Abstract
Although an increased frequency of CD4+CD8+ T cells has been observed in the peripheral blood during viral infections, their role, function, and biologic significance are still poorly understood. Here we demonstrate that the circulating CD4+CD8+ T-cell population contains mature effector memory lymphocytes specific for antigens of multiple past, latent, and high-level persistent viral infections. Upon in vitro antigenic challenge, a higher frequency of CD4+CD8+ than single-positive cells displayed a T helper 1/T cytotoxic 1 (Th1/Tc1) cytokine profile and proliferated. Ex vivo, more double-positive than single-positive cells exhibited a differentiated phenotype. Accordingly, their lower T-cell receptor excision circles (TREC) content and shorter telomeres proved they had divided more frequently than single-positive cells. Consistent with expression of the tissue-homing marker CXCR3, CD4+CD8+ T cells were demonstrated in situ at the site of persistent viral infection (ie, in the liver during chronic hepatitis C). Finally, a prospective analysis of hepatitis C virus (HCV) infection in a chimpanzee, the only animal model for HCV infection, showed a close correlation between the frequency of activated CD4+CD8+ T cells and viral kinetics. Collectively, these findings demonstrate that peripheral CD4+CD8+ T cells take part in the adaptive immune response against infectious pathogens and broaden the perception of the T-cell populations involved in antiviral immune responses. (Blood. 2004;104:478-486)
Introduction
T-cell development is an ordered process thought to take place exclusively in the thymus where CD4+CD8+ T cells develop into CD4+ and CD8+ T cells with mutually exclusive expression of these 2 receptors. Mature CD4+ and CD8+ T cells then leave the thymus and enter secondary lymphoid organs where they recognize their cognate antigen in the context of major histocompatibility complex (MHC) class II and class I molecules on antigen-presenting cells. Antigenic stimulation induces proliferation and differentiation into armed effector cells with the ability to home to infected organs and to participate in immune responses against intracellular pathogens.
Contrary to this conventional dichotomy, circulating CD4+CD8+ T cells have sporadically been reported in humans as well as in animals such as mice, chicken, swine, and monkeys.1-6 The existence of this unconventional and rare (< 5%) lymphocyte population in the peripheral blood was explained as a premature release of CD4+CD8+ T cells from the thymus into the periphery,7,8 where their maturation into functionally competent single-positive cells continues.7 There is, however, considerable evidence of an increased frequency of peripheral CD4+CD8+ T cells during viral infections.9-12 In human immunodeficiency virus (HIV) or Epstein-Barr virus (EBV) infections, for example, the percentage of CD4+CD8+ T cells can increase to 20% of all circulating lymphocytes.9,10 Yet, in humans, very little is known about their phenotype, antigen specificity, and function. This is particularly striking since in animal models CD4+CD8+ T cells have been described as antigen-specific memory cells that can be induced by vaccination.3,4,6 Thus, it is crucial to determine the role and biologic significance of peripheral CD4+CD8+ T cells in humans, as they could potentially contribute to the adaptive immune response against pathogens.
In this study, we addressed this issue with an extensive analysis of peripheral CD4+CD8+ T cells in both healthy individuals and patients with past or present viral infections. We determined the frequency and ex vivo phenotype of CD4+CD8+ T cells in regards to memory, activation, homing, differentiation, and maturation markers. Furthermore, we provide data on antigen specificity, proliferation, cytokine production, and cytotoxic potential of CD4+CD8+ T cells in response to tetanus toxoid and to a large variety of antigens (lysates of infected cells, overlapping viral peptides, minimal optimal viral epitopes) from past (influenza A virus [IV], measles virus [MV], vaccinia virus [VV]); latent (cytomegalovirus [CMV], EBV, varicella-zoster virus [VZV], herpes simplex virus [HSV]); and highly replicative, persistent (hepatitis C virus [HCV]) infections. We also demonstrate that CD4+CD8+ T cells are present in situ at the site of infection and provide evidence for their in vivo relevance during the course of HCV infection in a chimpanzee, as activation and antigen-specific proliferation of these cells in blood and liver correlated well with viral kinetics. This study shows that human peripheral CD4+CD8+ T cells are mature antigen-specific effector memory cells and that they may contribute to the adaptive immune response during viral infections.
Materials and methods
Blood samples
Peripheral blood mononuclear cells (PBMCs) were isolated from anticoagulant citrate dextrose (ACD)-anticoagulated blood of 10 healthy blood donors and 15 HCV-infected patients as described.13 Serum was tested for HCV antibodies by enzyme immunoassay (EIA) and for HCV RNA by quantitative polymerase chain reaction (PCR).14 All subjects gave written informed consent for research testing under protocols approved by the Institutional Review Boards of National Institutes of Health (NIH) Clinical Center or National Institute of Diabetes & Digestive & Kidney Diseases (NIDDK). For prospective analysis of CD4+CD8+ cells during the course of HCV infection, PBMCs and fine-needle liver biopsy samples were obtained from a chimpanzee (Ch1552) that had recovered from HCV genotype 1a infection and was intravenously rechallenged with 3 increasing doses (0.32, 3.2, 32 50% chimpanzee infectious dose [CID50]) homologous HCV under an approved animal protocol.15
Viral lysates, peptides, proteins, and antibodies
Lysates from CMV- or EBV-infected cells and the corresponding uninfected cells were purchased from Virion (Marristown, NJ). Lysates from HSV-1-, MV-, and VZV-infected cells and the corresponding uninfected cells were purchased from Advanced Biotechnologies (Columbia, MD).
Six hundred 15-mer peptides (Mimotopes, Clayton, Australia), overlapping by 10 amino acids and spanning the complete HCV genotype 1a polyprotein sequence, were resuspended in phosphate-buffered saline (PBS) in 5 mixes corresponding to Core (38 peptides), E1/E2/p7 (87 peptides), NS2-NS3 (84 peptides), NS4 (38 peptides), and NS5 (108 peptides) proteins and containing each peptide at 24 μg/mL. The HLA-A2-restricted minimal optimal epitopes EBVBMLF-1/280-288 GLCTLVAML,16 CMVpp65/495-503 NLVPMVATV,17 IVMA58-67 GILGFVFTLT,18 and VVVP35-43 SLSAYIIRV13 (Research Genetics, Huntsville, AL) were resuspended at 20 mg/mL in dimethyl sulfoxide (DMSO) and diluted to 1 mg/mL in PBS. Recombinant HCV core, NS3, helicase, NS4, NS5A, and NS5B proteins (genotype 1) were purchased from Mikrogen (Martinsried, Germany),13 and purified tetanus toxoid was from the University of Massachusetts Medical School Biologic Laboratories (Jamaica Plain, MA).
Anti-CD4-fluorescein isothiocyanate (FITC), anti-HLA-DR-FITC, anti-CD62L-FITC, anti-T-cell receptor αβ-FITC (anti-TCRαβ-FITC), anti-CD56-phycoerythrin (PE), anti-CD8α-peridinin chlorophyll A protein (PerCP), anti-CD4-PerCP, anti-CD69-PerCP (BD Pharmingen, San Diego, CA); anti-CD27-FITC, anti-CD28-FITC, anti-TNF-α-FITC (anti-tumor necrosis factor α-FITC), anti-CXCR3-PE, anti-CD45RO-allophycocyanin (APC), anti-interferon γ-APC (anti-IFN-γ-APC), anti-CD28-APC, anti-CD38-APC (Caltag Laboratories, Burlingame, CA); purified anti-CCR7, anti-CD57-FITC, anti-interleukin 4-PE (anti-IL-4-PE), anti-IL-10-APC, anti-TCRγδ-APC (BD Pharmingen); goat antimouse immunoglobulin M (IgM) μ chain-FITC (Jackson ImmunoResearch Laboratories, Baltimore, PA); anti-CD8-β chain-PE (Immunotech, Marseille, France); and IgG1/IgG2a isotype control antibodies were used for flow cytometry.
T-cell phenotyping
Phenotyping was performed on thawed PBMCs and either directly ex vivo or after cloning of chimpanzee liver-infiltrating lymphocytes (LILs). PBMCs (0.5-1 × 106) and LILs (0.5-1 × 105) were stained with antibodies against CD4, CD8, and other cell surface molecules for 30 minutes at 4°C. Staining with anti-CCR7 was followed by incubation with anti-IgM-FITC secondary antibody. Propidium iodide (0.5 μg/mL) was added to the cells for 5 minutes. Cells were then washed and 3 × 105 PBMCs or 104 LILs were acquired in the lymphogate on a FACSCalibur (Becton Dickinson, Mountain View, CA) and analyzed with FlowJo software (FlowJo, San Carlos, CA). Freezing/thawing did not significantly alter the expression of CD45RO, CD27, CD28, CD62L, CD1a, CD69, and HLA-DR. Although the expression of CCR7, CXCR3, CD56, and CD38 tended to be slightly higher on thawed (Figure 1) than on fresh (not shown) cells, the same trend was found for all 3 T-cell populations, so that CCR7, CXCR3, CD56, and CD38 expression on CD4+CD8+ cells relative to CD4+ and CD8+ cells was not affected.
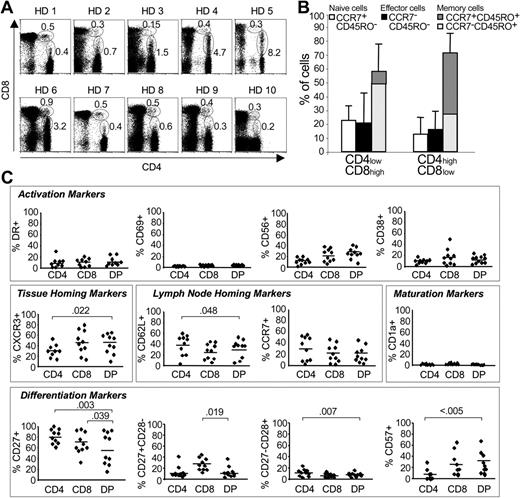
Frequency and phenotype of circulating CD4/CD8 double-positive cells in healthy blood donors. (A) Frequency of CD4+CD8+ cells in 10 healthy blood donors (HD). The percentage of CD4highCD8low and CD4lowCD8high double-positive cells in the PBMC population is indicated. (B) Percentage of naive, effector, and memory cells within the CD4highCD8low and CD4lowCD8high double-positive cell populations of 10 healthy blood donors. CCR7+CD45RO+ indicates central memory cells; and CCR7-CD45RO+, effector memory cells. Error bars indicate SD. (C) Phenotype of CD4 and CD8 single-positive cells and CD4+CD8+ cells (DP). The percentage of cells with the indicated marker is shown for each subpopulation. The solid line indicates the mean percentage of CD4 and CD8 single-positive and CD4+CD8+ cells with the indicated surface marker. Significant differences (P ≤ .05; Student t test) are indicated.
Frequency and phenotype of circulating CD4/CD8 double-positive cells in healthy blood donors. (A) Frequency of CD4+CD8+ cells in 10 healthy blood donors (HD). The percentage of CD4highCD8low and CD4lowCD8high double-positive cells in the PBMC population is indicated. (B) Percentage of naive, effector, and memory cells within the CD4highCD8low and CD4lowCD8high double-positive cell populations of 10 healthy blood donors. CCR7+CD45RO+ indicates central memory cells; and CCR7-CD45RO+, effector memory cells. Error bars indicate SD. (C) Phenotype of CD4 and CD8 single-positive cells and CD4+CD8+ cells (DP). The percentage of cells with the indicated marker is shown for each subpopulation. The solid line indicates the mean percentage of CD4 and CD8 single-positive and CD4+CD8+ cells with the indicated surface marker. Significant differences (P ≤ .05; Student t test) are indicated.
Intracellular cytokine staining
Two million PBMCs were stimulated with one of the following: 5 μg/mL VZV-, HSV-1-, or MV-infected cell lysates; a 1:4 dilution of CMV-infected cell lysate; a 1:8 dilution of EBV-infected cell lysate; 1 μg/mL HLA-A2-restricted EBV, IV, CMV, or VV peptides; 5 μg/mL tetanus toxoid; or the 5 HCV peptide mixes described above (final concentration of 1 μg/mL per peptide) in 500 μL RPMI1640 (Cellgro, Herndon, VA) supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, 100 μg/mL streptomycin, and 100 U/mL penicillin. Anti-CD28 and -CD49d antibodies (BD Pharmingen) were added at 1 μg/mL each. Appropriate negative (PBMCs stimulated with uninfected cell lysates or without peptide) and positive controls (50 ng/mL phorbol myristate acetate [PMA] and 500 ng/mL ionomycin [Sigma, St Louis, MO]) were included. After 2 hours, 2 μM monensin was added. After 4 additional hours, cells were treated with 2 mM ethylenediaminetetraacetic acid (EDTA) for 20 minutes at room temperature, washed, and stained with antibodies against cell surface molecules for 30 minutes at 4°C. After one additional wash followed by fixation and permeabilization with the cytofix/cytoperm kit (BD Pharmingen), cells were stained with antibodies against TNF-α and IL-4 for 30 minutes at 4°C, washed, resuspended in PBS, and immediately harvested on a FACSCalibur.
Cytokine secretion assay
PBMCs were plated in 48-well plates at 107 cells/mL and stimulated as described in the previous paragraph but without monensin. After 3 hours, IFN-γ and IL-10 cytokine secretion assays were performed according to the manufacturer's instructions (Miltenyi Biotec, Auburn, CA). At least 1.5 × 105 events were acquired in the lymphogate on a FACSCalibur (Becton Dickinson, San Jose, CA).
Isolation of T-cell subsets
CD4+ T cells were magnetically isolated from PBMCs with the CD4 MultiSort Kit (Miltenyi Biotec). Beads were detached with MultiSort release and stop reagents. CD4+CD8- T cells and CD4+CD8+ double-positive (DP) T cells were then isolated from the CD4+ population with CD8 microbeads (Miltenyi Biotec). The CD4- cell population obtained during the CD4 MultiSort procedure was used to negatively select T cells with the T-cell-negative isolation kit (Dynal, Oslo, Norway) followed by positive selection of CD4-CD8+ T cells with CD8 microbeads. All separation steps were performed manually with LS separation columns (Miltenyi Biotec) or automatically with the autoMACS (Miltenyi Biotec). The purity of CD3+CD4+CD8- and CD3+CD4-CD8+ T cells was greater than 96%, and that of CD3+CD4+CD8+ T cells was greater than 82%.
Quantification of TRECs
Genomic DNA was extracted from the isolated T-cell subsets using TRIzol reagent (Invitrogen, Carlsbad, CA). T-cell receptor excision circles (TREC) copy numbers were quantitated by real-time PCR with the primers CAC ATC CCT TTC AAC CAT GCT and GCC AGC TGC AGG GTT TAG G and the probe FAM-ACA CCT CTG GTT TTT GTA AAG GTG CCC ACT-TAMRA (Applied Biosystems, Foster City, CA) as previously described.19 A standard TREC-DNA plasmid was obtained by cloning the TREC-PCR product into the TOPO TA cloning vector (Invitrogen), and serial dilutions of quantitated numbers of the plasmid were included in the real-time PCR. Real-time PCR for β actin was done to normalize the cell number for each sample. All samples and standards were tested in duplicate and TRECs copy numbers were analyzed with Sequence Detector v1.6.3 software (Applied Biosystems).
Analysis of telomere length
Telomere length was determined with TeloTAGGG telomere length assay kit (Roche, Mannheim, Germany). Briefly, 1 μg genomic DNA and control DNA were digested with HinfI and RsaI, electrophoresed, and transferred onto Hybond-N+ nylon membranes (Amersham Pharmacia, Piscataway, NJ). The blotted membrane was prehybridized in hybridization buffer at 42°C, and digoxigenin-conjugated telomere probe was added for 3 hours at 42°C. After stringent washes, blocking, and staining with alkaline phosphatase-conjugated antidigoxigenin antibody (1:10 000) for 30 minutes at room temperature, blots were developed using enhanced chemiluminescence (Roche).
Immunohistochemistry
Anonymyzed, formalin-fixed, paraffin-embedded liver biopsy tissue was sectioned at 4 μm, deparaffinized in xylene, rehydrated in graded alcohol, and rinsed in distilled water. Target antigen retrieval for both CD4 and CD8 markers was achieved by heat-induced epitope retrieval with 1 mM EDTA (pH 8.0) for 15 minutes in a 1200-W microwave oven. After cooling for 30 minutes, slides were washed and double-label immunohistochemistry was performed using the NexES automated stainer and detection reagents (Ventana Medical Systems, Tucson, AZ). After antigen retrieval, endogenous peroxidase activity was blocked with hydrogen peroxide and endogenous biotin with unlabeled avidin biotin. Monoclonal anti-CD4 (clone 1F6) diluted 1:2 (Ventana/Nova Castra, Tucson, AZ) was applied for 30 minutes at 40°C and detected with biotinylated goat antimouse antibody and streptavidin-horseradish-peroxidase conjugate. The complex was visualized with diaminobenzidene and enhanced with copper sulfate. After extensive washing, monoclonal anti-CD8 (clone C8/144B) diluted 1:10 (DAKO-Cytomation, Carpinteria, CA) was applied for 32 minutes at 40°C and detected using biotinylated goat antimouse antibody and alkaline phosphatase-streptavidin conjugate. The complex was visualized with simultaneous application of Naphthol-AS-MX phosphatase and 4-chloro-2-methylbenzenediazonium salt in acetate buffer. Slides were washed in PBS, counterstained with hematoxylin, dehydrated, and mounted with permanent media. Appropriate positive and negative controls (multi-tissue block of tonsil, thymus, and spleen) were run in parallel. Images were acquired at ambient temperature using an Olympus BX40 microscope equipped with UplanF1 20 ×/0.50 and Plan 100 ×/1.25 oil objectives and an Olympus DP11 camera (Olympus America, Melville, NY). Digital images were processed with Adobe Photoshop 7.0 (Adobe Systems, Seattle, WA) applying the “Sharpen More” function once to the whole image and adjusting the background to white with the “Levels—white balance background” function.
Proliferation assay
Anti-CD3-stimulated T-cell lines20 were tested 3 weeks after the last restimulation in the presence of 100 000 irradiated (30 Gy [3000 rad]) autologous PBMCs and 1 μg HCV protein. [3H]thymidine (ICN, Costa Mesa, CA) was added on day 3 for 16 hours. Stimulation indices (counts per minute [cpm] in the presence of antigen/cpm in the absence of antigen) higher than 4 were significant.
Statistical analysis
Paired Student t tests were performed to analyze differences in cell surface marker expression between T-cell subpopulations. A 2-tailed P value of less than .05 was considered statistically significant.
Results
Frequency and phenotype of CD4+CD8+ cells in the blood of healthy donors
Frequency and phenotype of CD4+CD8+ T cells were analyzed directly ex vivo in blood samples of 10 healthy donors (Figure 1A). CD4+CD8+ T cells consisted of 2 populations: CD4lowCD8high accounting for 0.15% to 0.9% of PBMCs and CD4highCD8low representing 0.2% to 8.2% of PBMCs. Most CD4lowCD8high (> 55%) and CD4highCD8low (> 72%) cells had a memory phenotype (Figure 1B). Interestingly, CD4lowCD8high were mainly CCR7-CD45RO+ effector memory cells, whereas CD4highCD8low were predominantly CCR7+CD45RO+ central memory cells. Despite this difference in CCR7 expression, however, all other activation (HLA-DR, CD38, CD69, CD56), lymph node and tissue homing (CD62L, CCR7, CXCR3), differentiation (CD27, CD28), senescence (CD57), and maturation (CD1a) markers did not significantly differ between CD4lowCD8high and CD4highCD8low (not shown), and the following results are therefore presented for the entire double-positive cell population. Whereas no significant differences between CD4+CD8+ T cells and CD4+ or CD8+ T cells were observed for any activation markers (Figure 1C), significantly more CD4+CD8+ than CD4+ cells expressed the tissue-homing marker CXCR3 (mean, 44% versus 31%; P = .022), suggesting that these cells were more prone to migrate to the tissues than CD4+ cells. Accordingly, fewer CD4+CD8+ cells than CD4+ cells expressed the lymph node-homing marker CD62L (P < .05), and the same trend was observed for CCR7. CD4+CD8+ T cells appeared more differentiated than single-positive cells, as fewer double-positive cells expressed CD27 compared with both CD4+ (mean, 55% versus 80%; P = .003) and CD8+ (mean, 55% versus 65%; P = .039) cells. Within the CD4+CD8+ population, an equal percentage of cells had down-regulated either CD27 or CD28. Finally, significantly more CD4+CD8+ than CD4+ cells expressed CD57 (mean, 7% versus 28%; P < .005)21 and all had lost CD1a expression, indicating they had fully matured. Collectively, these results indicate that CD4+CD8+ T cells constituted a small, highly differentiated memory population in healthy blood donors.
Antigen specificity and effector functions of CD4+CD8+ cells in healthy blood donors
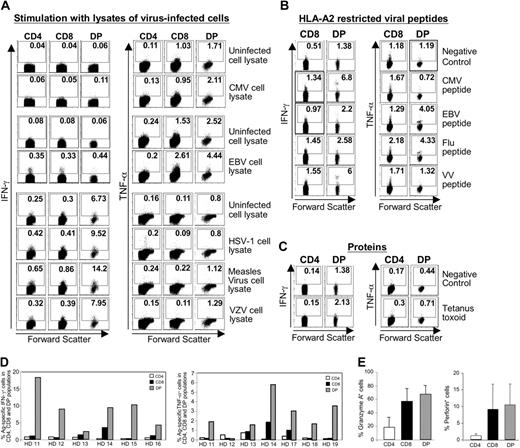
Because memory T cells vigorously respond to recall antigens, we compared the function of CD4+CD8+ cells to those of CD4+ and CD8+ cells using a panel of antigens from common self-limited (IV, VV, and MV) and latent (EBV, CMV, VZV, HSV-1) viral infections. We used lysates of virus-infected cells to study both CD8 and CD4 responses simultaneously (Figure 2A), a panel of HLA-A2-restricted minimal optimal viral epitopes to analyze MHC class I-restricted responses (Figure 2B), and tetanus toxoid to analyze MHC II-restricted responses (Figure 2C). As CD4lowCD8high and CD4highCD8low cells did not differ in their cytokine profile (not shown), the following results are presented for the entire CD4+CD8+ population. Interestingly, a significantly higher percentage of CD4+CD8+ than CD4+ and CD8+ cells were antigen specific and produced IFN-γ and TNF-α in response to virus-infected cell lysates, HLA-A2-restricted peptides, or tetanus toxoid protein (Figure 2A-C). For example, approximately 14% CD4+CD8+ T cells produced IFN-γ in response to measles virus-infected cell lysate compared with 0.65% and 0.86% CD4+ and CD8+ cells, respectively (Figure 2A left). Similarly, 4.4% CD4+CD8+ cells produced TNF-α in response to EBV-infected cell lysate compared with 2.6% of CD8+ cells (Figure 2A right). A higher percentage of cytokine-producing CD4+CD8+ cells was also observed in response to minimal optimal, HLA-A2-restricted epitopes (Figure 2B) and tetanus toxoid (Figure 2C). Accordingly, more double-positive (up to 39%) than single-positive cells (26% CD8 and 13% CD4) expressed CD69 after antigenic stimulation (not shown). In general, the cumulative frequency of all peptide-specific, cytokine-producing CD4+CD8+ cells was much higher than that of CD4+ and CD8+ cells (Figure 2D). Similar cumulative frequencies were obtained with virus-infected cell lysates and protein stimulation. In all cases, CD4+CD8+ cells displayed a predominant T helper 1/T cytotoxic 1 (Th1/Tc1) profile and only very few produced Th2/Tc2 cytokines (not shown).
Specificity and effector function ofCD4+CD8+ cells in healthy blood donors. (A-C) PBMCs of healthy blood donors (A) or HLA-A2-positive healthy blood donors (B-C) were ex vivo stimulated with the indicated virus-infected cell lysates (A) or HLA-A2-restricted peptides (B) or proteins (C) and analyzed by intracellular cytokine staining or cytokine secretion assay. The percentage of cytokine-producing cells is indicated in each quadrant. Dot plots are representative for 10 healthy blood donors tested. DP indicates CD4+CD8+ T cells. (D) Cumulative frequency of all CMV, EBV, IV, and VV peptide-specific, cytokine-producing CD4+, CD8+, and CD4+CD8+ cells in the PBMC population of each healthy blood donor (HD). Results are representative for 10 healthy donors. Similar results were obtained with virus-infected cell lysates and protein stimulation. (E) Mean granzyme A and perforin expression in the indicated cell populations of 5 healthy blood donors. Error bars indicate SD.
Specificity and effector function ofCD4+CD8+ cells in healthy blood donors. (A-C) PBMCs of healthy blood donors (A) or HLA-A2-positive healthy blood donors (B-C) were ex vivo stimulated with the indicated virus-infected cell lysates (A) or HLA-A2-restricted peptides (B) or proteins (C) and analyzed by intracellular cytokine staining or cytokine secretion assay. The percentage of cytokine-producing cells is indicated in each quadrant. Dot plots are representative for 10 healthy blood donors tested. DP indicates CD4+CD8+ T cells. (D) Cumulative frequency of all CMV, EBV, IV, and VV peptide-specific, cytokine-producing CD4+, CD8+, and CD4+CD8+ cells in the PBMC population of each healthy blood donor (HD). Results are representative for 10 healthy donors. Similar results were obtained with virus-infected cell lysates and protein stimulation. (E) Mean granzyme A and perforin expression in the indicated cell populations of 5 healthy blood donors. Error bars indicate SD.
To assess the cytotoxic potential of each cell population, we analyzed the presence of granzyme A and perforin, 2 prestored molecules, directly ex vivo by flow cytometry. As many CD4+CD8+ as CD8+ cells contained granzyme A and perforin (Figure 2E), indicating that CD4+CD8+ cells can potentially display cytotoxic functions against viral and vaccine antigens.
TREC content and telomere length of CD4+CD8+ T cells in healthy blood donors
Because most double-positive cells were memory cells (Figure 1B) and more differentiated than single-positive cells (Figure 1C; down-regulation of CD27), we hypothesized that they had divided more often than their single-positive counterparts. To test this hypothesis, CD4+ and CD8+ and CD4+CD8+ cells were purified with magnetic beads and evaluated for their TREC content and telomere length. TRECs are episomal DNA products generated during T-cell receptor rearrangement and not amplified during mitosis.22 Likewise, telomeres are not entirely replicated during the cell cycle.23 Therefore, the lowest TREC content and the shortest telomere length should be observed in those cells that have experienced the highest number of cell divisions. Indeed, CD4/CD8 double-positive cells displayed a lower TREC content (Figure 3B) and contain shorter telomeres (Figure 3C) than CD4+ and CD8+ cells, indicating that they had experienced more cell divisions than their single-positive counterparts. Collectively, these results show that the CD4+CD8+ cell population of healthy donors contained antigen-specific, differentiated memory cells that have taken part in the adaptive immune response to vaccines and past and latent viral pathogens.
TREC copy number and telomere length in different T-cell subsets. (A) Purity of purified CD4+ cells, CD8+ cells, and CD4+CD8+ cells after magnetic bead separation. (B) TREC copy number was determined by real-time PCR in each subpopulation and found to be lower in CD4+CD8+ (DP) than in CD4+ and CD8+ cell populations. (C) Telomere length was determined by Southern blot in each subpopulation. Arrows indicate long telomeres for CD4+ and CD8+ cells and short telomeres for the CD4+CD8+ population.
TREC copy number and telomere length in different T-cell subsets. (A) Purity of purified CD4+ cells, CD8+ cells, and CD4+CD8+ cells after magnetic bead separation. (B) TREC copy number was determined by real-time PCR in each subpopulation and found to be lower in CD4+CD8+ (DP) than in CD4+ and CD8+ cell populations. (C) Telomere length was determined by Southern blot in each subpopulation. Arrows indicate long telomeres for CD4+ and CD8+ cells and short telomeres for the CD4+CD8+ population.
Frequency and function of CD4+CD8+ T cells in HCV-infected patients
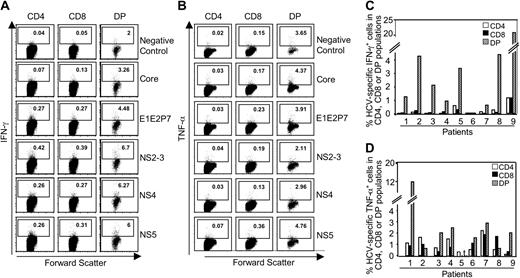
To assess whether CD4+CD8+ T cells also participated in the immune response in persistent viral infections and whether they were present at the site of infection, we analyzed their frequency and function in HCV infection. CD4+CD8+ cells represented an average of 1% of all CD3+ lymphocytes in both persistently HCV-infected patients and healthy donors (Table 1). Because a Th1/Tc1 response plays a critical role in control and clearance of HCV,24-29 we analyzed IFN-γ and TNF-α production of CD4, CD8, and CD4+CD8+ T cells with pools of overlapping peptides that covered the whole HCV polyprotein and therefore all potential HCV epitopes in the context of all given MHC class I and class II molecules. As illustrated in Figure 4A-B for a representative patient (patient 9), double-positive as well as single-positive cells responded to HCV antigenic stimulation and produced IFN-γ (Figure 4A) and TNF-α (Figure 4B). Interestingly, however, the frequency of HCV-specific cytokine-producing cells was much higher in the CD4+CD8+ than in the single-positive populations. For all tested patients (except patients 2 and 8 for TNF-α), the cumulative frequency of HCV peptide-specific, cytokine-producing cells in the CD4+CD8+ population was consistently equal to or higher than those in the single-positive populations (Figure 4C-D). In contrast to IFN-γ and TNF-α, IL-4 and IL-10 were produced at a much lower level (not shown). Corresponding to these cytokine responses, more than 3% of the CD4+CD8+ T cells proliferated after stimulation with HCV proteins compared with less than 1% for CD4 and CD8 single-positive cells in carboxyfluorescein succinimidyl ester (CFSE)-labeling assays (not shown).
Frequency of T-cell subpopulations in the blood of healthy donors and HCV-infected patients
. | % T-cell subpopulation in PBMCs, mean ± SD . | . | . | ||
|---|---|---|---|---|---|
. | CD4+ . | CD8+ . | CD4+CD8+ . | ||
| Healthy blood donors; n = 10 | 41 ± 6 | 17 ± 9 | 1.0 ± 0.6 | ||
| HCV-infected patients; n = 15 | 41 ± 10 | 27 ± 7 | 1.2 ± 0.5 | ||
. | % T-cell subpopulation in PBMCs, mean ± SD . | . | . | ||
|---|---|---|---|---|---|
. | CD4+ . | CD8+ . | CD4+CD8+ . | ||
| Healthy blood donors; n = 10 | 41 ± 6 | 17 ± 9 | 1.0 ± 0.6 | ||
| HCV-infected patients; n = 15 | 41 ± 10 | 27 ± 7 | 1.2 ± 0.5 | ||
Circulating CD4+CD8+ cells in HCV infection. (A-B) Representative dot plot of IFN-γ (A) and TNF-α (B) production by CD4+CD8+ cells and CD4+ and CD8+ cells after stimulation with HCV overlapping peptides. (C-D) Percentage of HCV-specific, IFN-γ-producing cells (C) or TNF-α-producing cells (D) in each cell subset for all patients tested. The bars indicate the cumulative response to all HCV peptide pools. nt indicates not tested.
Circulating CD4+CD8+ cells in HCV infection. (A-B) Representative dot plot of IFN-γ (A) and TNF-α (B) production by CD4+CD8+ cells and CD4+ and CD8+ cells after stimulation with HCV overlapping peptides. (C-D) Percentage of HCV-specific, IFN-γ-producing cells (C) or TNF-α-producing cells (D) in each cell subset for all patients tested. The bars indicate the cumulative response to all HCV peptide pools. nt indicates not tested.
After stimulation, virus-specific T cells are known to acquire tissue-homing capacities and to migrate to the site of infection. As HCV is a hepatotropic virus, we looked for the presence of CD4+CD8+ T cells at the site of infection, the liver, and identified CD4+CD8+ T cells in portal areas and hepatic sinuses by immunohistochemistry (Figure 5). Collectively, these results suggest that they may contribute to the immune response during persistent viral infections.
Intrahepatic CD4+CD8+ cells in HCV infection. (A) Liver histology (original magnification × 200) showing chronic hepatitis C, double stained for both CD4 (brown chromogen) and CD8 (red chromogen). T cells are clustered around the lymphoid aggregate and at the edges of the portal area where they are involved in interface hepatitis. The arrows indicate 2 CD4/CD8 double-stained cells. (B-D) High-magnification (original magnification × 1000) photomicrographs of double-stained cells in portal areas and hepatic sinuses. The arrows indicate CD4/CD8 double-stained cells.
Intrahepatic CD4+CD8+ cells in HCV infection. (A) Liver histology (original magnification × 200) showing chronic hepatitis C, double stained for both CD4 (brown chromogen) and CD8 (red chromogen). T cells are clustered around the lymphoid aggregate and at the edges of the portal area where they are involved in interface hepatitis. The arrows indicate 2 CD4/CD8 double-stained cells. (B-D) High-magnification (original magnification × 1000) photomicrographs of double-stained cells in portal areas and hepatic sinuses. The arrows indicate CD4/CD8 double-stained cells.
Prospective phenotypic and functional characterization of CD4+CD8+ T cells during the course of HCV infection
To provide further evidence of the in vivo relevance of CD4+CD8+ T cells, we prospectively analyzed these cells in blood and liver during the course of HCV infection in a chimpanzee, the only animal susceptible to HCV infection. Because most CD4+CD8+ T cells were memory cells, we chose a chimpanzee that had previously recovered from a primary HCV infection and was undergoing a secondary HCV rechallenge with 3 increasing doses of homologous virus (Figure 6A). The second injection (3.2 CID50) resulted in detectable viremia, which lasted for 4 weeks. After HCV clearance, the chimpanzee displayed sterilizing immunity against the 10-fold higher inoculation dose (32 CID50; Figure 6A). As expected, the brief period of viremia induced a significant infiltration of CD4+ and CD8+ cells in the liver. The absolute number of intrahepatic CD4+CD8+ T cells also increased but to a lesser extent (Figure 6A). Strikingly, however, more than 80% of these double-positive cells were activated and HLA-DR+ (Figure 6B right) compared with 50% of intrahepatic CD4 T cells (Figure 6B middle) and 20% to 40% CD8 cells (Figure 6B left). Moreover, more than 50% CD4+CD8+ but no single-positive cells were activated in the blood during viremia.
Phenotypic and functional characterization of CD4+CD8+ cells in a chimpanzee during the course of HCV infection. (A) Absolute number of CD4+ and CD8+ cells and CD4+CD8+ cells per centimeter liver biopsy during the course of HCV infection. The 3 arrows indicate the time point of intravenous HCV inoculation. CID50 indicates 50% chimpanzee infectious dose. (B) Activation status of CD4+ and CD8+ cells and CD4+CD8+ cells as assessed by expression of HLA-DR. + and - indicate presence and absence of serum HCV RNA as determined by RT-PCR. (C) Phenotype of CD4+CD8+ liver-derived cell lines. In the left panel, cells were gated on CD3+ T cells. In the middle panel, CD3+CD4+ T cells were gated and examined for CD8 α and β chain expression. The anti-CD8α antibody recognizes the α chain either in the αα homodimer or in the αβ heterodimer. The anti-CD8β antibody recognizes only the β chain. In the right panel, cells were gated on CD4+CD8+ cells and examined for TCR αβ or γδ expression. (D) Proliferative responses of the respective CD4+CD8+ T-cell lines to HCV proteins. Dotted lines represent the cutoff of positivity.
Phenotypic and functional characterization of CD4+CD8+ cells in a chimpanzee during the course of HCV infection. (A) Absolute number of CD4+ and CD8+ cells and CD4+CD8+ cells per centimeter liver biopsy during the course of HCV infection. The 3 arrows indicate the time point of intravenous HCV inoculation. CID50 indicates 50% chimpanzee infectious dose. (B) Activation status of CD4+ and CD8+ cells and CD4+CD8+ cells as assessed by expression of HLA-DR. + and - indicate presence and absence of serum HCV RNA as determined by RT-PCR. (C) Phenotype of CD4+CD8+ liver-derived cell lines. In the left panel, cells were gated on CD3+ T cells. In the middle panel, CD3+CD4+ T cells were gated and examined for CD8 α and β chain expression. The anti-CD8α antibody recognizes the α chain either in the αα homodimer or in the αβ heterodimer. The anti-CD8β antibody recognizes only the β chain. In the right panel, cells were gated on CD4+CD8+ cells and examined for TCR αβ or γδ expression. (D) Proliferative responses of the respective CD4+CD8+ T-cell lines to HCV proteins. Dotted lines represent the cutoff of positivity.
Liver-infiltrating lymphocytes isolated from liver biopsies during viremia were then nonspecifically expanded with anti-CD3 and IL-2. In 7 of 66 cell lines, more than 70% of the cells were CD4+CD8+; in 24 of 66 cell lines, most (> 70%) cells were CD4+; and in 8 of 66 cell lines, most (> 70%) cells were CD8 single-positive. These predominantly CD4+CD8+ cell lines were not an artifact of in vitro culture because sorted (> 98% purity) CD4high and CD8high single-positive cells of the same chimpanzee did not generate any double-positive cell lines during 4 months of identical in vitro culture (not shown). Representative cell lines that consisted predominantly of double-positive cells are shown in Figure 6C-D. In all cell lines, the majority of the CD4+CD8+ T-cell population expressed the conventional CD8αβ molecule (Figure 6C middle) and all double-positive cells expressed αβTCR (Figure 6C right). In addition, CD4+CD8+ T-cell lines were HCV specific and proliferated vigorously after stimulation with HCV proteins (Figure 6D). In total, 87.5% of the CD4+CD8+ cell lines obtained during viremia and 53% of the CD4+CD8+ cell lines obtained after viral clearance were HCV specific. These results demonstrate that CD4+CD8+ T cells are present at the site of infection and may play a role in the HCV-specific immune response.
Discussion
This study demonstrates that circulating CD4+CD8+ T cells are mature memory cells that mount rapid Th1/Tc1 recall responses to vaccine and viral antigens from self-limited, past, or highly replicative, persistent infections. Our study also proves that CD4+CD8+ T cells truly exist in vivo in extrathymic sites, as these cells were detected in situ at the site of inflammation and viral replication and could be cloned from liver biopsies of a chimpanzee during the course of HCV infection. Undoubtedly, the presence of mature, functional CD4+CD8+ T cells in peripheral blood and tissues challenges the perception of the T-cell populations involved in adaptive immune responses during viral infections.
For several reasons, we are confident that the CD4+CD8+ T-cell population was not a flow cytometry or an in vitro culture artifact: (1) we included propidium iodide in our experiments to exclude dead cells (not shown); (2) we separated peripheral CD4+CD8+ T cells from CD4+ and CD8+ cells by magnetic beads and also cloned CD4+CD8+ T cells from liver biopsies and maintained them in culture for several months, thereby excluding the possibility of aggregates of single-positive cells; and (3) we detected CD4 and CD8 mRNA in double-positive cells (not shown), thereby excluding a passive acquisition of CD4 and CD8 molecules by single-positive cells. Thus, CD4+CD8+ cells exist as a distinct T-cell population in the blood and at the site of infection.
Circulating CD4+CD8+ T cells were detectable at equal frequency in healthy blood donors and HCV-infected patients. This result differs from published reports that showed an increased frequency of double-positive cells in mice and swine after vaccination with reovirus or pseudorabies virus.3,4 However, these discrepancies can be reconciled by our observation that the increased frequency of CD4+CD8+ T cells is of transient nature and that the cells are sequestrated to the site of infection, as clearly shown during the course of HCV infection in a chimpanzee (Figure 5A). This is particularly important, as we show for the first time that appearance, activation, and function of circulating and intrahepatic CD4+CD8+ T cells correlate with viral kinetics.
Interestingly, double-positive cells displayed lower TREC content and shorter telomeres than single-positive cells. To our knowledge, this is the first report demonstrating at the molecular level that CD4+CD8+ T cells experienced more cell divisions than their single-positive counterparts. In fact, our results may even underestimate the proliferative history of double-positive cells because of an approximately 20% contamination with CD4+ cells (Figure 3A left), suggesting that the TREC content would even be lower if the CD4+CD8+ cell population was purer. Pure double-positive (> 96%) cell populations were obtained after cell sorting, but cell yields were too low to perform these experiments with sorted cells. This molecular evidence of the replicative history of CD4+CD8+ cells was consistent with the expression of CD57, a marker of replicative senescence.21
Tightly linked to antigen-specific stimulation and T-cell proliferation is the differentiation status of T cells. Here, fewer CD4+CD8+ than single-positive cells expressed CD27. This was associated with loss of lymph node-homing molecules (CCR7, CD62L) and increased expression of tissue-homing markers (CXCR3) compared with CD4+ cells. This is in accordance with other studies showing down-regulation of differentiation markers on single-positive effector cells concomitantly with gain of memory markers and cytotoxic activity.16 We then demonstrated that CD4+CD8+ T cells were effector memory cells detectable in situ in the HCV-infected liver. This is consistent with reports that single-positive effector memory cells localize preferentially in nonlymphoid peripheral tissues and provide rapid effector function upon stimulation.30,31 Thus, our results suggest that CD4+CD8+ T cells promptly participate in the immune response in viral infections.
The function and role of circulating CD4+CD8+ T cells as part of the adaptive immune response is still a matter of debate. Increasing numbers of studies describe CD4+CD8+ T cells in the blood of patients infected with different pathogens9,10,32-36 but only 3 have analyzed the function of CD4+CD8+ T cells in humans10,32,33 and only one used specific antigens to stimulate CD4+CD8+ T cells.33 This report, however, is limited to CMV and HIV infections, compares responses of CD4+CD8+ T cells only to those of CD4+ T cells, and classified double-positive cells as a subset of CD4+ effector cells. In contrast, here we used antigens from a large panel of viruses that cause self-limited, latent, or highly replicative, persistent infections and demonstrated that double-positive cells constitute a distinct T-cell population exhibiting typical CD4 and CD8 T-cell functions. Specifically, CD4+CD8+ T cells secreted Th1/Tc1 cytokines in response to MHC class II- and MHC class I-restricted antigens. In addition, the perforin and granzyme A content of CD4+CD8+ T cells was comparable to that of CD8+ cells, suggesting that the double-positive cell population may lead to rapid lysis of virus-infected cells. This correlates with other studies showing cytolytic activity of CD4+CD8+ T cells in an anti-CD3 antibody-redirected cytotoxic assay and directly ex vivo after incubation with CMV-loaded antigen-presenting cells.5,33 Although preliminary experiments demonstrated that cytokine responses of HCV-peptide-stimulated PBMCs could be blocked by anti-MHC class I antibodies (not shown), it would be necessary to examine the effect of anti-MHC class I and II antibodies at the single cell (ie, clonal) level to determine if any given double-positive cell can respond to peptides presented by either MHC molecule.
The origin of peripheral CD4+CD8+ T cells is still controversial. A thymic origin is supported by our finding that the majority of CD4+CD8+ liver-derived T-cell lines expressed the conventional CD8αβ molecule. In addition, published reports describe that thymus-derived single-positive cells can up-regulate either CD4 or CD8 upon activation in HIV infection33,35 and that thymus-derived, mature naive CD1a- double-positive cells in neonatal cord blood can generate mature memory double-positive cells in the periphery.37 On the other hand, an extrathymic development in immuno-logic organs such as gut or liver may also be possible, as both organs contain their own lymphoid system and represent immuno-logically distinct organs with a high number of resident natural killer (NK) and NKT cells, γδ T cells, and CD4+CD8+ T cells.38-40 This last hypothesis is very attractive especially in the case of hepatitis C, as liver-resident CD4+CD8+ T cells could immediately act to control HCV replication in the liver and induce and enhance adaptive immune responses.
In any case, our results also have important technical implications for the analysis of virus-specific immune responses. Whereas we were able to distinguish between cytokine-producing single-positive and double-positive cells by flow cytometry, many other studies use EliSpot assays with purified CD4 and CD8 T cells.25,26 Depending on the cell isolation method, CD4+CD8+ T cells are contained in either one or the other T-cell subset and their function cannot be evaluated separately. In light of our results, however, CD4+CD8+ T cells should be tested separately in further immuno-logic analyses.
Prepublished online as Blood First Edition Paper, March 25, 2004; DOI 10.1182/blood-2003-12-4395.
Supported by an intramural NIH research program.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank all blood donors and patients for participation in the study; Dr Stephen Feinstone for chimpanzee biopsies and blood samples; and Drs Tobias Manigold, Vito Racanelli, and Andy Talal for excellent scientific discussions.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal