Abstract
CD8+ T cells play a crucial role in the control of viral infections by direct elimination of infected cells and secretion of a number of soluble factors. Recent data suggest that HIV-1-specific CD8+ T cell subsets may differ in their ability to exert these effector functions. Here, we directly compared the cytokine secretion patterns and cytotoxic capacity of HIV-1-specific CD8+ T cells, using a flow-cytometric cytotoxicity assay based on caspase-3 activation in dying target cells. These experiments revealed considerable intraindividual and interindividual differences among epitope-specific T-cell effector functions: while the frequency of HIV-1-specific CD8+ T cells secreting interferon-γ but no tumor necrosis factor-α (TNF-α) following antigenic stimulation was only weakly correlated to their cytotoxic activity (R = 0.05, P = .57), a subset of CD8+ T cells secreting both inter-feron-γ and TNF-α was substantially more strongly associated with cytotoxicity (R = 0.67, P < .001). This subset of CD8+ T cells also exhibited stronger intracellular perforin expression and more pronounced direct ex vivo HIV-1-specific cytoxicity than CD8+ T cells secreting solely interferon-γ following sorting of these subpopulations according to their cytokine profile. These results suggest that HIV-1-specific cytotoxicity of CD8+ T cells is preferentially mediated by a subset of CD8+ T cells secreting both interferon-γ and TNF-α. (Blood. 2004;104:487-494)
Introduction
HIV-1-specific CD8+ T cells emerge shortly after acute HIV-1 infection and are vigorously expanded during the ensuing disease process.1 The T-cell receptor of these cells can recognize viral epitopes bound to major histocompatibility complex (MHC) class I molecules, which results in the execution of a variety of antiviral effector functions impairing viral replication and curtailing the generation of new viral progeny. While direct eradication of HIV-1-infected cells by MHC class I-restricted cytolysis, using either a granule-dependent (perforin/granzyme) or ligand-induced (fas-fasL) pathway, is the hallmark of CD8+ T-cell function, the secretion of cytokines, chemokines, or other soluble factors can also contribute to the reduction of viral replication.2,3
While the cytokine secretion pattern of HIV-1-specific CD8+ T cells has been analyzed in a variety of studies,4-6 only limited data are available that address the direct ex vivo cytotoxic activity of these cells on a single epitope level. This is largely due to the limited sensitivity of currently available experimental tools for the direct ex vivo quantification of HIV-1-specific cytotoxic activity. Yet, recent studies have shown that under conditions of continuous antigen exposure in chronic infection, the cytotoxic activity of virus-specific CD8+ T cells might be selectively impaired,4,7-10 whereas their cytokine secretion ability seems to be largely preserved. Moreover, it has been suggested that certain effector functions of HIV-1-specific CD8+ T cells are selectively executed by distinct subsets within the same epitope-specific T-cell population.11,12 Therefore, a more precise characterization of the HIV-1-specific CD8+ T-cell subsets exhibiting cytotoxic capacity is warranted to better understand the antiviral activity mediated by HIV-1-specific CD8+ T cells.
In the present study, we applied a newly adapted cytotoxicity assay13,14 using caspase-3 substrates as indicators of target cell death to study the cytolytic activity of HIV-1-specific CD8+ T cells on the single epitope level directly ex vivo. Fluorogenic caspase-3 substrates emit fluorescence signals upon specific enzymatic cleavage by caspase-3 and have been recently proposed as sensitive tools to quantify and visualize the cytotoxic activity of antigen-specific CD8+ T cells by flow cytometry.14 The fluorogenic caspase-3 substrates selectively indicate the intracellular activation of caspase-3 in target cells, which represents one of the earliest molecular events that occurs in cells dying of CD8+ T-cell-mediated cytotoxicity by either the granzyme/perforin-dependent or the ligand-induced pathway.15 Using this sensitive assay, we compared the epitope-specific cytokine secretion profile of HIV-1-specific CD8+ T-cell subsets to their cytotoxic activity and identified a subset of CD8+ T cells secreting both interferon-γ and TNF-α that exhibited strong cytotoxic capacity.
Patients, materials, and methods
Study individuals
This study included 9 HIV-1-infected individuals followed at the Massachusetts General Hospital (MGH; Boston) and the Fenway Community Health Care Center (Boston). At the time of the study, none of the study subjects were treated with antiretroviral drugs; 7 of them had previously been subjected to antiretroviral therapy but were off therapy for more than 8 months; 2 study subjects had never been treated. The clinical and demographic characteristics of the study subjects are summarized in Table 1. The study was approved by the respective institutional review boards and was conducted in accordance with the human experimentation guidelines of the Massachusetts General Hospital.
Clinical and demographic characteristics of the study individuals
Patient ID . | Sex . | Age, y . | Ethnicity . | Time since HIV-1 diagnosis, mo. . | CD4+ T-cell count/μL . | HIV-1 RNA load, coples/mL . | HLA class I type . | Peptides tested . |
|---|---|---|---|---|---|---|---|---|
| AC-04 | M | 41 | White | 69 | 398 | 14 400 | A2, 11; B18, 44; Cw5, 12 | A11-AK9 (Nef) (AVDLSHFLK) |
| A11-AK9 (RT) (AIFQSSMTK) | ||||||||
| A11-QVK9 (QIYAGIKVK) | ||||||||
| A11-KK11 (KTKPPLPSVKK) | ||||||||
| B44-AW11 (AEQASQDVKNW) | ||||||||
| AC-14 | M | 49 | White | 53 | 500 | 3 650 | A2, 3; B8, 62; Cw7, 10 | A3-RK11 (RMRGAHTNDVK) |
| A2-IV9 (ILKEPVHGV) | ||||||||
| A3-RK9 (RLRPGGKKK) | ||||||||
| A3-RK10 (RIRTWKSLVK) | ||||||||
| B8-EI8 (EIYKRWII) | ||||||||
| B8-FL8 (FLKEKGGL) | ||||||||
| AC-02 | M | 48 | White | 72 | 706 | 269 | A11, 29; B8, 44; Cw4, 7 | B8-EI8 (EIYKRWII) |
| B8-FL8 (FLKEKGGL) | ||||||||
| B8-EV9 (ELRSLYNTV) | ||||||||
| A11-AK11 (ACQGVGGPGHK) | ||||||||
| B44-AY9 (AENLWVTVY) | ||||||||
| AC-16 | M | 40 | White | 51 | 1049 | 56 700 | A28, 29; B14, 44; Cw8 | A29-SY9 (SFEPIPIHY) |
| A29-SY10 (SFNCGGEFFY) | ||||||||
| B14-DA9 (DRFYKTLRA) | ||||||||
| B14-CC9 (CRAPRKKGC) | ||||||||
| B14-EL9 (ERYLKDQQL) | ||||||||
| B44-AW11 (AEQASQDVKNW) | ||||||||
| AC-06 | M | 42 | White | 54 | 542 | 51 800 | A3; B7; Cw7 | B7-SM9 (SPAIFQSSM) |
| B7-GL9 (GPGHKARVL) | ||||||||
| B7-TL9 (TPQDLNTML) | ||||||||
| B7-HA9 (HPVHAGPVA) | ||||||||
| B7-HI10 (HPRISSEVHI) | ||||||||
| B7-HKI10 (HPKISSEVHI) | ||||||||
| A3-KK10 (KLVDFRELNK) | ||||||||
| A3-GK9 (GIPHPAGLK) | ||||||||
| A3-RK10 (RIRTWKSLVK) | ||||||||
| CO-06 | M | 50 | White | 92 | 672 | 1 200 | A25, 32; B8, 14; Cw7, 8 | B8-EI8 (EIYKRWII) |
| B8-FL8 (FLKEKGGL) | ||||||||
| B8-GL9 (GPKVKQWPL) | ||||||||
| Cw8-KL10 (KAAVDLSHFL) | ||||||||
| B14-DA9 (DRFYKTLRA) | ||||||||
| B14-EL9 (ERYLKDQQL) | ||||||||
| AC-05 | M | 43 | White | 62 | 387 | 14 100 | A3; B14, 60; Cw3, 7 | A3-ATK9 (AIFQSSMTK) |
| B14-DA9 (DRFYKTLRA) | ||||||||
| A3-QK10 (QVPLRPMTYK) | ||||||||
| A3-KK9 (KIRLRPGGK) | ||||||||
| A3-AK9 (AVDLSHFLK) | ||||||||
| B40-KL10 (KEKGGLEGL) | ||||||||
| FW-003 | M | 41 | White | 36 | 649 | 4 180 | A1, 3; B8, 14; Cw7, 8 | B8-EI8 (EIYKRWII) |
| B8-FL8 (FLKEKGGL) | ||||||||
| A3-QK10 (QVPLRPMTYK) | ||||||||
| A3-ATK9 (AIFQSSMTK) | ||||||||
| A3-RK9 (RLRPGGKKK) | ||||||||
| B8-DL9 (DCKTILKAL) | ||||||||
| B8-GL8 (GGKKKYKL) | ||||||||
| FW-009 | M | 43 | White | 37 | 322 | 68 880 | A31, 68; B7, 70; Cw7, 1 | B7-TL10 (TPGPGVRYPL) |
| B7-IL9 (IPRRIRQGL) | ||||||||
| A68-DL9 (DTVLEEMNL) |
Patient ID . | Sex . | Age, y . | Ethnicity . | Time since HIV-1 diagnosis, mo. . | CD4+ T-cell count/μL . | HIV-1 RNA load, coples/mL . | HLA class I type . | Peptides tested . |
|---|---|---|---|---|---|---|---|---|
| AC-04 | M | 41 | White | 69 | 398 | 14 400 | A2, 11; B18, 44; Cw5, 12 | A11-AK9 (Nef) (AVDLSHFLK) |
| A11-AK9 (RT) (AIFQSSMTK) | ||||||||
| A11-QVK9 (QIYAGIKVK) | ||||||||
| A11-KK11 (KTKPPLPSVKK) | ||||||||
| B44-AW11 (AEQASQDVKNW) | ||||||||
| AC-14 | M | 49 | White | 53 | 500 | 3 650 | A2, 3; B8, 62; Cw7, 10 | A3-RK11 (RMRGAHTNDVK) |
| A2-IV9 (ILKEPVHGV) | ||||||||
| A3-RK9 (RLRPGGKKK) | ||||||||
| A3-RK10 (RIRTWKSLVK) | ||||||||
| B8-EI8 (EIYKRWII) | ||||||||
| B8-FL8 (FLKEKGGL) | ||||||||
| AC-02 | M | 48 | White | 72 | 706 | 269 | A11, 29; B8, 44; Cw4, 7 | B8-EI8 (EIYKRWII) |
| B8-FL8 (FLKEKGGL) | ||||||||
| B8-EV9 (ELRSLYNTV) | ||||||||
| A11-AK11 (ACQGVGGPGHK) | ||||||||
| B44-AY9 (AENLWVTVY) | ||||||||
| AC-16 | M | 40 | White | 51 | 1049 | 56 700 | A28, 29; B14, 44; Cw8 | A29-SY9 (SFEPIPIHY) |
| A29-SY10 (SFNCGGEFFY) | ||||||||
| B14-DA9 (DRFYKTLRA) | ||||||||
| B14-CC9 (CRAPRKKGC) | ||||||||
| B14-EL9 (ERYLKDQQL) | ||||||||
| B44-AW11 (AEQASQDVKNW) | ||||||||
| AC-06 | M | 42 | White | 54 | 542 | 51 800 | A3; B7; Cw7 | B7-SM9 (SPAIFQSSM) |
| B7-GL9 (GPGHKARVL) | ||||||||
| B7-TL9 (TPQDLNTML) | ||||||||
| B7-HA9 (HPVHAGPVA) | ||||||||
| B7-HI10 (HPRISSEVHI) | ||||||||
| B7-HKI10 (HPKISSEVHI) | ||||||||
| A3-KK10 (KLVDFRELNK) | ||||||||
| A3-GK9 (GIPHPAGLK) | ||||||||
| A3-RK10 (RIRTWKSLVK) | ||||||||
| CO-06 | M | 50 | White | 92 | 672 | 1 200 | A25, 32; B8, 14; Cw7, 8 | B8-EI8 (EIYKRWII) |
| B8-FL8 (FLKEKGGL) | ||||||||
| B8-GL9 (GPKVKQWPL) | ||||||||
| Cw8-KL10 (KAAVDLSHFL) | ||||||||
| B14-DA9 (DRFYKTLRA) | ||||||||
| B14-EL9 (ERYLKDQQL) | ||||||||
| AC-05 | M | 43 | White | 62 | 387 | 14 100 | A3; B14, 60; Cw3, 7 | A3-ATK9 (AIFQSSMTK) |
| B14-DA9 (DRFYKTLRA) | ||||||||
| A3-QK10 (QVPLRPMTYK) | ||||||||
| A3-KK9 (KIRLRPGGK) | ||||||||
| A3-AK9 (AVDLSHFLK) | ||||||||
| B40-KL10 (KEKGGLEGL) | ||||||||
| FW-003 | M | 41 | White | 36 | 649 | 4 180 | A1, 3; B8, 14; Cw7, 8 | B8-EI8 (EIYKRWII) |
| B8-FL8 (FLKEKGGL) | ||||||||
| A3-QK10 (QVPLRPMTYK) | ||||||||
| A3-ATK9 (AIFQSSMTK) | ||||||||
| A3-RK9 (RLRPGGKKK) | ||||||||
| B8-DL9 (DCKTILKAL) | ||||||||
| B8-GL8 (GGKKKYKL) | ||||||||
| FW-009 | M | 43 | White | 37 | 322 | 68 880 | A31, 68; B7, 70; Cw7, 1 | B7-TL10 (TPGPGVRYPL) |
| B7-IL9 (IPRRIRQGL) | ||||||||
| A68-DL9 (DTVLEEMNL) |
HLA typing
HLA class I typing was performed at a commercial laboratory (Dynal Biotech, Oxford, United Kingdom) by sequence-specific polymerase chain reactions (PCRs) according to standard procedures. DNA for typing was extracted using the Purgene DNA Isolation kit for peripheral blood mononuclear cell (PBMC) samples (Gentra Systems, Minneapolis, MN).
Synthetic HIV-1 peptides
Synthetic HIV-1 clade B peptides corresponding to a previously described optimal CD8+ T-cell epitopes sequence (http://hiv-web.lanl.gov) were synthesized at the MGH Peptide Core Facility on an automated peptide synthesizer using F-moc technology.
Lymphocyte separation
Blood specimens were drawn in acid-citrate-dextrose (ACD) tubes (Becton Dickinson, San Jose, CA). PBMCs were separated from whole blood by Ficoll-Hypaque (Histopaque 1077; Sigma, St Louis, MO) density gradient centrifugation, as described.6
Cell lines and media
Epstein-Barr virus (EBV)-transformed B lymphoblastoid cell lines (BCLs) were established and maintained in R20 medium (RPMI 1640 medium [Sigma], supplemented with 2 mM l-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, 10 mM Hepes [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], and 20% heat-inactivated fetal calf serum [FCS]), as described previously.16
Cytotoxicity assay with fluorogenic caspase-3 substrates
Cytotoxicity assays with fluorogenic caspase-3 substrates were adapted according to a protocol of Liu et al,14 using the Cytoxilux cytotoxicity kit (OncoImmunin, Gaithersburg, MD). BCLs were labeled with the peptide of interest (2 μg/mL) in phosphate-buffered saline (PBS) supplemented with a target cell fluorophore (provided by OncoImmunin). After 1 hour of incubation at 37°C, BCLs were washed twice with PBS and mixed with freshly harvested PBMCs at an effector-to-target ratio of 50:1 (or as indicated) for one hour at 37°C, 5% CO2. Subsequently, the entire medium was removed and 70 μL fluorescein isothiocyanate (FITC)-labeled caspase-3 substrates were added. Following an additional incubation period (30 minutes, 37°C, 5% CO2), cells were washed twice with reagent buffer supplied by the manufacturer (OncoImmunin) and immediately processed to flow cytometric analysis on a FACSCalibur instrument (BD Biosciences, San Jose, CA). In some assays, antibodies blocking TNF-α (clone Mab11; BD Biosciences), TNF-α receptors (clone 16803; R&D Systems, Minneapolis, MN), fas receptors (clone ZB4; Immunotech, Marseille, France), or fas-ligands (clone Nok-2; BD Biosciences) were added or effector cells were treated with concanamycin A (0.2 μM; Sigma) for 30 minutes to deplete them from intracellular perforin granules.17 Target cell populations were identified by positive staining for the target cell fluorophore and gated according to forward- and side-scatter (FCS/SSC) characteristics. Target cells mixed with effector cells but lacking previous loading with HIV-1-specific peptides as well as peptide-labeled target cells without subsequent exposure to effector PBMCs were used as controls in each assay. The proportion of target cells dying of MHC class I-restricted cytolysis was calculated by subtracting the proportion of caspase-3-positive target cells in the control samples from the total proportion of caspase-3-positive target cells labeled with a specific viral peptide. The proportion of target cells positive for caspase-3 was consistently lower than 4% (median: 2.3%, range: 0%-4%) in the negative control samples. HIV-1-specific CD8+ T-cell responses were considered positive when the proportion of caspase-3-expressing target cells reached at least 5% after subtracting background cytotoxic activity and was at least twice as high as in negative control samples.
Flow cytometric detection of antigen-induced intracellular cytokine expression
Intracellular staining assays were carried out as described previously.18 Briefly, autologous BCLs were loaded with the peptide of interest (2 μg/mL) and incubated for one hour at 37°C. After 2 washes, these cells were mixed with freshly harvested PBMCs at an effector-to-target ratio of 5:1, which yielded optimal stimulation results.18 One microgram per milliliter anti-CD28 and anti-CD49d antibodies (BD Biosciences) was added. Cells were incubated for one hour at 37°C, 5% CO2, followed by additional 5 hours in the presence of a secretion inhibitor (Brefeldin A, 10 μg/mL; Sigma). Cells were then stained with surface antibodies (CD8, CD3, CCR7, CD45RA; BD Biosciences) at 4°C for 30 minutes. After 2 washes, cells were fixed and permeabilized using the Caltag Cell Fixation and Permeabilization kit according to the manufacturer's protocol (Caltag, Burlingame, CA). Subsequently, intracellular antigen staining was performed using 15 μL anti-interferon-γ- and anti-TNF-α-specific monoclonal antibodies (BD Biosciences). Cells were then washed and analyzed on a FACS Calibur flowcytometry instrument (BD Biosciences) using FITC, phycoerythrin (PE), peridinin chlorophyll-alpha protein (PerCP), and allophycocyanin (APC) as fluorescent parameters. Control conditions were established by the use of autologous PBMCs, which had not been stimulated with peptide, but otherwise had been treated identically. Samples with isotype-control antibodies were used to determine the levels of unspecific antibody binding and cell autofluorescence.
For multiparameter flow cytometric analysis, we used a panel of 3 different monoclonal surface antibodies (CD45RA PE-Cy5, CD8-APCCy7, CCR7-cascade yellow) in addition to 3 intracellular antibodies (interferon-γ PE-Cy7, TNF-α APC, perforin PE; all antibodies purchased from BD Biosciences). Staining procedures were carried out as described, except for the fact that colchicin (10 μM; Sigma) was added to the samples to block the release of cytotoxic granules. Samples were acquired on a LSRII flow cytometric device (BD Biosciences), using the FACS DiVa software (BD Biosciences) according to the manufacturer's instructions. Data analysis was performed with the FlowJo software package (Treestar, Ashland, OR).
Cytokine secretion assays and cell sorting
Cytokine secretion assays were run using interferon-γ- or TNF-α-catching assays (Miltenyi Biotech, Bergisch Gladbach, Germany) as described previously16 to isolate viable cytokine-secreting CD8+ T-cell populations. Briefly, PBMCs were stimulated with viral antigens and costimulatory antibodies as described. After 3 hours of incubation at 37°C, 5% CO2, cells were labeled with anti-interferon-γ/CD45 or anti-TNF-α/CD45 bispecific antibodies (kindly provided by Miltenyi Biotech) and incubated for an additional 45 minutes under steady rotation at 37°C, 5% CO2. APC/PE-labeled anti-interferon-γ or anti-TNF-α antibodies were then added together with anti-CD8 PE-Cy5 antibodies (Beckman Coulter, Fullerton, CA) and the entire sample was incubated for an additional 10 minutes on ice, followed by 2 washes with PBS. Cell sorting was performed on a FACSVantage flow cytometer according to standard procedures under appropriate biosafety conditions.19
Statistical analysis
Data are indicated as median and range or means and standard deviations. Linear correlations were calculated using standard Pearson correlation coefficients, but significance levels have been assessed based on a repeated measures model.20 Differences between nominal data were tested for statistical significance by use of a 2-tailed Student t test and a P value of less than .05 was considered significant. Data analysis and graphical presentation were performed using the GraphPad Prism software package (Graph-Pad Software, San Diego, CA).
Results
Evaluation of the cytotoxic activity of freshly harvested HIV-1-specific CD8+ T cells using fluorogenic caspase-3 substrates
Fluorogenic caspase-3 substrates, which emit fluorescence signals upon specific enzymatic cleavage by caspase-3, have been recently shown to be a sensitive tool to quantify the cytotoxic activity of antigen-specific CD8+ T cells by flow cytometry.14 Here, we adapted this technique for the direct ex vivo assessment of the cytotoxic activity of HIV-1-specific CD8+ T cells in freshly isolated PBMC samples on the single epitope level. Figure 1A shows caspase-3-specific staining of an HLA-B8-expressing BCL after a one-hour incubation with freshly harvested PBMCs from an HLA-B8-positive individual. A total of 18% of all target cells previously loaded with the HLA-B8-restricted HIV-1 Gag peptide EI8 (EIYKRWII) emitted caspase-3-specific fluorescence signals following exposure to PBMCs. Yet, only negligible quantities of caspase-3-specific fluorescence signals were observed in control BCLs that either lacked previous labeling with the B8-EI8 peptide, were pulsed with an HLA class I-mismatched peptide, or were not exposed to effector PBMCs. In addition, target cell apoptosis was completely abrogated when PBMC samples were depleted of CD8+ T cells, indicating that the observed cytotoxicity was mediated by CD8+ T cells (data not shown). Finally, in line with previous results,14 we observed a significant correlation between the cytotoxic activity of HIV-1-specific CD8+ T-cell clones measured comparatively in a standard 4-hour chromium-release test and the one-hour caspase-3 cleavage assay (R = 0.9, P < .05), although the caspase-based cytotoxicity assay was up to 10-fold more sensitive.
Evaluation of the cytotoxic activity of HIV-1-specific CD8+T cells by use of fluorogenic caspase-3 substrates. (A) Untreated or peptide-pulsed autologous BCLs were coincubated for 60 minutes with freshly isolated PBMCs at an effector-to-target (E/T) ratio of 50:1 and subsequently stained with FITC-labeled caspase-3 substrates. Gating was performed according to fluorescence signals of the target cell fluorophore and FSC/SSC characteristics. Percentages indicate the proportion of caspase-3-positive cells. Only HLA class I-matched BCLs labeled with the specific peptide were lysed by CD8+ T cells. (B) Kinetic analysis of B8-EI8-labeled target cell apoptosis after addition of PBMCs at an E/T ratio of 50:1. The assay was run in triplicate; results indicate the mean and standard deviation (error bars), demonstrating a low (< 10%) intra-assay variability. (C) Evolution of B8-EI8-labeled target cell apoptosis following 60 minutes of coincubation with freshly isolated PBMCs from an additional study person at various E/T ratios. (D) Simultaneous assessment of caspase-3 activation and 7-aminoactinomycin uptake in B8-EI8-labeled target cells at various time points after exposure to PBMCs at an E/T ratio of 50:1.
Evaluation of the cytotoxic activity of HIV-1-specific CD8+T cells by use of fluorogenic caspase-3 substrates. (A) Untreated or peptide-pulsed autologous BCLs were coincubated for 60 minutes with freshly isolated PBMCs at an effector-to-target (E/T) ratio of 50:1 and subsequently stained with FITC-labeled caspase-3 substrates. Gating was performed according to fluorescence signals of the target cell fluorophore and FSC/SSC characteristics. Percentages indicate the proportion of caspase-3-positive cells. Only HLA class I-matched BCLs labeled with the specific peptide were lysed by CD8+ T cells. (B) Kinetic analysis of B8-EI8-labeled target cell apoptosis after addition of PBMCs at an E/T ratio of 50:1. The assay was run in triplicate; results indicate the mean and standard deviation (error bars), demonstrating a low (< 10%) intra-assay variability. (C) Evolution of B8-EI8-labeled target cell apoptosis following 60 minutes of coincubation with freshly isolated PBMCs from an additional study person at various E/T ratios. (D) Simultaneous assessment of caspase-3 activation and 7-aminoactinomycin uptake in B8-EI8-labeled target cells at various time points after exposure to PBMCs at an E/T ratio of 50:1.
In an attempt to define optimal conditions for this cytotoxicity assay, we analyzed the time course of target cell apoptosis following exposure to effector cells as well as the effector-to-target ratio yielding maximal rates of caspase-3 activation in target cells. As shown in Figure 1B, MHC class I-restricted target cell killing was observed as early as 30 minutes after effector-target coincubation and subsequently tended to plateau. Moreover, an effector-to-target ratio of 10:1 was sufficient to reveal MHC class I-restricted cytotoxic activity, yet the peak level of cytotoxicity was observed at an effector-to-target ratio of 50:1 (Figure 1C). Finally, we observed that target cells emitting caspase-3-specific fluorescence signals subsequently stained brightly positive for the DNA-dye 7-aminoactinomycin D (7-AAD), which selectively enters cells with disrupted outer cell membranes (Figure 1D). This observation indicated that caspase-3 activation in target cells is preceding the physical markers of cell demise. Taken together, these data show that caspase-3 substrates represent highly sensitive and specific tools for the direct ex vivo quantification of the cytotoxic activity of HIV-1-specific CD8+ T cells using freshly isolated PBMCs.
Discrepancies between cytotoxicity and interferon-γ secretion of HIV-1-specific CD8+ T cells
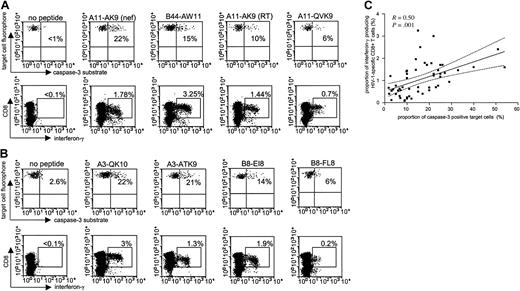
Numerous studies have identified and analyzed HIV-1-specific CD8+ T cells based on their ability to secrete interferon-γ following antigen-specific stimulation; however, it is currently not known if interferon-γ secretion is directly correlated to the cytotoxic activity of these cells. To address this question, we used the cytotoxicity assay with fluorogenic caspase-3 substrates for a direct head-to-head comparison of the cytotoxic and cytokine-secretion capacity of HIV-1-specific CD8+ T cells at the single epitope level for a number of different epitope-specific responses in 9 study subjects. We used peptide-pulsed BCLs for PBMC stimulation both in the cytotoxicity and the cytokine-secretion assay in order to insure an equal mode of antigen presentation. Figure 2A-B demonstrates that there was no direct correlation between interferon-γ production and cytotoxicity for different epitope-specific CD8+ T-cell responses within the same individual. Instead, the hierarchy of immune responses assessed by inter-feron-γ production and cytotoxicity differed, as demonstrated for 2 representative study subjects, AC-04 (Figure 2A) and FW-003 (Figure 2B). Overall, interferon-γ secretion and cytolysis after stimulation with a total of 53 different HIV-1-specific CD8+ T-cell epitopic peptides in the 9 study subjects (median of 6 peptides per subject; range, 3-9 peptides, Table 1) correlated significantly (R = 0.50, P = .001; Figure 2C). However, when plotting the proportion of caspase-3-expressing target cells against the proportion of interferon-γ-secreting CD8+ T cells, more than 64% (n = 34) of all data points were located outside of the 95% confidence interval of the regression line (Figure 2C), 19 of these below the 2.5% cutoff lines and 15 above the 97.5% cut-off lines, respectively. Taken together, these data indicate that a given level of interferon-γ secretion can be associated with both high or low levels of cytotoxicity, and suggest that interferon-γ secretion of HIV-1-specific CD8+ T cells alone is not directly predictive of their cytotoxic activity.
Comparison of interferon-γ secretion and cytotoxic activity of HIV-1-specific CD8+T cells at the single epitope level. HIV-1-specific CD8+ T-cell responses to 5 optimal CD8+ T-cell epitopes are shown in study individuals AC-04 (A) and FW-003 (B), respectively. Percentages reflect the proportion of caspase-3-expressing target cells (top rows) and the corresponding proportion of interferon-γ-secreting CD8+ T cells (bottom rows) following co-incubation of peptide-pulsed BCL and freshly harvested PBMCs. (C) Correlation between epitope-specific interferon-γ secretion and cytotoxic activity in 53 different immune responses from 9 different study persons. Dashed lines indicate the 95% confidence intervals of the regression line.
Comparison of interferon-γ secretion and cytotoxic activity of HIV-1-specific CD8+T cells at the single epitope level. HIV-1-specific CD8+ T-cell responses to 5 optimal CD8+ T-cell epitopes are shown in study individuals AC-04 (A) and FW-003 (B), respectively. Percentages reflect the proportion of caspase-3-expressing target cells (top rows) and the corresponding proportion of interferon-γ-secreting CD8+ T cells (bottom rows) following co-incubation of peptide-pulsed BCL and freshly harvested PBMCs. (C) Correlation between epitope-specific interferon-γ secretion and cytotoxic activity in 53 different immune responses from 9 different study persons. Dashed lines indicate the 95% confidence intervals of the regression line.
Discrepancies between cytotoxicity and interferon-γ secretion of HIV-1-specific CD8+ T cells are not related to a heterogeneous maturation phenotype
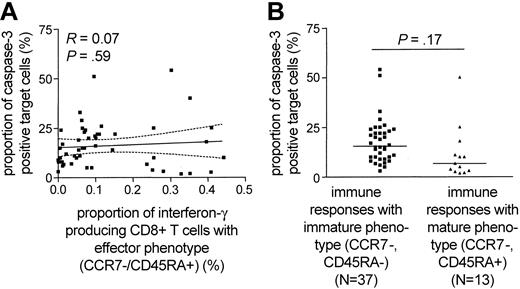
Recent studies proposed that the maturation of HIV-1-specific CD8+ T cells occurs in several sequential stages, each of them corresponding to a specific pattern of CD45RA and CCR7 surface expression.21 Based on this model, the functional repertoire of HIV-1-specific CD8+ T cells has been linked to distinct maturation stages, as defined by CD45RA and CCR7 expression.21,22 In previous studies,8,23 the majority of HIV-1-specific CD8+ T cells exhibited an “effector-memory” pheno-type (CCR7-/CD45RA-), while a smaller proportion of these cells shared a terminally differentiated “effector” phenotype (CCR7-/CD45RA+). Reasoning that the observed discrepancies between cytolytic activity and cytokine secretion of HIV-1-specific CD8+ T cells might be related to a divergent maturation stage, we correlated the cytotoxic potential of HIV-1-specific CD8+ T cells with the proportion of interferon-γ-secreting CD8+ T cells that share the terminally differentiated “effector” phenotype. Overall, of the 50 different HIV-1-specific CD8+ T-cell populations from 8 of the 9 study subjects for which sufficient numbers of PBMCs were available, the major proportion (median of 93%, range: 12%-99%) shared an “effector-memory” phenotype, whereas a median proportion of 6.75% (range: 1%-88%) exhibited a terminally differentiated “effector” phenotype. When selectively plotting the proportion of terminally differentiated (CCR7-/CD45RA+) interferon-γ-secreting CD8+ T cells against the corresponding level of antigen-dependent cytotoxicity, no statistical correlation between those parameters was observed (R = 0.07, P = .59; Figure 3A). Moreover, there was no significant difference between the cytotoxic potential of terminally differentiated (defined as > 20% of epitope-specific CD8+ T cells CCR7-/CD45RA+) or preterminally differentiated (defined as < 20% of epitope-specific CD8+ T cells CCR7-/CD45RA+) HIV-1-specific CD8+ T cells (Figure 3B). These data indicate that discrepancies between the cytotoxicity and interferon-γ secretion of HIV-1-specific CD8+ T cells are not directly related to a heterogeneous maturation phenotype.
No relationship between the epitope-specific cytotoxic activity of HIV-1-specific CD8+T cells and their maturation phenotype. (A) Correlation between the proportion of HIV-1-specific interferon-γ secreting CD8+ T cells with effector phenotype and the corresponding epitope-specific CD8+ T-cell-mediated cytotoxic activity. Dashed lines represent the 95% confidence intervals of the regression line. (B) Epitope-specific cytotoxic activity of HIV-1-specific CD8+ T cells stratified according to their maturation phenotype. HIV-1-specific CD8+ T-cell populations with more than 20% of CCR7-/CD45RA+ cells were classified as terminally differentiated immune responses, whereas populations with less than 20% of CCR7-/CD45RA+ cells were regarded as preterminally differentiated immune responses.
No relationship between the epitope-specific cytotoxic activity of HIV-1-specific CD8+T cells and their maturation phenotype. (A) Correlation between the proportion of HIV-1-specific interferon-γ secreting CD8+ T cells with effector phenotype and the corresponding epitope-specific CD8+ T-cell-mediated cytotoxic activity. Dashed lines represent the 95% confidence intervals of the regression line. (B) Epitope-specific cytotoxic activity of HIV-1-specific CD8+ T cells stratified according to their maturation phenotype. HIV-1-specific CD8+ T-cell populations with more than 20% of CCR7-/CD45RA+ cells were classified as terminally differentiated immune responses, whereas populations with less than 20% of CCR7-/CD45RA+ cells were regarded as preterminally differentiated immune responses.
Close correlation between the cytotoxic activity of HIV-1-specific CD8+ T cells and their ability to secrete both interferon-γ and TNF-α
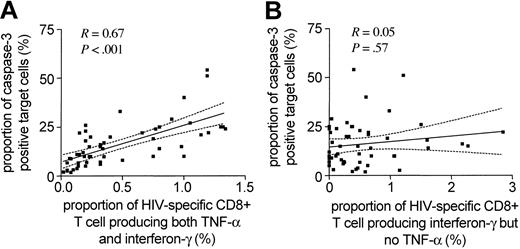
While HIV-1-specific CD8+ T cells have most commonly been analyzed and identified by their ability to secrete interferon-γ, they can produce an array of additional cytokines and chemokines following viral stimulation.4,24 Yet, it is currently unclear if the pattern of antigen-specific cytokine secretion by epitope-specific CD8+ T-cell subsets is directly associated with a distinct functional activity of these cells. In an effort to address this question, we extended the above studies that assessed interferon-γ only to simultaneously analyze the antigen-specific secretion of interferon-γ, TNF-α, and interleukin 2 (IL-2) in HIV-1-specific CD8+ T cells to determine if the concomitant release of these cytokines might be more predictive of the cytolytic activity of these cells than interferon-γ secretion alone. While antigen-dependent release of IL-2 was rarely observed in HIV-1-specific CD8+ T cells (median of 0.085%, range: 0.05%-0.16%), TNF-α was produced to a variable extent in all analyzed populations of HIV-1-specific CD8+ T cells. Overall, the subset of HIV-1-specific CD8+ T cells secreting both interferon-γ and TNF-α in response to viral antigen stimulation constituted between 15% and 100% (median: 52%) of the total number of epitope-specific interferon-γ-producing CD8+ T cells. This proportion differed for distinct epitope-specific responses both intraindividually and interindividually, reaching its highest level in study subject AC-06 (median: 100%, range: 21%-100%) and its lowest level in study subject AC-02 (median: 12%, range: 5%-21%). Interestingly, when we selectively plotted the subset of epitope-specific CD8+ T cells secreting both interferon-γ and TNF-α after specific stimulation against the level of epitope-specific target cell lysis, the degree of correlation was dramatically greater (R = 0.67, P < .001) than the one observed for CD8+ T cells releasing interferon-γ but not TNF-α (R = 0.05, P = .57; Figure 4).
Close association between the cytotoxic activity of HIV-1-specific CD8+T cells and their ability to produce both interferon-γ and TNF-α following viral antigen exposure. Correlation between the epitope-specific cytotoxic activity of HIV-1-specific CD8+ T cells and the corresponding proportion of HIV-1-specific CD8+ T cells producing both interferon-γ and TNF-α (A) or interferon-γ but no TNF-α (B) following antigenic stimulation. Dashed lines indicate the 95% confidence intervals of the regression line.
Close association between the cytotoxic activity of HIV-1-specific CD8+T cells and their ability to produce both interferon-γ and TNF-α following viral antigen exposure. Correlation between the epitope-specific cytotoxic activity of HIV-1-specific CD8+ T cells and the corresponding proportion of HIV-1-specific CD8+ T cells producing both interferon-γ and TNF-α (A) or interferon-γ but no TNF-α (B) following antigenic stimulation. Dashed lines indicate the 95% confidence intervals of the regression line.
The cytotoxic effector function of HIV-1-specific CD8+ T cells is preferentially mediated by a subset of TNF-α- and interferon-γ-producing cells
To directly compare the cytotoxic effects of HIV-1-specific CD8+ T cells producing both interferon-γ and TNF-α or interferon-γ but no TNF-α, we sorted epitope-specific CD8+ T-cell subsets according to their cytokine secretion profile, using bispecific CD45/interferon-γ and CD45/TNF-α antibodies. Figure 5 shows results from study subject AC-16: after stimulation with the HIV-1 Gag peptide B44-AW11 (AEQASQDVKNW), a total of 1.4% of all CD8+ T cells secreted interferon-γ. Of these cells, 62% released TNF-α in conjunction with interferon-γ, whereas 38% of these cells secreted interferon-γ only. After sorting these 2 populations, the coincubation of AW11-labeled BCLs with AW11-specific CD8+ T cells secreting both interferon-γ and TNF-α at an effector-to-target ratio of 1:1 resulted in caspase-3 activation in 25% of target cells. In contrast, a substantially smaller proportion of dying target cells (2.9%) was observed after incubating AW11-loaded BCLs with AW11-specific CD8+ T cells secreting inter-feron-γ but no TNF-α (Figure 5A). Overall, we compared the cytotoxic properties of epitope-specific CD8+ T cells secreting both interferon-γ and TNF-α or interferon-γ only in a total of 5 different HIV-1-specific CD8+ T-cell populations from 4 different study individuals. As indicated in Figure 5B, the subset of cells secreting both TNF-α and interferon-γ consistently induced apoptosis in a substantial proportion of peptide-labeled target cells (median of 26%, range: 18%-53%), while the corresponding subgroups of CD8+ T cells secreting interferon-γ but not TNF-α elicited apoptosis in a significantly smaller fraction of target cells (median of 5.5%, range: 2.9%-26%, P = .02). Interestingly, the cytotoxic effects of HIV-1-specific CD8+ T lymphocytes secreting both TNF-α and interferon-γ were almost entirely abrogated when these cells were treated with concanamycin A, which selectively depletes cells of intracellular perforin17 (Figure 5C). In line with this observation, we observed that HIV-1-specific CD8+ lymphocytes that simultaneously release interferon-γ and TNF-α have substantially higher degrees of intracellular perforin expression when compared with the subset of CD8+ T cells secreting interferon-γ only (Figure 5D), suggesting that the preferential cytotoxic effects of this cellular subgroup are predominantly mediated by enhanced perforin release. In contrast, using a 6-color flow cytometric analysis, no difference was found between the maturation phenotype (as defined by CD45RA/CCR7 expression) of these 2 distinct cellular subsets (Figure 5E). Taken together, these experiments demonstrate that a subset of HIV-1-specific CD8+ T cells secreting both TNF-α and interferon-γ can exhibit stronger direct ex vivo cytotoxic activity than those cells secreting only interferon-γ, and not TNF-α.
Cytotoxic effects of HIV-1-specific CD8+ T cells can be preferentially mediated by a subset of cells producing both interferon-γ and TNF-α in responses to viral antigen stimulation. (A) PBMCs from study subject AC-16 were stimulated for 3 hours with the viral peptide B44-AW11 (AEQASQDVKNW). After labeling with fluorogenic interferon-γ- and TNF-α-catching reagents, HIV-1-specific CD8+ T cells were sorted according to their cytokine secretion pattern. The CD8+ T-cell populations producing both interferon-γ and TNF-α or interferon-γ but no TNF-α were then selectively tested in caspase-3-based cytotoxicity assays with peptide-pulsed BCLs at an E/T ratio of 1:1. Quadrants were set according to the caspase-3 activation in unloaded target cells. (B) Target cell apoptosis following incubation with sorted subsets of HIV-1-specific CD8+ T cells secreting either interferon-γ and TNF-α or interferon-γ only. Data reflect the means and standard deviations from 5 different epitope-specific CD8+ T-cell populations in 4 study subjects. (C) Target cell apoptosis induced by sorted TNF-α- and IFN-γ-secreting HIV-1-specific CD8+ T cells that were treated with concanamycin A (con A) or not. Data indicate means and standard deviations from 3 different experiments. (D) Intracellular expression of perforin in HIV-1-specific CD8+ T cells secreting either interferon-γ and TNF-α or interferon-γ only. Means and standard deviations from 12 different CD8+ T-cell populations are shown. (E) Proportion of HIV-1-specific CD8+ T cells with “effector” phenotype (CD45RA+/CCR7-) within the subsets of cells secreting TNF-α and interferon-γ or interferon-γ alone. Data reflect means and standard deviations of 12 different CD8+ T-cell populations.
Cytotoxic effects of HIV-1-specific CD8+ T cells can be preferentially mediated by a subset of cells producing both interferon-γ and TNF-α in responses to viral antigen stimulation. (A) PBMCs from study subject AC-16 were stimulated for 3 hours with the viral peptide B44-AW11 (AEQASQDVKNW). After labeling with fluorogenic interferon-γ- and TNF-α-catching reagents, HIV-1-specific CD8+ T cells were sorted according to their cytokine secretion pattern. The CD8+ T-cell populations producing both interferon-γ and TNF-α or interferon-γ but no TNF-α were then selectively tested in caspase-3-based cytotoxicity assays with peptide-pulsed BCLs at an E/T ratio of 1:1. Quadrants were set according to the caspase-3 activation in unloaded target cells. (B) Target cell apoptosis following incubation with sorted subsets of HIV-1-specific CD8+ T cells secreting either interferon-γ and TNF-α or interferon-γ only. Data reflect the means and standard deviations from 5 different epitope-specific CD8+ T-cell populations in 4 study subjects. (C) Target cell apoptosis induced by sorted TNF-α- and IFN-γ-secreting HIV-1-specific CD8+ T cells that were treated with concanamycin A (con A) or not. Data indicate means and standard deviations from 3 different experiments. (D) Intracellular expression of perforin in HIV-1-specific CD8+ T cells secreting either interferon-γ and TNF-α or interferon-γ only. Means and standard deviations from 12 different CD8+ T-cell populations are shown. (E) Proportion of HIV-1-specific CD8+ T cells with “effector” phenotype (CD45RA+/CCR7-) within the subsets of cells secreting TNF-α and interferon-γ or interferon-γ alone. Data reflect means and standard deviations of 12 different CD8+ T-cell populations.
Discussion
HIV-1-specific CD8+ T cells have been shown to execute a variety of different effector functions, resulting in the control of viral replication.1,25,26 However, subsets within an oligoclonal HIV-1-specific CD8+ T-cell population may differ in their ability to exert these effector mechanisms. The methods used to examine HIV-1-specific CD8+ T cells include their numeric quantification by tetramer staining as well as the functional assessment of their cytokine secretion pattern, but each of these assays is limited in that they do not directly detect CD8+ T-cell-mediated cytotoxic activity. Nevertheless, the cytolytic function of antigen-specific CD8+ T cells has been demonstrated to be essential for the clearance of a number of viral infections, such as lymphocytic choriomeningitis virus (LCMV),27 and is likely to significantly determine the antiviral effects of HIV-1-specific CD8+ T cells.2 Here, we used fluorogenic caspase-3 substrates as indicators of target cell death in a flow-cytometry-based cytotoxicity assay,14 allowing for a highly sensitive visualization and quantification of the cytototoxic activity of HIV-1-specific CD8+ T cells in freshly harvested PBMC samples. Applying this technique, we show substantial discrepancies between interferon-γ secretion of HIV-1-specific CD8+ T cells and their cytotoxic activity and identified a subset of HIV-1-specific CD8+ T cells producing both interferon-γ and TNF-α with strong cytotoxic activity.
Our studies demonstrate considerable intraindividual and inter-individual discrepancies between cytokine secretion and cytotoxic activity of HIV-1-specific CD8+ T cells at the single epitope level. Similar numbers of interferon-γ-secreting epitope-specific CD8+ T cells were associated with both high and low levels of cytotoxicity, suggesting a significant functional heterogeneity between interferon-γ secretion and MHC class I-restricted cytotoxicity in HIV-1 infection. This is in line with a number of recent studies challenging the idea of a homogenous execution of virus-specific CD8+ T-cell effector functions. It has been shown that within human CD8+ T cells directed against cytomegalovirus (CMV), the ratio of interferon-γ secretion to target cell lysis can vary widely when both functions were assessed in parallel.28 In addition, recent studies demonstrated that both antigen-dependent perforin degranulation29 and proliferation11 of HIV-1-specific CD8+ T cells can occur independently from interferon-γ secretion, indicating discrepancies between these CD8+ T-cell effector functions in HIV-1. Taken together, these data suggest that the assessment of inter-feron-γ secretion alone does not accurately reflect the HIV-1-specific cytotoxic activity of CD8+ T cells.
The underlying mechanisms for the functional heterogeneity between interferon-γ secretion and cytotoxicity in HIV-1-specific CD8+ T cells are not well understood. In vitro studies using HIV-1-specific CD8+ T-cell clones have shown that HIV-1 Nef-mediated down-regulation of HLA class I molecules can abrogate the cytolytic effects of CD8+ T-cell clones, while preserving their antigen-dependent cytokine secretion activity.30 Similarly, the replicative senescence of HIV-1-specific CD8+ T-cell clones seems to alter their cytotoxic activity, while it apparently does not interfere with their cytokine secretion function.31 These studies indicate that HIV-1 infection itself as well as the resulting chronic immune activation might preferentially impair the cytotoxic function of CD8+ T cells in vitro. Moreover, recent data suggest that the maturation of HIV-1-specific CD8+ T cells is skewed in chronic HIV-1 infection, resulting in impairment of their functional repertoire.21,32,33 In 8 study subjects, we simultaneously assessed the cytotoxic activity and the maturation status of HIV-1-specific CD8+ T cells directed against a number of different epitopes (median: 6, range: 3-9). In line with previous studies, the majority of HIV-1-specific CD8+ T-cell responses shared an “effector-memory” phenotype (CCR7-/CD45RA-); however, this pheno-type varied between different individuals, as well as between the different epitope-specific responses in the same study subject. We observed no association between the maturation phenotype of interferon-γ-secreting HIV-1-specific CD8+ T cells and the cytotoxic activity of these cells. This is in line with recent studies demonstrating the absence of an association between HIV-1-specific CD8+ T-cell maturation phenotypes and their intracellular expression of perforin as a surrogate marker for CD8+ T-cell cytotoxicity.8,34
A prominent observation of this study is that the epitopespecific cytotoxic activity of HIV-1-specific CD8+ T cells is substantially more closely correlated to the proportion of CD8+ T cells with concomitant antigen-specific interferon-γ and TNF-α secretion than to the proportion of cells producing interferon-γ alone following stimulation with viral antigen. In addition, the subset of HIV-1-specific CD8+ T cells simultaneously secreting interferon-γ and TNF-α mediated significantly stronger cytotoxic effects than the subset of CD8+ T cells producing interferon-γ alone following the sorting of freshly harvested HIV-1-specific CD8+ T cells according to their cytokine secretion profile. The stronger cytotoxicity mediated by interferon-γ- and TNF-α-secreting CD8+ T cells did not reflect a direct cytotoxic effect of secreted TNF-α,35 as in the experimental design of the one-hour cytotoxicity assay, neither exogenously added recombinant human TNF-α nor TNF-α specific monoclonal antibodies were found to specifically alter the CD8+ T-cell-mediated cytotoxic activity (data not shown). In addition, antibodies blocking fas receptors or fas ligands had no specific impact on the experimental outcome of the cytotoxicity assays. In contrast, the cytotoxic capacity of sorted TNF-α- and interferon-γ-secreting HIV-1-specific CD8+ T cells was almost completely abrogated when cells were treated with concanamycin A, which selectively depletes cells from intracellular perforin granules, suggesting that the preferential cytotoxic effects of this cellular subset were predominantly mediated by the release of perforin. Indeed, this subset of CD8+ T cells had substantially higher degrees of detectable intracellular perforin expression than HIV-1-specific CD8+ T cells secreting inter-feron-γ but no TNF-α. Overall, these data suggest that the ability to secrete TNF-α is closely associated with the cytotoxic activity of HIV-1-specific CD8+ T cells. This is in line with observations in chronically LCMV-infected mice demonstrating a hierarchical loss of effector functions in virus-specific CD8+ T cells, with TNF-α secretion and cytotoxic activity being exhausted significantly earlier during the disease course than interferon-γ secretion.7 Further studies are therefore warranted to analyze the subset of interferon-γ- and TNF-α-secreting HIV-1-specific CD8+ T cells in individuals with slow or rapid disease progression to determine if this subgroup of cells might represent a more precise correlate of protective immunity against HIV-1 than interferon-γ-secreting HIV-1-specific CD8+ T cells in general.
In summary, the results of this study demonstrate wide variations between interferon-γ secretion and cytotoxic activity among HIV-1-specific CD8+ T cells. In addition, we show that HIV-1-specific CD8+ T cells secreting both TNF-α and interferon-γ in response to stimulation with HIV-1-specific CD8+ T-cell epitopic peptides exhibit strong cytotoxic activity. Thus, these data show for the first time the existence of cellular subsets with distinct cytokine secretion and cytotoxic capacities within HIV-1-specific CD8+ T cells targeting the same viral epitope. These results will be relevant for a more precise evaluation of HIV-1-specific CD8+ T-cell responses generated in natural infection or following immunization with HIV-1-specific vaccines.
Prepublished online as Blood First Edition Paper, April 1, 2004; DOI 10.1182/blood-2003-12-4341.
Supported by grants from the National Institutes of Health (M.A., E.S.R., J.Z., T.M.A., B.D.W.), the Doris Duke Charitable Foundation (M.A., E.S.R., B.D.W.), the Foundation for AIDS and Immunology Research (X.G.Y., M.A.), and the Deutsche Forschungsgemeinschaft (M.L.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Dr Ronald J. Bosch, Center for Biostatistics in AIDS Research, Harvard School of Public Health, Boston, MA, for excellent advice on statistical data analysis.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal