Abstract
CD4+CD25+ T-regulatory (Treg) cells have been shown to critically regulate self- and allograft tolerance in several model systems. Studies of human Treg cells have been restricted by the small number present in peripheral blood and their naturally hypoproliferative state. To better characterize Treg suppressor cell function, we determined methods for the isolation and expansion of these cells. Stringent magnetic microbead-based purification was required for potent suppressor cell line generation. Culture stimulation with cell-sized Dynabeads coated with anti-CD3 and anti-CD28 monoclonal antibodies, CD4+ feeder cells, and interleukin 2, provided for marked expansion in cell number (100-fold), with retention and enhancement of suppressor function. The potent Treg cell lines suppressed proliferation in dendritic cell-driven allo-mixed lymphocyte reaction (MLR) cultures by more than 90%. The Treg-derived suppressor cells functioned early in allo-MLR because expression of activation antigens and accumulation of cytokines was nearly completely prevented. Importantly, cultured Treg cells also suppressed activated and matured dendritic cell-driven responses. These results demonstrate that short-term suppressor cell lines can be generated, and they can express a very potent suppressive activity. This approach will enable more detailed biologic studies of Treg cells and facilitate the evaluation of cultured Treg cells as a novel form of immunosuppressive therapy. (Blood. 2004;104:453-461)
Introduction
It is becoming increasingly clear that naturally arising CD4+CD25+ T regulatory (Treg) cells can influence most immune responses.1,2 Initially they were described to be critical for the control of autoimmunity,3-5 and more recently, they have been shown to regulate transplantation tolerance,6,7 antitumor immunity,8,9 and anti-infectious responses.10,11 Thus, these cells appear to be central control elements of immunoregulation, and understanding their biology will be important for efforts aimed at therapeutically manipulating immune responses.
Treg cells were first characterized in mice, where they constitute 6% to 10% of lymph node and splenic CD4+ T-cell populations. They are generated both through central thymic developmental mechanisms in pathogen-free mice12 and also arise by poorly defined peripheral generation or expansion mechanisms.13-15 It has been demonstrated that after antigen-specific activation, Treg cells can nonspecifically suppress proliferation of either CD4+ or CD8+ T cells.15,16 The mechanism of suppression is unclear and, in vitro, appears to require cell-cell contact. A significant subsequent result of suppression is impaired production of interleukin 2 (IL-2).15-17 In vivo, the suppression mechanism is more controversial, with some studies showing dependence on immunosuppressive cytokines,15,18 which are not required for in vitro suppression.
In tissue culture, CD4+CD25+ cells are naturally anergic and hyporesponsive even with strong antibody-based stimulation.16,17 However, with the provision of exogenous IL-2, they can proliferate to antigen-loaded antigen-presenting cells (APCs) or to anti-CD3-based stimulation.16,17 Initial studies revealed that anti-CD3 plus IL-2-based culture enabled limited expansion of functional murine Treg cells over the course of a 7-day culture.19,20 On a per cell basis these cells were 4- to 6-fold more potent at suppression.19 Later work has demonstrated that multiple restimulations of murine Treg cells can lead to loss of function.21 Studies in mouse models of bone marrow transplantation (BMT) have shown that CD4+CD25+ cells can prevent graft-versus-host disease (GVHD) across major histocompatibility complex barriers.22-24 Our previous studies have shown that ex vivo polyclonally expanded Treg cells, with anti-CD3 plus IL-2 (for 10 days), can be effective in preventing GVHD.22 Studies by others have shown that ex vivo expansion of Treg cells with irradiated allogeneic APCs plus exogenous IL-2 can suppress GVHD.24,25 Subsequent studies have shown that Treg cells can prevent GVHD and still allow for antitumor or graft-versus-leukemia (GVL) effects.25-27 Thus, Treg cells could have a potential role in clinical immunosuppressive therapy in transplantation, provided human Treg cells could be isolated and expanded in culture to provide sufficient numbers for in vivo infusion.
Studies of human CD4+CD25+ Treg cells have lagged behind those of murine cells but are becoming increasingly reported. These cells are present in smaller numbers in human peripheral blood than in murine lymph node or spleen, and in addition, the population of CD25+ cells is less distinct in human blood because there is an overlapping population of CD25dim cells. It has been reported that only the brightest 1% to 2% of CD25 expressers, which after isolation by fluorescence-activated cell sorting (FACS), have suppressor function (in low-dose immobilized anti-CD3 monoclonal antibody [mAb]-based coculture suppressor assays).28 The potency of suppression exhibited by freshly isolated human CD4+CD25+ cells has been modest in phytohemagglutinin or soluble anti-CD3 mAb-based assays.29-31 The best suppressor activity has been obtained by 2 groups who used dendritic cell (DC)-stimulated mixed lymphocyte reactions (MLRs) for assay.32,33 In the only report of human CD4+CD25+ polyclonal expansion, using soluble anti-CD3, and lymphoblastoid cells and peripheral blood mononuclear cells (PBMCs) as feeder cells, plus IL-2, the suppressive function noted (a 65% reduction of proliferation at a suppressor-responder cell ratio of 1:1) was less than typically observed with mouse Treg cells.34 Short-term antigen-specific cultures have been reported using DCs plus cytokine supplementation, but these lines have generally had limited expansion capability even after several rounds of stimulation.35
To better characterize CD4+CD25+ Treg cell function, we sought to develop improved means to isolate and culture these important cells. Here we show that stringently purified, polyclonally activated (with anti-CD3/28 mAb-coated beads and CD4+ feeder cells), human CD4+CD25+ cells can form cell lines that exhibit enhanced suppressor function. Hence, we characterized the phenotype and function of these culture-expanded potent Treg cells. These cell lines exhibit a profound suppressive bioactivity and form a model system for the study of Treg cell biology.
Materials and methods
Magnetic-activated cell sorting purification of CD4+ T-cell subsets
T cells were isolated from buffy coat preparations derived from the whole blood of healthy volunteer donors (Memorial Blood Centers, Minneapolis, MN). Leukocyte-rich buffy coat cells were centrifuged over Ficoll-Hypaque layers to collect PBMCs. CD25+ cells were isolated by positive selection from PBMCs with directly conjugated anti-CD25 magnetic microbeads (2 μL/107 cells; Miltenyi Biotec, Auburn, CA), and purified over an LS+ column. Cells were then applied to a second magnetic column, washed, and eluted again. After the double-column procedure, cells were routinely more than 93% pure (for CD25) by FACS analysis. Alternatively, cells were indirectly stained with anti-CD25-fluorescein isothiocyanate (FITC), clone 2A3 (Becton Dickinson Immunocytometry Systems, San Jose, CA), washed, and bound to anti-FITC multisort microbeads (3 μL/107 cells; Miltenyi Biotec) and positively selected. As with the direct microbead system, cells were reapplied to a second column. After column purification, multisort beads were detached, and the CD25+ cells were depleted of cells expressing CD8, CD14, CD19, CD20, and CD56 with a cocktail of mAb-coated microbeads for lineage depletion.
The non-CD25 fraction of PBMCs was further depleted of CD25+ cells with more anti-CD25 microbeads (10 μL/107 cells). CD4+ T cells were then isolated from the CD25 fraction by positive selection with anti-CD4 mAb-coated magnetic microbeads (10 μL/107 cells; Miltenyi Biotec). Cells were routinely 96% to 98% pure CD4+CD25- by FACS analysis.
Culture of T cells
Isolated CD4+CD25+ cells or control CD4+CD25- cells were cultured with anti-CD3/CD28 mAb-coated Dynabeads (University of Pennsylvania, Philadelphia)36,37 at a 2:1 bead-total cell ratio. CD4+CD25- feeder cells were irradiated at 30 Gy and added at a 1:1 ratio to CD4+CD25+ cells. Cells were cultured at 1 million (nonirradiated) cells/mL in 24-well plates. IL-2 was added on day 3 at 50 IU/mL (Chiron, Emeryville, CA). Cells were split as needed, approximately one third every 3 days during the fast-growth phase. Culture media was RPMI 1640 (Gibco, Grand Island, NY) supplemented with 10% fetal calf serum (FCS; Gibco), l-glutamine, penicillin, and streptomycin.
Stimulator cells for MLR cultures
Immature DCs were generated from CD14+ monocytes,38,39 isolated from PBMCs, by magnetic bead-based purification (Miltenyi Biotec), and were cultured in X-Vivo-15 (BioWhittaker, Walkersville, MD) media at 1 million cells/mL supplemented with granulocyte-macrophage colony-stimulating factor (GM-CSF; 50 ng/mL final) and IL-4 (20 ng/mL final) cytokines from R&D Systems (Minneapolis, MN). Cells were cultured for 5 to 10 days before use as stimulators in MLRs. For some experiments, DCs were matured with tumor necrosis factor α (TNF-α; 20 ng/mL final) and poly (I:C), a Toll-like receptor 3 (TLR-3) agonist ligand (20 μg/mL final; Sigma, St Louis, MO) for 2 days.40-42 In other experiments, the TNF and Poly (I:C) (at the same concentrations), or lipopolysaccharide (LPS; 10-1000 ng/mL; Sigma) were added directly to MLRs. DC stimulators were irradiated at 30 Gy.
MLR assay culture
A total of 5 × 104 responding CD4+CD25- T cells and 5 × 103 DC stimulator APCs were cultured per well in 96-well U-bottom plates. Test cultured suppressor or conventional T-cell lines were added at 2.5 × 104/well for standard assays or in graded numbers for titration experiments. For antibody-blocking experiments, 1 × 104 suppressor cells were used. Culture media was RPMI 1640 (Gibco) supplemented with 10% FCS (Gibco), l-glutamine, penicillin, and streptomycin. Wells were pulsed on days 3, 5, 6, and 7 with 3H-thymidine for the last 16 hours of culture. All time points had 6 replicates. Results are expressed in counts per minute. Data are collected with direct beta counter; hence, our reported counts per minute are lower than typically reported with liquid scintillation amplification. Thus, the absolute magnitude of counts is lower, but the comparative differences between experimental samples remain the same.
Cytokine analysis
Culture supernatants were spun free of cells and aliquots were frozen at -80°C. Supernatants were evaluated by the Luminex assay system with a latex bead-based multianalyte system (R&D Systems).
Cytotoxicity
Cultured suppressor cell lines were tested for cytotoxicity against allogeneic DCs or the natural killer (NK) cell-sensitive cell line K562 in 4-hour 51Cr-release assays. Effector-to-target ratios ranged from 20:1 to 0.6:1. Target cells were labeled with sodium chromate-51Cr (DuPont, Wilmington, DE) for 60 minutes. All determinations were performed in triplicate and the percentage of lysis was determined. Positive control lytic NK92 cells were obtained from the American Type Culture Collection (Rockville, MD) and maintained in the presence of 500 U/mL recombinant human IL-2 (Chiron).
mAbs
To follow purification, cells were stained with an anti-CD25-phycoerythrin (PE), which is not blocked by anti-CD25 microbeads, clone M-A251 (BD Pharmingen, San Diego, CA). Other antibodies for flow cytometry included anti-CD4-PerCP (clone SK3), anti-CD8-PerCP (SK1), and anti-CD19-APC (4G7) from Becton Dickinson Immunocytometry Systems; anti-CD27-FITC (M-T271), anti-CD62L-PE (Dreg 56), anti-CD69-FITC (FN50), anti-CD152-PE (BNI3), anti-CD122-PE (Mik-b2), anti-CD132-PE (AG184), and anti-CD134 (ACT35) from BD Pharmingen; and anti-CCR7-PE (no. 150503) and anti-glucocorticoid-induced TNF receptor (anti-GITR)-PE (no. 110416) from R&D Systems. In functional experiments aimed at blocking suppression, neutralizing antibodies were used at titered amounts up to 20 μg/mL. Antibodies included anti-IL-10 (23738), anti-IL10-receptor-α (37607), and anti-transforming growth factor β-1, -2, -3 (anti-TGF-β-1,-2,-3) (1D11) from R&D Systems.
Flow cytometry
For immunofluorescence staining, cells were stained for 30 minutes at 4°C, with titered amount of each antibody. Cells were washed again and analyzed on a FACS Calibur cytometer (BD Immunocytometry Systems). Cell line subsets were sorted on a FACS Vantage. Data were analyzed by FloJo software version 4.4 (Treestar, Ashland, OR). Intracellular staining was done using paraformaldehyde-fixed cells, 2% at room temperature for 30 minutes, followed by permeabilization and staining for 1 hour, and washing in 0.1% saponin-containing buffer, phosphate-buffered saline (PBS) plus 5% FCS-5% human AB serum.
Statistics
All error bars represent 1 SD above and below the mean. A paired, 2-tailed Student t test was used to determine the statistical significance of differences between proliferative responses. P values less than .05 were considered significant.
Results
MACS-based CD4+CD25+ Treg cell isolation
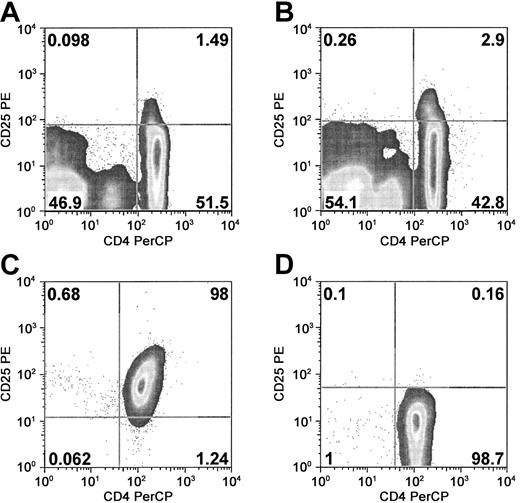
Human peripheral blood contains a small population of cells that express the Treg phenotype, positive for both CD4 and CD25 antigens (and distinctly positive for CD25; Figure 1A). This population represents approximately 1% to 2% of PBMCs, or 3% to 5% of CD4+ T cells (mean, 3.8%; range, 2.4%-6.3% of CD4+ T cells; n = 20). Some donors exhibit a more distinct population of CD4+ and CD25+ cells (Figure 1B). However, there exists a large population of partially overlapping CD25dim cells, which necessitates stringent purification for the isolation of the CD25+ Treg cells. To more specifically isolate the CD25+ Treg cells we developed a modified magnetic-activated cell sorting (MACS) purification. Using a lower titer of anti-CD25 mAb-coated microbeads led to isolation of cells with higher mean channel fluorescent intensity of CD25 expression. In addition, reapplication of the magnetically isolated cells to a second column for additional purification further increased the enrichment of CD25+ cells. The use of cleavable microbeads allowed for removal of beads after CD25 purification, which then permitted subsequent lineage depletion (of CD8, CD14, CD19, and CD56 cells). This strategy led to the generation of highly purified CD4+CD25+ cell populations (Figure 1C). The CD25- fraction of cells was further depleted of CD25+ cells and used as the source for purification of CD4+CD25- cells, for isolation of conventional T-cell controls (Figure 1D).
Purification of CD4+CD25+ cells from peripheral blood. Representative 2-color FACS plots of PBMCs and purified CD4+CD25+ and CD4+CD25- cells. (A) Examination of peripheral blood reveals CD4+CD25+ cells constitute 1% to 3% of PBMCs. There are a variable number of non-CD4+ cells that express CD25, generally of lower intensity expression (mostly B cells). (B) Some donors evidence a more distinct CD4+CD25+ population. (C) CD4+CD25+ cells. Cells were purified by anti-CD25-FITC and anti-FITC cleavable microbeads and subsequently lineage depleted. Intensity of CD25-PE staining is slightly decreased by prior staining with anti-CD25-FITC. (D) CD4+CD25- cells. Cells were purified by CD25 depletion of PBMCs, followed by CD4+ positive selection. Data are representative of 20 donor evaluations and 10 cell purification experiments.
Purification of CD4+CD25+ cells from peripheral blood. Representative 2-color FACS plots of PBMCs and purified CD4+CD25+ and CD4+CD25- cells. (A) Examination of peripheral blood reveals CD4+CD25+ cells constitute 1% to 3% of PBMCs. There are a variable number of non-CD4+ cells that express CD25, generally of lower intensity expression (mostly B cells). (B) Some donors evidence a more distinct CD4+CD25+ population. (C) CD4+CD25+ cells. Cells were purified by anti-CD25-FITC and anti-FITC cleavable microbeads and subsequently lineage depleted. Intensity of CD25-PE staining is slightly decreased by prior staining with anti-CD25-FITC. (D) CD4+CD25- cells. Cells were purified by CD25 depletion of PBMCs, followed by CD4+ positive selection. Data are representative of 20 donor evaluations and 10 cell purification experiments.
Anti-CD3/CD28 beads and IL-2 facilitate expansion of Treg cells
Human CD4+CD25+ Treg cells are hyporesponsive to stimulation with anti-CD3 mAb or DCs. However, they can proliferate when given these stimuli plus IL-2 or IL-15, albeit to a much lower extent than CD4+CD25- T cells.32,33 For our initial experiments, purified CD4+CD25+ cells were expanded with immobilized anti-CD3 plus IL-2, which allowed for a 5- to 10-fold expansion over 2 weeks. To further augment expansion potential, a costimulatory molecule-based stimulation was investigated. To do this, we used cell-sized Dynabeads with anti-CD3 and anti-CD28 mAbs covalently attached (designated 3/28 beads). This reagent has been used successfully for clinical-scale expansion of conventional T cells for immunotherapy trials,36,37 and has enabled more than one million-fold expansion of T cells. Quite dramatically, however, we found that the stringently purified CD25+ Treg cells proliferated poorly with stimulation with the 3/28 beads alone. This contrasts with the vigorous response generated by the CD4+CD25- cells (Figure 2A). The weak response of CD4+CD25+ cells is significantly augmented by IL-2 supplementation, and this combination is sufficient for modest Treg expansion from most donors (Figure 2B). However, the more stringently the CD4+CD25+ cells were purified, the less well they grew in culture, even with stimulation with 3/28 beads and IL-2.
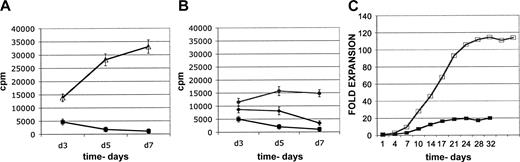
Expansion of CD4+CD25+ suppressor cell lines. Proliferation of CD4+CD25+ cells in short-term assays and accumulation in long-term cultures. (A) Proliferation of highly purified CD4+CD25- cells (▵) and double-column lineage-negative CD4+CD25+ cells (▪), in short-term 96-well 3H-thymidine incorporation assays. CD4+CD25- cells markedly proliferate, whereas CD4+CD25+ cells minimally and transiently proliferate. (B) Augmentation of proliferation of highly purified double-column lineage-negative CD4+CD25+ cells (▪), in short-term 96-well 3H-thymidine incorporation assays. IL-2 at 100 IU/mL augments expansion (♦). However, irradiated CD4+CD25- feeder cell supplementation (1:1 ratio; •) provides for increased expansion, which is more sustained. Representative of 4 experiments. (C) Long-term culture accumulation of CD4+CD25+ cell lines. Cell lines stimulated once with anti-CD3/anti-CD28 mAb-coated beads (□), or with immobilized anti-CD3 (▪), both supplemented with feeder cells. Cells were split and fed IL-2 every 3 to 4 days as needed. Data are reported as fold expansion of cell number and are representative of 22 cultures for anti-CD3/CD28 mAb-coated beads and 3 cultures for plastic-immobilized anti-CD3 mAbs. Error bars represent 1 SD above and below the mean.
Expansion of CD4+CD25+ suppressor cell lines. Proliferation of CD4+CD25+ cells in short-term assays and accumulation in long-term cultures. (A) Proliferation of highly purified CD4+CD25- cells (▵) and double-column lineage-negative CD4+CD25+ cells (▪), in short-term 96-well 3H-thymidine incorporation assays. CD4+CD25- cells markedly proliferate, whereas CD4+CD25+ cells minimally and transiently proliferate. (B) Augmentation of proliferation of highly purified double-column lineage-negative CD4+CD25+ cells (▪), in short-term 96-well 3H-thymidine incorporation assays. IL-2 at 100 IU/mL augments expansion (♦). However, irradiated CD4+CD25- feeder cell supplementation (1:1 ratio; •) provides for increased expansion, which is more sustained. Representative of 4 experiments. (C) Long-term culture accumulation of CD4+CD25+ cell lines. Cell lines stimulated once with anti-CD3/anti-CD28 mAb-coated beads (□), or with immobilized anti-CD3 (▪), both supplemented with feeder cells. Cells were split and fed IL-2 every 3 to 4 days as needed. Data are reported as fold expansion of cell number and are representative of 22 cultures for anti-CD3/CD28 mAb-coated beads and 3 cultures for plastic-immobilized anti-CD3 mAbs. Error bars represent 1 SD above and below the mean.
Because CD4+CD25+ T cells appear to have defects in cytokine production,32,33 we reasoned that conventional T cells could complement this deficiency and provide for augmented expansion. Irradiated CD4+CD25- feeder cells were added to 3/28 bead-stimulated Treg cultures at initiation (1:1 ratio) and provided for a sustained increase in proliferative response (Figure 2B). This augmentation was significantly greater than that with IL-2 alone at 100 IU/mL. Interestingly, supplementation of Treg cultures with conditioned media (20% vol/vol; derived from activated conventional CD4+CD25- T cells on day 5 after 3/28 bead stimulation) could largely (but not completely) reproduce the effects of feeder cells. This suggests that the activated conventional T cells produce soluble growth factors for CD4+CD25+ cell expansion (primarily IL-2, but possibly other factors). Importantly, these feeder cell-supplemented cell lines maintained potent suppressor function.
With the use of 3/28 beads, IL-2, and CD4+CD25- feeders, the CD4+CD25+-derived cell lines exhibited significant growth, and over 100-fold expansion was readily obtainable. The expansion occurred as a classical sigmoid growth curve, beginning slowly, rapidly expanding over 1 to 2 weeks, and then reaching a plateau phase (Figure 2C). After reaching a growth plateau phase, cell lines were maintained in IL-2, and suppressor function was sustained for 3 to 6 weeks. In some cases cell lines in culture for up to 3 months had potent suppressive activity; however, typically suppressor function diminished over time (not shown).
Functional assessment of CD4+CD25+ suppressor cell lines in MLR assays
All cell lines were initially screened for suppressor activity in MLRs after 2 to 3 weeks of culture and then further analyzed over the next 3 to 4 weeks. To evaluate suppressor function, we used an HLA-mismatched allo-MLR assay as a functional readout. Purified freshly isolated CD4+CD25- T cells were reacted with irradiated immature DCs from unrelated donors. Test cells (cultured CD4+CD25+ and CD4+CD25--derived cell lines) were added to the MLR at day 0 with a regulator-responder ratio of 1:2. Suppression was reflected by decreased proliferation and was most evident on day 6 to 7, peak of control MLR. These assays are very robust and consistent among donors and therefore served as our standard measure of suppression.
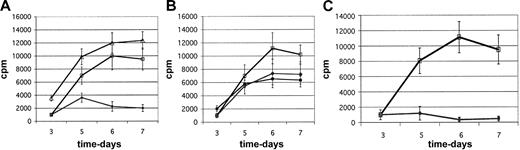
The majority of cell lines derived from CD4+CD25+ cells (19 of 25, 76%) had clear suppressive function (> 65% inhibition of proliferation) at a suppressor-responder ratio of 1:2 (Figure 3A). In contrast, cell lines derived from CD4+CD25- cells were found invariantly to augment the MLR (Figure 3A). The remaining CD4+CD25+-derived cell lines (6 of 25, 24%) had weak suppressive function (20%-65% inhibition of proliferation). However, none augmented the MLR (Figure 3B). Consistent with prior observations,32 freshly isolated MACS-purified CD4+CD25+ cells have only modest suppressive activity in these MLR assays, equivalent to the weak suppressor cell lines (20%-65% inhibition of proliferation; Figure 3B). Importantly, 9 (47%) of 19 of the suppressive cell lines had potent suppressor activity, and these cell lines almost completely inhibit MLR cultures (> 90% inhibition of proliferation; Figure 3C). The level of suppressive activity was an intrinsic characteristic of each line, in that weak or potent suppressive cell lines had consistently similar activity when tested in multiple independent MLR experiments over several weeks of analysis (see “Characterization of suppressor cell function in MLR assays”).
Purified cultured CD4+CD25+ cells markedly suppress MLRs. The MLR cultures contain various test-cell populations at a 1:2 suppressor-responder cell ratio. Shown are kinetic curves of proliferation over a 1-week MLR. Cultures were pulsed daily with 3H-thymidine for last 16 hours of culture. (A) Representative cell lines derived from CD4+CD25+ cells (double-column lineage-depletion purification) are good suppressors (•). In contrast, cell lines derived from CD25- cells (▵) augment the MLR versus control MLR cultures (□). Results are representative of 22 experiments. (B) Fresh standard MACS-purified CD4+CD25+ cells (♦), added to the MLR, compared with a representative weakly suppressive CD4+CD25+ cell line (•) versus control MLR (□). Results are representative of 4 experiments. (C) The MLR was nearly completely blocked by addition of potently suppressive cultured CD4+CD25+ cells (•) versus control MLR (□). Representative of 7 potent suppressor cell lines, tested in 14 MLRs. Error bars represent 1 SD above and below the mean.
Purified cultured CD4+CD25+ cells markedly suppress MLRs. The MLR cultures contain various test-cell populations at a 1:2 suppressor-responder cell ratio. Shown are kinetic curves of proliferation over a 1-week MLR. Cultures were pulsed daily with 3H-thymidine for last 16 hours of culture. (A) Representative cell lines derived from CD4+CD25+ cells (double-column lineage-depletion purification) are good suppressors (•). In contrast, cell lines derived from CD25- cells (▵) augment the MLR versus control MLR cultures (□). Results are representative of 22 experiments. (B) Fresh standard MACS-purified CD4+CD25+ cells (♦), added to the MLR, compared with a representative weakly suppressive CD4+CD25+ cell line (•) versus control MLR (□). Results are representative of 4 experiments. (C) The MLR was nearly completely blocked by addition of potently suppressive cultured CD4+CD25+ cells (•) versus control MLR (□). Representative of 7 potent suppressor cell lines, tested in 14 MLRs. Error bars represent 1 SD above and below the mean.
Characterization of CD4+CD25+ and CD4+CD25- cell lines
Potent suppressor cell lines were cultured in parallel with CD4+ CD25--derived cell lines from the same individual, which served as conventional T-cell controls. The weakly suppressive cell lines were also characterized and compared to determine the distinguishing characteristics versus the potently suppressive cell lines. Interestingly, it was often possible to predict suppressor function based simply on growth characteristics in culture, where the most rapidly growing CD4+CD25+ lines generally had the least suppressive function (not shown).
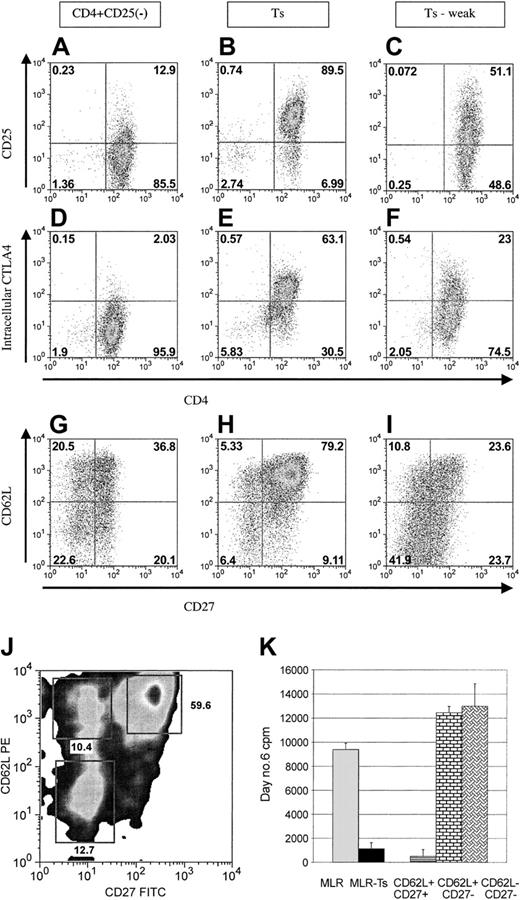
All cell lines initially expressed a somewhat typical activated T-cell phenotype after stimulation with the 3/28 beads. The cell lines transiently expressed relatively equivalent amounts of activation antigens, which quickly diminished over the 2 to 3 weeks after activation. These include CD122, CD132, GITR, OX40 (CD134), and cell surface CTLA4 (CD152). However, after several weeks of culture, when the cells become relatively quiescent (maintained in IL-2), the phenotypes become clearly divergent. Compared with T-cell lines derived from CD25- cells, the strongly suppressive Treg cell lines expressed higher levels of CD25, and the elevated expression was sustained. The levels after 3 weeks of culture (mean fluorescence intensity [MFI] 22 versus 210) are shown (Figure 4A-B). In addition, intracellular staining for CTLA4 demonstrated enhanced expression in the suppressor cell lines (MFI 8 versus 64; Figure 4D-E). The differences in CD25 and intracellular CTLA4 expression were the most distinct phenotypic characteristics identified in our studies that distinguish conventional versus CD25+-derived cell lines. The weakly suppressive cell lines expressed intermediate amounts of these 2 key descriptive antigens (Figure 4C,F).
Flow cytometric comparison of CD25+ versus CD25- cell lines. After 3 to 4 weeks of culture expansion, antigen expression was profiled by FACS analysis. Shown are representative plots of CD25--derived cell lines compared with potent suppressor cell lines and weakly suppressive cell lines. (A) Cell lines derived from CD25- cells express low levels of CD25 as they revert to a more quiescent state. (B) Potent suppressor cell lines maintain high levels of CD25 expression. (C) Cell lines derived from CD4+CD25+ cells that have weak suppressor function express intermediate levels of CD25. (D) CD4+CD25--derived cell lines express minimal intracellular CTLA4. (E) Potent suppressor cell lines maintain high intracellular expression of CTLA4. (F) Weak suppressor lines express intermediate levels. (G) CD25--derived cell lines express variable levels of CD62L and diminished CD27. (H) Potent suppressor cell lines contain a higher percentage of cells that express both CD62L and CD27. (I) Weak suppressor cell lines contain a lower percentage of cells that express CD62L and CD27. (J-K) Cell sorting of suppressor cell line subsets reveals potent suppressor cells to express CD62L and CD27. (J) FACS plot showing sorting gates for CD62L and CD27 subsets. (K) Functional analysis in MLRs reveals suppressor activity solely within the CD62L and CD27 double-positive subset (▤). Control MLR cultures (▦) and suppressed MLR (▪). The CD62L+/CD27- subset (brick bar) and the CD62L-/CD27- subset (weaved bar) both augment the MLR. Error bars represent 1 SD above and below the mean.
Flow cytometric comparison of CD25+ versus CD25- cell lines. After 3 to 4 weeks of culture expansion, antigen expression was profiled by FACS analysis. Shown are representative plots of CD25--derived cell lines compared with potent suppressor cell lines and weakly suppressive cell lines. (A) Cell lines derived from CD25- cells express low levels of CD25 as they revert to a more quiescent state. (B) Potent suppressor cell lines maintain high levels of CD25 expression. (C) Cell lines derived from CD4+CD25+ cells that have weak suppressor function express intermediate levels of CD25. (D) CD4+CD25--derived cell lines express minimal intracellular CTLA4. (E) Potent suppressor cell lines maintain high intracellular expression of CTLA4. (F) Weak suppressor lines express intermediate levels. (G) CD25--derived cell lines express variable levels of CD62L and diminished CD27. (H) Potent suppressor cell lines contain a higher percentage of cells that express both CD62L and CD27. (I) Weak suppressor cell lines contain a lower percentage of cells that express CD62L and CD27. (J-K) Cell sorting of suppressor cell line subsets reveals potent suppressor cells to express CD62L and CD27. (J) FACS plot showing sorting gates for CD62L and CD27 subsets. (K) Functional analysis in MLRs reveals suppressor activity solely within the CD62L and CD27 double-positive subset (▤). Control MLR cultures (▦) and suppressed MLR (▪). The CD62L+/CD27- subset (brick bar) and the CD62L-/CD27- subset (weaved bar) both augment the MLR. Error bars represent 1 SD above and below the mean.
To determine further differences between the weakly and potently suppressive cell lines, additional cell surface antigen analysis was undertaken. We noted a correlation of 3 antigens (CD62L, CCR7, and CD27) that were expressed on a higher percentage of cells in the potent suppressor cell lines compared with weak lines (Figure 4G-I). To further evaluate for functional relevance, we used magnetic beads to isolate CD62L+ or CD27+ cells (the brightest 2 antigens) and found enrichment for suppressor activity in both of the positive subsets (not shown). To more definitively determine the function of these cell line subsets, cell lines were sorted for CD62L+/CD27+, CD62L+/CD27-, and CD62L-/CD27+ cells (Figure 4J) and each population was tested for suppressor activity in MLRs. Suppressor function was solely within the CD62L+/CD27+ subset. In contrast, the other subsets were found to augment the MLR (Figure 4K). Thus, CD4+CD25+ cell lines can contain mixtures of suppressive and nonsuppressive cells, and the suppressive effects can be dominant over the augmenting effects of the nonsuppressors. CD62L and CD27 coexpression can be used to distinguish these subsets and facilitate selection of cell lines (or cell line subsets) with potent suppressive potential.
Characterization of suppressor cell function in MLR assays
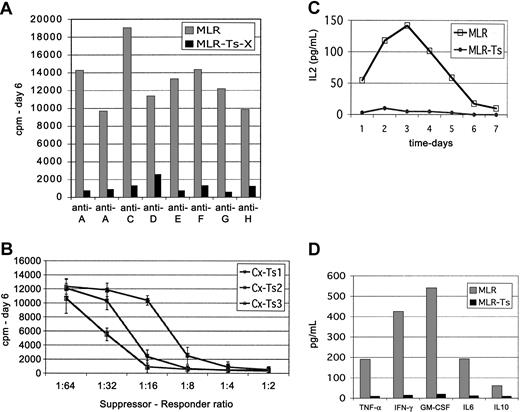
To determine the cellular mechanism of suppression in the MLR assays, potent cell lines (> 90% day 6 MLR suppression) were selected for further analysis. They were first shown to be consistently suppressive and then titered to determine minimum number required for potent suppression. To determine how broadly reactive the cultured Treg cells are, several independent lines were tested for suppression in 8 separate HLA-mismatched MLR cultures. In all cases, the cultured Treg cells markedly suppressed all MLR cultures analyzed (Figure 5A). Although there was some variation in the magnitude of suppression (mean, 92%; range, 81%-98%; n = 16), potent Treg cell lines were effective in inhibiting almost all MLR cultures. To quantitate the minimum number of suppressor cells required for potent inhibition, titrated numbers of suppressor cells were added to indicator MLR cultures. The titration curves (Figure 5B) revealed an approximate break point at a suppressor-responder ratio of less than 1:10 (5000 suppressors to 50 000 responders). The titration curves were nonlinear, possibly indicating cooperative effects in the overriding of suppression with low Treg cell dose.
Cultured CD4+CD25+ cells consistently and markedly suppress MLR proliferation and cytokine secretion. Potent CD25+ suppressor cell lines were tested in multiple MLRs from various unrelated donors. (A) Shown are 8 separate MLRs displaying variance of control and suppressed proliferation. Most all donor combinations were markedly impaired. Control MLR cultures (▦) and suppressed MLR (▪). Results are representative of more than 20 experiments with 7 different potent suppressor cell lines. (B) Graded numbers of potent cultured suppressor cells were added to the MLR to determine the minimum number needed for inhibition. Up to a 1:16 dilution (roughly 3125 suppressors) still would markedly impair the MLR when using the most potent suppressor cell lines. Three plots are shown representative of 6 potent suppressor cell lines. Error bars represent 1 SD above and below the mean. (C) Daily assessment of IL-2 levels in culture supernatant reveals profound block in IL-2 accumulation in suppressed MLR cultures (•) versus control MLR cultures (□). Representative of 4 MLRs. (D) Assessment of other cytokines produced by activated T cells reveals profound impairment of accumulation. Significant levels of TNF-α, IFN-γ, GM-CSF, IL-6, or IL-10 are not produced. Shown are levels on day 6, peak of accumulation in control MLR cultures (▦) versus suppressed MLR cultures (▪). Representative of 4 MLRs.
Cultured CD4+CD25+ cells consistently and markedly suppress MLR proliferation and cytokine secretion. Potent CD25+ suppressor cell lines were tested in multiple MLRs from various unrelated donors. (A) Shown are 8 separate MLRs displaying variance of control and suppressed proliferation. Most all donor combinations were markedly impaired. Control MLR cultures (▦) and suppressed MLR (▪). Results are representative of more than 20 experiments with 7 different potent suppressor cell lines. (B) Graded numbers of potent cultured suppressor cells were added to the MLR to determine the minimum number needed for inhibition. Up to a 1:16 dilution (roughly 3125 suppressors) still would markedly impair the MLR when using the most potent suppressor cell lines. Three plots are shown representative of 6 potent suppressor cell lines. Error bars represent 1 SD above and below the mean. (C) Daily assessment of IL-2 levels in culture supernatant reveals profound block in IL-2 accumulation in suppressed MLR cultures (•) versus control MLR cultures (□). Representative of 4 MLRs. (D) Assessment of other cytokines produced by activated T cells reveals profound impairment of accumulation. Significant levels of TNF-α, IFN-γ, GM-CSF, IL-6, or IL-10 are not produced. Shown are levels on day 6, peak of accumulation in control MLR cultures (▦) versus suppressed MLR cultures (▪). Representative of 4 MLRs.
Proliferation was nearly completely impaired in suppressed MLRs at all time points, suggesting that the suppressor effects occur within the first 3 days, that is, prior to the proliferative burst in MLRs. To evaluate for earlier effects and search for potential regulatory cell deviation of the quality of immune response, MLR supernatants were evaluated for cytokine content. There was a profound suppression of cytokine accumulation. Suppressed MLRs make a minimally detectable small early wave of IL-2 (at the threshold of sensitivity of assay), with no late production detectable (Figure 5C). Because the control MLR manifested IL-2 accumulation in the supernatant, even 1 day after initiation of culture, the suppressor effect was detectable and already profound as early as the first day of the suppressed MLR. In addition, accumulation of late cytokines (peaks typically day 5-7), such as TNF-α, interferon γ (IFN-γ), GM-CSF, and IL-6, was nearly completely prevented throughout the culture (Figure 5D). There was no induction of IL-4 or IL-10 to indicate deviation to a TH2 or T-regulatory type 1 (Tr1/IL-10 producing) differentiation. In fact, IL-10 accumulation is prevented as well.
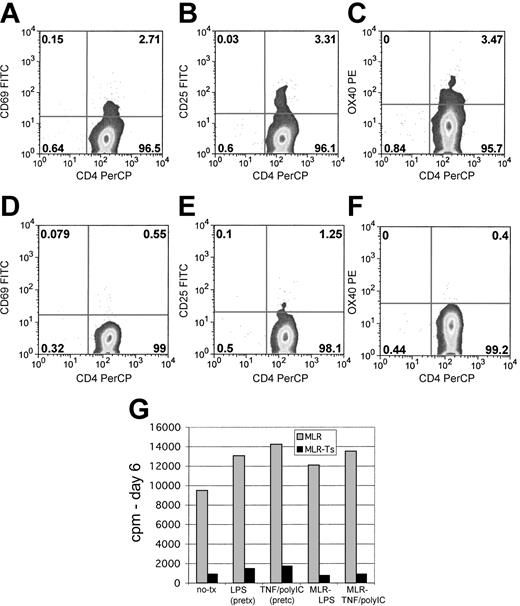
To examine responder T-cell reactivity early in the MLR, cells were assayed by flow cytometry for activation marker expression. Using responder cells derived from an HLA-A2+ donor, and suppressor cells derived from an HLA-A2- donor, the responding cells could be distinguished during coculture. Control and suppressed MLR cells were evaluated 24 or 48 hours after initiation of culture for induction of CD69, CD25, and OX40 (CD134). In the control MLR, the 2% to 4% of responder T cells showed expression of these activation antigens (Figure 6A-C), consistent with the expected alloreactive T-cell frequency for HLA-mismatched MLRs. Notably, in the suppressed MLRs very few responder T cells showed expression of these activation antigens (Figure 6D-F). These data further suggest a very early block in T-cell activation as a mechanism of Treg cell action.
Cultured Treg cells impair the activation of responding T cells and can suppress MLRs driven by mature DCs. MLRs were evaluated for expression of activation antigens after 1 day of culture. Shown are control MLRs stained for CD69 (A), CD25 (B), and OX40 (CD134; C). In suppressed MLRs responding T cells were first gated on HLA-A2 to distinguish them from the HLA-A2- suppressor cells. Shown are responder cells from suppressed MLRs stained for CD69 (D), CD25 (E), and OX40 (CD134; F). Results are representative of 3 experiments. (G) Maturation of DCs prior to MLR by LPS or TNF/poly (I:C) combination, or inclusion of these stimulating factors in MLRs fails to bypass suppression. Control MLR cultures (▦) and suppressed MLR (▪).
Cultured Treg cells impair the activation of responding T cells and can suppress MLRs driven by mature DCs. MLRs were evaluated for expression of activation antigens after 1 day of culture. Shown are control MLRs stained for CD69 (A), CD25 (B), and OX40 (CD134; C). In suppressed MLRs responding T cells were first gated on HLA-A2 to distinguish them from the HLA-A2- suppressor cells. Shown are responder cells from suppressed MLRs stained for CD69 (D), CD25 (E), and OX40 (CD134; F). Results are representative of 3 experiments. (G) Maturation of DCs prior to MLR by LPS or TNF/poly (I:C) combination, or inclusion of these stimulating factors in MLRs fails to bypass suppression. Control MLR cultures (▦) and suppressed MLR (▪).
The potency of suppression was remarkably retained if the DCs stimulating the MLRs were activated or matured. Maturation/activation of DCs with lipopolysaccharide (LPS), a TLR4 ligand, or the combination of TNF/poly (I:C), a double-stranded RNA analog-TLR 3 ligand,41,42 did not lead to bypass of suppression (Figure 6G). Inclusion of LPS or TNF/poly (I:C) in the MLR culture also did not bypass suppression. Thus, the cultured human Treg cells were very potent, and activated DCs, which express abundant costimulatory molecules and cytokines, were not able to bypass their suppressive effect.
The potent and early inhibition of MLRs suggests APC inactivation or elimination as a possible mechanism of suppression. However, by microscopic evaluation, the DCs appear to persist for the duration of culture. In addition, the suppressor cell lines did not have cytotoxicity for allogeneic DCs (Figure 7A) or NK/LAK-sensitive targets (K562; Figure 7B) in chromium-release assays.
Functional analysis of suppression in MLRs. (A) Suppressor cell lines lack significant cytotoxicity for DCs in chromium release assays (•). Control lysis mediated by NK cell line NK92 (▪); 5000 labeled targets were used, with up to a 20:1 effector-target ratio. (B). Suppressor cell lines lack NK or lymphokine-activated killing (LAK)-type activity and show no lytic activity against K562 in chromium release assays (•). Control lysis mediated by NK cell line NK92 (▪); 5000 labeled targets were used, with up to a 20:1 effector-target ratio. (C) In MLR assays, neutralizing antibodies to immunosuppressive factors IL-10 and TGF-β, as well as anti-IL-10R, or combinations of all 3 fail to reverse suppression mediated by cultured suppressor cell lines. (D) Potent suppressor cell lines have minimal inhibitory activity added to MLRs driven by DCs that are autologous to the suppressor (and allogeneic to the responder). Suppressor cell line Ts-A (▤) was used to suppress MLR cultures driven by DC-A (from the same donor as the suppressor) or by DC-B (from a different donor from suppressor). Suppressor cell line Ts-B (checkered bars) was also tested against DC-A and DC-B. Representative of 4 experiments.
Functional analysis of suppression in MLRs. (A) Suppressor cell lines lack significant cytotoxicity for DCs in chromium release assays (•). Control lysis mediated by NK cell line NK92 (▪); 5000 labeled targets were used, with up to a 20:1 effector-target ratio. (B). Suppressor cell lines lack NK or lymphokine-activated killing (LAK)-type activity and show no lytic activity against K562 in chromium release assays (•). Control lysis mediated by NK cell line NK92 (▪); 5000 labeled targets were used, with up to a 20:1 effector-target ratio. (C) In MLR assays, neutralizing antibodies to immunosuppressive factors IL-10 and TGF-β, as well as anti-IL-10R, or combinations of all 3 fail to reverse suppression mediated by cultured suppressor cell lines. (D) Potent suppressor cell lines have minimal inhibitory activity added to MLRs driven by DCs that are autologous to the suppressor (and allogeneic to the responder). Suppressor cell line Ts-A (▤) was used to suppress MLR cultures driven by DC-A (from the same donor as the suppressor) or by DC-B (from a different donor from suppressor). Suppressor cell line Ts-B (checkered bars) was also tested against DC-A and DC-B. Representative of 4 experiments.
To determine if known immunosuppressive factors were mediating the action of the cultured Treg cells, antibodies capable of neutralizing IL-10 or TGF-β were added to control and suppressed MLR cultures. Because of the potency of the suppressive effect, lower numbers of suppressors were added to make the assay more sensitive to reversal of suppression. Despite this, antibodies reactive with IL-10, IL10R-α, or TGF-β-1, -2, -3 failed to reverse suppression and the inclusion of all 3 antibodies together had a very modest effect (Figure 7C). Doses of 1, 10, or 20 μg/mL were tested with the effects only noted at the highest dose levels. In addition, transwell studies were undertaken to determine if soluble factors released by Treg cells could convey suppression. Indicator MLR cells were cultured above resting or activated Treg cells or suppressed MLR cultures, separated by membranes with 0.4-μm pores. No suppressive effect was found to pass through the membrane (not shown).
Importantly, when cultured Treg cells were added to allo-MLRs driven by APCs that were derived from the same donor as the suppressors, but still allogeneic to responder T cells, minimal suppression was noted (Figure 7D). Thus, when cultured Treg cells derived from donor A are added to an allo-MLR driven by DCs from the same donor A, there is minimal suppression. However, when these same Treg cells are added to an MLR driven by DCs from donor B, there is suppression. These results indicate that the cultured Treg cells are not constitutively suppressive of all MLRs. The cultured Treg cells need some form of specific additional stimulation that can be provided by allo-DCs (and not by autologous DCs).
Discussion
In this study, we demonstrate that suppressor cells can be isolated and expanded in culture from human blood. Importantly, these cultured Treg cells can be expanded over 100-fold and, when pure, express enhanced and potent suppressive activity. We have characterized the suppressive function of these cells in MLR assays and shown that they can nearly completely block HLA-mismatched MLRs. These culture-expanded suppressor cells have many of the hallmark features of the freshly isolated CD4+CD25+ Treg cells. They highly express CD25 and cytoplasmic CTLA4, their activity is dependent on cell-cell contact, and suppression does not seem to be dependent on cytolytic activity or on the immunosuppressive cytokines IL-10 or TGF-β.
Prior work has noted the need in the human system of isolating the CD25+bright subset of CD4+CD25+ cells to detect suppressor activity (with freshly isolated cells) in antibody-based coculture assays.28 We have found a similar situation in our culture system, where the most stringently purified CD4+CD25+ cells form the best suppressor cell line precursors. Contaminating CD25dim cells in CD25+ fractions can grow faster and overgrow the CD25+bright cells, and thereby preclude the full manifestation of suppressor cell function. Thus, we emphasized stringent purification. We found that lower titers of anti-CD25 magnetic microbeads (one fifth of the manufacturer's recommendation) and a repurification over a second column greatly facilitated the generation of Treg cell lines with potent suppressive capabilities. The use of even lower titers of anti-CD25 mAb-coated magnetic micobeads or addition of a third column step to the purification did not significantly improve results and had the disadvantage of decreasing yields.
To our knowledge, these data are the first to demonstrate the feasibility of anti-CD3/28 mAb-coated bead-based approach for Treg cell activation and expansion. When anti-CD28 is combined with anti-CD3 mAb a more efficient expansion strategy results. There was some initial concern that anti-CD28 mAb costimulation may lead to reversal of suppression, as noted in short-term murine suppressor assays.17,19 Our findings indicate that anti-CD28 mAb-mediated expansion of human Treg cells does not impair suppressor ability directly over the long-term and may actually increase it. In addition, we and others have recently demonstrated that soluble anti-CD28 mAb does not lead to bypass of suppression in anti-CD3 mAb-based suppression assays in the human system (not shown).14 Interestingly, in the rat, anti-CD28 superagonist mAbs (particular epitope-specific anti-CD28 mAbs alone signal as a mitogen, and not just as a costimulator) alone can preferentially enhance expansion of CD4+CD25+ cells in vivo.43 Thus, CD28 signals may not only be important for homeostatic expansion of Treg cells but may also be used to overdrive their accumulation. Despite anti-CD3/CD28 mAb-coated bead-based and IL-2-supplemented activation, stringently purified CD4+CD25+ cells (or FACS sorted CD4+CD25+bright cells) did not grow well. Growth was better with the addition of CD4+CD25- feeders, which provide IL-2 and other growth factors.
CD4+CD25+ cell line growth and suppressor function were variable and inversely correlated. We favor the interpretation that cell lines with poor suppressor function were the result of inclusion of small numbers of conventional T cells in the CD4+CD25+ cultures. Cell lines derived from more stringently purified cells did not grow as well but were more potent suppressors. In addition, the immunophenotype of the weakly suppressive cell lines (CD25 moderate and split CD62L or CD27) was consistent with a mixed population of conventional and suppressive cells. Interestingly, after culture, the functionally active suppressor cells were exclusively within the CD62L+ and CD27+ double-positive population, and the potent suppressor cell lines nearly uniformly expressed these antigens.
In our functional analysis we demonstrated that the potent cultured Treg cells can nearly completely block an allo-MLR. With these cell lines, the marked suppressive effects could occur with as little as 3125 suppressors (1:16 ratio) in a culture of 50 000 responding CD4+ T cells and 5000 DC stimulators. These profound effects are more potent than what has previously been reported for freshly isolated32-34 or expanded human CD4+CD25+ cells.34 The potent suppressor cell lines prevented accumulation of all the cytokines tested that were produced in control cultures (IL-2, TNF-α, IFN-γ, GM-CSF, IL-6, and IL-10). The block in T-cell activation appeared to occur very early because a marked suppressive effect on activation antigen expression and IL-2 accumulation was clearly evident on the first day of culture. This suggests the hypothesis that Treg function in MLR cultures predominantly reflects impairment of T-cell activation.
Surprisingly, the activation or maturation of the DCs did not lead to the bypass of suppression. This finding is in contrast to what has been recently reported for freshly isolated murine Treg cells, where LPS or CpG-containing DNA oligodeoxynucleotide-mediated signaling of spleen-derived DCs led to the bypass of suppression.44 However, in that system the Treg cells were not culture activated and expanded. In this system, the activated and expanded human Treg cells can override the cytokines and costimulatory molecules expressed by activated DCs and still block the MLR response. The finding of increased potency of suppressor function after culture is consistent with what has been shown with activation of murine Treg cells. Suppressor function is activation dependent,16 and short-term culture with anti-CD3 and IL-2 augments suppressive ability.20 We favor the interpretation that the cultured Treg cells are primed (more sensitive) to reactivation of T-cell receptor (TCR), and hence TCR-induced suppressor function is more readily expressed. IL-2 (in conjunction with TCR signaling) has also recently been shown to be important for up-regulation of suppressor function.45
Murine studies have revealed that under a number of conditions tested, freshly isolated or cultured Treg cells have been able to prevent GVHD and still allow for GVL activity,25-27 or even immune reconstitution.25 It has been suggested that Treg cells can inhibit alloreactive T-cell expansion and cytokine production (more important for GVHD induction) more so than CTL activity (more important for antitumor activity), and some data supporting this model have been presented.27 If these concepts and mechanisms accurately reflect human disease pathogenesis, then the provision of cultured Tregs to patients may provide for improved outcomes in BMT.
The availability of large numbers of cultured Treg cells will enable more detailed immunologic, biochemical, and molecular characterization of these important cells. In addition, because our procedure is adaptable for good manufacturing practice (GMP) conditions, clinical testing may soon be feasible, and cultured Treg cells may be useful as a novel form of immunosuppressive therapy.
Prepublished online as Blood First Edition Paper, March 18, 2004; DOI 10.1182/blood-2004-01-0151.
Supported in part by grants from the Vikings Children's Fund, American Cancer Society, Children's Cancer Research Fund, Leukemia and Lymphoma Society no. 6220-04 (W.R.G.) and National Institutes of Health grants R01 AI34495 and R37 HL56067 (B.R.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal