Abstract
Nitric oxide (NO) is a potent radical produced by nitric oxide synthase (NOS) and has pleiotrophic activities in health and disease. As mast cells (MCs) play a central role in both homeostasis and pathology, we investigated NOS expression and NO production in human MC populations. Endothelial NOS (eNOS) was ubiquitously expressed in both human MC lines and skin-derived MCs, while neuronal NOS (nNOS) was variably expressed in the MC populations studied. The inducible (iNOS) isoform was not detected in human MCs. Both growth factor-independent (HMC-1) and -dependent (LAD 2) MC lines showed predominant nuclear eNOS protein localization, with weaker cytoplasmic expression. nNOS showed exclusive cytoplasmic localization in HMC-1. Activation with Ca2+ ionophore (A23187) or IgE-anti-IgE induced eNOS phosphorylation and translocation to the nucleus and nuclear and cytoplasmic NO formation. eNOS colocalizes with the leukotriene (LT)-initiating enzyme 5-lipoxygenase (5-LO) in the MC nucleus. The NO donor, S-nitrosoglutathione (SNOG), inhibited, whereas the NOS inhibitor, NG-nitro-l-arginine methyl ester (L-NAME), potentiated LT release in a dose-dependent manner. Thus, human MC lines produce NO in both cytoplasmic and nuclear compartments, and endogenously produced NO can regulate LT production by MCs. (Blood. 2004;104: 462-469)
Introduction
Mast cells (MCs) are tissue-resident effector cells that arise from bone marrow precursors.1 They produce and secrete numerous bioactive agents and have been implicated in diverse homeostatic functions such as angiogenesis, wound healing, and tissue remodeling.2 Due to their plethora of mediators and strategic localization, MCs also play central roles in various disease states, including multiple sclerosis and T-helper type 2 (TH2)-driven inflammatory conditions such as asthma.3,4
Nitric oxide (NO) is a potent radical with diverse roles in regulating cellular activation.5 NO is derived from L-arginine by the nitric oxide synthase (NOS) family of enzymes. The Ca2+-dependent members include endothelial (eNOS) and neuronal (nNOS), characterized by constitutive expression and low NO production. Inducible NOS (iNOS) is up-regulated by a variety of inflammatory mediators and functions independently of cellular Ca2+ levels and releases large amounts of NO.6 Numerous investigators have shown that rodent MCs are regulated by endogenous NO from both constitutive and inducible sources.7,8 However, little is known about the production of NO by human MCs or the potential involvement of NO as modulator of human MC function.
The aim of this study was to investigate the expression of NOS and production of NO in human MCs. Furthermore, we studied the regulation of NOS and the resulting functional effects on the release of leukotrienes. Our results indicate that NO may be a novel modulator that determines the functional phenotype of human MCs.
Materials and methods
Reagents
The NOS inhibitor NG-nitro-l-arginine methyl ester (L-NAME), inactivate enantiomer NG-nitro-d-arginine methyl ester (D-NAME), and NO donor S-nitrosoglutathione (SNOG) were obtained from Calbiochem (San Diego, CA). DAF-FM (4,5-diaminofluorescein diacetate) was obtained from Molecular Probes (Eugene, OR). Rabbit polyclonal antibodies against nNOS, iNOS, and eNOS were obtained from Santa Cruz (Santa Cruz, CA), and mouse monoclonals were from BD Transduction Laboratories (San Diego, CA). Rabbit polyclonal antibody against phosphorylated eNOS (Ser1177) was from Upstate Biotechnology (Lake Placid, NY), and rabbit anti-5-LO was from Cayman Chemical (Ann Arbor, MI).
Cell lines
HMC-1, an immature human MC line (a kind gift from J. H. Butterfield, Minneapolis, MN), was cultured in Iscove medium (Life Technologies, Grand Island, NY) with 10% fetal bovine serum (FBS), 2 mM glutamine, 40 U/mL penicillin/streptomycin, and 1.0 mM thioglycerol. The human basophilic leukemia cell, KU812 (a kind gift from J. S. Marshall, Halifax, NS, Canada), was cultured in RPMI 1640 (Life Technologies) supplemented with 10% FBS, 2 mM glutamine, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 40 U/mL penicillin/streptomycin. KU812 cells were differentiated by culture with 0.3 mM sodium butyrate for 3 days before use. As a source of in vitro-derived mature human MCs, the LAD 2 growth factor-dependent human MC line (obtained from Dr A. S. Kirshenbaum and D. D. Metcalfe, National Institutes of Health, Bethesda, MD)9 was cultured in StemPro-34 medium (Life Technologies) supplemented with 100 ng/mL recombinant human stem cell factor (rhSCF; Peprotech, Rocky Hill, NY). LAD 2 cells were subcultured every 7 days. The LAD 2 cells are homologous to other sources of in vitro-derived normal mature human MCs, including CD34 derived, and are used as an alternative to primary human MCs, as they can be activated by IgE cross-linking.9 All cells were maintained at 37°C in a humidified incubator at 5% CO2.
Human skin mast cell isolation
Human skin MCs (HSMCs) were dispersed from 5 foreskin samples (mean age, 2.4 ± 0.7 years, wet weight 0.7 ± 0.1 g) as previously described.10 Briefly, dissected samples were incubated for 1 hour at 37°C in collagenase (318 U/mL; Life Technologies). Cells were passed through nylon gauze (150 μm) and washed with complete RPMI 1640 (150 g, 10 minutes). MCs were purified by positive magnetic selection using monoclonal anti-c-kit antibody (15 μg/mL; YB5.B8; BD Pharmingen, Mississauga, ON, Candada) and goat anti-mouse Ig-coated magnetic beads (15 μg/mL; Miltenyi Biotec, Sunnyvale, CA) as described.11 The average yields were 4.5 ± 0.7 × 105 MCs per foreskin sample with 93% ± 2.1% purity and 94% ± 1.3% viability assessed after staining with Kimura or trypan blue, respectively.
Sensitization of human mast cells
LAD 2 cells were sensitized with human IgE (2 μg/mL; Serotec, Raleigh, NC) for 2 hours at 37°C. Cells were washed and resuspended in HEPES-buffered Tyrode solution, then stimulated with mouse anti-human IgE antibody (1 μg/mL; Serotec) for 30 minutes.
Reverse-transcriptase polymerase chain reaction
Total RNA was isolated from MCs as previously described.12 One microgram of RNA was converted to cDNA by reverse transcription reaction (M.MLv reverse transcriptase; Invitrogen, Burlington, ON, Canada) in a total volume of 20 μL. Polymerase chain reaction (PCR) amplification was performed on a PTC-100 Thermal Cycler (MJ Research, Boston, MA). The primers were designed to be intron-spanning based on published sequence data: nNOS, sense 5′-AAGGGCAATGTGCCTGTCGT-3′, antisense 5′-ATTGCCGTTGGCCTGAAGCA-3′ (PCR product, 826 bp); iNOS, sense 5′-AGTTTCTGGCAGCAACGG-3′, antisense 5′-TTAAGTTCTGTGCCGGCAG-3′ (PCR product, 532 bp); eNOS, sense 5′-ACCTGCAAAGCAGCAAGTCCACG-3′, antisense 5′-CCGAACACCAAAGTCATGGGAGT-3′ (PCR product, 837 bp). PCR conditions were as follows: denaturing at 95°C for 45 seconds, annealing for 45 seconds, and extension at 72°C for 1 minute. The optimized annealing temperature was 49°C for nNOS, 47°C for iNOS, and 52°C for eNOS. Products were run on a 1.2% agarose gel and stained with ethidium bromide (Sigma, St Louis, MO). RNA for positive controls was from human brain (Ambion, Houston, TX) and cultured human endothelial cells.
Cloning and sequencing of cDNA bands
The amplified PCR products were subcloned into pCR2.1 plasmid vector using the T/A cloning kit (Invitrogen). Double-stranded DNA sequencing was conducted using an ABI 373A automated sequencer (Applied Biosystems, Foster City, CA).
Western blot
MCs were incubated in 24-well plates at 1 × 106 cells/well. Cells were dissociated in radioimmunoprecipitation assay (RIPA) buffer (phosphate-buffered saline [PBS], 1% NP-40). The total protein content was determined by the Bradford technique (Bio-Rad, Hercules, CA). Protein samples (15 μg) were mixed with Lamelli buffer and separated on a 6% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride membrane (Bio-Rad). The membrane was incubated with primary antibodies for 1 hour at room temperature. Dilutions of the primary antibodies are polyclonal nNOS (1:1000), iNOS (1:500), eNOS (1:500), phospho-eNOS (1:500), and 5-LO (1:1000). The secondary antibody, 1:5000 horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG (Serotec) was added and incubated for 1 hour at room temperature. Labeling was detected by chemiluminescence by addition of SuperSignal substrate solution (Pierce, Rockford, IL). The resulting bands were scanned and quantified in a gel scanner (ImageMaster DTS; Pharmacia, Uppsala, Sweden).
Subcellular fractionation
HMC-1 cells were fractionated as previously described.13 Briefly, cells were pelleted by centrifugation (150g, 5 minutes) then resuspended in lysis buffer (10 mM HEPES, 2 mM MgCl2, 15 mM KCl, 0.1 mM EDTA [ethylenediaminetetraacetic acid], and 0.2% Nonidet P-40 [NP-40]) with protease inhibitors for 15 minutes on ice. Cells were ruptured with a Dounce homogenizer, then centrifuged 10 000g for 20 minutes. The supernatant was frozen as the cytoplasmic fraction. The pellet was resuspended in extraction buffer (20 mM HEPES, 1.5 mM MgCl2, 0.4 M NaCl, 20% glycerol, 0.2% EDTA, and 0.2% NP-40), then centrifuged at 10 000g for 20 minutes to produce a nuclear fraction. The purity of nuclear extracts was tested by Western blot using an α-tubulin antibody (Molecular Probes). All nuclear preps used in this study were negative for this cytoskeletal protein, indicating minimal cytoplasmic contamination.
Confocal microscopy
Localization of NOS in MCs was performed on HMC-1 or LAD 2 cells fixed in 4% paraformaldehyde for 20 minutes, then permeabilized with 0.1% Triton X-100 in PBS. All slides were incubated overnight at 4°C with primary antibody. Specific antibody binding was detected with rhodamine red-labeled goat antirabbit or 4,4-difluoro-4-bora-3a, 4a-diaza-s-indacene (BODIPY)-labeled rabbit antimouse secondary antibodies (Molecular Probes). Nuclei were stained with propidium iodide (PI; 500 nM; Molecular Probes). Negative controls with rabbit serum or mouse isotype immunoglobulin were run concurrently. To insure specificity in colocalization studies, further controls included cross-checking labeled secondary antibodies with the above control immunoglobulins. All cell images were obtained using a 40 × 1.3 oil Plan Neofluar objective on a Zeiss confocal microscope (Heidelberg, Germany) with laser scanning microscope (LSM) 510 software. Images were obtained using a Zeiss Axiocam HR. To determine nuclear colocalization of eNOS, fluorescein isothiocyanate (FITC)-stained images were combined with PI-stained images of the same cell.
Assay for NOS activity
To assess NO production in MCs, NOS activity in HMC-1 cells was measured by the conversion of l-[14C] arginine to l-[14C] citrulline, using a NOS assay kit (Calbiochem) according to manufacturer's procedures and as described previously.7 Each sample was run in the presence of EGTA (ethylene glycol tetraacetic acid) (2 mM) to determine the levels of calcium-dependent (constitutive) NOS activity. Calcium-independent (inducible) NOS activity was determined by subtracting the constitutive activity from the total NOS activity in the sample. The level of citrulline produced was expressed as pmol/min/mg of protein.
Diaminofluorescein assay for nitric oxide in human mast cells
NO production and localization in MCs were assayed using DAF-FM, a cell-permeable NO-sensitive fluorescent dye as previously described.14,15 In brief, MCs were loaded with 10 μM of DAF-FM for 1 hour at 37°C, then pipetted onto coverslip-bottomed petri dishes (Falcon BD Biosciences, San Jose, CA). Cells were stimulated by either A23187 (0.1 μM) for HMC-1 cells or IgE cross-linking (1 μg/mL) for LAD 2 cells. Images were obtained at 0 and 10 minutes using a Zeiss confocal laser scanning microscope. Images were collected using 488-nm (excitation) and 505- to 530-nm (emission) filter set, as outlined in the previous paragraph. In some experiments the NOS inhibitor L-NAME or the NO donor SNOG was added 30 minutes before loading with DAF, as negative and positive controls, respectively.
Enzyme-linked immunosorbent assay (ELISA) for cysteinyl leukotrienes
HMC or LAD 2 cells (1 × 106/mL) were stimulated with A23187 (0.1 μM) or anti-IgE (1 μg/mL), respectively, for 30 minutes at 37°C. Culture supernatants were collected and cysteinyl leukotrienes (LTC4, D4, E4, C5, D5, E5) quantified using Enzyme Immuno Assay (EIA; Cayman Chemical) according to the manufacturer's protocols.
Statistical analysis
Data were analyzed using analysis of variance (ANOVA) followed by the Bonferroni test for comparisons. P values less than .01 were considered significant.
Results
NOS mRNA expression by human mast cells
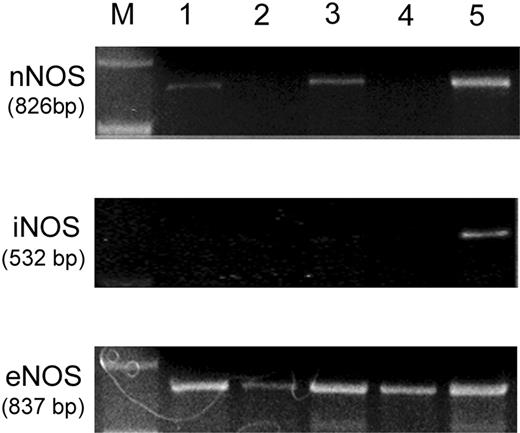
Few studies have looked at NOS expression in human MCs, and little data exists.16,17 Therefore, we characterized the expression of nNOS, iNOS, and eNOS mRNA in HMC-1, KU812, LAD 2, and HSMC cells using RT-PCR analysis. At 30 cycles all human MC types studied expressed eNOS at high levels (Figure 1), while iNOS expression was negative in all samples even when cycle numbers were increased to 45. RT-PCR studies in HMC-1 or LAD 2 cells after stimulation (15 minutes) with A23187 or IgE cross-linking, respectively, showed no iNOS production (data not shown). nNOS expression was variable, with KU812 and LAD 2 cells being negative while HMCs and HSMC were positive (Figure 1). However, nNOS levels were weaker when directly compared to eNOS at 30 cycles. NOS mRNA was detected with the appropriate controls; human brain RNA for nNOS and iNOS, and human endothelial cell RNA for eNOS. The identity of all products was confirmed by cloning and sequencing. All PCR products were absent when the RT step was eliminated (data not shown).
Expression of NOS isoforms in human mast cell populations. nNOS: RT-PCR analysis of nNOS mRNA in HMC-1 (lane 1), KU812 (lane 2), skin MCs (lane 3), and LAD 2 cells (lane 4). Human brain RNA was used as a positive control (lane 5). iNOS: RT-PCR analysis of iNOS mRNA in HMC-1 (lane 1), KU812 (lane 2), skin MCs (lane 3), and LAD 2 cells (lane 4). Human brain RNA was used as a positive control (lane 5). eNOS: RT-PCR analysis of eNOS mRNA in HMC-1 (lane 1), KU812 (lane 2), skin MCs (lane 3), and LAD 2 cells (lane 4). Human endothelial cell RNA was used as a positive control (lane 5). The sizes of expected amplified products are indicated. Molecular weight markers (M) are indicated. Results are representative of at least 3 independent RNA isolations.
Expression of NOS isoforms in human mast cell populations. nNOS: RT-PCR analysis of nNOS mRNA in HMC-1 (lane 1), KU812 (lane 2), skin MCs (lane 3), and LAD 2 cells (lane 4). Human brain RNA was used as a positive control (lane 5). iNOS: RT-PCR analysis of iNOS mRNA in HMC-1 (lane 1), KU812 (lane 2), skin MCs (lane 3), and LAD 2 cells (lane 4). Human brain RNA was used as a positive control (lane 5). eNOS: RT-PCR analysis of eNOS mRNA in HMC-1 (lane 1), KU812 (lane 2), skin MCs (lane 3), and LAD 2 cells (lane 4). Human endothelial cell RNA was used as a positive control (lane 5). The sizes of expected amplified products are indicated. Molecular weight markers (M) are indicated. Results are representative of at least 3 independent RNA isolations.
Confocal analysis of NOS expression
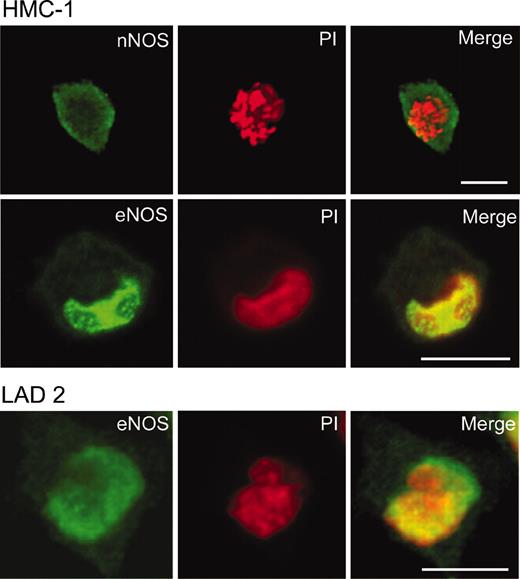
To determine if the expression of NOS mRNA could be correlated with the presence of NOS protein and to resolve the cellular localization patterns, laser scanning confocal microscopy using both polyclonal and monoclonal antibodies was performed. HMC-1 cells stained weakly for nNOS protein with a diffuse pattern present throughout the cytoplasm but with no nuclear staining (Figure 2). LAD 2 cells and KU812 were negative for nNOS as expected from the PCR results (data not shown). Strikingly, HMC-1 cells stained with anti-eNOS antibody showed weak positive staining in the cytoplasm, whereas the nucleus showed strong staining in most (96% ± 1.5%, n = 3) cells. LAD 2 cells showed nuclear positivity in fewer (87% ± 2.6%, n = 3) cells (Figure 2). iNOS protein was not detected in any cell type studied. Cells stained in parallel with nonimmune rabbit serum also were negative. The results with polyclonal anti-NOS antibodies were confirmed with monoclonal antibodies.
Localization of NOS in HMC-1 and LAD 2 cells. HMC-1 or LAD 2 cells were fixed in 4% paraformaldehyde and incubated with rabbit anti-nNOS antibody or rabbit anti-eNOS. Antibody labeling was detected with BODIPY-conjugated goat antirabbit antibodies (green). Nuclei of cells were detected with propidium iodide (red); overlapping fluorescence is indicated in yellow. Original magnification × 600; bar = 10 μm.
Localization of NOS in HMC-1 and LAD 2 cells. HMC-1 or LAD 2 cells were fixed in 4% paraformaldehyde and incubated with rabbit anti-nNOS antibody or rabbit anti-eNOS. Antibody labeling was detected with BODIPY-conjugated goat antirabbit antibodies (green). Nuclei of cells were detected with propidium iodide (red); overlapping fluorescence is indicated in yellow. Original magnification × 600; bar = 10 μm.
As eNOS has been shown to localize to Golgi, as well as to the perinuclear region in other cell types,18 colocalization in combination with the nuclear stain PI was used to confirm the nuclear eNOS staining. When the eNOS fluorescence signal was overlaid with the PI signal, there was strong colocalization in the nucleus/nuclear membrane as indicated by the yellow fluorescence in the merged image (Figure 2). Interestingly, analysis of Z-stack section images taken every 2 μM throughout the cells showed that the localization pattern was heterogeneous, with regions of high eNOS/DNA colocalization interspersed with regions of almost no colocalization.
NOS protein
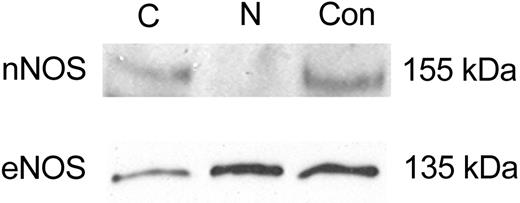
To further confirm the confocal localization results, Western blot analysis on highly enriched nuclear and cytoplasmic extracts were investigated. HMC-1 cells contained cytoplasmic and nuclear eNOS, confirming the subcellular eNOS localization (Figure 3). In addition, nNOS protein was abundant only in cytoplasmic fractions (Figure 3). Rehybridization of the Western blot with antibody against the cytoplasmic protein α-tubulin validated the fractionation procedure (data not shown).
Immunoblot analysis of NOS protein expression in subcellular fractions from HMC-1 cells. HMC-1 cells were lysed and separated into cytosolic (Cy) or nuclear (N) fractions as described in “Materials and methods”; proteins from each fraction were separated by SDS-PAGE under reducing conditions. Control (Con) proteins (human brain and endothelial cell lysate, respectively) were run concurrently. Representative immunoblot of nNOS and eNOS content in subcellular fractions. For all blots, equal amounts of protein (15 μg) were loaded. Molecular weight markers indicated at right. All blots are representative of at least 3 independent experiments.
Immunoblot analysis of NOS protein expression in subcellular fractions from HMC-1 cells. HMC-1 cells were lysed and separated into cytosolic (Cy) or nuclear (N) fractions as described in “Materials and methods”; proteins from each fraction were separated by SDS-PAGE under reducing conditions. Control (Con) proteins (human brain and endothelial cell lysate, respectively) were run concurrently. Representative immunoblot of nNOS and eNOS content in subcellular fractions. For all blots, equal amounts of protein (15 μg) were loaded. Molecular weight markers indicated at right. All blots are representative of at least 3 independent experiments.
NOS activity and NO formation in human MCs
We investigated whether HMC-1 exhibited NOS activity using the citrulline assay to determine the conversion of radioactive L-arginine. HMC-1 extracts showed significant citrulline generation (54.3 ± 8.6 pmol/min/mg) that could be attributed to cNOS (nNOS and/or eNOS) but not iNOS activity, as chelation of Ca2+ from the reaction mixture reduced citrulline formation (7.4 ± 5.1 pmol/min/mg) (Figure 4A). Interestingly, the levels of NOS activity in HMC-1 were similar to that seen in brain homogenates (rat) used as a positive control (Figure 4A). These results establish that human HMC-1 cells have the potential for cNOS activity.
NOS activity and nitric oxide localization in human mast cells. (A) NOS activity in HMC-1 cells, as measured using the [14C]-l-arginine conversion assay. Brain homogenate was used as a positive control. Each sample was run in the presence of EGTA (2 mM) to determine the levels of calcium-dependent (constitutive) NOS activity. The results are expressed as picomoles l-citrulline formed per minute, per milligram of protein. Data shown as mean ± SEM for 3 independent experiments. (B) Measurement of endogenous NO formation in HMC-1 and LAD 2 cells as detected by DAF fluorescence. Cells were loaded with DAF, basal fluorescence images were obtained, and then HMC-1 cells were stimulated with A23187 (0.1 μM) or LAD 2 cells stimulated by IgE cross-linking (1 μg/mL). Changes in fluorescence were captured using confocal microscopy 10 minutes after activation. Most HMC-1 cells (75% ± 6.3%; n = 4) were positive, whereas fewer LAD 2 cells (42% ± 1.6%, n = 3) were positive. White arrowheads show nuclear/perinuclear DAF fluorescence. White arrows indicate DAF-negative cells that also show cytoplasmic ruffling (HMC-1 and LAD 2) or granule release (LAD 2). Original magnification × 800; bar = 10 μm. Results are representative of at least 3 independent experiments.
NOS activity and nitric oxide localization in human mast cells. (A) NOS activity in HMC-1 cells, as measured using the [14C]-l-arginine conversion assay. Brain homogenate was used as a positive control. Each sample was run in the presence of EGTA (2 mM) to determine the levels of calcium-dependent (constitutive) NOS activity. The results are expressed as picomoles l-citrulline formed per minute, per milligram of protein. Data shown as mean ± SEM for 3 independent experiments. (B) Measurement of endogenous NO formation in HMC-1 and LAD 2 cells as detected by DAF fluorescence. Cells were loaded with DAF, basal fluorescence images were obtained, and then HMC-1 cells were stimulated with A23187 (0.1 μM) or LAD 2 cells stimulated by IgE cross-linking (1 μg/mL). Changes in fluorescence were captured using confocal microscopy 10 minutes after activation. Most HMC-1 cells (75% ± 6.3%; n = 4) were positive, whereas fewer LAD 2 cells (42% ± 1.6%, n = 3) were positive. White arrowheads show nuclear/perinuclear DAF fluorescence. White arrows indicate DAF-negative cells that also show cytoplasmic ruffling (HMC-1 and LAD 2) or granule release (LAD 2). Original magnification × 800; bar = 10 μm. Results are representative of at least 3 independent experiments.
Because of the large number of LAD 2 cells required for citrulline determinations and the slow growth rate of these cells in culture, we used the NO-specific fluorescent dye diaminofluorescein (DAF-2) combined with live-cell confocal analysis to directly measure the dynamic production and localization of NO in both LAD 2 and HMC-1 cells. We recently developed this methodology for analyzing NO production in rat MCs.14 Confocal images of DAF-loaded HMC-1 cells showed minimal fluorescence. Upon stimulation with A23187 (1 μM) (10 minutes), most (75% ± 6.3%, n = 4) HMC-1 cells showed a moderate increase in cytoplasmic fluorescence combined with pronounced nuclear localization (Figure 4B). No significant increase in intracellular fluorescence was seen in time control experiments or if HMC-1 cells were pretreated with NOS inhibitor L-NAME (data not shown).
In parallel experiments performed on IgE-sensitized LAD 2 cells we saw a similar pattern of NO formation following IgE cross-linking (42% ± 1.6%, n = 3) with similar rapid kinetics (< 10 minutes). Notably, the localization of NO was heterogenous, with some cells showing only cytoplasmic NO while others showed both cytoplasmic and nuclear positivity (Figure 4B). Thus, both HMC-1 and LAD 2 cells can produce NO rapidly (< 10 minutes) upon stimulation.
Interestingly, both HMC-1 and LAD 2 exhibited heterogenous NO formation with populations of NOHi and NOLow cells. Cells that were NOLow showed more pronounced cytoplasmic changes (eg, ruffling), with LAD 2 NOLow cells showing granule release compared to NOHi cells. These results are similar to that obtained with rat MCs.14
Activation and codistribution of eNOS and 5-LO
MCs can produce both LT and NO, and interplay between the 2 pathways has been demonstrated in other cell types.19,20 LT synthesis is initiated by the activation of 5-lipoxygenase (5-LO), which localizes to the nucleus in MCs.19 Since eNOS and 5-LO appear to reside in similar subcellular compartments and both require Ca2+ for activation, we investigated their distribution in MCs.
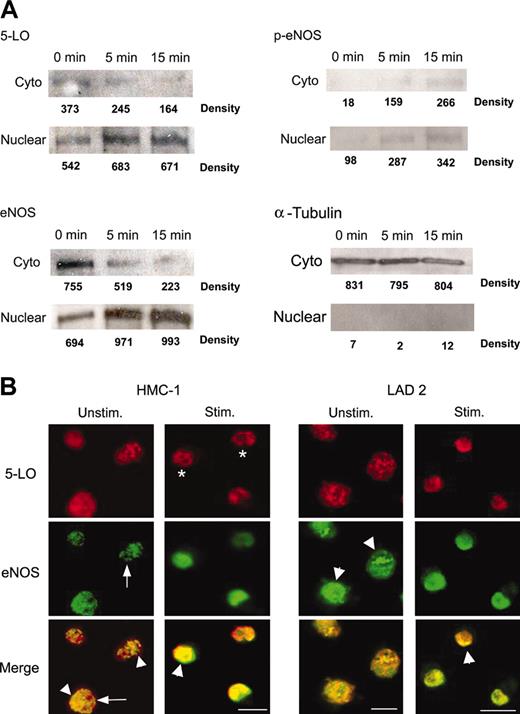
As has been previously shown in rat basophilic leukemia (RBL) cells,21 5-LO in HMC-1 cells is mobilized to nuclear compartments upon activation with A23187 (1 μM) in a time-dependent manner (Figure 5A). As constitutive NOS enzymes are dependent on Ca2+ for full enzymatic activity, we investigated the effects of elevated intracellular Ca2+ on eNOS and nNOS localization in HMC-1 cells. HMC-1 cells were treated with 1 μM A23187 for various time points prior to cell fractionation. There was a moderate change in eNOS localization with movement of cytosolic eNOS into the nuclear fraction after A23187 treatment (1.5-fold increase) as determined by band densitometry. Control blots using α-tubulin demonstrate no change in distribution upon activation, showing purity of the nuclear extracts and lack of overall “shift” in MC proteins following stimulation (Figure 5A). There was no appreciable change in nNOS localization (data not shown).
Translocation and colocalization of eNOS and 5-LO in HMC-1. (A) Western blot analysis of 5-LO, eNOS, phospho-eNOS (Ser1177), and α-tubulin in subcellular fractions from HMC-1 cells. Cells were stimulated with A23187 (0.1 μM) for the indicated times. Cell fractionation and Western blotting were performed as described in “Materials and methods.” Equal quantities (15 μg) of protein were added to each lane, and the relative band intensities (numbers below the bands) were determined by densitometry (arbitrary units). Results are representative of 3 independent experiments. (B) Colocalization of eNOS and 5-LO in human mast cells. HMC-1 or LAD 2 cells were untreated or stimulated with A23187 (0.1 μM) or IgE cross-linking (1 μg/mL) for 15 minutes, respectively. Cells were fixed in 4% paraformaldehyde and incubated with rabbit anti-5-LO (red) and mouse anti-eNOS (green). Antibody labeling was detected with rhodamine red-conjugated goat antirabbit and BODIPY-conjugated rabbit antimouse antibodies; overlapping fluorescence is indicated in yellow. White arrowheads indicate nuclear localization, white arrows indicate cytoplasmic staining, and white stars indicate nuclear envelope localization. Original magnification × 800; bar = 10 μm.
Translocation and colocalization of eNOS and 5-LO in HMC-1. (A) Western blot analysis of 5-LO, eNOS, phospho-eNOS (Ser1177), and α-tubulin in subcellular fractions from HMC-1 cells. Cells were stimulated with A23187 (0.1 μM) for the indicated times. Cell fractionation and Western blotting were performed as described in “Materials and methods.” Equal quantities (15 μg) of protein were added to each lane, and the relative band intensities (numbers below the bands) were determined by densitometry (arbitrary units). Results are representative of 3 independent experiments. (B) Colocalization of eNOS and 5-LO in human mast cells. HMC-1 or LAD 2 cells were untreated or stimulated with A23187 (0.1 μM) or IgE cross-linking (1 μg/mL) for 15 minutes, respectively. Cells were fixed in 4% paraformaldehyde and incubated with rabbit anti-5-LO (red) and mouse anti-eNOS (green). Antibody labeling was detected with rhodamine red-conjugated goat antirabbit and BODIPY-conjugated rabbit antimouse antibodies; overlapping fluorescence is indicated in yellow. White arrowheads indicate nuclear localization, white arrows indicate cytoplasmic staining, and white stars indicate nuclear envelope localization. Original magnification × 800; bar = 10 μm.
Previous studies showed that the presence of eNOS protein does not always correlate with NO production, as eNOS activity can be regulated by multiple posttranslational modifications, including phosphorylation.22 As phosphorylation at Ser1177 is considered to be an important mechanism to increase eNOS activity,23 we measured phospho-eNOS (Ser1177) levels in HMC-1 cells. After 5 minutes of treatment with A23187 (1 μM), HMC-1 cells showed increased phospho-eNOS in both cytoplasmic (10-fold) and nuclear (3.5-fold) fractions compared with 0 time points. These data suggest that increased Ca2+ levels potentiate eNOS phosphorylation at Ser1177, an indicator of increased eNOS activity (Figure 5A). This increased activity correlates well with the time course (10 minutes) of DAF fluorescence (NO synthesis) seen in the confocal experiments.
These data indicate that MCs contain cytoplasmic and nuclear pools of NOS and that eNOS can be mobilized and activated upon stimulation.
5-LO and eNOS colocalization
Given the similar expression pattern of 5-LO and eNOS in MCs, we investigated the colocalization of these enzymes using confocal microscopy. In unstimulated HMC-1 or LAD 2 cells, 5-LO colocalized with eNOS in both cytoplasmic and nuclear compartments, as shown by the similar patterns of anti-5-LO (red) and anti-eNOS (green) staining and confirmed by the yellow pattern in the merged overlays (Figure 5B). However, the colocalization is not complete, as noted by regions in both nucleus and cytoplasm that are free of colocalized signal.
Upon activation with A23187 or IgE cross-linking, there is significant migration of 5-LO to the nucleus in the time course studied (15 minutes). There is also some migration of eNOS, although levels, particularly in HMC-1 cells, are difficult to delineate due to high levels of constitutive nuclear expression. Colocalization of both proteins is much stronger after stimulation, with decreased cytoplasmic staining and with some cells showing nuclear envelope patterning, as has been previously noted for 5-LO in activated rat MCs.21 Thus, 5-LO and eNOS protein colocalize and may form a functional regulatory unit based on this interaction.
Leukotriene ELISA/NO inhibitors
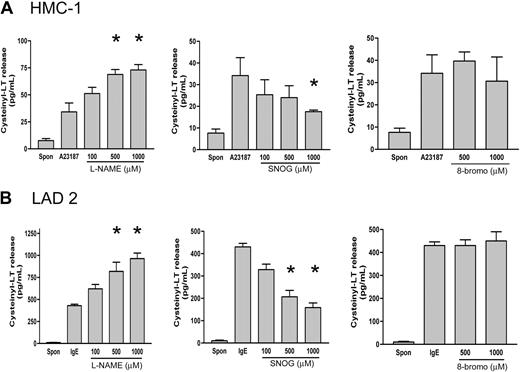
To confirm a biologic role for NO in modulating human MC LT formation, we analyzed the release of LT in stimulated HMC-1 or LAD 2 supernatants after pretreatment with an NO donor (SNOG) or NOS inhibitor (L-NAME). Treatment of HMC-1 cells with A23187 (1 μM) (33.1 ± 8.6 pg/mL, n = 3) or cross-linking of IgE on LAD 2 cells (448 ± 11.1 pg/mL, n = 3) significantly increased cysteinyl leukotriene production over basal levels (HMC-1, 6.2 ± 1.3 pg/mL, n = 3; LAD 2, 4.6 ± 2.8 pg/mL, n = 3) in unstimulated cells (Figure 6). The levels of LT release were significantly lower in HMC-1 than in LAD 2 cells, suggesting important differences between immature and mature MC phenotypes. Pretreatment of cells with the NO donor SNOG for 30 minutes prior to activation caused a significant (P < .01) and dose-dependent inhibition of LT release in both cell types (HMC-1, 51%; LAD-2, 63% inhibition) (Figure 6). Conversely, pretreatment with the NOS inhibitor L-NAME resulted in a significant (P < .01) dose-dependent potentiation of LT release (HMC-1, 55%; LAD 2, 51%). Pretreatment with the inactive enantiomer D-NAME (1000 μM) showed no change in leukotriene formation, showing the specificity of the L-NAME effect (data not shown). Interestingly, although the amounts of LT release varied greatly between the 2 cell types, the NO donor and NOS inhibitor effected release to a similar extent in both cells. As NO can modulate cellular activities by activating soluble guanylate cyclase and increasing levels of cyclic guanosine monophosphate (cGMP),6 we examined the effect of a membrane permeant analog of cGMP, 8-bromo-cGMP. 8-bromo-cGMP (1 mM) had no effect on A23187 or IgE-driven LT release in either cell type (Figure 6).
Effect of NOS inhibition and exogenous NO on cysteinyl leukotriene production in human MCs. (A) Concentrations of cysteinyl leukotrienes in medium from HMC-1 cells were determined by ELISA. Cells were preincubated (30 minutes) with the NOS inhibitor (L-NAME), NO donor (SNOG), or cGMP analog (8-bromocGMP), then stimulated with A23187 (1 μM) for 30 minutes. Results are expressed as mean ± SEM for 3 independent experiments. Asterisk indicates P < .01 by comparison with untreated cells. (B) Concentrations of cysteinyl leukotrienes in medium from LAD 2 cells was determined by ELISA. Cells were preincubated (30 minutes) with the NOS inhibitor (L-NAME), NO donor (SNOG), or cGMP analog (8-bromo-cGMP), then stimulated with anti-IgE (1 μg/mL) for 30 minutes. Results are expressed as mean ± SEM for 3 independent experiments. *P < .01 by comparison with untreated cells.
Effect of NOS inhibition and exogenous NO on cysteinyl leukotriene production in human MCs. (A) Concentrations of cysteinyl leukotrienes in medium from HMC-1 cells were determined by ELISA. Cells were preincubated (30 minutes) with the NOS inhibitor (L-NAME), NO donor (SNOG), or cGMP analog (8-bromocGMP), then stimulated with A23187 (1 μM) for 30 minutes. Results are expressed as mean ± SEM for 3 independent experiments. Asterisk indicates P < .01 by comparison with untreated cells. (B) Concentrations of cysteinyl leukotrienes in medium from LAD 2 cells was determined by ELISA. Cells were preincubated (30 minutes) with the NOS inhibitor (L-NAME), NO donor (SNOG), or cGMP analog (8-bromo-cGMP), then stimulated with anti-IgE (1 μg/mL) for 30 minutes. Results are expressed as mean ± SEM for 3 independent experiments. *P < .01 by comparison with untreated cells.
Discussion
In rodent MCs it previously has been shown that MC responsiveness and MC phenotype can be determined by NO.24 However, there is little data concerning the production and effects of NO in human MCs. Our results show distinct differences in NOS expression and localization in a variety of human MC populations. All MC types studied were positive for eNOS but showed variable expression of nNOS and no detectable iNOS.
Discerning patterns is difficult, as the immortalized cell lines HMC-1 and KU812 show differential expression of nNOS. The nature of the genetic phenotype of these cell lines may account for these differences, as HMC-1 cells have a mutation in the c-kit receptor.9 Furthermore, as HMC-1 cells represent immature MCs whereas KU812 expresses both MCs and basophilic characteristics, more detailed studies in blood basophils and in vivo MC populations are required to determine if the differences between HMC-1 and KU812 cells in nNOS expression reflect in vivo differences between MCs and basophils. Indeed, additional studies with human MCs derived from normal tissues will further define if our observed pattern of NOS expression heterogeneity is not exclusively an in vitro phenomenon.
The “mature” MC, namely HSMC (nNOS positive) and LAD-2 (nNOS negative), also showed different nNOS expression. It previously has been shown by immunohistochemistry that both human skin and nasal mucosal MCs also are positive for nNOS.17,25 Thus, our nNOS results with ex vivo HSMCs are compatible with those in vivo studies, and there are significant differences in NOS expression in human MC populations contributing further to MC heterogeneity.
With the lack of constitutive iNOS expression, human MCs appear to rely on constitutive (cNOS) in their quiescent state. This pattern is reiterated in other human immune cells, with neutrophils and eosinophils also being identified as cNOS expressors, employing eNOS and nNOS isoforms.26,27 Interestingly, nNOS was expressed in eosinophils infiltrating inflammatory sites in the skin, suggesting that there may be specific factors that induce nNOS usage in this cellular environment.27
The lack of iNOS expression is not surprising, given the “basal” status of the MC populations studied and the need for appropriate stimuli for iNOS transcription in other systems.6 Indeed, the time course of DAF (NO) fluorescence (< 10 minutes) in HMC-1 cells stimulated with A23187 and LAD 2 cells stimulated with IgE cross-linking was very similar to that seen in rat MCs,14 which is associated with Ca2+ flux and cNOS activity. Absence of any iNOS expression following stimulation with A23187 or IgE cross-linking gives more evidence that a cNOS isoform is also functional in human MCs. Notably, studies in other human cell types, including monocytes, macrophages, and neutrophils, also showed no iNOS expression, even when activated with known iNOS inducers such as interferon-γ (IFN-γ) and tumor necrosis factor (TNF).26,28 However, iNOS expression now has been noted in all these cell types from patients with various diseases.29 However, the in vitro conditions necessary to stimulate NO production remain incompletely characterized, and appropriate stimulation also may induce iNOS expression in human MCs.
As little is known about NOS distribution in human MCs, the pattern of NOS localization was interesting, with isoforms showing distinct and overlapping distributions. Because eNOS has sites for lipid modification at is amino terminal that can direct its localization, we expected plasma membrane compartmentalization as seen in endothelial cells.23,30 Instead, we saw diffuse cytoplasmic staining along with a strong presence in the nucleus. Since other cell types have shown eNOS localization in the perinuclear or Golgi regions,18 we confirmed nuclear eNOS by colocalization with PI in confocal analysis and with subcellular fractionation and Western blot. Moreover, following MC activation, eNOS translocated from the cytoplasmic compartment to the nuclear fraction. Since 5-LO also can translocate to the nucleus upon activation,31 these enzymes may share a common translocation mechanism.
The possibility exists, however, that there are other explanations for our observations. In nuclear fractions there is a plethora of charged proteins that could bind antibodies nonspecifically.32 To address these drawbacks, we used multiple techniques to determine NOS localization. Furthermore, we used antibodies from differing species to confirm our confocal and Western blot results, using multiple fixation, permeabilization, and staining routines. Despite these precautions, limitations still exist, and further investigations of fluorescent-tagged eNOS or 5-LO, IP using purified proteins, or immunolabeling with electron microscopy will help confirm and define the distribution and interaction of these proteins in human MCs.
Previous studies have shown that eNOS protein can be detected in nuclear and perinuclear regions and that eNOS is active in such sites.18,23,33 Furthermore, both Ca2+ and calmodulin accumulate at nuclear sites in activated MCs,34,35 suggesting that the nucleus is a favorable environment for NO production. While defined roles for NO production in the nucleus are unknown, our data showing colocalization of eNOS with 5-LO, nuclear NO production, and NO regulation of LT formation offer novel insights into potential functions. However, as NOS and 5-LO do not completely co-associate in the nucleus, there are clearly other potential roles for each, including control of gene transcription by regulation of transcription factors.32 In fact, ongoing studies using NOS inhibitors have shown that nuclear NO may regulate chemokine expression in these same cell types (M.G., unpublished data, April 2003).
The mechanisms involved in NO modulation of LT release from human MCs is likely complex. The best-known target for cellular NO is soluble guanylate cyclase, which increases cellular cGMP levels.6 Indeed, rapid signaling through eNOS-derived NO is modulated to a great extent by cGMP. However, our results with 8-bromo-cGMP showed no effect on short-term LT release, implying that there are mechanisms independent of cGMP by which MC NO regulates LT release. Nitrosylation or nitration of tyrosine residues may be involved.20 These data are consistent with those seen in other studies of the cGMP-independent effects of NO on MC secretion (degranulation)36 or adhesion.37 Interestingly, these results were obtained using either NO donors or iNOS-driven systems to produce sustained NO and effect MC responses38 and proposed that other NO/protein interactions (including nitrosylation) are responsible for the effects.
Indeed, NO derived from iNOS or NO donors previously has been shown to inhibit LT activity in macrophages by nitrosylation of 5-LO.20 Given the interaction of eNOS and 5-LO we observed, such a mechanism also may be active in MCs, although through eNOS activity. It has been proposed that 5-LO exists in a “metabolon” of sequential enzymes to facilitate efficient production of LT,19 and it is attractive to postulate that eNOS may be a regulatory component within this complex. Our results show that eNOS and 5-LO appear to be regulated in a similar pattern with close association in cytoplasmic and nuclear domains. Thus, similar mechanisms may be employed in their trafficking and activation.
Our results and those of others have shown that HMC-1 cells exhibit weak and variable LT release compared to mature MCs.39 In fact, LT release in human MC populations is heterogeneous and regulated by environmental factors.40 Interestingly, LAD 2 cells showed fewer DAF (NO)-positive cells than HMC-1 upon activation and released significantly more LT. Environmental influences undoubtedly modulate NOS expression, including increased nNOS production, with potential outcomes of tonic NO production on MC function and mediator release.
These results highlight the tenant of human MC heterogeneity, properties that are likely underestimated due to the difficulty in obtaining large cell numbers in vivo.1 We predominantly employed 2 cell types in these studies, HMC-1 and the newly identified growth factor-dependent LAD 2.9 HMC-1 cells contain a constitutively active mutation in the c-kit receptor, which propagates growth factor-independent expansion. However, limited information can be derived from these cells, as they represent immature MCs that produce limited amounts of granule mediators, and a lack of FcϵRI expression negates activation through IgE cross-linking.9 LAD 2 cells are used as a “normal” MC homolog and differ significantly from HMC-1 cells, expressing a similar mature phenotype and responsiveness as CD34-derived human MCs.9 LAD 2 cells lack c-kit mutation and are dependent on stem cell factor (SCF) for growth and, most importantly, express FcϵRI and can be activated by IgE cross-linking. It is interesting to note that despite the spectrum of MC phenotype shown by these cells, NO modulation of LT synthesis was observed in both cell types. Thus, NOS-derived NO may be an important general facet of LT regulation in MC populations.
Much research has centered on the role of iNOS in inflammation and the effects caused by high levels of NO.6 However, there is considerable recent interest in the evolving role of eNOS in immune regulation. Using NOS knockout mice, a vital role for eNOS in propagating immune processes in macrophages41 and initiating leukocyte influx into inflammatory sites42 has been identified. In addition, an unexpected role for nNOS, but not iNOS, was identified in mouse models of asthmatic inflammation.43 Our studies expand this understanding and indicate that there are differences in the roles played by cNOS in various cells. Unfortunately, our results do not identify discreet roles for eNOS or nNOS in MCs expressing both isoforms, and investigations with knockdown strategies are needed to help delineate the role of each isoform in inflammation. Furthermore, our DAF/live-cell studies also define a cytoplasmic partition of immediate (< 10 minutes) NO formation, suggesting that there also is selective delivery of this potent molecule to cytoplasmic targets. Defining roles for this cytoplasmic compartmentalized requires further study. Indeed, similar to our results in rat MCs,14 we have recently identified a role for NO in regulating granule release in HMC-1 and LAD 2 cells (M.G. and S.D.M., unpublished data, December 2003).
The hallmarks of the MCs are its armamentarium of granule-stored mediators, de novo lipid-derived molecules, and newly formed cytokines that can be differentially and rapidly released.44 Such complex regulation must arise from involved and necessarily overlapping control machinery.44 Previous studies in rodent MCs7,38 and human basophils45 have shown a role for endogenous and exogenous NO in inhibition of granule mediator secretion. We have shown here for the first time that MC-derived NO, localized to the nuclear membrane, modulates LT produced immediately (< 30 minutes) after activation. In addition, we have shown in this and previous studies14 NO formation and NOS localization within the nucleus, which may account for the NO regulation of chemokine and cytokine production seen in other cell types.46 NO thus appears to be strategically placed to impinge on many pathways associated with MC activation. Further knowledge of the factors that control the expression of NOS isoforms in human MCs and that regulate NO production and its intracellular functions may uncover novel therapeutic targets for allergic and other inflammatory diseases.
Prepublished online as Blood First Edition Paper, March 25, 2004; DOI 10.1182/blood-2003-08-2990.
Supported by the Canadian Institutes of Health Research grant MT 7034, an Alberta Heritage Foundation for Medical Research Studentship (M.G.), and an Alberta Lung Association Studentship (S.D.M.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
A. Dean Befus is the AstraZeneca Canada Inc Chair in Asthma Research.

![Figure 4. NOS activity and nitric oxide localization in human mast cells. (A) NOS activity in HMC-1 cells, as measured using the [14C]-l-arginine conversion assay. Brain homogenate was used as a positive control. Each sample was run in the presence of EGTA (2 mM) to determine the levels of calcium-dependent (constitutive) NOS activity. The results are expressed as picomoles l-citrulline formed per minute, per milligram of protein. Data shown as mean ± SEM for 3 independent experiments. (B) Measurement of endogenous NO formation in HMC-1 and LAD 2 cells as detected by DAF fluorescence. Cells were loaded with DAF, basal fluorescence images were obtained, and then HMC-1 cells were stimulated with A23187 (0.1 μM) or LAD 2 cells stimulated by IgE cross-linking (1 μg/mL). Changes in fluorescence were captured using confocal microscopy 10 minutes after activation. Most HMC-1 cells (75% ± 6.3%; n = 4) were positive, whereas fewer LAD 2 cells (42% ± 1.6%, n = 3) were positive. White arrowheads show nuclear/perinuclear DAF fluorescence. White arrows indicate DAF-negative cells that also show cytoplasmic ruffling (HMC-1 and LAD 2) or granule release (LAD 2). Original magnification × 800; bar = 10 μm. Results are representative of at least 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/2/10.1182_blood-2003-08-2990/6/m_zh80140463900004.jpeg?Expires=1767773956&Signature=JBFJdy5HMwFgul1pGJOo9Ibz~FhTOeV395ZzBkY~ygSoxCKU9nbaMkWEIhEVzzmqih3mcrk1yHSSwEdN-VSuIWxMdy9CP-BQxYMSrxbrz6XIlgJzU0gTk7b99o9IC6Boe8OULHLpyf7N3H5J8rRCZPX9mvIRSfE6LTRVShrp9KjNf02dnE7r4Gal2cONuqry74MwES3qJPaTR8SSCPNOA9hEZ4FQ3Z~Sc-~0wd~UwARjDzdQx9wCKpj4TvAEIjcR3RkeiRfgagctEAnXlln2MW7tD9jYdpHrG8EK~zmNVI7t9s7GxxQNr0ij1StF141cimOl-IHiHvTvP-0nSY8eig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal