Abstract
Arsenic trioxide (ATO) induces differentiation and apoptosis of malignant cells in vitro and in vivo and has been used in the treatment of a variety of hematologic malignancies. We found that in NB4 acute promyelocytic and in K562 erythroleukemia cell lines treatment with the MEK1 inhibitors PD98059 and PD184352 greatly enhances apoptotic cell death induced by ATO alone. Combined treatment results in the induction of the p53AIP1 (p53-regulated apoptosis-inducing protein 1) gene in both cell lines. Because NB4 and K562 cell lines carry an inactive p53, we investigated the possible role of p73, a p53 paralogue that has been shown to regulate several p53 target genes including p21, Bax, and p53AIP1. We found that MEK1 inhibitors reduce the levels of dominant-negative (ΔN) p73 proteins and promote the accumulation of endogenous p73α through its transcriptional activation and its tyrosine phosphorylation, resulting in p21 up-regulation and significant inhibition of cell growth. ATO reduces ΔNp73 levels and promotes a p300-mediated acetylation of endogenous p73, thus favoring cell cycle arrest and apoptosis. Finally, the combined treatment with MEK1 inhibitors and ATO enhances the affinity of phosphoacetylated p73 for the p53AIP1 promoter in vivo, as determined by chromatin immunoprecipitation experiments, leading to p53AIP1 up-regulation and increased apoptosis. (Blood. 2004; 104:519-525)
Introduction
Arsenic trioxide (ATO) suppresses neoplastic cells growth in vitro and in vivo by inducing both apoptosis and cell cycle arrest. Under certain conditions, ATO also induces differentiation of leukemia cells.1 Based on the results of multicenter clinical trials,2,3 ATO is considered the treatment of choice for patients with relapsed acute promyelocytic leukemia (APL), particularly in patients exposed to all-trans-retinoic acid (ATRA) within the prior 12 months.4
Despite the well-documented clinical efficacy of ATO, the precise mechanisms regulating arsenic-dependent induction of apoptosis have not been elucidated and several molecular targets have been proposed (for a review, see Zhu et al5 ), including PML and other nuclear bodies proteins,6,7 nuclear factor-κB (NF-κB),8 glucocorticoid nuclear receptors,9 as well as components of the mitogen-activated protein kinase (MAPK) signaling cascade.10 The p53-mdm2 pathway has also been shown to be targeted by ATO.6 Whereas the role of p53 in stress responses is well established, recent advances strongly support a pivotal role for the p53 paralogues p73 and p6311,12 in the execution of drug-induced cell death and chemosensitivity of cancer cells in both p53 wild-type and p53 null tumors (for a review, see Melino et al13 ). Indeed, p73 is sufficient to trigger cell death independently of the status of p5314-16 and, conversely, p53 requires p63 and p73 to induce apoptosis.17
Multiple TA (transactivation competent, proapoptotic and anti-proliferative) p73 COOH-terminal splicing isoforms (α,β,γ,δ,ϵ,ζ) exist.18 In addition, dominant-negative (ΔN) variants, expressed from a second promoter, lack the amino-terminal transactivation domain, act as trans-repressors of p53- and p73-dependent transcription, and possess antiapoptotic and pro-proliferative potential.13,19-23 ΔNp73 inhibits both TAp73 and p53-induced apoptosis.13,19-23 ΔNp73 is induced by TAp73 and p53, thus creating a dominant-negative feedback loop that regulates p53 and p73 function.24,25 Enhanced expression of ΔNp73, rather than inactivating mutations within the TP73 gene, has been associated with tumor development.26 The complex expression strategy of the p53 family of tumor suppressors is consistent with the failure to correlate p53 status alone with prognosis and response to anticancer treatments.
The ability of TAp73 proteins to induce cell cycle arrest and apoptosis in cells exposed to anticancer drugs relies on their ability to activate a number of p53-responsive elements (p53-REs) containing target genes. The p53-regulated apoptosis-inducing protein 1 (p53AIP1), whose expression is induced by p53 and p73 under apoptotic conditions,27,28 has been recognized as a primary effector gene of wild-type p53 and TAp73-induced apoptosis.29
We show here that the p73-p53AIP1 pathway plays an important role in the apoptotic response of NB4 and K562 leukemic cells to ATO and that inhibition of MEK1 activity greatly enhances this response by acting on the same pathway.
Materials and methods
Reagents
ATO was purchased from Sigma (St Louis, MO). A 1-mM stock solution was obtained by dissolving ATO in phosphate-buffered saline (PBS); the solution was diluted to working concentration immediately before use. The 100-mM stock solutions of the MEK-1 inhibitors PD98059 (2′-amino-3′-methoxyflavone; Cell Signaling Technology, Beverly, MA) and PD184352 (2-[chloro-4-iodo-phenylamino]-N-cyclopropylmethoxy-3,4-difluoro-benz-amide), kindly provided to us by Dr J. S. Sebolt-Leopold (Cancer Molecular Sciences, Pfizer Global Research & Development, Ann Arbor, MI), were prepared in dimethyl sulfoxide (DMSO). These reagents are highly selective inhibitors of MEK-1 phosphorylation and activation.30-32 We used 1 μM PD184352 and 10 to 40 μM PD98059 concentrations that were proved to be effective in vitro in leukemic cells as documented by ourselves and other authors.33,34 The specific phosphatidylinositol 3-kinase (PI3-K) inhibitor wortmannin was purchased from Sigma and prepared in DMSO.
Apoptosis detection, immunoprecipitation, and immunoblotting
To evaluate apoptosis, after in vitro treatment with PD98059 or ATO (or both), cells were collected by centrifugation, washed twice in cold PBS, and permeabilized in 90% ethanol and 10% PBS prior to DNA staining. The permeabilized cells were incubated with 50 μg/mL propidium iodide, 100 U/mL RNase A (Sigma), 0.1% Nonidet P-40, and 0.1% trisodium citrate for 30 minutes prior to analysis using a Becton Dickinson (Heidelberg, Germany) fluorescence-activated cell sorting (FACS) analyzer. Cells with a hypodiploid DNA content (< 2n, > 0.2n) were counted as apoptotic. Flow cytometry was performed with a FACSCalibur apparatus (Becton Dickinson). Data were analyzed using FlowJo 3.4 software (Tree Star, San Carlos, CA). Immunoblotting and immunoprecipitations assays were performed essentially as described.33,34 Immunoprecipitations were performed by incubating 2000 to 3000 μg total cell lysate as described.28,33,35
RT-PCR assay
After 24 hours of treatment, total RNAs were extracted (Trizol reagent; Gibco-BRL Life Technologies, Burlington, ON, Canada) and reverse transcribed using the SuperScript One-Step reverse transcription-polymerase chain reaction (RT-PCR) with platinum Taq kit (Gibco-BRL Life Technologies) and the resulting cDNAs were amplified with primers specific for ΔNp73, TAp73, and β-actin. The primer sequences were as follows: p73TA forward: TTG CTA TGG ACG TCT TCC ACC TGG; p73TA reverse: AGA GCT GGG TTG TGC GAA GGG CGA GTG GGT GG-, p73ΔN forward: AGT TGA CAG AAC TAA GGG AGA TGG G-; p73ΔN reverse: TGC TCA GCA GAT TGA ACT GGG.
Chromatin immunoprecipitation assay
Protein complexes were cross-linked to DNA in living nuclei by adding formaldehyde (Merck, Darmstadt, Germany) directly to tissue culture medium to a final concentration of 1%. Cross-linking was allowed to proceed for 10 minutes at room temperature and was then stopped by the addition of glycine to a final concentration of 0.125 M. Cross-linked cells were washed with PBS and swelled in RSB buffer (3 mM MgCl2,10 mM NaCl, 10 mM Tris [tris(hydroxymethyl)aminomethane]--chloride [pH 7.4], and 0.1% IGEPAL CA-330 [Sigma]). Nuclei were pelleted by microcentrifugation and lysed by incubation in nuclear lysis buffer (1% sodium dodecyl sulfate, 10 mM EDTA [ethylenediaminetetraacetic acid], 50 mM Trischloride [pH 8.1], 0.5 mM phenylmethylsulfonyl fluoride [PMSF], 100 ng leupeptin/mL, and 100 ng aprotinin/mL). The resulting chromatin solution was sonicated for 10 pulses of 20 seconds at 80% power to generate 300 to 2000 base pair (bp) DNA fragments. After microcentrifugation, the supernatant was precleared with blocked protein A-positive Staphylococcus aureus (Staph) cells (Boehringer Mannheim, Mannheim, Germany), diluted 1:5 with dilution buffer (0.01% sodium dodecyl sulfate, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris-chloride [pH 8.1], 167 mM NaCl, 0.5 mM PMSF, 100 ng leupeptin/mL, and 100 ng aprotinin/mL), and divided into aliquots. Then 5 μg anti-p73 (H79) antibody was added to each aliquot of chromatin and incubated on a rotating platform for 12 to 16 hours at 4°C. Antibody-protein-DNA complexes were isolated by immunoprecipitation with blocked protein A-positive Staph A cells. Following extensive washing, bound DNA fragments were eluted and analyzed by subsequent PCR using primers specific for the bax, p21, and p53AIP1 promoters. Primers sequences are available on request.
siRNA transfections
Prior to electroporation, NB4 and K562 cells were washed twice with serum-free Opti-MEM (Gibco BRL, Paisley, United Kingdom) and resus-pended to a final concentration of 5 × 106 cells/mL in Opti-MEM (Gibco BRL). Subsequently, 0.5 mL cell suspension was mixed with 10 μg fluorescein-labeled double-stranded RNA oligonucleotides (siRNA) specific for either green fluorescent protein (GFP) and TAp73 and electropo-rated in a 0.4-cm cuvette using the Gene Pulser electroporation apparatus (Bio-Rad Laboratories, Hercules, CA) using a single-pulse protocol (voltage 260 V and capacitance 1050 μF). Under these conditions we consistently reached a transfection efficiency of 80% or more without significant reduction of viability in both cell lines (data not shown and Van Tendeloo et al36 ). Twenty-four hours after transfection cells were treated with the different compounds for 72 hours and fluorescein isothiocyanate (FITC)-positive cells were analyzed for sub-G1 DNA content. To check for siRNA efficiency (Figure 4Bi) we transfected NB4 cells with an expression vector encoding for an HA-tagged version of TAp7328 alone or in the presence of siRNAp73 or siRNAGFP. Cells were lysed 24 hours after transfection and TAp73 expression was assessed by anti-HA immunoblot. The sequences of GFP and TAp7-specific fluorescein-labeled siRNAs are available on request.
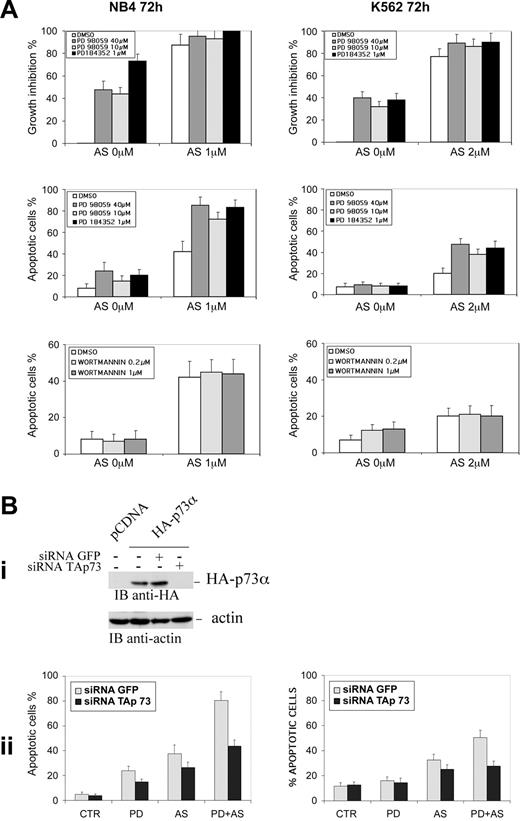
Abrogation of TAp73 expression inhibits PD plus ATO-induced apoptosis in leukemia cells. (A) NB4 and K562 cells seeded at 1 × 105 cells/mL were pretreated for 3 hours with MEK1 inhibitors PD98059 (40 μM or 10 μM) or PD184352 (1 μM) or with the PI3K inhibitor wortmannin (0.2 μM and 1 μM) and then incubated for 72 hours with the indicated concentrations of ATO. Viable cells were counted by the trypan blue dye exclusion method and apoptosis was measured as the percentage of cells with hypodiploid DNA content. Each value represents the mean ± SD of 4 independent experiments. (Bi) NB4 cells were transfected with an expression vector encoding for an HA-tagged version of TAp73 alone or in the presence of siRNAp73 or siRNAGFP. Cells were lysed 24 hours after transfection and TAp73 expression was assessed by anti-HA immunoblot. (Bii) NB4 (left) and K562 (right) cells were transfected with the indicated siRNAs and subsequently treated with PD184352 (1 μM), ATO (1 μM for NB4 and 2 μM for K562), or PD plus ATO for 72 hours prior to apoptosis analysis. Values are the mean ± SD of 3 independent experiments. AS = ATO.
Abrogation of TAp73 expression inhibits PD plus ATO-induced apoptosis in leukemia cells. (A) NB4 and K562 cells seeded at 1 × 105 cells/mL were pretreated for 3 hours with MEK1 inhibitors PD98059 (40 μM or 10 μM) or PD184352 (1 μM) or with the PI3K inhibitor wortmannin (0.2 μM and 1 μM) and then incubated for 72 hours with the indicated concentrations of ATO. Viable cells were counted by the trypan blue dye exclusion method and apoptosis was measured as the percentage of cells with hypodiploid DNA content. Each value represents the mean ± SD of 4 independent experiments. (Bi) NB4 cells were transfected with an expression vector encoding for an HA-tagged version of TAp73 alone or in the presence of siRNAp73 or siRNAGFP. Cells were lysed 24 hours after transfection and TAp73 expression was assessed by anti-HA immunoblot. (Bii) NB4 (left) and K562 (right) cells were transfected with the indicated siRNAs and subsequently treated with PD184352 (1 μM), ATO (1 μM for NB4 and 2 μM for K562), or PD plus ATO for 72 hours prior to apoptosis analysis. Values are the mean ± SD of 3 independent experiments. AS = ATO.
Statistical analysis
The statistical analysis was performed using the Dunnet test.
Results and discussion
Recent studies suggest that components of the prosurvival signal transduction pathways involving Ras and the mitogen-activated protein kinases (MAPKs) can confer an aggressive, apoptosis-resistant phenotype to leukemia cells. The use of small molecule MEK1 inhibitors, such as PD98059 or PD184352, can induce apoptosis in vitro and in vivo and could sensitize leukemia cells to drug-induced apoptosis33,34,37 and are reviewed by Platanias38 and Lee and McCubrey.39
We observed that the combined treatment with MEK1 inhibitors and ATO significantly increased the amount of apoptotic cells, as compared to ATO alone, in both the NB4 promyelocytic leukemia and K562 erythroleukemia cell lines. The percentage of sub-G1 apoptotic cells after 72 hours of treatment with MEK1 inhibitor PD98059 (40 μM) and ATO (1 μM in NB4 cells, 2 μM in K562 cells) was 2.7- or 3.2-fold higher than in cells treated with ATO alone (Figure 1A). Similar results were obtained with PD184352 (1 μM; Figure 4A).
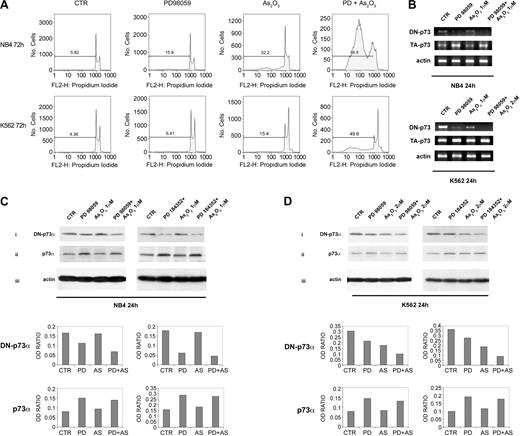
MEK-1 inhibition sensitizes leukemic cells to ATO-induced apoptosis. (A) NB4 and K562 cell lines were seeded at 1 × 105 in the presence of DMSO (vehicle) or PD98059 for 3 hours and then incubated for 72 hours with the indicated concentration of ATO. Apoptosis was then measured as percentage of cells with hypodiploid DNA content. Results are representative of one of 3 independent experiments. (B) Total RNAs extracted from leukemic cells treated for 24 hours were reverse transcribed using the specific primers for ΔNp73 and TAp73. NB4 (C) and K562 (D) cell lines were seeded at 1 × 105 in the presence of DMSO (vehicle), PD98059 (40 μM), or PD184352 (1 μM) for 3 hours, and then incubated for 24 hours with the indicated concentrations of ATO. Endogenous p73α and ΔNp73 proteins were revealed by immunoblotting analysis using a mouse monoclonal anti-p73 (clone 1288; Imgenex, San Diego, CA), or a mouse monoclonal anti-ΔNp73 (clone 38C674; Imgenex). Antiactin (Santa Cruz Biotechnology, Santa Cruz, CA) immunoblotting was performed as loading control. β-actin, ΔN-p73, and TA-p73 bands were subjected to densitometric scanning using TINA 2 software (Raytest Isotopenmessgerate, Germany) and the ΔNp73/β-actin or TAp73/β-actin ratio was calculated. AS = ATO.
MEK-1 inhibition sensitizes leukemic cells to ATO-induced apoptosis. (A) NB4 and K562 cell lines were seeded at 1 × 105 in the presence of DMSO (vehicle) or PD98059 for 3 hours and then incubated for 72 hours with the indicated concentration of ATO. Apoptosis was then measured as percentage of cells with hypodiploid DNA content. Results are representative of one of 3 independent experiments. (B) Total RNAs extracted from leukemic cells treated for 24 hours were reverse transcribed using the specific primers for ΔNp73 and TAp73. NB4 (C) and K562 (D) cell lines were seeded at 1 × 105 in the presence of DMSO (vehicle), PD98059 (40 μM), or PD184352 (1 μM) for 3 hours, and then incubated for 24 hours with the indicated concentrations of ATO. Endogenous p73α and ΔNp73 proteins were revealed by immunoblotting analysis using a mouse monoclonal anti-p73 (clone 1288; Imgenex, San Diego, CA), or a mouse monoclonal anti-ΔNp73 (clone 38C674; Imgenex). Antiactin (Santa Cruz Biotechnology, Santa Cruz, CA) immunoblotting was performed as loading control. β-actin, ΔN-p73, and TA-p73 bands were subjected to densitometric scanning using TINA 2 software (Raytest Isotopenmessgerate, Germany) and the ΔNp73/β-actin or TAp73/β-actin ratio was calculated. AS = ATO.
Apoptosis and cell cycle arrest are the major tumor suppression functions of p53 but in K562 and NB4 leukemic cells this protein has lost the ability to bind and activate its target genes.11,40 Given the well-recognized ability of p73 to transactivate p53 target genes,11,13 irrespective of p53 status, we investigated the role of p73 in ATO-induced apoptosis.
First, we assessed the effect of MEK1 inhibitors and ATO treatment on ΔNp73 and TAp73 expression. We found that ΔNp73 expression was decreased, both at the mRNA (Figure 1B) and the protein level (Figure 1Ci,Di), after PD98059 40 μM or PD184352 1 μM treatment in NB4 and in K562 cells. Under ATO exposure NB4 showed only a slight decrement in ΔNp73 expression, whereas a more marked decrement in ΔNp73 protein levels was observed in ATO-treated K562 cells (Figure 1Ci,Di). When NB4 and K562 cells were exposed to a combination of PDs and ATO a striking cooperative effect was observed (Figure 1Ci,Di). Endogenous TAp73α protein expression was sharply increased after MEK1 inhibition, whereas only a slight increment of TAp73α was observed in both cell lines after ATO treatment (Figure 1Cii,Dii). Although both cell lines showed a MEK1 inhibition-mediated increment of TAp73α protein, a significant TAp73 transcriptional activation after PD treatment was observed only in NB4 cell line (Figure 1B).
Altogether, our analysis indicates that the treatment with PD98059 or PD184352 promotes the accumulation of endogenous TAp73α and the reduction of ΔNp73, both events contributing to the observed p73-dependent cell cycle arrest (Figure 1A). Because ATO, which is able to induce NB4 and K562 apoptotic cell death (Figure 1A), was relatively less efficient than MEK1 inhibitors in elevating the TA/ΔNp73 ratio, we sought to compare the ability of ATO and MEK1 inhibitors to induce p73 acetylation, a posttranslational modification that is known to boost TAp73 proapoptotic activity.28 Specific phosphorylation and acetylation events contribute to the activation of p73 gene products in response to DNA damage and bolster p73 apoptotic functions by potentiating the selective recruitment of p73 onto the promoters of apoptotic target genes versus gene involved in cell cycle arrest and re-entry.14-16,28 Because ATO has been shown to induce DNA strand breaks and DNA-protein cross-links in a variety of cell lines,41-45 we asked whether apoptotic concentrations of ATO were able to induce TAp73α acetylation and tyrosine phosphorylation. We found that endogenous TAp73α was strongly acetylated in response to apoptotic concentrations of ATO, but not after PD98059 treatment, in both NB4 and K562 cell lines (Figure 2A). Similar results were observed with the MEK1 inhibitor PD184352 (1 μM; data not shown). To investigate the mechanisms underlying the increased levels of p73 acetylation in response to ATO treatment, we first evaluated if ATO treatment influenced the physical interaction between p73 and the acetyltransferase p300, which is known to acetylate p73 in response to doxorubicin and cisplatin treatment.28 As shown in Figure 2Bi, treatment of both NB4 and K562 cells with ATO or ATO plus PD increases the levels of p73-p300 interaction as detected by anti-p73 immunoprecipitation experiments followed by anti-p300 immunoblotting. Endogenous p73 is both activated and stabilized in response to γ irradiation, doxorubicin, and cisplatin through a c-abl-dependent pathway, participating in the apoptotic response to DNA damage.14-16 To investigate whether the accumulation of p73 correlated with its tyrosine phosphorylation, we immunoblotted the anti-p73 immunoprecipitates from ATO and PD-treated cells with antiphosphotyrosine antibodies. A very slight increment in TAp73α tyrosine phosphorylation was observed in NB4 and K562 cells after ATO treatment (Figure 2Bii). By contrast, a strong tyrosine phosphorylation of p73α was observed after PD treatment in both cell lines (Figure 2Bii). The stronger tyrosine phosphorylation of p73α observed after PD compared to ATO treatment correlated well with the accumulation of p73α observed in PD-treated cells (Figure 1Cii,Dii and Figure 2Biii). Altogether, these results strongly suggests that the high levels of apoptosis we observed in ATO-plus PD-treated cells is the result of the increased levels of phosphoacetylated p73 species. It is noteworthy to underline that the kinetics of p73 phosphorylation/acetylation in NB4 and K562 cells are quite different. The phosphorylation of p73 as well as its interaction with acetyltransferase p300 are earlier events in NB4 (2 hours) as compared to K562 (24 hours; Figure 2Bi-ii). It is tempting to speculate that this differential behavior might be the consequence of an altered DNA damage-cAbl-p300-p73 pathway in the K562 cells due to the presence of p210 BCR-Abl.46
Apoptotic doses of ATO induce TAp73α acetylation and MEK1 inhibition promotes TAp73α tyrosine phosphorylation in leukemic cells. (A) Leukemic cells were pretreated with either DMSO or PD98059 (40 μM) for 3 hours and then treated with ATO (1 μM, NB4; or 2 μM, K562) for 24 hours. Lysates from treated cells were subsequently immunoprecipitated with a rabbit polyclonal anti-p73 (H79; Santa Cruz Biotechnology) or with a control antibody from lysates of NB4 and K562 treated with ATO. The immunoprecipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted using a rabbit polyclonal antiacetylated lysine (Upstate Biotechnology, Lake Placid, NY). To evaluate the relative levels of p73 acetylation, bands were subjected to densitometric scanning using TINA 2 software (Raytest Isotopenmessgerate, Staubenhardt, Germany). (B) Leukemic cells were pretreated with either DMSO or PD184352 (1 μM) for 3 hours and then treated with ATO for 2 hours (1 μM; NB4) or for 24 hours (2 μM; K562). Extracts from mock and treated NB4 and K562 cells were immunoprecipitated with a rabbit polyclonal anti-p300 (N-15; Santa Cruz Biotechnology) or a rabbit polyclonal anti-p73 (H79; Santa Cruz Biotechnology) or with a control antibody. The immunoprecipitates were analyzed by SDS-PAGE and immunoblotted using rabbit polyclonal anti-p300 antibodies (N-15; Santa Cruz Biotechnology; subpanel i), or a mouse monoclonal antiphosphotyrosine antibody (clone 4G10; Upstate Biotechnology; subpanel ii). A 1:5 aliquot of the immunoprecipitated material was immunoblotted with the anti-p73 monoclonal antibody (clone 1288; Imgenex, San Diego, CA; subpanel iii).
Apoptotic doses of ATO induce TAp73α acetylation and MEK1 inhibition promotes TAp73α tyrosine phosphorylation in leukemic cells. (A) Leukemic cells were pretreated with either DMSO or PD98059 (40 μM) for 3 hours and then treated with ATO (1 μM, NB4; or 2 μM, K562) for 24 hours. Lysates from treated cells were subsequently immunoprecipitated with a rabbit polyclonal anti-p73 (H79; Santa Cruz Biotechnology) or with a control antibody from lysates of NB4 and K562 treated with ATO. The immunoprecipitates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted using a rabbit polyclonal antiacetylated lysine (Upstate Biotechnology, Lake Placid, NY). To evaluate the relative levels of p73 acetylation, bands were subjected to densitometric scanning using TINA 2 software (Raytest Isotopenmessgerate, Staubenhardt, Germany). (B) Leukemic cells were pretreated with either DMSO or PD184352 (1 μM) for 3 hours and then treated with ATO for 2 hours (1 μM; NB4) or for 24 hours (2 μM; K562). Extracts from mock and treated NB4 and K562 cells were immunoprecipitated with a rabbit polyclonal anti-p300 (N-15; Santa Cruz Biotechnology) or a rabbit polyclonal anti-p73 (H79; Santa Cruz Biotechnology) or with a control antibody. The immunoprecipitates were analyzed by SDS-PAGE and immunoblotted using rabbit polyclonal anti-p300 antibodies (N-15; Santa Cruz Biotechnology; subpanel i), or a mouse monoclonal antiphosphotyrosine antibody (clone 4G10; Upstate Biotechnology; subpanel ii). A 1:5 aliquot of the immunoprecipitated material was immunoblotted with the anti-p73 monoclonal antibody (clone 1288; Imgenex, San Diego, CA; subpanel iii).
To evaluate whether ATO-induced acetylation/phosphorylation of p73 modulates its affinity to cell cycle arrest and apoptotic target genes, we performed a chromatin immunoprecipitation (ChIP) assay using anti-p73-specific antibodies to immunoprecipitate cross-linked chromatin from untreated or cells treated with PD98059, ATO, and PD98059 plus ATO. As shown in Figure 3A, after 24 hours of PD98059 treatment p73 was readily recruited to the p21 promoter, whereas after either ATO or PD98059 plus ATO treatment p73α was strongly recruited to the promoters of proapoptotic genes Bax and p53AIP1 and, conversely, its affinity to the p21 was reduced.
Endogenous p73α is recruited onto apoptotic target genes in vivo in response to ATO. (A) A chromatin immunoprecipitation assay was performed from either untreated or NB4 and K562 leukemic cells treated with PD98059, ATO, and PD98059 plus ATO. gDNA obtained from untreated or treated cells was used to normalize the DNA to immunoprecipitation. An anti-p73 (H79 polyclonal antiserum from Santa Cruz Biotechnology) or control unrelated antibodies were used. Immunoprecipitated material was amplified using primers specific for p21 or p53AIP1 or bax promoters. (B) NB4 or K562 cells, after 3 hours of pretreatment with PD98059, were incubated with the indicated concentrations of ATO. Expression of PARP, p53AIP1, and Bax was revealed after 24 and 48 hours in NB4 and after 24, 48, and 72 hours of treatment in K562 cells. (C) Levels of p21 protein were assessed by immunoblotting after 24 hours in NB4 and after 48 hours of treatment in K562 cells. (D) Expression of Bcr-Abl after 24 hours of treatment. Cell lysates were analyzed by immunoblotting analysis using a mouse monoclonal anti-PARP (F2; Santa Cruz Biotechnology), rabbit polyclonal anti-p53AIP1 (CT; AnaSpec, San Jose, CA), rabbit polyclonal, anti-Bax (Cell Signaling Technology), horseradish peroxidase conjugate anti-p21WAF1/CIP1 (Santa Cruz Biotechnology), mouse monoclonal anti-c-Abl (24-11; Santa Cruz Biotechnology), and goat polyclonal antihuman actin (Santa Cruz Biotechnology). Antiactin immunoblotting (Santa Cruz Biotechnology) was performed as loading control. AS = ATO.
Endogenous p73α is recruited onto apoptotic target genes in vivo in response to ATO. (A) A chromatin immunoprecipitation assay was performed from either untreated or NB4 and K562 leukemic cells treated with PD98059, ATO, and PD98059 plus ATO. gDNA obtained from untreated or treated cells was used to normalize the DNA to immunoprecipitation. An anti-p73 (H79 polyclonal antiserum from Santa Cruz Biotechnology) or control unrelated antibodies were used. Immunoprecipitated material was amplified using primers specific for p21 or p53AIP1 or bax promoters. (B) NB4 or K562 cells, after 3 hours of pretreatment with PD98059, were incubated with the indicated concentrations of ATO. Expression of PARP, p53AIP1, and Bax was revealed after 24 and 48 hours in NB4 and after 24, 48, and 72 hours of treatment in K562 cells. (C) Levels of p21 protein were assessed by immunoblotting after 24 hours in NB4 and after 48 hours of treatment in K562 cells. (D) Expression of Bcr-Abl after 24 hours of treatment. Cell lysates were analyzed by immunoblotting analysis using a mouse monoclonal anti-PARP (F2; Santa Cruz Biotechnology), rabbit polyclonal anti-p53AIP1 (CT; AnaSpec, San Jose, CA), rabbit polyclonal, anti-Bax (Cell Signaling Technology), horseradish peroxidase conjugate anti-p21WAF1/CIP1 (Santa Cruz Biotechnology), mouse monoclonal anti-c-Abl (24-11; Santa Cruz Biotechnology), and goat polyclonal antihuman actin (Santa Cruz Biotechnology). Antiactin immunoblotting (Santa Cruz Biotechnology) was performed as loading control. AS = ATO.
The recruitment of p73 to the apoptotic target genes Bax and p53AIP1 (Figure 3A) correlates with p73 acetylation status (Figure 2A). Moreover, after ATO treatment p73 was acetylated and recruited somehow more efficiently on Bax as compared to p53AIP1 promoter. When ATO was associated with PD98059 the further increase of p73 recruitment to the p53AIP1 correlates well with the striking increase of the sub-G1 population observed after PD98059 plus ATO combined treatment (Figures 1A and 4A). The combined treatment also led to an increased poly (ADP-ribose) polymerase (PARP) fragmentation that reflects increased apoptosis (Figure 3B). Interestingly, we found that Bax protein accumulated to a greater extent after ATO treatment than after PD98059 plus ATO treatment in both cell lines (Figure 3B), whereas p53AIP1 expression was greatly enhanced after PD98059 plus ATO treatment compared to ATO alone (2.4- and 4.0-fold increase in K562 and NB4 cells, respectively). Similar results were obtained with PD184352 plus ATO (data not shown). Interestingly, also in these experiments we observed a delay in Bax and p53AIP1 up-regulation in ATO and PD plus ATO-treated K562 (72 hours) versus NB4 (48 hours) cell line (Figure 3B). Because the Bcr-Abl kinase is known to exert resistance against apoptosis46,47 and given the capability of ATO to down-regulate Bcr-Abl protein levels,48,49 we postulate that the delay that occurred in K562 was dependent on Bcr-Abl. As shown in Figure 3D, an exposure interval of 24 hours of ATO treatment was necessary to sensibly decrease the level of Bcr-Abl protein in K562. In addition, the longer interval in PD-mediated p73 tyrosine phosphorylation and its interaction with p300 that occurred in ATO-treated K562 (24 hours) versus NB4 (2 hours), as shown in Figure 2B, might be explained by the same mechanism.
Finally, we have performed a combined analysis of cell growth inhibition and induction of apoptosis in NB4 and K562 cells treated with the different drug combinations. PD98059 (40 or 10 μM) or PD184352 (1 μM) alone triggers significant cell growth inhibition and only slight induction of apoptosis, which is more evident in NB4 cells (Figure 4A). This biologic effect correlates well with the ability of the PD to promote both the accumulation of endogenous p73α, through its transcriptional up-regulation and tyrosine phosphorylation, and the reduction of ΔNp73 (Figure 1B-D), with p21 gene up-regulation (Figure 3C), and with the inability of PDs to induce p73 acetylation (Figure 2A) and p73 recruitment onto the promoters of p53/p73 apoptotic target genes (Figure 3A). ATO alone induces both growth inhibition and apoptosis (Figure 4A) as a result of an increased expression of both p21 and Bax (Figure 3B-C), the down-regulation of ΔNp73 expression, more relevant in K562 (Figure 1C-D), and the acetylation of endogenous p73 (Figure 2A). When PD98059 and ATO were combined p73 acetylation was strongly induced (Figure 2A), the affinity of p73 for the p53AIP1 promoter was boosted (Figure 3A) resulting in the up-regulation of the p53AIP1 expression (Figure 3B), and a greatly enhanced apoptosis of treated cells was seen (Figures 1A and 4A). As shown in Figure 4A, the percentage of sub-G1 apoptotic cells after 72 hours of treatment with MEK1 inhibitors, PD98059 (40 μM and 10 μM) or PD184352 (1 μM), in combination with ATO (1 μM in NB4 cells, 2 μM in K562 cells) was significantly higher than in cells treated with ATO alone; mean % ± SD of apoptotic cells in PD plus ATO versus ATO-treated cells were 85.4% ± 8.2% (PD98059 40 μM + ATO), 72.6% ± 7.7% (PD98059 10 μM + ATO), 83.2% ± 7.0% (PD 184352 1 μM + ATO) versus 41.3% ± 9.8% (ATO 1 μM; P < .001) and 47.6% ± 4.8% (PD98059 40 μM + ATO), 38.2% ± 3.9% (PD98059 10 μM + ATO), 44.5% ± 6.7% (PD 184352 1 μM+ ATO) versus 19.3% ± 4.8% (ATO 2 μM; P < .001), respectively, in NB4 and K562 cells.
To test the specificity of the pathways involved in the activation of p73-dependent pathways in our system, we also inhibited the PI3K pathway with the specific inhibitor wortmannin.30 No relevant effects on induction of apoptosis in NB4 and K562 cells were observed when ATO was associated with the PI3K inhibitor wortmannin (0.2 and 1 μM; Figure 4A).
Collectively, our results support a model in which an elevated TA/ΔNp73 ratio, together with an increased recruitment of TAp73 onto its apoptotic target genes promoters due to TAp73 acetylation and its tyrosine phosphorylation both contribute to ATO-induced apoptosis and its enhancement by cotreatment with PDs. Further investigation will be necessary to determine whether additional posttranslational modifications and the kinases are involved in these mechanisms. Given the homology between p53 and p73, it is not excluded that homeodomain-interacting protein kinase 2 (HIPK2) could play an important role in these processes. This serine/threonine kinase binds to and phosphorylates at Ser46 p5350 inducing a subtle change of p53 conformation that enhances the affinity to the promoters of apoptosis-related genes such as P53AIP127 ; moreover HIPK2 interacts with p73 in vivo.51
Finally, to determine the contribution of p73-p53AIP1 pathway activation in mediating PD plus ATO-induced apoptosis, the TAp73 mRNA was selectively knocked-down by means of specific fluorescein-labeled double-stranded RNA oligonucleotides (siRNA). Recently, siRNA has been shown to achieve a high degree of specificity with low toxicity also in mammalian cells52,53 acting through a degradative chain reaction catalyzed by the activation of a cellular RNA-dependent RNA polymerase.54-56 The efficiency of siRNA-mediated TAp73 down-regulation was evaluated by transient transfection experiments. Transfection of TAp73 siRNA, but not the unrelated GFP siRNA, led to decreased TAp73 in NB4 cells (Figure 4Bi) without affecting the levels of the unrelated protein actin (Figure 4Bi). The percentage of sub-G1 apoptotic NB4 and K562 cells after 72 hours of treatment with MEK1 inhibitor PD184352 (1 μM) and ATO (1 μM in NB4 cells, 2 μM in K562 cells) was significantly diminished in cells transfected with TAp73 siRNA relative to cells transfected with control siRNA (Figure 4Bii). These findings indicate that p73 is a major determinant of PD plus ATO efficacy in leukemia cells carrying an inactive p53. A number of recent reports have shown the importance of a functional p73 for tumor cells chemosensitivity.57 Our observations further confirm this concept and suggest that modulation of p73 proteins expression and function might represent in the future a new molecular target for leukemia treatment.
Prepublished online as Blood First Edition Paper, March 18, 2004; DOI 10.1182/blood-2003-08-2743.
Supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC; A.B., A.C., and M.L.), Ministero dell'Istruzione dell'Università e della Ricerca Scientifica (MIUR FIN, FIL, and Progetto Strategico Oncologia SP/4: Terapia preclinica molecolare in oncologia; A.B.), MIUR-FIRB (M.L.), and Associazione Chiara Tassoni per la Lotta contro la Leucemia e il Cancro-Parma (P.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal