Abstract
Cb2, the gene encoding the peripheral cannabinoid receptor, is located in a common virus integration site and is overex-pressed in retrovirally induced murine myeloid leukemias. Here we show that this G protein-coupled receptor (GPCR) is also aberrantly expressed in a high percentage of human acute myeloid leukemias. We investigated the mechanism of transformation by Cb2 and demonstrate that aberrant expression of this receptor on hematopoietic precursor cells results in distinct effects depending on the ligand used. Cb2-expressing myeloid precursors migrate upon stimulation by the endocannabinoid 2-arachidonoylglycerol and are blocked in neutrophilic differentiation upon exposure to another ligand, CP55940. Both effects depend on the activation of Gαi proteins and require the mitogen-induced extracellular kinase/extracellular signal-regulated kinase (MEK/ERK) pathway. Down-regulation of cyclic adenosine monophosphate (cAMP) levels upon Gαi activation is important for migration induction but is irrelevant for the maturation arrest. Moreover, the highly conserved G protein-interacting DRY motif, present in the second intracellular loop of GPCRs, is critical for migration but unimportant for the differentiation block. This suggests that the Cb2-mediated differentiation block requires interaction of Gαi proteins with other currently unknown motifs. This indicates a unique mechanism by which a transforming GPCR, in a ligand-dependent manner, causes 2 distinct oncogenic effects: altered migration and block of neutrophilic development. (Blood. 2004;104:526-534)
Introduction
Using retroviral insertional mutagenesis we recently demonstrated that Cb2, the gene encoding the peripheral cannabinoid receptor, is located in a common virus integration site (Evi11) in Cas-Br-M murine leukemia virus (MuLV)-induced myeloid leukemias, suggesting that Cb2 is a protooncogene involved in transformation.1,2 Cb2 encodes a 7-transmembrane (7TM) protein belonging to the family of Gαi protein-coupled receptors (GαiPCRs).3 This receptor is normally expressed in areas enriched for B lymphocytes—that is, marginal zone of the spleen, in the cortex of lymph nodes, in the nodular corona of Peyer patches, and in the mantle zones of secondary follicles in tonsils.3-6 Cb2 receptor is involved in B-cell differentiation and migration of splenic B lymphocytes, suggesting a role for this receptor in the immune response.5,7 The natural activator of Cb2 has been demonstrated to be 2-arachidonoylglycerol (2-AG),7-11 although a number of alternative Cb2 ligands have been reported (reviewed by Howlett et al12 ).
Acute myeloid leukemia (AML) is characterized by an accumulation of immature nonfunctional cells in the bone marrow and blood.13 Myeloid leukemia is considered to be a multigenetic disease involving cooperation between several disease genes.14-16 The genetic abnormalities in AML may result in aberrant expression of protooncogenes or inactivation of tumor suppressor genes, and consequently leukemia cells escape from regulatory signals, resulting in altered proliferation, aberrant survival, and a maturation arrest. Our previous observation that Cb2 is overexpressed in myeloid cell lines containing a retroviral insertion near Cb2 suggests that it may be involved in leukemic transformation in certain mouse leukemias. In the present study we demonstrate that the CB2 receptor is aberrantly expressed in several human myeloid cell lines and primary AML samples, whereas normal bone marrow precursor cells do not express this G protein-coupled receptor (GPCR).
We generated a Cb2-EGFP fusion construct,17 which was introduced into murine normal bone marrow cells and into 32D/granulocyte colony-stimulating factor receptor (G-CSF-R) cells. 32D/G-CSF-R cells proliferate in vitro in the presence of interleukin-3 (IL-3) and are capable of terminally differentiating toward mature neutrophils upon G-CSF stimulation. Furthermore, this cell line is a useful in vitro model to study molecular mechanisms involved in granulocytic differentiation18-20 and to perform functional analysis of transforming genes causing a block of neutrophilic differentiation.17,21 The Cb2-EGFP fusion protein appears fully functional, because the Cb2-expressing marrow cells and 32D/G-CSF-R/Cb2 cells migrate in response to the endocannabinoid 2-AG. In the present study we assessed whether 2-AG was capable of inducing a neutrophilic differentiation block of 32D/G-CSF-R/Cb2 cells. We demonstrate that the endocannabinoid 2-AG, although being a potent stimulator of migration of Cb2-expressing cells, could not block G-CSF-induced neutrophilic differentiation. Next, we tested whether another potent cannabinoid ligand, CP55940,22,23 could affect neutrophilic differentiation of Cb2-expressing cells. Interestingly, CP55940 failed to induce migration but evoked a complete arrest of neutrophilic differentiation.
Classical signaling by GPCRs is based on transduction of extracellular signals to downstream effectors via intracellular, heterotrimeric G protein complexes, which comprise α, β, and γ subunits.24,25 The recruitment of G proteins to GPCRs may require several motifs present in 7TM receptors. A well-characterized domain involving G protein recruitment and activation is the so-called DRY motif.26-29 The DRY (Asp-Arg-Tyr) box is a highly conserved region in 7TM receptors, located N-terminally in the second intracellular loop of most GPCRs. To analyze whether the DRY motif present in Cb2 receptor is crucial for migration and/or block of differentiation, we generated 2 different DRY mutants, DRA-Cb2 and DAY-Cb2. Finally, we demonstrated that mitogen-induced extracellular kinase/extracellular signal-regulated kinase (MEK/ERK) signaling is involved in both Cb2 functions whereas down-regulation of the intracellular cAMP levels is only required for migration.
Materials and methods
Cannabinoid ligands, cytokines, and inhibitors of intracellular signaling
The Cb2 ligands 2-arachidonoylglycerol (2-AG), anandamide (AEA), WIN 55212-2, cannabinol, cannabidiol, Δ8-tetrahydrocannabinol (Δ8-THC), and Δ9-tetrahydrocannabinol (Δ9-THC) were obtained from Sigma (Zwijndrecht, The Netherlands). N-palmitoylethanolamine (PEA) and N-acylethanolamine (POEA) were from ICN Biomedicals (Zoetermeer, The Netherlands) and CP55940 from Pfizer (Groton, CT). Cb1 inverse agonist SR141716 and Cb2 inverse agonist SR144528 were kindly donated by Dr Casellas (Sanofi Recherche, Montpellier, France). Recombinant human stromal cell-derived factor-1α (SDF-1α) was obtained from R&D Systems (Uithoorn, The Netherlands). Murine IL-3 was obtained from an IL-3-producing Chinese hamster ovary (CHO) cell line, and G-CSF was from Amgen (Thousand Oaks, CA). Dibutyryl cyclic adenosine monophosphate (dbcAMP) and U0126 (MEK inhibitor) were from Kordia Life Sciences (Leiden, The Netherlands) whereas PD98059 (MEK inhibitor) was obtained from Omnilabo International (Breda, The Netherlands). The inhibitors were dissolved in dimethyl sulfoxide (DMSO) and added to the cultures at the indicated concentrations and were refreshed daily.
Cb2-EGFP expression construct, site-directed mutagenesis, and infection of 32D/G-CSF-R cells
A Cb2-EGFP fusion construct was generated and cloned into pLNCX (Clontech, Palo Alto, CA) as described previously.17 A QuikChange Site-Directed Mutagenesis Kit was used to mutate the DRY motif present in Cb2-EGFP receptor as indicated by the supplier (Stratagene Europe, Amsterdam, The Netherlands). The primers 5′-GCTGTTGACCGCGCCCTATGTCTGTG-3′ and 5′-CACAGACATAGGGCGCGGTCAACAGC-3′ were used to mutate the wild-type (wt) DRY motif into a DRA motif, and the primers 5′-CCGCTGTTGACGCCTACCTATGTCTG-3′ and 5′-CAGACATAGGTAGGCGTCAACAGCGG-3′ were used to mutate the wt DRY motif into a DAY motif (Figure 5). The Cb2-EGFP and the DRY mutated constructs were verified by nucleotide sequence. These expression constructs were transfected into Phoenix cells type E (gift from G. Nolan, Stanford, CA), and the viral supernatants were used for infection of 32D/G-CSF receptor (32D/G-CSF-R) cells. Single clones were obtained using limiting dilution in 96-well microtiter trays (Becton Dickinson, Mountain View, CA), and infected clones were selected on 0.8 mg/mL G418 (Gibco, Breda, The Netherlands). Cb2-EGFP fusion protein and DRY mutant expression was analyzed by Leica DMRXA microscopy (Leica Microsystems, Rijswijk, The Netherlands) and flow cytometric analysis of enhanced green fluorescent protein (EGFP) fluorescence.
Mutation of the DRY motif in Cb2 and analysis of 32D/G-CSF-R/Cb2 mutant clones. (A) Location of the DRY motif in Cb2. Introduced mutations are indicated in bold. The box shows the results of ligand binding assays. The dissociation constant (Kd) is expressed for CP55940 in picomolars and for 2-AG in nanomolars. Maximum binding (Bmax) is expressed as femtomoles per milligram of protein. Data were pooled from independent experiments performed on 2 clones of the same cell type. (B) Flow cytometric analysis of representative 32D/G-CSF-R clones expressing the distinct constructs. The insets show cell fluorescence distribution in the infected cells by microscopy; original magnification × 63. (C) Four representative mutant clones cultured for 8 days in the presence of G-CSF with or without CP (100 nM). (D) Four representative 32D/G-CSF-R clones expressing Cb2 mutants cultured in G-CSF with or without CP, Cb2 (C2), and Cb1 (C1) inverse agonist (100 nM). Counts were carried out on day 8 of culture. □ indicates blast cells; ▪, intermediate forms; and ▦, terminally differentiated neutrophils. (E) In vitro migration of cells containing a DRY, DRA, or DAY motif. Cells were exposed to medium with 300 nM 2-AG or control medium; 100 nM C1 or C2 was added to the upper chamber.The percentage of migration is the average of 3 clones.
Mutation of the DRY motif in Cb2 and analysis of 32D/G-CSF-R/Cb2 mutant clones. (A) Location of the DRY motif in Cb2. Introduced mutations are indicated in bold. The box shows the results of ligand binding assays. The dissociation constant (Kd) is expressed for CP55940 in picomolars and for 2-AG in nanomolars. Maximum binding (Bmax) is expressed as femtomoles per milligram of protein. Data were pooled from independent experiments performed on 2 clones of the same cell type. (B) Flow cytometric analysis of representative 32D/G-CSF-R clones expressing the distinct constructs. The insets show cell fluorescence distribution in the infected cells by microscopy; original magnification × 63. (C) Four representative mutant clones cultured for 8 days in the presence of G-CSF with or without CP (100 nM). (D) Four representative 32D/G-CSF-R clones expressing Cb2 mutants cultured in G-CSF with or without CP, Cb2 (C2), and Cb1 (C1) inverse agonist (100 nM). Counts were carried out on day 8 of culture. □ indicates blast cells; ▪, intermediate forms; and ▦, terminally differentiated neutrophils. (E) In vitro migration of cells containing a DRY, DRA, or DAY motif. Cells were exposed to medium with 300 nM 2-AG or control medium; 100 nM C1 or C2 was added to the upper chamber.The percentage of migration is the average of 3 clones.
Flow cytometric analysis
32D/G-CSF-R cells transduced with Cb2-EGFP,17 as well as with Cb2-EGFP DRA mutant, Cb2-EGFP DAY mutant, and EGFP control, were analyzed by flow cytometric analysis by means of EGFP (FACScan flow cytometer; Becton Dickinson, Mountain View, CA) as described previously.17 EGFP cell fluorescence was visualized using a fluorescence microscope (DMRXA; Leica, Wetzlar, Germany) with PL APO 63 ×/1.32 lenses. The images were captured with a Leica DMRXA camera, and Leica QFISH software was used.
The myeloid cell lines HL60 (ATCC CCL 240; American Type Culture Collection, Manassas, VA), MV 4-11 (ATCC CRL 9591), U937 (ATCC CRL 1593), KG1 (ATCC CCL 246), KG1a (ATCC CCL 246.1), K562 (ATCC CCL 243), NB-4,30 and ME-1 31 as well as primary AML samples and CD34+ cells were used for immunofluorescence analyses. Bone marrow AML samples at diagnosis and from healthy volunteers were obtained after informed consent. Blasts from AML patients and healthy bone marrow specimens were isolated from the samples by Ficoll-Hypaque (Nygaard, Oslo, Norway) centrifugation.32 The cells were then cryopre-served as described.33
Normal umbilical cord blood CD34 cells were purified using a magnetic cell sorting system (MACS cell isolation kits; Miltenyi Biotec, Bergisch Gladbach, Germany). In brief, cells were thawed, washed twice in RPMI 1640 medium (Life Technologies, Breda, The Netherlands), and cultured in this medium supplemented with penicillin (100 U/mL), streptomycin (100 ng/mL), and 10% fetal calf serum (Life Technologies) for 1 hour at 37°C and 10% CO2. After washing, cells were incubated on ice with the polyclonal N-terminal anti-CB2 antibody (1:50) (Affinity Bioreagents, Golden, CO) for 1 hour, followed by 30 minutes of incubation with the fluorescein isothiocyanate (FITC)-conjugated secondary rabbit antibody (1:200) goat antirabbit (GAR)-FITC/immunoglobulin G (IgG) (Nordic Immunological Laboratories, Tilburg, The Netherlands). In case of dual staining, cells were incubated next for 30 minutes with phycoerythrin (PE)-conjugated primary or secondary control antibodies (GAR-FITC/IgG and IgG1 PE). CD34 PE and CD14 PE were obtained from Becton Dickinson (Franklin Lakes, NJ), CD33 PE and IgG1 PE were from Beckman Coulter (Fullerton, CA), and CD66 PE was obtained from CLB Laboratories (Amsterdam, The Netherlands). Cells were washed twice with phosphate-buffered saline (PBS), resuspended in 500 μL PBS containing 0.5% bovine serum albumin (BSA), and analyzed using a FACScan flow cytometer (Becton Dickinson, Mountain View, CA).
Ligand binding analysis
[3H]2-AG was synthesized from 1,3-dibenzyloxy-2-propanol and [3H]arachidonic acid (200 Ci/mmol [7400 GBq/mmol]; ARC, St Louis, MO), as reported,34 and [3H]CP55940 (5-(1,1′-dimethyheptyl)-2-(1R,5R-hydroxy-2R-[3-hydroxypropyl] cyclohexyl)-phenol; 126 Ci/mmol [4662 GBq/mmol]) was from NEN DuPont de Nemours (Köln, Germany). Membrane fractions were prepared from the different clones (100 × 106 per test) as reported35 and were used in rapid filtration assays with the synthetic cannabinoid [3H]CP55940. Apparent dissociation constant (Kd) and maximum binding (Bmax) values of [3H]CP55940 were calculated from saturation curves through nonlinear regression analysis with the Prism 3 program (GraphPAD Software for Science, San Diego, CA). Binding of [3H]2-AG was evaluated with the same filtration assays used for [3H]CP55940, and apparent Kd and Bmax values were calculated through nonlinear regression analysis of saturation curves.35 In all experiments, unspecific binding was determined in the presence of 10 μM nonlabeled agonist. Data reported are the mean (± SD) of at least 3 independent determinations, each in duplicate. Statistical analysis was performed by the nonparametric Mann-Whitney test with the InStat 3 program (GraphPAD Software for Science).
In vitro proliferation and neutrophilic differentiation of 32D/G-CSF-R cells
The 32D/G-CSF receptor (32D/G-CSF-R) cell line19 was cultured in RPMI 1640 medium (Life Technologies) supplemented with penicillin (100 IU/mL), streptomycin (100 ng/mL), 10% fetal calf serum (FCS), and human G-CSF (100 ng/mL) for 9 days. Cell counting was performed using a CASY1/TTC cell counter (Schärfe System, Reutlingen, Germany), and the cell density was readjusted to 2 × 105/mL daily. Morphologic analysis was done by microscopy on May-Grünwald Giemsa-stained cytospins (Shandon Holland, Amsterdam, The Netherlands). Images were acquired with a telemicroscope system (Zeiss, Koln, Germany) consisting of an Axioplan 2 microscope equipped with Plan Apo and Plan Neofluor objectives. The proprietary Software Autocyte Link version 1.1 was run on a personal computer equipped with an Intel Pentium class IV processor and Windows 2000. Magnification, × 40.
Migration assay
Migration assays were performed using 5 μm pore size and 6.5-mm diameter transwells (Corning Costar, Amsterdam, The Netherlands) as previously described.7 In brief, cells were washed twice with Hanks balanced salt solution (HBSS) medium, resuspended in 100 μL migration medium (Iscove modified Dulbecco medium [IMDM] plus 0.5% BSA), and placed in the upper chamber of the transwells. In the lower chamber, 600 μL migration medium with or without ligand was placed. After 4 hours of incubation at 37°C and 5% CO2, the upper chamber was removed and the numbers of migrated cells were determined using a CASY1/TTC cell counter (Schärfe System). Cb1- and Cb2-specific antagonists (100 nM) were added to the upper chamber when tested. PD98059, U0126, and dbcAMP were added to the cells, incubated 30 minutes at 37°C, and then transferred to the upper well.
Cb2-EGFP retroviral vectors, virus production, and infection of mouse bone marrow progenitor cells
Cb2-EGFP was obtained by Eco47III/NotI digestion from pEGFP-N1 vector and subcloned as a blunt fragment into HpaI site of pBabe retroviral vector. Correct insertion of Cb2-EGFP was verified by nucleotide sequencing. The expression constructs were transfected into Phoenix cells type E (gift from G. Nolan), and the virus-containing supernatants were used for infection of bone marrow progenitor cells as described previously.21 Transduction efficiency was determined by fluorescence-activated cell sorter (FACS) analysis of EGFP fluorescence. To study migration, cells were cultured in Cell Gro medium supplemented as before plus 2.5 μg/mL puromycin (Sigma) for 4 to 5 days and then used in a migration assay. Bone marrow suspension cultures were performed in RPMI 1640 medium supplemented with 10% FCS, 2.5 μg/mL puromycin (Sigma), and human G-CSF (100 ng/mL). Cultures were carried out in the presence or absence of CP55940, the Cb1 inverse agonist, the Cb2 inverse agonist, or combinations of these agents. Cell countings were performed every 3 to 4 days, and cytospins were prepared for morphologic analysis.
Results
CB2 frequently is expressed on human acute myeloid leukemia cells but absent on normal myeloid precursors
Cb2 is frequently targeted in retrovirally induced leukemia, resulting in overexpression of this receptor.1,7 To investigate whether CB2 may be involved in human malignancies as well, we studied expression of this receptor on malignant and normal myeloid precursor cells using specific antibodies and flow cytometric analysis. High receptor levels were observed in HL60, NB4 (Figure 1A), U937, and MV 4-11 (data not shown). The cell lines KG1, KG1a, K562, and ME-1 did not show any CB2 protein expression (data not shown). High CB2 expression was observed on AML blasts in 14 of 30 patient samples. Two typical examples are demonstrated in Figure 1B. Flow cytometric analysis of CD34-purified fractions from normal bone marrow revealed no expression of CB2 on these cells (Figure 1B). Moreover, double labeling of normal marrow cells using CB2-specific antibodies in combination with CD34, CD33, CD66, or CD14 revealed no detectable CB2 levels on myeloid cells at any differentiation stage (Figure 1C). These data suggest that CB2 expression on myeloid leukemia cells in humans as well as in mice is an abnormal feature.
CB2 expression in human acute myeloid leukemia cells and normal myeloid precursors. (A) Flow cytometric analysis of a representative CB2-positive and a CB2-negative myeloid cell line. Staining was performed using a CB2 N-terminal antibody followed by FITC-conjugated secondary rabbit antibody. (B) CB2 cell surface expression analysis on primary AML patient samples and normal CD34+ bone marrow cells using the CB2 N-terminal antibody. (C) Immunophenotyping of normal total bone marrow using flow cytometric analysis.
CB2 expression in human acute myeloid leukemia cells and normal myeloid precursors. (A) Flow cytometric analysis of a representative CB2-positive and a CB2-negative myeloid cell line. Staining was performed using a CB2 N-terminal antibody followed by FITC-conjugated secondary rabbit antibody. (B) CB2 cell surface expression analysis on primary AML patient samples and normal CD34+ bone marrow cells using the CB2 N-terminal antibody. (C) Immunophenotyping of normal total bone marrow using flow cytometric analysis.
CP55940 mediates a decrease of neutrophilic differentiation, and 2-AG induces migration of Cb2-expressing bone marrow precursors
To study the mechanism of transformation by this GPCR, we introduced the Cb2 gene fused in frame to EGFP as previously described7 into Percoll-separated normal murine bone marrow cells. Cb2-expressing bone marrow cells migrated significantly in response to the endocannabinoid 2-AG (Figure 2). These 2-AG-migrated CB2-EGFP-expressing bone marrow cells when placed in an in vitro colony assay were capable of generating high numbers of G-CSF-, granulocyte-macrophage CSF (GM-CSF)-, and IL-3-stimulated colonies (data not shown). 2-AG-induced migration could be fully abolished by addition of Cb2 inverse agonists, whereas Cb1 inverse agonist did not affect migration. EGFP control-infected bone marrow cells weakly migrated upon 2-AG stimulation. The low numbers of 2-AG-migrated EGFP-expressing control cells were not capable of forming any colonies in vitro. Although we did not investigate which cell types were 2-AG responsive, our previous data7 would suggest that these cells may be B lymphocytes.
2-AG-induced migration of Cb2- or EGFP-transduced murine bone marrow cells. Cb2-transduced (left) or EGFP-transduced (right) bone marrow precursors were exposed to medium with or without 300 nM 2-AG. Cells were placed in the upper well in the presence or absence of 100 nM of either Cb2 (C2) or Cb1 (C1) inverse agonist. Data represent the mean values of 3 independent experiments. Error bars indicate SD.
2-AG-induced migration of Cb2- or EGFP-transduced murine bone marrow cells. Cb2-transduced (left) or EGFP-transduced (right) bone marrow precursors were exposed to medium with or without 300 nM 2-AG. Cells were placed in the upper well in the presence or absence of 100 nM of either Cb2 (C2) or Cb1 (C1) inverse agonist. Data represent the mean values of 3 independent experiments. Error bars indicate SD.
In vitro culture using IL-3 or G-CSF revealed no effect of 2-AG on proliferation or differentiation of Cb2-expressing marrow cells (data not shown). We next studied whether another well-known Cb2 agonist, CP55940, had an effect on marrow precursors expressing Cb2. A decrease in neutrophilic differentiation, although statistically not significant (analysis of variance [ANOVA] test, P = .11), of Cb2-expressing cells was observed when cultured in suspension with G-CSF plus CP55940 as compared with cultures with G-CSF only (Table 1). Moreover, addition of Cb2 inverse agonist recovered the appearance of mature neutrophils (Table 1), whereas the Cb1 inverse agonist had no effects (data not shown). EGFP-transduced bone marrow cells do not show any response to CP55940 or inverse agonists in an in vitro differentiation assay (Table 1).
Morphologic analysis of Cb2- and EGFP-transduced murine bone marrow cells cultured for 6 days
. | Experiment 1 . | . | . | Experiment 2 . | . | . | Experiment 3 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Immature* . | Premature† . | Mature‡ . | Immature* . | Premature† . | Mature‡ . | Immature* . | Premature† . | Mature‡ . | ||||||
| Cb2 | |||||||||||||||
| G§ | 18 | 30 | 52 | 15 | 17 | 68 | 5 | 11 | 84 | ||||||
| G + CP∥ | 17 | 39 | 44 | 29 | 32 | 39 | 13 | 30 | 57 | ||||||
| G + CP + C2¶ | 8 | 11 | 81 | 9 | 21 | 70 | 3 | 10 | 87 | ||||||
| EGFP | |||||||||||||||
| G§ | 6 | 11 | 83 | 17 | 17 | 66 | 7 | 6 | 87 | ||||||
| G + CP∥ | 5 | 16 | 79 | 13 | 25 | 62 | 2 | 15 | 83 | ||||||
| G + CP + C2¶ | 10 | 20 | 70 | 7 | 20 | 73 | 2 | 8 | 90 | ||||||
. | Experiment 1 . | . | . | Experiment 2 . | . | . | Experiment 3 . | . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | Immature* . | Premature† . | Mature‡ . | Immature* . | Premature† . | Mature‡ . | Immature* . | Premature† . | Mature‡ . | ||||||
| Cb2 | |||||||||||||||
| G§ | 18 | 30 | 52 | 15 | 17 | 68 | 5 | 11 | 84 | ||||||
| G + CP∥ | 17 | 39 | 44 | 29 | 32 | 39 | 13 | 30 | 57 | ||||||
| G + CP + C2¶ | 8 | 11 | 81 | 9 | 21 | 70 | 3 | 10 | 87 | ||||||
| EGFP | |||||||||||||||
| G§ | 6 | 11 | 83 | 17 | 17 | 66 | 7 | 6 | 87 | ||||||
| G + CP∥ | 5 | 16 | 79 | 13 | 25 | 62 | 2 | 15 | 83 | ||||||
| G + CP + C2¶ | 10 | 20 | 70 | 7 | 20 | 73 | 2 | 8 | 90 | ||||||
Percentage of myeloblasts and promyelocytes.
Percentage of myelocytes and metamyelocytes.
Percentage of band and segmented neutrophils.
G-CSF (100 ng/mL).
G-CSF + 100 nM CP55940.
G-CSF + 100 nM CP55940 + 1 μM Cb2 inverse agonist.
The endocannabinoid 2-AG stimulates migration, and CP55940 induces a full block of neutrophilic differentiation of Cb2-expressing 32D/G-CSF-R cells
To further analyze the effects of Cb2 and the distinct ligands in detail, Cb2-EGFP or EGFP constructs were introduced into 32D/G-CSF-R cells. Eight Cb2-expressing clones and 8 EGFP control clones were first cultured in the presence of G-CSF and different concentrations of 2-AG (100 nM to 1 μM). 2-AG did not affect neutrophilic differentiation at any of the concentrations tested (Figure 3A-B). On the other hand, 2-AG was shown to be an efficient stimulator of migration of the 32D/G-CSF-R/Cb2 cells as determined in a transwell assay (Figure 3A). This effect was receptor specific, because 2-AG-induced migration was fully counteracted by the Cb2 inverse agonist SR144528 but not by the Cb1 inverse agonist SR141716 (Figure 3A). In contrast, the other Cb2 ligand, CP55940, fully blocked G-CSF-induced neutrophilic differentiation of Cb2-expressing 32D/G-CSF-R clones (Figure 3A-B). CP55940 did not affect maturation of EGFP control 32D/G-CSF-R cells (Figure 3B). Addition of Cb2 inverse agonist to the G-CSF/CP55940-containing cultures completely restored neutrophilic differentiation of Cb2-expressing 32D/G-CSF-R cells, whereas the Cb1 inverse agonist had no effect (Figure 3A). Titration experiments revealed that picomolar concentrations of CP55940 were sufficient to significantly stimulate a differentiation block (Figure 3C). Using 100 nM CP55940 and different concentrations of Cb2 inverse agonist, we observed that neutrophilic differentiation of Cb2-expressing cells could be recovered in a dose-dependent manner (Figure 3D). In contrast to 2-AG, CP55940 could not induce migration of Cb2-expressing cells (Figure 3A).
Effects of distinct cannabinoids on the G-CSF-induced neutrophilic differentiation and migration of 32D/G-CSF-R cells. (A) Morphologic analysis of May-Grünwald Giemsa-stained cytospins of 1 representative Cb2-expressing clone cultured in G-CSF and 100 nM 2-AG or CP55940 (CP) in the presence or absence of 1 μM Cb2 (C2) or Cb1 (C1) inverse agonist. In vitro migration of 32D/G-CSF-R/Cb2 cells upon 2-AG or CP stimulation; 100 nM C2 or C1 was added to the upper well when tested. (B) Two representative Cb2-expressing and 2 representative EGFP-expressing clones were cultured in G-CSF and 100 nM 2-AG or CP. (C) Differential counts of a representative CP titration experiment in the presence of G-CSF (day 8 of culture). □ indicates blast cells; ▪, intermediate forms; and ▦, terminally differentiated neutrophils. (D) Differential counts of a 32D/G-CSF-R/Cb2 clone cultured with G-CSF, 100 nM CP, and different concentrations of C2.
Effects of distinct cannabinoids on the G-CSF-induced neutrophilic differentiation and migration of 32D/G-CSF-R cells. (A) Morphologic analysis of May-Grünwald Giemsa-stained cytospins of 1 representative Cb2-expressing clone cultured in G-CSF and 100 nM 2-AG or CP55940 (CP) in the presence or absence of 1 μM Cb2 (C2) or Cb1 (C1) inverse agonist. In vitro migration of 32D/G-CSF-R/Cb2 cells upon 2-AG or CP stimulation; 100 nM C2 or C1 was added to the upper well when tested. (B) Two representative Cb2-expressing and 2 representative EGFP-expressing clones were cultured in G-CSF and 100 nM 2-AG or CP. (C) Differential counts of a representative CP titration experiment in the presence of G-CSF (day 8 of culture). □ indicates blast cells; ▪, intermediate forms; and ▦, terminally differentiated neutrophils. (D) Differential counts of a 32D/G-CSF-R/Cb2 clone cultured with G-CSF, 100 nM CP, and different concentrations of C2.
CP55940-mediated block of differentiation and 2-AG-induced migration are pertussis toxin (PTX) sensitive
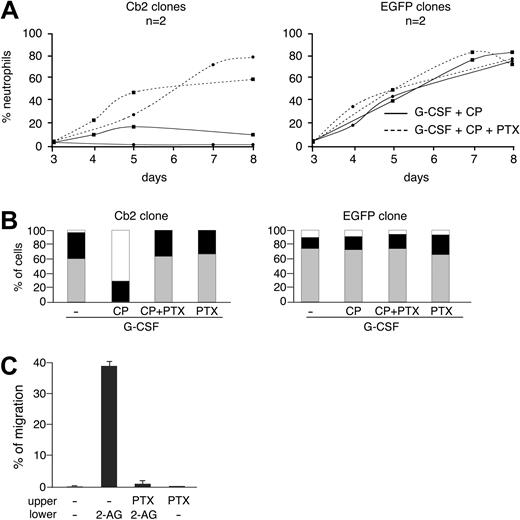
Cb2 receptor belongs to the GαiPCR subfamily. To study whether Cb2 requires Gαi proteins to stimulate migration or block neutrophilic differentiation, we used PTX, a molecule that prevents heteromer formation between the G protein and the receptor. We observed full differentiation of Cb2-expressing 32D/G-CSF-R cells when PTX (100 ng/mL) was added to the G-CSF/CP55940 cultures (Figure 4A-B). Addition of PTX to the EGFP control clones had no effect on the neutrophilic differentiation of these cells (Figure 4A-B). Moreover, 2-AG-induced migration of 32D/G-CSF-R/Cb2 cells was completely abolished by 300 ng/mL PTX (Figure 4C).
Effect of pertussis toxin (PTX) on the CP55940-evoked block of differentiation and the 2-AG-induced migration of 32D/G-CSF-R cells. (A) Two representative Cb2- and EGFP-expressing 32D/G-CSF-R clones were cultured for 8 days in the presence of G-CSF plus CP559940 (CP) with or without PTX (100 ng/mL). (B) Differential counts of a representative Cb2- and EGFP-expressing 32D/G-CSF-R clone at day 7 of culture in the presence of G-CSF with or without CP and PTX. □ indicates blast cells; ▪, intermediate forms; and ▦, terminally differentiated neutrophils. (C) Effect of 300 ng/mL PTX on 2-AG-induced migration of 32D/G-CSF-R/Cb2 cells. PTX was added to the cells and preincubated for 1 hour at 37°C before the cells were placed in the upper chamber of a transwell assay. Values indicate the average of 3 representative clones. Error bars indicate SD.
Effect of pertussis toxin (PTX) on the CP55940-evoked block of differentiation and the 2-AG-induced migration of 32D/G-CSF-R cells. (A) Two representative Cb2- and EGFP-expressing 32D/G-CSF-R clones were cultured for 8 days in the presence of G-CSF plus CP559940 (CP) with or without PTX (100 ng/mL). (B) Differential counts of a representative Cb2- and EGFP-expressing 32D/G-CSF-R clone at day 7 of culture in the presence of G-CSF with or without CP and PTX. □ indicates blast cells; ▪, intermediate forms; and ▦, terminally differentiated neutrophils. (C) Effect of 300 ng/mL PTX on 2-AG-induced migration of 32D/G-CSF-R/Cb2 cells. PTX was added to the cells and preincubated for 1 hour at 37°C before the cells were placed in the upper chamber of a transwell assay. Values indicate the average of 3 representative clones. Error bars indicate SD.
Mutation of the DRY motif in Cb2 causes a reduced migration response to 2-AG but does not affect CP55940-mediated block of differentiation
To assess whether the Cb2 DRY motif is important to recruit and activate G proteins in the CP55940-mediated block of differentiation and/or the 2-AG-induced migration, 32D/G-CSF-R cells were infected with retrovirus carrying different Cb2-EGFP DRY mutants. Distinct constructs (ie, Cb2-DRY [wt], Cb2-DRA mutant, and Cb2-DAY mutant) were generated (Figure 5A), introduced into 32D/G-CSF-R cells, and studied in transwell and differentiation assays. Following G418 selection, 6 32D/G-CSF-R clones for each construct were obtained. Expression of the distinct Cb2-EGFP variants introduced into 32D/G-CSF-R cells was analyzed by means of fluorescence microscopy and flow cytometric analysis. A representative clone for each transfected construct is shown in Figure 5B. Equal levels of fluorescence were detected in the 3 Cb2 clone types, and receptor membrane distribution was similar in all cell types (Figure 5B). Binding of 2-AG and CP55940 to the different clones was assessed by ligand binding assays. Figure 5A indicates that receptor levels (Bmax) as well as affinities (Kd) for 2-AG and CP55940 on 32D/G-CSF-R cells were comparable between clones transduced with the distinct constructs. Cb2 mutants cultured in the presence of G-CSF plus CP55940 showed a block in neutrophilic differentiation (Figure 5C-D) comparable to the differentiation block observed with the nonmutated Cb2 wt transduced cells. This block of differentiation was reversible by the Cb2 inverse agonist but not by addition of Cb1 inverse agonist (Figure 5D). In contrast, the 2-AG-induced migration of 32D/G-CSF-R/Cb2 mutants was significantly reduced in comparison with the nonmutated Cb2 control clones (Figure 5E). The reduced levels of 2-AG-induced migration could still be abolished by addition of Cb2, but not Cb1, inverse agonist (Figure 5E).
dbcAMP interferes with migration but not with the neutrophilic differentiation block of Cb2-expressing 32D/G-CSF-R cells
Because activation of GαiPCRs inhibits adenyl cyclase activity, we investigated whether down-regulation of the intracellular cAMP levels was necessary to drive the distinct Cb2 effects. Addition of dbcAMP, a cAMP analog, to the G-CSF plus CP55940-containing cultures did not recover neutrophilic differentiation of 32D/G-CSF-R/Cb2 cells (Figure 6A-B). dbcAMP did not alter neutrophilic maturation of EGFP control clones (Figure 6A-B). Increasing concentrations of dbcAMP partially blocked 2-AG-induced migration of Cb2-expressing 32D/G-CSF-R cells (Figure 6C). Thus, down-regulation of intracellular cAMP levels seems to be partially responsible for Cb2-mediated migration but appears unimportant for the block of neutrophilic differentiation following Cb2 receptor stimulation.
Effects of dbcAMP and MEK/ERK inhibitors on 2-AG-induced migration and the CP55940-stimulated block of differentiation. (A) A representative Cb2 and EGFP control 32D/G-CSF-R clone cultured with G-CSF with or without 100 nM CP55940 (CP), 100 μM dbcAMP, or 25 μM PD98059 (PD). (B) Pictures of May-Grünwald Giemsa-stained cytospins (day 8) of a representative Cb2-expressing 32D/G-CSF-R clone cultured under the different conditions. Original magnification × 63. (C) Effects of different concentrations of dbcAMP and U0126 on the 2-AG-induced (300 nM) migration of a representative Cb2-expressing 32D/G-CSF-R clone. The y-axis indicates the percentage of migrated cells in relation to the nontreated cells.
Effects of dbcAMP and MEK/ERK inhibitors on 2-AG-induced migration and the CP55940-stimulated block of differentiation. (A) A representative Cb2 and EGFP control 32D/G-CSF-R clone cultured with G-CSF with or without 100 nM CP55940 (CP), 100 μM dbcAMP, or 25 μM PD98059 (PD). (B) Pictures of May-Grünwald Giemsa-stained cytospins (day 8) of a representative Cb2-expressing 32D/G-CSF-R clone cultured under the different conditions. Original magnification × 63. (C) Effects of different concentrations of dbcAMP and U0126 on the 2-AG-induced (300 nM) migration of a representative Cb2-expressing 32D/G-CSF-R clone. The y-axis indicates the percentage of migrated cells in relation to the nontreated cells.
Interference of CP55940-mediated block of differentiation as well as 2-AG-induced migration by MEK/ERK pathway inhibitors
We next studied whether signaling via MEK/ERK pathway is critical for the distinct Cb2-mediated effects. MEK inhibitors, PD98059 (Figure 6A-B) or U0126 (data not shown), fully recovered neutrophilic differentiation of 32D/G-CSF-R/Cb2 cells cultured with G-CSF plus CP55940. MEK inhibitors did not alter differentiation of EGFP control clones (Figure 6A-B). Addition of U0126 to transwell assays revealed a dose-dependent inhibition of 2-AG-induced migration of Cb2-expressing 32D/G-CSF-R cells (Figure 6C). The same results were observed when the cells were exposed to PD98059 in a transwell assay (data not shown). This effect appeared highly specific because stimulation of migration of 32D/G-CSF-R cells by SDF-1, the ligand for CXCR4, could not be inhibited by U0126 (data not shown). These data indicate that MEK/ERK signaling is critical in 2-AG-induced migration as well as for CP55940-induced block of neutrophilic differentiation.
Discussion
The peripheral cannabinoid receptor gene, Cb2, encodes a 7-trans-membrane (7TM) G protein-coupled receptor (GPCR).3 Using retroviral insertional mutagenesis, we identified Cb2 as the target gene in the Evi11 locus, indicating that Cb2 may be a protoonco-gene involved in leukemogenesis.1,2 We previously observed that Cb2 is highly expressed in myeloid cell lines containing a retroviral insertion in Cb2,1,7 and here we showed that CB2 is overexpressed in several human myeloid leukemia cell lines. Interestingly, here we report that CB2 is frequently overexpressed in AML blasts, whereas normal bone marrow fractions are CB2-negative. It is unclear why CB2 is so highly expressed in particular AML samples and cell lines. We did not find any correlation between the different morphologic AML subtypes and CB2 expression levels. Likewise, no correlation was found between the origin of the distinct cell lines and Cb2 protein levels. Real-time polymerase chain reaction studies revealed high levels of CB2 mRNA in the CB2-positive cell lines and not in the negative lines, suggesting altered transcription (R.D. et al, unpublished observation, March 2003). The cause of these differences in transcription remains to be elucidated. When overexpressed in the myeloid precursors, Cb2 induces a block in neutrophilic development17 and stimulates migration of Cb2-expressing cells in vitro.7 The endocannabinoid 2-AG is the most potent agonist capable of inducing migration of cannabinoid receptor-expressing cells.7,9-11 Here we report that 2-AG has no effect on the G-CSF-induced differentiation of 32D/G-CSF-R/Cb2 cells in vitro, whereas another well-described ligand, CP55940,22,23 stimulates a neutrophilic differentiation block. The fact that the Cb2 inverse agonist SR144528 could fully counteract the 2 functions demonstrates receptor specificity. To our knowledge this is the first example of a GPCR that, when overexpressed in myeloid precursors cells, causes 2 different effects depending on the ligand.
Normal murine bone marrow precursors aberrantly expressing Cb2 show a moderate but steady impairment of maturation when cultured with G-CSF plus CP55940. Leukemia is a multigenic disease, meaning that a combination of genetic defects is required to obtain a full leukemia.15,16 For instance, we previously demonstrated that aberrant Cb2 expression frequently coincides with aberrant expression of Evi1,36,37 another transforming gene shown to be involved in impairment of neutrophilic development.38-40 The observation that in 32D/G-CSF-R cells Cb2 overexpression causes a complete block of neutrophilic differentiation most likely reflects cooperation between Cb2 and other genetic defects present in this myeloid precursor cell line. Interestingly, Evi1 retroviral insertions as well as overexpression have been reported for 32D cells.41 It would be of interest to study whether overexpression of Cb2 in combination with Evi1 in normal marrow precursors would lead to a more severe block of neutrophilic differentiation.
The experiments presented in this paper demonstrate that Cb2 receptor may evoke 2 distinct biologic effects depending on the ligand used for stimulation (ie, migration upon 2-AG presentation and block in neutrophilic differentiation following exposure to CP55940). How the interaction of distinct Cb2 ligands to Cb2 receptor may result in activation of different processes in the same cell is an interesting question. Several examples exist of one receptor interacting with distinct ligands. The cytokines IL-3, IL-5, as well as GM-CSF interact with different receptor complexes that all share a common β receptor chain. The specificity of these distinct ligands for the different complexes is determined by the ligand-specific receptor α chains.42,43 Similarly, IL-2, IL4, IL-7, IL-9, and IL-15 each interact with unique receptor complexes that all share a common γ chain.44,45 The distinct effects that we observed upon 2-AG or CP55940 stimulation may be explained by stimulation of 2 distinct receptor complexes that both contain the Cb2 receptor. For a long time it was generally believed that GPCRs function as monomers. However, GPCRs may be involved in high-molecular weight complexes formed by homodimers as well as heterodimers. Interestingly, dimer formation may affect receptor-ligand binding, signaling, and function.46-48 Another intriguing observation is that agonist presentation can promote or decrease receptor dimerization in several GPCRs.49-51 In addition, receptor heterodimerization between GPCRs may result in generation of novel ligand binding sites52 or novel ligand binding properties.53 GPCRs may even interact with non-G protein-coupled receptors, such as tyrosine kinase receptors.54 In this respect it is interesting that a functional interaction between CB1 receptor and fibroblast growth factor receptor as well as with the insulin-like growth factor 1 receptor has been demonstrated.55,56 Our observation that binding of distinct ligands to Cb2 receptors evoked different effects may be explained by the Cb2 receptor being present in distinct complexes depending on the ligand or acting as a monomer with one agonist and as a dimer or multimer with the other.
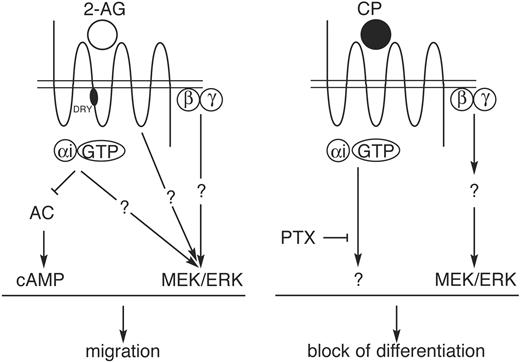
Cb2 belongs to the family of GPCRs,3,57,58 suggesting that signaling upon receptor stimulation may require G proteins. The inhibition of migration by PTX administration, as well as the inefficient chemotaxis of cells expressing DRY-Cb2 mutants, indicates that Cb2 receptor requires G protein activity to induce migration upon 2-AG stimulation. In addition, most chemokine receptors belong to the subgroup of GαiPCRs,59-61 suggesting that adenyl cyclase inactivation and cAMP down-modulation via Gαi proteins may be critical for induction of migration. The decrease of 2-AG-induced migration of 32D/G-CSF-R/Cb2 cells following dbcAMP administration demonstrates that this pathway is critical for Cb2-mediated cell motility. On the other hand, our data show that down-regulation of the intracellular cAMP levels is not the only pathway involved in migration of Cb2-expressing cells. Transwell studies using MEK inhibitors demonstrate that this route plays a critical role in 2-AG-stimulated chemotaxis as well. Previous studies demonstrated that stimulation of cannabinoid receptors Cb1 and Cb2 causes phosphorylation of ERK and, consequently, activation of this pathway.62-64 Although critical for Cb2-induced chemotaxis, MEK/ERK signaling is not a prerequisite for GPCR-induced migration in general. For instance, SDF-1-induced migration of 32D/G-CSF-R/Cb2 cells that endogenously express CXCR4 receptor is insensitive to the addition of MEK inhibitors (data not shown). We clearly demonstrate that the 2-AG-induced migration involves MEK/ERK pathway, but the mechanism of activation remains unclear. Multiple examples show that the MEK/ERK signaling route may be activated through the βγ complex,65 although G protein-independent manners of ERK activation have been proposed as well for GPCRs.65,66 Summarizing, Cb2-mediated migration depends on at least 2 distinct signaling pathways that both appear to be indispensable (Figure 7).
Schematic representation of Cb2 signaling. Signal transduction pathways linked to Cb2 leading to the 2-AG-induced migration and the CP55940-evoked block of neutrophilic differentiation.
Schematic representation of Cb2 signaling. Signal transduction pathways linked to Cb2 leading to the 2-AG-induced migration and the CP55940-evoked block of neutrophilic differentiation.
As with migration induction, our data suggest that multiple pathways are involved in the Cb2-induced block of differentiation (Figure 7). MEK/ERK signaling has been shown to be critical, because MEK inhibitors fully recovered G-CSF-induced differentiation in the presence of CP55940. Previously, we also demonstrated involvement of phosphatidylinositol-3 kinase (PI-3K) in the Cb2-mediated block of differentiation.17 On the other hand, intracellular cAMP down-regulation is unimportant for this effect. Moreover, the DRY motif, which is critical for the 2-AG-induced migration, was completely unnecessary for the induction of a maturation arrest. These experiments would suggest that G protein signaling is dispensable for the Cb2-induced differentiation block. However, we demonstrated that the CP55940-evoked block in differentiation of 32D/G-CSF-R/Cb2 cells could be fully reversed by the addition of PTX. Therefore, these data suggest that activated Cb2 receptors may induce the proper signals via G proteins, but because we observed that the DRY motif is unnecessary for the CP55940-induced block of differentiation, we suggest involvement of another currently unknown G protein interaction domain in Cb2.
Multiple GPCRs have previously been reported to have transforming abilities—for example, the α1B-adrenergic,67 thrombin,68 and serotonin 1C receptors69 and the receptor encoded by the Mas oncogene.70,71 In contrast to Cb2, which interacts with Gαi subunits, many of these previously identified transforming GPCRs can associate with Gαs subunits.72 An interesting question is whether transformation of myeloid precursor cells by Cb2 is a feature unique for this particular GPCR or whether the peripheral cannabinoid receptor is a paradigm for a novel class of transforming GPCRs. The fact that we have shown that stimulation of migration as well as the interference with differentiation by Cb2 are PTX sensitive, meaning involvement of Gαi, may indicate that other Gαi-interacting GPCRs with transforming abilities may exist. Interestingly, using retroviral insertional mutagenesis, we and others recently identified, among a large panel of novel leukemia disease genes, 4 GPCR-encoding genes (Suzuki et al73 ; Joosten et al74 ; and R.D. et al, unpublished observation, November 2002). Three of those genes encode GPCR that may interact with Gαi subunits: endothelial differentiation gene 3-R (Edg3-R), chemokine-R7 (CCR7), and vomeronasal1-R (V1-R). Introduction of these transforming receptors or other potentially interesting GPCRs into 32D/G-CSF-R cells will be a valid approach to determine whether Cb2 is a paradigm for a novel class of transforming GPCRs.
Prepublished online as Blood First Edition Paper, March 23, 2004; DOI 10.1182/blood-2003-12-4357.
Supported by the Dutch Cancer Foundation Koningin Wilhelmina Fonds.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof Dr I. P. Touw (Erasmus MC, Rotterdam) for donation of the 32D/G-CSF-R cell line. We thank Dr P. Casellas (Sanofi Recherche, Montpellier, France) for donation of the Cb1 and Cb2 inverse agonist. We thank I. van Ostaijen, E. de Wee, and C. M. Dicke for technical assistance and J. Prasher for critical reading of the manuscript. We thank K. van Rooyen for preparation of the figures. We thank the Department of Pathology at Erasmus MC for the use of the telemicroscope system.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal