. The data presented in this paper show that integrins αMβ2 (CD11b/CD18, Mac-1, CR3) and α5β1 (CD49e/CD29, very late antigen-5 [VLA-5]) on leukocytes can bind Glu-Pg and that α5β1 is involved in Pg activation.
Materials and methods
Proteins, peptides, and monoclonal antibodies
Human Glu-Pg was purified from fresh human plasma using affinity chromatography on lysine-Sepharose followed by gel filtration as described.26 The N-terminal fragment of Pg (residues 1 to 78) was generated during spontaneous conversion of native Glu-Pg into Lys-Pg due to the traces of active Pm. For that purpose, Glu-Pg (5 to 10 mg/mL) purified without inhibitors was incubated at 4° C for 24 to 26 hours, and the progress of Glu-Pg to Lys-Pg conversion was monitored by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) using 7.5% homogeneous gel (Pharmacia Fast Gel; Pharmacia, Uppsala, Sweden). Its identity was confirmed by N-terminal sequence analysis, and a single EPLDDYVNTQ sequence was determined. Lys-Pg was prepared as described.26 Lys-Pm was from Enzyme Research Laboratories (South Bend, IN). Glu-Pg was labeled with 125I using IODO-GEN according to the manufacturer's protocol (Pierce, Rockford, IL). The expression, purification, and characterization of the αMI-domain as a fusion protein with glutathione S-transferase (GST) have been previously described.27 The free αMI-domain was prepared by cleavage of the fusion construct with thrombin (Enzyme Research Laboratories, South Bend, IL). Streptokinase, t-PA, N-formyl-methionyl-leucyl-phenylalanine (fMLP), tranexamic acid, and ϵ-aminocaproic acid (ϵ-ACA) were from Sigma (St Louis, MO). The chromogenic plasmin substrate Val-Leu-Lys-p-nitroanilide (S-2251) was from Chromogenix Diapharma Group (Franklin, OH). The peptide P2, corresponding to the γ-chain residues γ377 to γ395 of human fibrinogen (YSMKKTTMKIIPFNRLTIG), was described previously.28 Anti-αMβ2 function-blocking monoclonal antibodies (mAbs) 44a and IB4 recognize the epitopes within the αMI-domain29 and the β2 subunit,30 respectively. The mechanism by which mAb IB4 blocks adhesion is not direct and apparently involves the allosteric mechanism.31 Monoclonal antibody 1965 (clone JBA1), directed against the β1 integrin subunit, and polyclonal anti-α5β1 antibody 1950 were from Chemicon (Temecula, CA). Polyclonal anti-Pg antibody was from Accurate Chemicals (Westbury, NY), and anti-GST mAb (clone DG122-2A7) was from Upstate Biotechnology (Lake Placid, NY). Monoclonal antibody 8A11, directed against human fibronectin,32 and mAb 4-2,33 recognizing human fibrinogen, were described previously.
Cell culture
Human embryonic kidney (HEK) 293 cells expressing αMβ2 and mock-transfected cells were described previously.34 To generate cells that express the “I-less” form of αMβ2, the construct in which the αM sequence Glu131-Gly321 was deleted from the αM reading frame was generated by overlap extension mutagenesis.35 Briefly, the 2 pairs of primers were designed, and αM coding regions 73 to 509 bp and 1083 to 3543 bp were amplified by polymerase chain reaction (PCR) and then fused together in the subsequent PCR reaction. The modified αM sequence was cloned into pcDNA3.1. HEK 293 cells were stably transfected with a created plasmid and pcDNA3.1-β2 vector as previously described.25 Erythroleukemic K562 cells were obtained from American Type Culture Collection (ATCC) (Rockville, MD). The cell lines were maintained in Dulbecco modified Eagle medium (DMEM)–F12 (BioWhittaker, Walkersville, MD) supplemented with 10% fetal bovine serum (FBS) and 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid). U937 monocytoid cells were obtained from ATCC and cultured in RPMI 1640 supplemented with 10% FBS. Neutrophils were isolated under sterile conditions from peripheral blood obtained from consenting volunteers and anticoagulated with acid-citrate-dextrose essentially as described.36
Cell adhesion assays
The wells of polysterene microtiter plates (Immulon 4HBX, Dynex Technologies, Chantilly, VA) or Costar (Bethesda, MD) were coated with various concentrations of protein ligands for 3 hours at 37° C and postcoated with 0.5% polyvinylpyrrolidone (PVP) for 1 hour at 37° C. The cells were labeled with 10 μM calcein acetoxymethyl ester (calcein-AM) (Molecular Probes, Eugene, OR), and assays were performed as described previously.37 Fluorescence was measured in a fluorescence plate reader (CytoFluorII, Applied Biosystems, Foster City, CA), and the number of adherent cells was determined from a standard curve constructed using the fluorescence of aliquots with a known number of labeled cells.
Immunofluorescent studies
The binding of Pg to the ECM deposited by fibroblasts was analyzed by immunofluorescent staining. WI38 human lung fibroblasts were obtained from ATCC and were grown in DMEM supplemented with 10% FBS, 2 mM glutamine, and 2 mM sodium pyruvate. Cells were harvested from the flasks with trypsin-EDTA (trypsin–ethylenediaminetetraacetic acid), washed, and cultured on glass coverslips in 12-well plates in DMEM-F12 supplemented with 10% FBS and 2 mM sodium pyruvate for 72 hours. Cells in selected wells were rinsed, and fresh medium supplemented with 0.25 mg/mL fibrinogen was added. A thin film of fibrin that formed on the top of the fibroblast layer after 1 hour was removed, and 0.5 mL of 0.1 mg/mL Pg was added to the wells with and without added fibrinogen. After 1 hour of incubation at 37° C, the media were removed and cells were fixed in 3.7% paraformaldehyde for 20 minutes. After blocking with 3% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) (blocking buffer), primary antibodies directed against human fibronectin, fibrinogen, and Pg diluted in the blocking buffer were added. After 2 hours of incubation, the slides were washed with triethanolamine-buffered saline (TBS) plus 0.05% Tween 20, and the secondary goat antimouse immunoglobulin G (IgG) conjugated to Alexa 594 (1:1500 dilution) or goat antirabbit IgG conjugated to Alexa 488 (1:1500 dilution) was added for 30 minutes. Images of the fluorescent-labeled ECM were obtained using a Leica TCS-NT laser scanning confocal microscope (Leica, Heidelberg, Germany) and HCX Plan Apo × 63 NA 1.4 oil (λ) lenses. Leica's Confocal software was used for image acquisition, and Corel Photo-Paint 8.0 software (Jericho, NY) was used for image processing.
Solid-phase binding assays
To test the interaction of immobilized Pg or its derivatives with soluble αMI-domain, 96-well microtiter plates were coated overnight at 4° C with Pg and Pm (100 μL, 5 μg/mL in the presence of 1 mM phenylmethylsulfonyl fluoride [PMSF]) or the N-terminal Pg peptide (100 μL, 20 μM) and postcoated with 0.4% polyvinyl alcohol (PVA). The αMI-domain (100 μL, 10 μg/mL) as a fusion with GST was added for 1 hour at 25° C, the plates were washed, and anti-GST mAb was added. The bound αMI-domain was quantitated after the reaction with goat antimouse IgG conjugated with alkaline phosphatase. Nonspecific binding of the αMI-domain to PVA-coated wells was subtracted.
To measure the binding of soluble Pg to the immobilized αMI-domain, microtiter plates were coated with free αMI-domain (100 μL, 5.0 μg/mL) and postcoated with 0.4% PVA. 125I-Pg (100 μL per well, 20 μg/mL, 106 cpm) was added with or without the peptide P2 (100 μM), tranexamic acid (5 mM), or ϵ-ACA (50 mM). After 60 minutes of incubation at 25° C, the wells were washed with TBS–Tween 20, the bound Pg was eluted with 200 μLof1%SDS–5 mM dithiothreitol (DTT) at 60° C for 30 minutes, and the radioactivity was measured.
Surface plasmon resonance (SPR) studies
Binding parameters for the interaction of soluble Glu-Pg with the αMI-domain were measured by using a Biacore 3000 SPR-based biosensor (Biacore, Uppsala, Sweden). The αMI-domain was coupled with a CM5 chip (Biacore) at a concentration of 1100 response units according to the manufacturer's protocol. Different concentrations of Glu-Pg in HSB-P buffer (Biacore; 10 mM HEPES [pH 7.4], 150 mM NaCl, 0.005% polysorbate 20 adjusted to 1 mM MgCl2) were flowed over the chip containing the αMI-domain. All data were corrected for the response obtained using a blank reference flow cell that was activated with 1-ethyl-3-(3-dimethyl aminopropyl) carbodiimide/N-hydroxysuccinimide (EDC/NHS) and then blocked with ethanolamine. Steady-state experiments were performed by injecting the αMI-domain at 10 μL/min for 8 minutes. The chip surface was regenerated with 2 M NaCl and 50 mM NaOH. Data were analyzed using the BIAevaluation 3.1 program. Nonspecific binding to the blank flow cell was subtracted.
Binding of 125I-Pg to cells in suspension
Neutrophils, αMβ2-expressing HEK 293, or U937 cells were washed in Hanks balanced salt solution (HBSS) and resuspended in the same media containing 0.1% BSA. Cells (107/mL) were incubated with 125I-Pg (10 μg/mL, 5 × 105 cpm/mL) in the presence or absence of mAb 44a (40 μg/mL), mAb IB4 (30 μg/mL), tranexamic acid (5 mM), and phorbol myristate acetate (PMA) (10 nM) in a total volume 150 μLat 25° C for 30 minutes; 50 μL aliquots were layered over 300 μL of 20% sucrose in HBSS-BSA and centrifuged for 3 minutes in a Beckman microfuge (Beckman Instruments, Fullerton, CA). The tube tips were amputated, and radioactivity was counted in a gamma counter.
Pg activation by cultured cells.
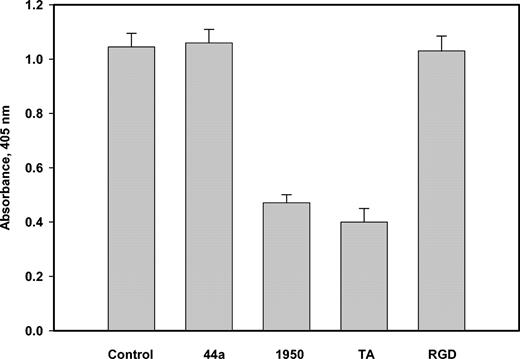
A total of 1.5 × 106 U937 monocytoid, αMβ2-expressing, or mock-transfected HEK 293 cells in HBSS were incubated in the solution containing the chromogenic substrate S-2251 (0.7 mM) in the presence or absence of Glu-Pg (7.0 μg/mL), anti-αM (mAb 44a), anti-α5β1 (mAb 1950), anti-β1 (1965), and t-PA (70 ng/mL) in a total volume of 150 μL at 25° C. Absorbance was measured at 405 nm after 180 minutes, and absorbance of the samples that did not contain cells or Pg was subtracted.
Results
Immunostaining of Pg deposited into the ECM
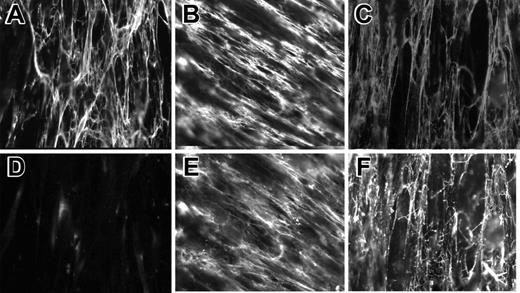
Previous in vitro studies demonstrated that Pg is capable of interacting with ECM proteins, including fibronectin, laminin, collagen IV, and fibrin.7,8,23,24,38 In 2 recent reports, Pg was found in the atherosclerotic plaque11 and in the zona pellucida of mammalian oocytes.39 However, the pattern of Pg deposition within the ECM has not been investigated. Therefore, we have examined whether Pg localizes with fibronectin and fibrinogen within the ECM produced by live cells using immunofluorescence. WI38 human lung fibroblasts were cultured to confluence in the presence or absence of fibrinogen, and then soluble Glu-Pg was added to cell layers. After several days in culture, fibroblasts develop an extensive extracellular fibronectin matrix (Figure 1A-B). Coculturing of fibrinogen with cells resulted in deposition of fibrin(ogen) into the matrix (Figure 1C). Binding of exogenous Glu-Pg to fibronectin- or fibrinogen-containing matrices was demonstrated by staining the matrix with anti-Pg antibodies (Figure 1E-F). The presence of endogenous Pg was not detected (Figure 1D). The fibrillar pattern of Pg staining was similar to that of fibronectin and fibrinogen, indicating that Glu-Pg binds to these proteins within the ECM and potentially can serve as an adhesive ligand for cell surface integrins.
Immunofluorescent staining of Pg incorporated into the matrix produced by fibroblasts. WI38 fibroblasts were cultured in DMEM for 72 hours to produce ECM. Cells in selected wells were cultured with soluble plasma fibrinogen for an additional 2 hours, and the fibrin film formed on the surface of the cell layer was removed. Glu-Pg was added for 1 hour to wells with and without added fibrinogen. Cells were fixed and then incubated with mAb 8A11 against fibronectin (5 μg/mL), mAb 4-2 against fibrinogen (5 μg/mL), and polyclonal Ab against Pg (1:1000). Double immunostaining with anti-Fn (A) and anti-Pg (D). (B,E) Staining with anti-Fn and anti-Pg, respectively. (C,F) Staining with anti-Fg and anti-Pg, respectively.
Immunofluorescent staining of Pg incorporated into the matrix produced by fibroblasts. WI38 fibroblasts were cultured in DMEM for 72 hours to produce ECM. Cells in selected wells were cultured with soluble plasma fibrinogen for an additional 2 hours, and the fibrin film formed on the surface of the cell layer was removed. Glu-Pg was added for 1 hour to wells with and without added fibrinogen. Cells were fixed and then incubated with mAb 8A11 against fibronectin (5 μg/mL), mAb 4-2 against fibrinogen (5 μg/mL), and polyclonal Ab against Pg (1:1000). Double immunostaining with anti-Fn (A) and anti-Pg (D). (B,E) Staining with anti-Fn and anti-Pg, respectively. (C,F) Staining with anti-Fg and anti-Pg, respectively.
Adhesion of αMβ2-expressing cells to Pg and its derivatives
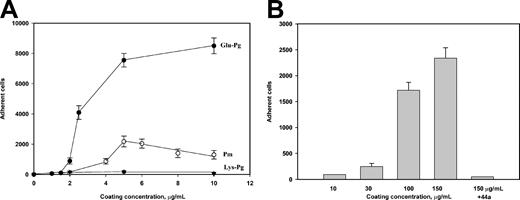
We have previously demonstrated that integrin αMβ2 can bind Glu-Pg.25 Therefore, adhesion of the αMβ2-expressing HEK 293 cells to immobilized Glu-Pg was examined further. As shown in Figure 2, Pg supported efficient adhesion, which was dependent on the concentration of Pg used. The specificity of the αMβ2-Pg interaction was determined using function-blocking antibodies directed to the αM (mAb 44a) and β2 (mAb IB4) subunits. Cell adhesion was reduced by both mAbs (Figure 2, shown for mAb 44a). Monoclonal antibodies inhibited adhesion more effectively at the low Pg concentrations: at 1.5 to 2 μg/mL Pg, mAb 44a inhibited adhesion by about 90% to 75%, whereas about 50% inhibition was achieved at higher coating concentrations of Pg. Specificity of the interaction between αMβ2 and Pg was also confirmed by the finding that at 100 μM, the fibrinogen peptide P2, a specific inhibitor of αMβ2-ligand interactions,25,28 abolished adhesion to Glu-Pg by 90% (not shown). Mock-transfected cells also adhered to Glu-Pg, but the level of adhesion was about 1.6 times lower than that of the αMβ2-expressing cells, and mAb 44a did not block adhesion. Thus, these results suggest that αMβ2 can mediate adhesion to Pg and that other cell surface molecules may contribute to adhesion (see below).
Adhesion of αMβ2- and mock-transfected cells to Glu-Pg. αMβ2-expressing (•) and mock-transfected (▴) HEK 293 cells (5 × 104) labeled with calcein-AM in HBSS/HEPES were added to wells coated with different concentrations of Glu-Pg and postcoated with 0.5% PVP. Some αMβ2-expressing (○) and mock-transfected (▵) cells were preincubated with mAb 44a (40 μg/mL). Glu-Pg was pretreated with 2 mM PMSF for 15 minutes at 22°C to prevent its activation on the surface. After 25 minutes at 37°C, nonadherent cells were removed by 3 washes with PBS. Fluorescence of adherent cells was measured in a fluorescent plate reader and converted to cell numbers. A representative of 3 independent experiments is shown. Data shown are means of triplicate determinations, and error bars represent SE.
Adhesion of αMβ2- and mock-transfected cells to Glu-Pg. αMβ2-expressing (•) and mock-transfected (▴) HEK 293 cells (5 × 104) labeled with calcein-AM in HBSS/HEPES were added to wells coated with different concentrations of Glu-Pg and postcoated with 0.5% PVP. Some αMβ2-expressing (○) and mock-transfected (▵) cells were preincubated with mAb 44a (40 μg/mL). Glu-Pg was pretreated with 2 mM PMSF for 15 minutes at 22°C to prevent its activation on the surface. After 25 minutes at 37°C, nonadherent cells were removed by 3 washes with PBS. Fluorescence of adherent cells was measured in a fluorescent plate reader and converted to cell numbers. A representative of 3 independent experiments is shown. Data shown are means of triplicate determinations, and error bars represent SE.
We next examined whether Pm, the proteolytically active form of Pg, supports αMβ2-mediated adhesion. As shown in Figure 3A, Pm supported a much lower extent of adhesion than Glu-Pg; at 5 μg/mL coating concentration, about 3.5-fold fewer cells adhered to Pm. The low level of cell adhesion mediated by Pm could not be attributed to its proteolytic activity because Pm was inactivated by phenylmethylsulfonyl fluoride (PMSF) prior to immobilization onto the plastic. Because Pm varies from its native zymogen form, Glu-Pg, by the absence of the N-terminal peptide (residues 1 to about 78), we tested cell adhesion to the Lys-Pg form, which also lacks this segment. No cell adhesion to immobilized Lys-Pg was detected at any concentration of coated protein (Figure 3A). In parallel experiments, almost equal signals were obtained using anti-Pg polyclonal antibodies, indicating that similar amounts of Glu-Pg, Lys-Pm, and Lys-Pg were immobilized (not shown). The difference in the adhesion-promoting activity between Glu-Pg and its N-terminally truncated derivatives prompted us to examine the ability of the 78-residue N-terminal Pg domain to support adhesion. When immobilized on microtiter plates, the N-terminal fragment supported efficient adhesion of the αMβ2-expressing cells, and the cell treatment with mAb 44a inhibited adhesion completely (Figure 3B). These data suggested that the N-terminal domain of Pg contains the αMβ2-binding site. However, on a molar basis, about 340-fold higher coating concentrations of the N-terminal fragment were required for maximal adhesion than that of Pg, suggesting that other site(s) in Pg can contribute to its binding to αMβ2.
Adhesion of αMβ2-expressing cells to Pg and its derivatives. The αMβ2-expressing cells were added to wells coated with different concentrations of Glu-Pg, Lys-Pg, and Pm (A) or the N-terminal fragment of Glu-Pg (B). Pm was inactivated with 2 mM PMSF for 15 minutes at 22° C. Some cells were preincubated with 40 μg/mL mAb 44a for 20 minutes at 22° C before cell aliquots were added to microtiter plates coated with the N-terminal Pg fragment. Adhesion was quantitated as described in the legend to Figure 2. Results are the total numbers of adherent cells and the mean ± SE values of 4 to 7 individual experiments.
Adhesion of αMβ2-expressing cells to Pg and its derivatives. The αMβ2-expressing cells were added to wells coated with different concentrations of Glu-Pg, Lys-Pg, and Pm (A) or the N-terminal fragment of Glu-Pg (B). Pm was inactivated with 2 mM PMSF for 15 minutes at 22° C. Some cells were preincubated with 40 μg/mL mAb 44a for 20 minutes at 22° C before cell aliquots were added to microtiter plates coated with the N-terminal Pg fragment. Adhesion was quantitated as described in the legend to Figure 2. Results are the total numbers of adherent cells and the mean ± SE values of 4 to 7 individual experiments.
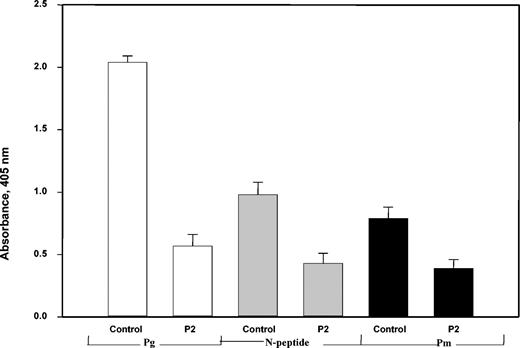
To probe the structural requirements of αMβ2 binding, the interactions of Glu-Pg, Pm, and the N-terminal fragment with the αMI-domain were tested. Previous data demonstrated that within αMβ2, the independently folded 24 kDa αMI-domain is responsible for binding of several ligands.40 Initially, we examined the ability of the integrin, in which the αMI-domain was deleted by genetic manipulation, to interact with Glu-Pg. Adhesion of cells expressing the “I-less” integrin was about 10% of that of wild-type αMβ2-expressing cells, suggesting the requirement of the αMI-domain in Pg recognition (these experiments were performed in the presence of anti-α5β1; not shown). To substantiate the role of the αMI-domain, Glu-Pg, Lys-Pm, and the N-terminal fragment were immobilized on microtiter plates, and their interactions with soluble αMI-domain were tested. Each of the proteins bound the αMI-domain (Figure 4), with Glu-Pg being the most active substrate, and the interactions were inhibited by the fibrinogen peptide P2. Because this peptide binds to the site within the αMI-domain implicated in binding of several αMβ2 ligands,25 these data suggest that the common ligand-binding site of the αMI-domain is involved in binding to Pg. However, the additional interactions of the αMI-domain may account for the incomplete inhibition. Taken together, these observations demonstrate that the αMI-domain within αMβ2 is the critical structure for Glu-Pg binding.
Interaction of αMβ2 with Glu-Pg and its derivatives is mediated by theαMI-domain. The αMI-domain as a fusion protein with GST at 10 μg/mL in TBS containing 1 mM MgCl2, 1 mM CaCl2 in the presence or absence of the P2 peptide (100 μM) was added to microtiter plates coated with 5 μM each Glu-Pg and Pm or 10 μM N-terminal Pg fragment. After incubation for 3 hours at 37° C, anti-GST mAb was added for 1.5 hours. Binding of the αMI-domain was then detected with a secondary goat antimouse IgG conjugated to alkaline phosphatase, with subsequent development of the reaction with p-nitrophenyl phosphate.
Interaction of αMβ2 with Glu-Pg and its derivatives is mediated by theαMI-domain. The αMI-domain as a fusion protein with GST at 10 μg/mL in TBS containing 1 mM MgCl2, 1 mM CaCl2 in the presence or absence of the P2 peptide (100 μM) was added to microtiter plates coated with 5 μM each Glu-Pg and Pm or 10 μM N-terminal Pg fragment. After incubation for 3 hours at 37° C, anti-GST mAb was added for 1.5 hours. Binding of the αMI-domain was then detected with a secondary goat antimouse IgG conjugated to alkaline phosphatase, with subsequent development of the reaction with p-nitrophenyl phosphate.
The expression of cell surface binding sites for Pg was shown previously for many cells, including leukocytes, and was implicated in generating the proteolytic activity required for degradation of the ECM during leukocyte migration (reviewed by Plow et al6 ). Therefore, we have investigated whether αMβ2 serves as a cell surface receptor for soluble Pg by measuring the binding of Glu-Pg to the αMβ2-expressing cells in suspension. As shown in Figure 5A, the incubation of cells with 125I-Glu-Pg resulted in its binding, but neither anti-αMβ2 mAb inhibited this interaction. Similar results were obtained when the binding of Glu-Pg was tested indirectly by measuring the activity of Pm generated upon the conversion of cell-bound Glu-Pg in the presence of t-PA (not shown). In contrast, the carboxy-terminal lysine analog tranexamic acid, at 5 mM, inhibited the Pg binding completely. This is in agreement with previous findings that the binding of soluble Pg to cell surfaces is mediated by its LBS.41 Also, when the immobilized αMI-domain was tested for its ability to bind soluble 125I-Glu-Pg, only tranexamic acid and ϵ-ACA inhibited the interaction whereas the P2 peptide was not effective (Figure 5B). To characterize the binding of soluble Glu-Pg with the αMI-domain further, we have determined the dissociation constant (Kd) for this interaction using surface plasmon resonance (SPR). Figure 5C shows that the binding of Glu-Pg to the αMI-domain was dose dependent and saturable. The Scatchard plot constructed from the binding data (Figure 5C, inset in the bottom panel) demonstrated the presence of a major class of binding sites with a Kd of 0.3 ± 0.01 μM, which falls within the range reported for the interactions of Pg LBSs with cells (reviewed by Plow et al42 ). Taken together, these analyses suggest that soluble Glu-Pg can bind the αMI-domain entirely through the Pg LBSs and that the specific ligand-binding site of the αMI-domain is engaged with the immobilized Glu-Pg only. This pattern of binding is consistent with the conclusion that αMβ2 utilizes different mechanisms for binding of the 2 forms of Glu-Pg (immobilized versus soluble). Such differential ligand recognition is a hallmark of many integrins, including αMβ2.43,44
The interaction of soluble Glu-Pg with αMβ2-expressing cells and the recombinant αMI-domain. (A) The αMβ2-expressing HEK 293 cells (106) were incubated with mAb 44a (40 μg/mL), mAb IB4 (30 μg/mL), or tranexamic acid (5 mM) in HBSS containing 0.1% BSA and 2 mM MnCl2 for 20 minutes at 22° C. Pg (10 μg/mL, 5 × 105 cpm/mL) was added, and the incubation was continued for another 20 minutes. Cell suspensions (50 μL) were layered over 300 μL of 20% sucrose in HBSS-BSA followed by centrifugation at 12 400g for 3 minutes. Radioactivity was measured as described in “Materials and methods.” (B) Binding of the radiolabeled Glu-Pg to immobilized αMI-domain.125I-Glu-Pg (20 μg/mL) was pretreated with P2 (100 μM), ϵ-ACA (50 mM), or tranexamic acid (5 mM) for 20 minutes at 22° C before being added to microtiter wells coated with αMI-domain (5 μg/mL) in the GST-free form and postcoated with 0.4% PVA. Binding of125I-Glu-Pg to the αMI-domain was quantitated. (C) Equilibrium analysis of the interaction of the αMI-domain with Pg. (i) Representative profiles of the SPR responses (RU) for Glu-Pg concentrations ranging from 0.1 μMto11 μM binding to the αMI-domain coupled to the chip surface. Pg was inactivated by incubating with diisopropylfluorophosphate. RU indicates response units. (ii) Saturable binding curve and (inset) Scatchard plot. Req indicates the response at equilibrium; C, the Glu-Pg concentration.
The interaction of soluble Glu-Pg with αMβ2-expressing cells and the recombinant αMI-domain. (A) The αMβ2-expressing HEK 293 cells (106) were incubated with mAb 44a (40 μg/mL), mAb IB4 (30 μg/mL), or tranexamic acid (5 mM) in HBSS containing 0.1% BSA and 2 mM MnCl2 for 20 minutes at 22° C. Pg (10 μg/mL, 5 × 105 cpm/mL) was added, and the incubation was continued for another 20 minutes. Cell suspensions (50 μL) were layered over 300 μL of 20% sucrose in HBSS-BSA followed by centrifugation at 12 400g for 3 minutes. Radioactivity was measured as described in “Materials and methods.” (B) Binding of the radiolabeled Glu-Pg to immobilized αMI-domain.125I-Glu-Pg (20 μg/mL) was pretreated with P2 (100 μM), ϵ-ACA (50 mM), or tranexamic acid (5 mM) for 20 minutes at 22° C before being added to microtiter wells coated with αMI-domain (5 μg/mL) in the GST-free form and postcoated with 0.4% PVA. Binding of125I-Glu-Pg to the αMI-domain was quantitated. (C) Equilibrium analysis of the interaction of the αMI-domain with Pg. (i) Representative profiles of the SPR responses (RU) for Glu-Pg concentrations ranging from 0.1 μMto11 μM binding to the αMI-domain coupled to the chip surface. Pg was inactivated by incubating with diisopropylfluorophosphate. RU indicates response units. (ii) Saturable binding curve and (inset) Scatchard plot. Req indicates the response at equilibrium; C, the Glu-Pg concentration.
Integrin α5β1 contributes to cell adhesion to Pg
The above findings (Figure 2) showed that function-blocking antibodies directed against αMβ2 were not able to inhibit adhesion of the αMβ2-expressing cells to Pg completely. To test whether other integrins are involved in cell adhesion to Pg, mock-transfected HEK 293 cells were tested. The cells efficiently adhered to Glu-Pg but not Pm (Figure 6A). Because HEK 293 cells express several β1 integrins, with α5β1 being the predominant integrin species,34 the effect of function-blocking antibodies against these integrins was tested. Anti-α5β1 and anti-β1 antibodies inhibited adhesion to Glu-Pg by about 50% (shown for anti-α5β1 antibody 1950). These antibodies inhibited cell adhesion to fibronectin completely. As a control, anti-αMβ2 blocking reagents (mAb 44a and P2) had no effect on adhesion of HEK 293 cells. To ascribe the contribution of the anti-integrin–resistant component of adhesion to the function of Pg LBSs, cell adhesion was conducted in the presence of tranexamic acid. At 5 mM, tranexamic acid inhibited cell adhesion by 50%, and a combination of anti-α5β1 Ab 1950 and tranexamic acid blocked adhesion by 90%. To determine if α5β1-mediated adhesion to Glu-Pg occurs with cells other than HEK 293 cells, we tested K562 human erythroleukemic cells. These cells express integrin α5β1 and do not express αMβ2. As shown in Figure 6B, Glu-Pg supported efficient adhesion of K562 cells, and adding anti-α5β1 antibody 1950 inhibited adhesion by about 75%. In addition, the LBS of Glu-Pg participated in K562 cell adhesion because tranexamic acid alone blocked adhesion by 35%. A combination of anti-α5β1 and tranexamic acid decreased adhesion more effectively (95%) than either agent separately.
Adhesion of the α5β1-expressing cells to Glu-Pg and Pm. (A) Calcein-labeled mock-transfected HEK 293 cells were preincubated for 20 minutes at 22° C with P2 (200 μM), anti-β1 mAb 1965 (20 μg/mL), anti-α5β1 polyclonal antibody (1:200 dilution), or tranexamic acid (5 mM) and then added to wells coated with Glu-Pg (5 μg/mL), Pm (5 mg/mL), or fibronectin (3 μg/mL). Adhesion was performed as described in the legend to Figure 2. (B) Calcein-labeled K562 cells activated with 10 nM PMA were incubated with mAb 44a (20 μg/mL), tranexamic acid (5 mM), anti-α5β1 mAb 1950 (1:200), or a combination of the inhibitors, for 20 minutes, and then added to wells coated with Glu-Pg (3 μg/mL). After 30 minutes of incubation at 37° C, nonadherent cells were removed and adhesion was quantitated.
Adhesion of the α5β1-expressing cells to Glu-Pg and Pm. (A) Calcein-labeled mock-transfected HEK 293 cells were preincubated for 20 minutes at 22° C with P2 (200 μM), anti-β1 mAb 1965 (20 μg/mL), anti-α5β1 polyclonal antibody (1:200 dilution), or tranexamic acid (5 mM) and then added to wells coated with Glu-Pg (5 μg/mL), Pm (5 mg/mL), or fibronectin (3 μg/mL). Adhesion was performed as described in the legend to Figure 2. (B) Calcein-labeled K562 cells activated with 10 nM PMA were incubated with mAb 44a (20 μg/mL), tranexamic acid (5 mM), anti-α5β1 mAb 1950 (1:200), or a combination of the inhibitors, for 20 minutes, and then added to wells coated with Glu-Pg (3 μg/mL). After 30 minutes of incubation at 37° C, nonadherent cells were removed and adhesion was quantitated.
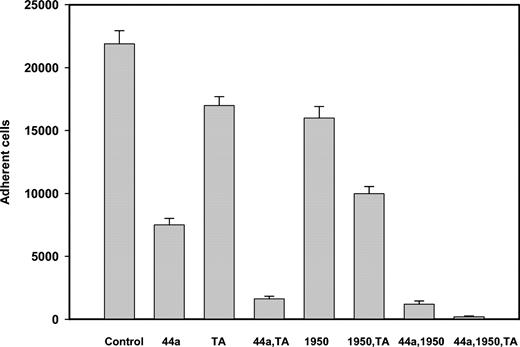
To extend our findings with transfected cells, we examined whether Glu-Pg and Pm can support adhesion of blood neutrophils. Neutrophils express α5β1 and αMβ2, and the latter can be up-regulated further by agonist stimulation. Neutrophils isolated from fresh human blood adhered to both Glu-Pg and Pm, with Glu-Pg being a 2- to 3-fold better substrate. Activation with 100 nM fMLP increased adhesion to Pg and Pm by about 5-fold (not shown). To dissect the contribution of specific integrin-binding sites and LBSs, neutrophil adhesion to Glu-Pg was tested in the presence of tranexamic acid and integrin-specific mAbs. As shown in Figure 7, inhibition of the LBSs decreased adhesion of fMLP-activated neutrophils to Glu-Pg by only 23%, and anti-αMβ2 and anti-α5β1 alone produced 66% and 28% inhibition, respectively. The combination of each mAb with tranexamic acid inhibited adhesion more effectively, and the treatment of neutrophils with a mixture of 2 mAbs and tranexamic acid resulted in the almost complete inhibition of adhesion. These results suggest that adhesion of neutrophils to Glu-Pg depends on both integrins αMβ2 and α5β1 and that the LBSs of Glu-Pg contribute to adhesion by binding to cell surface proteins. The participation of the 2 integrins and LBSs in adhesion to Glu-Pg was also demonstrable with U937 cells (not shown).
Adhesion of neutrophils to Glu-Pg. Neutrophils were isolated from human blood, resuspended in HBSS, and labeled with calcein. FMLP-(100 nM) activated neutrophils were preincubated with anti-αM mAb 44a (40 μg/mL), anti-α5β1 Ab 1950 (1:200 dilution), or tranexamic acid (5 mM) for 20 minutes at 25° C. Cells were then distributed into the wells coated with Glu-Pg (3.0 μg/mL). Fluorescence of adherent cells was measured as described in “Materials and methods.”
Adhesion of neutrophils to Glu-Pg. Neutrophils were isolated from human blood, resuspended in HBSS, and labeled with calcein. FMLP-(100 nM) activated neutrophils were preincubated with anti-αM mAb 44a (40 μg/mL), anti-α5β1 Ab 1950 (1:200 dilution), or tranexamic acid (5 mM) for 20 minutes at 25° C. Cells were then distributed into the wells coated with Glu-Pg (3.0 μg/mL). Fluorescence of adherent cells was measured as described in “Materials and methods.”
α5β1-mediated Pg activation
Previous studies demonstrated that Pg binding to cells through the LBSs enhanced its activation by t-PA. To test whether Pg-integrin interactions are accompanied by Pg activation, we have measured the Pm activity generated in the cell suspension containing Glu-Pg in the presence or absence of anti-αM, anti-α5β1, or anti-β1 function-blocking antibodies. Anti-α5β1 and anti-β1 inhibited Glu-Pg activation in a concentration-dependent manner on the 3 cell lines tested, including the αMβ2-expressing, mock-transfected HEK 293, and U937 monocytoid cells (Figure 8, shown for U937 cells). The maximal inhibition achieved with both antibodies was about 50% to 55%. The binding of Glu-Pg to α5β1 on these cells appears to be RGD independent because the RGD peptide at 100 μM did not inhibit Glu-Pg activation. With each cell line, the function-blocking mAb 44a (against αM) did not affect Glu-Pg activation whereas tranexamic acid decreased the activation by 55% ± 6%. Although addition of t-PA stimulated Glu-Pg activation, it did not change the inhibitory effect of anti-α5β1 (not shown). The ability of anti-α5β1 antibody to interfere with Glu-Pg activation indicates that in contrast to αMβ2, α5β1 can recognize both immobilized and soluble Glu-Pg. Moreover, because generation of the Pm activity was detected in the absence of exogenously added Pg activators, the data suggest that α5β1 is involved in Pg activation.
Activation of Pg by U937 cells. U937 cells (1.5 × 106/mL) were incubated in HBSS containing Pg (7.0 μg/mL) and S-2251 (0.7 mM) in the presence or absence of anti-αM mAb 44a, anti-α5β1 Ab 1950 (1:200), tranexamic acid (5 mM), or GRGDSP (0.1 mM), and the enzymatic activity of generated Pm was determined by measuring absorbance at 405 nm after 180 minutes.
Activation of Pg by U937 cells. U937 cells (1.5 × 106/mL) were incubated in HBSS containing Pg (7.0 μg/mL) and S-2251 (0.7 mM) in the presence or absence of anti-αM mAb 44a, anti-α5β1 Ab 1950 (1:200), tranexamic acid (5 mM), or GRGDSP (0.1 mM), and the enzymatic activity of generated Pm was determined by measuring absorbance at 405 nm after 180 minutes.
Discussion
This study was designed to examine the ability of human Pg to support cell adhesion by binding to the cell surface integrin receptors. The main findings are that (1) Glu-Pg can be deposited into the extracellular matrix in which it localizes with the 2 major matrix proteins, fibronectin and fibrinogen; (2) Glu-Pg is capable of supporting cell adhesion through integrins αMβ2 and α5β1; (3) the Pg LBSs contribute to adhesion by binding cell surface lysines; and (4) cell-associated Glu-Pg activation is inhibited by anti-α5β1 mAb. The ability of Pg to support adhesion has been demonstrated with various cells expressing both αMβ2 and α5β1, including neutrophils, cultured U937 monocytoid cells, and genetically engineered cells.
Recent studies of Pg interactions have focused on the binding of soluble Pg to cells and provided a good understanding of events that occur during the assembly of the Pg system on cell surfaces. Similar to other proteins such as fibrinogen, vitronectin, fibronectin, and others, plasminogen can exist in 2 states: as a soluble plasma protein and in association with insoluble extracellular matrix. Previous in vitro studies demonstrated that Pg and its activators, t-PA and u-PA, bind to the components of the ECM and the basement membrane.7,8,23,24 Pg also can be found within inflamed tissues11 where it was apparently derived from circulation. Although the predominant source of Pg is the liver, recent studies demonstrated a broad expression of Pg mRNA in murine45 and several human tissues46 and hypothesized that the extrahepatic organs may be local sources of Pg in vivo. Therefore, cell binding to Pg incorporated into the ECM could have been envisioned; however, this potential mechanism of cell interactions with Pg has not been distinguished in previous studies. Accordingly, we have examined the role of Pg in supporting cell adhesion mediated by integrins.
We demonstrated that immobilized Pg supports efficient cell adhesion, and integrins αMβ2 and α5β1 contribute to this process. Identification of αMβ2 as a receptor for Pg was based on inhibition of cell adhesion by function-blocking mAbs to this receptor and on the interaction of Pg with the recombinant αMI-domain. The integrin αMβ2 is expressed mainly on phagocytic leukocytes and mediates important leukocyte functions during the inflammatory responses.47 αMβ2 is a multiligand receptor that recognizes a variety of different molecules, including many ECM proteins (reviewed by Yakubenko et al25 ). Several proteases, including neutrophil elastase,48 myeloperoxidase,49 and catalase,44 have been previously demonstrated to bind αMβ2. The present study extends this group of αMβ2 ligands to include Pg. Within αMβ2, the αMI-domain is primarily responsible for its ligand-binding properties40 and, accordingly, Pg falls into the category of ligands that bind this domain. Also, consistent with previous findings of ligand-binding properties of β2 integrins, the related leukocyte integrin αLβ2 (CD11a/CD18, lymphocyte function-associated antigen-1 [LFA-1]), which has a narrow ligand-binding specificity, does not bind Pg.25
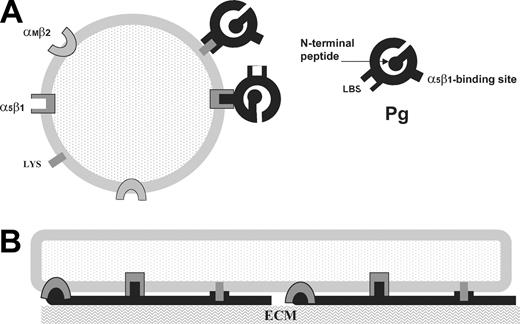
We have found a distinction in the way immobilized and soluble Pg engage the αMI-domain. The interaction between the immobilized Pg and αMβ2 was mediated by a specific ligand-binding site in the αMI-domain and the complementary site in Pg inasmuch as it was blocked by the specific inhibitor of αMβ2, the peptide P2. In addition, the Pg LBSs contribute to the interaction with the αMI-domain because lysine analogs tranexamic acid or ϵ-ACA blocked cell adhesion to Pg. In contrast, binding of soluble Pg to immobilized αMI-domain was mediated only by the Pg LBSs because tranexamic acid and ϵ-ACA completely inhibited this interaction whereas αMβ2 blocking agents were not effective. Tranexamic acid also fully blocked the binding of soluble Pg to cells in suspension, suggesting that even if αMβ2 does participate in Pg binding, this should involve αMβ2 lysines. The proposed molecular interactions of αMβ2 with 2 forms of Pg are depicted in Figure 9. Previous studies demonstrated that stimulation of neutrophils with PMA enhanced their Pg binding rapidly and substantially.50 This up-regulation was not dependent upon the proteolytic remodeling of cell surface proteins, leading to the increased availability of the C-terminal lysine residues. Notably, neutrophil surface expression of αMβ2 is up-regulated 3- to 10-fold as a result of its mobilization from internal pools in response to agonist stimulation. However, although αMβ2 may potentially contribute to accumulation of Pg on the cell surface, this appears to involve the Pg LBSs because anti-αMβ2 mAbs did not block the binding of soluble Pg to cells and did not interfere with generation of the Pm activity by cells.
Diagram of the proposed molecular interactions of Pg with cell surface receptors. (A) In cell suspension, soluble Pg (black) binds lysines (Lys) via its LBS. Integrin α5β1 is involved in Pg activation presumably by binding soluble Pg. (All cell receptors are in gray.) (B) An adherent cell spreads on Pg bound to the ECM. Unfolded immobilized Pg interacts with all 3 cell surface receptors.
Diagram of the proposed molecular interactions of Pg with cell surface receptors. (A) In cell suspension, soluble Pg (black) binds lysines (Lys) via its LBS. Integrin α5β1 is involved in Pg activation presumably by binding soluble Pg. (All cell receptors are in gray.) (B) An adherent cell spreads on Pg bound to the ECM. Unfolded immobilized Pg interacts with all 3 cell surface receptors.
The αMβ2-mediated adhesion to Pg was markedly higher to Glu-Pg than that to Lys-Pg and Lys-Pm. The Lys-Pg form is generated by the cleavage of an about 8 kDa N-terminal fragment from intact Glu-Pg by Pm. The 2 forms of Pg have different conformations,51 and Lys-Pg is more readily activated by activators.52,53 When tested with the αMβ2-expressing cells, the N-terminal Pg fragment supported efficient adhesion and also directly bound to the recombinant αMI-domain. Because the N-terminal fragment does not contain the LBS, its interaction with the receptor appears to be mediated by a specific integrin-binding site. However, the N-terminal domain may not be the only binding site for αMβ2 in Glu-Pg because higher molar concentrations of the N-terminal fragment than those of Glu-Pg were required to support effective adhesion. The difference in adhesion supported by Glu-Pg and Lys-Pg speculatively can be attributed to alterations in the conformation. The precedents of modulation of cell adhesion by the conformational states of proteins are numerous (reviewed by Lishko et al33 ). For example, thrombospondin supports platelet adhesion when coated onto the surface in the presence of Ca2+, whereas it becomes essentially nonadhesive as a calcium-depleted conformer.54
Integrin α5β1 was identified as the second integrin on the surface of HEK 293 cells that contributes to adhesion to Pg. The role of this integrin in adhesion to Glu-Pg was extended with neutrophils, U937 monocytoid cells, and K562 erythroleukemic cells. Integrin α5β1 is a receptor for fibronectin55 and fibrin(ogen),56 and α5β1-mediated cell adhesion to these ligands is inhibited by RGD-containing peptides that duplicate sequences in these ligands. In addition, recent studies demonstrated the non-RGD–dependent binding of α5β1 to fibrinogen.57 Glu-Pg does not contain RGD and, thus, further studies are needed to define the binding specificity of this novel α5β1 ligand. In contrast to αMβ2, α5β1 appears to engage both immobilized and soluble Glu-Pg (Figure 9) because anti-α5β1 but not anti-αMβ2 mAbs inhibited generation of Pm activity by cells. Although the α5β1-dependent conversion of Pg to Pm by cultured cells was detected, the mechanism by which α5β1 contributes to Pg activation remains to be elucidated. In addition, it is unlikely that this process would occur on the surface of normal nonstimulated circulating leukocytes in the absence of Pg activators.
Previous studies provided evidence of a direct involvement of Pg in migration of phagocytic leukocytes in response to inflammatory stimuli,3,4 and the Pg system was also implicated in assisting migration of other cells in a variety of physiopathological processes.1,2 However, the precise mechanism by which Pg participates in cell migration is unknown. The relationships between Pg in the ECM and Pg bound to cell surface receptors, including integrins, is also not clear. Because both cell surface receptors and the ECM proteins can bind Pg, a question arises as to whether migrating leukocytes, which carry large amounts of Pg, are capable of exchanging it with the ECM. The interactions of Pg with cells and the ECM are mediated primarily by weak LBSs and, therefore, it is interesting to speculate that Pg can be carried over from the cell surface onto the ECM and/or in the opposite direction. In this regard, we recently observed that fMLP-activated neutrophils picked 125I-Pg from the fibrin/125I-Pg substrate to which they adhered in the presence of nonlabeled Pg (V.K.L., unpublished results, 2002).
In summary, the present studies revealed the interaction of Pg with integrins αMβ2 and α5β1. The physiological role of this association remains unclear, and further studies should determine the relationships between Pg and integrins in leukocyte adhesion and migration and the role of integrins in Pg activation.
Prepublished online as Blood First Edition Paper, April 13, 2004; DOI 10.1182/blood-2003-09-3016.
Supported by National Institutes of Health (NIH) grant HL 6399 and the Established Investigator Grant from the American Heart Association.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal