Abstract
In this study we describe the first monoclonal antibody, integrin activated conformation-1 (IAC-1), to recognize the active form of the platelet-collagen receptor, the integrin α2β1. IAC-1 has the following properties: (1) IAC-1 fails to bind to resting platelets but readily interacts with platelets stimulated by the glycoprotein VI-specific agonist, convulxin, and by other agonists; (2) similar concentration response relationships for binding of IAC-1 and soluble collagen were observed in convulxin-stimulated platelets; (3) the epitope for IAC-1 is T199Y200K201, which is located at the opposite site of the metal ion-dependent adhesion site in a region not involved in the I-domain “shifts” that occur upon ligand binding; (4) IAC-1 strongly binds to recombinant α2 I-domain, therefore suggesting that the neo-epitope appears to be exposed by an “unmasking” of I-domain-covering regions upon activation; (5) IAC-1 binds to platelets during adhesion to collagen under shear conditions, demonstrating activation of α2β1; (6) as IAC-1 does not interfere with platelet-collagen binding, it defines a new class of antibodies that is distinct from those belonging to the “cation- and ligand-induced binding sites” (CLIBSs) and the “ligand mimetic” group. These characteristics make IAC-1 a very powerful tool to study α2β1 activation under dynamic and physiologically relevant conditions. (Blood. 2004;104:390-396)
Introduction
Integrins are a large family of receptor molecules that mediate attachment of cells to each other and to the surrounding extracellular matrix (ECM). Integrin engagement activates signaling pathways involved in adhesion-dependent processes including cell migration, proliferation, survival, and differentiation. Consequently, they participate in diverse (patho)physiologic processes as diverse as hemostasis, immunity, and inflammatory disorders such as atherosclerosis. A unique aspect of integrin function consists of affinity regulation of their extracellular domain for biologic ligands by signals from within the cell (inside-out signaling).1
The α2β1 integrin is expressed on a variety of cell types,2 mainly serving as a collagen receptor.3 The I-domain, inserted into the head region of the α2 subunit, plays a central role in ligand binding and recapitulates many of the ligand-binding properties of the intact integrin.4-6 The crystal structure of the α2 I-domain, determined both in the absence7 and presence of an α2β1-specific collagen-related peptide,8 has revealed that ligand binding to the I-domain results in a change in metal coordination leading to reorganization of the upper surface and propagation of conformational changes to the opposite pole of the domain.8 These structural differences are thought to reflect different conformations of α2β1. However, no information is available on how such changes in the I-domain might be induced through inside-out activation.
Kainoh et al9 were the first to demonstrate α2β1-mediated, activation-dependent platelet adhesion to collagen. This was later extended by Jung and Moroi10-13 and Wang et al,14 who demonstrated platelet activation-dependent binding of soluble collagen to α2β1. Interestingly, the apparent affinity for soluble collagen was lower in platelets stimulated by adenosine diphosphate (ADP) compared with platelets stimulated by thrombin or a glycoprotein VI (GPVI)-specific collagen-related peptide, suggesting 2 different activation states of α2β1.
In the present study, we describe for the first time the identification of an anti-α2 I-domain monoclonal antibody (mAb) that binds to activated but not to resting platelets. Thus, these data provide direct evidence of a conformational switch for α2β1 during platelet activation. The mAb is termed integrin activated conformation-1 (IAC-1). We further show that IAC-1 recognizes both the intermediate and high-affinity states of α2β1, as its binding was induced by ADP and by strong agonists. Using chimeric α2 I-domains, we mapped the IAC-1 epitope to amino acids T199Y200K201 in the loop between β-strand C and α-helix 3 at the bottom of the I-domain, distant from the metal ion-dependent adhesion site (MIDAS). This region is not involved in the I-domain shifts observed upon ligand binding. Taken together, our findings indicate that a neo-epitope is generated upon platelet activation by “unmasking” areas of the α2 I-domain covered by other regions of α2β1 rather than by a shift in the I-domain itself.
Materials and methods
Materials
Gi9, an inhibitory anti α2 I-domain mAb, was purchased from Immunotech (Marseille, France). Fluorescein isothiocyanate (FITC)-conjugated CD49b mAb (AK-7, anti-α2 I), phycoerythrin (PE)-conjugated CD62P (anti-P-selectin), and FITC-conjugated PAC-1 (anti-activated αIIbβ3) mAbs were purchased from BD Bioscience (San Diego, CA). An unrelated mouse monoclonal immunoglobulin G 1 (IgG1), MOPC-21-FITC, and horse-radish peroxidase (HRP)-conjugated goat antimouse IgG (GAM-HRP) were purchased from Sigma (St Louis, MO); FITC-conjugated rabbit antimouse (RAM-FITC) was purchased from Dako (Glostrup, Denmark); and streptavidin-HRP was purchased from Roche (Mannheim, Germany). Platelet agonists were Horm collagen (Nycomed, Munich, Germany), acid soluble human collagen type I (Sigma), convulxin (Kordia, Leiden, The Netherlands), ADP and U46619 (Sigma), collagen-related peptide (CRP; a kind gift from Dr Farndale, Cambridge, United Kingdom), and thrombin (Kordia).
Collagen type I and anti-α2 I-domain mAbs were labeled with FITC or NHS-SS-biotin (sulfosuccinimidyl-2-(biotinamido)ethyl-1,3-dithiopropionate) according to the instructions of the supplier (Pierce, Rockford, IL).
Production of anti-α2 I-domain mAbs
Anti-α2 I-domain mAbs were produced essentially as described.15 Briefly, BALB/c mice were immunized by injection with purified α2 I-MBP. Two days after the final intraperitoneal antigen boost, the spleen cells were fused with Sp2/0 mouse myeloma cells and cultured in HAT (hypoxanthine aminopterin thymidine) selection medium (Gibco, Paisley, United Kingdom). Cloning was performed by a limiting dilution method using 96-well microtiter plates with mouse peritoneal macrophage feeder layers. Hybrid-omas producing anti-α2 I-MBP mAbs were identified in an enzyme-linked immunosorbent assay (ELISA), essentially as described in “Binding of mAbs to the α2 I-domain” using plates coated with purified recombinant α2 I-MBP (5 μg/mL in phosphate-buffered saline [PBS]). Ascitic fluid of the positive clones was prepared by intraperitoneal injection of 5 × 106 hybrid cells into BALB/c mice pretreated with pristane (Sigma). IgG was purified from ascites fluid by chromatography on a Protein A Sepharose column (Amersham Biosciences, Uppsala, Sweden) and antibody concentration was measured spectrophotometrically.
Binding of mAbs to α2 I-domain
Microtiter plates were coated with recombinant α2 I-His (5 μg/mL in PBS) and, after washing and blocking with 3% milk powder, a dilution series of mAbs was added for 90 minutes. Bound mAbs were detected with GAM-HRP (1:10 000 in PBS) and visualization was performed using orthophenylenediamine (OPD; Sigma) and H2O2. The reaction was stopped with 4 M H2SO4 and absorbance was measured at 490 nm. Nonspecific binding was determined by parallel incubation of the mAb dilution series on uncoated blocked wells of the same microtiter plate.
Epitope mapping using human/mouse chimeras
DH5αF' cells containing plasmids with human/mouse chimeras of the α2 I-domain were kindly given by Dr D. Tuckwell (Manchester, United Kingdom). Mutant α2 I-domains were expressed and purified as described.5 Binding of mAbs to wild-type and mutant α2 I-domains was examined in an ELISA in which the I-domains (25 μg/mL) were coated and a serial dilution of biotinylated mAb was applied. mAb binding to the I-domains was detected with streptavidin-HRP (1:5000 in PBS) and OPD as substrate. The binding specificity of these mAbs was confirmed by Western blot analysis with I-domains applied on a 10% sodium dodecyl sulfate (SDS)-polyacrylamide gel and blotted on nitrocellulose membranes. After blocking with 5% milk powder, anti-α2 I-domain mAbs (1 μg/mL in PBS with 0.5% milk powder and 0.05% Tween 20) were added and incubated for 90 minutes. mAb binding was detected with GAM-HRP (1:10 000 in PBS) and enhanced chemiluminescence (ECL) Western blotting detection reagents on a high-performance chemiluminescence film (Amersham Biosciences).
Platelet preparation
Blood samples from healthy volunteers, free of acetylsalicylic acid for the last 10 days, were collected on acid citrate dextrose (ACD). Platelet-rich plasma (PRP) was obtained by centrifugation. Washed platelets were prepared essentially as described.16
Static adhesion and aggregation of platelets
Static adhesion of washed human platelets (3 × 105/μL) to human collagen type I (25 μg/mL in PBS) coated on a microtiter plate was tested in the presence or absence of various concentrations of anti-α2 I-domain mAbs. Platelet binding was determined by measuring endogenous phosphatase activity after lysis of the platelets with Triton X-100 using P-nitrophenyl phosphate (Merck, Darmstadt, Germany) as substrate.6
Platelet aggregation was monitored using an ELVI-400 platelet aggregometer (Elvi Logos, Milano, Italy) at 37°C under stirring conditions. Aggregation of washed platelets was induced by human collagen type I (threshold collagen concentration to induce full aggregation). In inhibition assays, platelets were preincubated with the mAbs (10 μg/mL final concentration) for 5 minutes before agonist addition.
Flow cytometry
Reaction mixtures of PRP or washed platelets (5 μL in a total volume of 50 μL), agonist, CD62P-PE, and FITC-labeled mAb or FITC-labeled collagen in buffer A (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 150 mM NaCl, 1 mM MgCl2, 5 mM KCl, pH 7.4) were incubated for 15 minutes. The reaction was stopped by adding 10 volumes of 0.2% formyl saline, and a 1:10 dilution was analyzed on a FACS Calibur flow cytometer (Becton Dickinson). Convulxin (50 ng/mL), ADP (50 μM), U46619 (5 μM), CRP (500 ng/mL), thrombin (1 U/mL), and von Willebrand factor (VWF; 10 μg/mL) were used as agonists. Data are represented by the median value of fluorescence (MF), and the ratio of MF of stimulated platelets to that of resting platelets.
Binding assay with 125I-labeled mAb
Anti-α2 I-domain mAb IAC-1 and 15D7 were radiolabeled with iodine-125 (125I; Amersham Biosciences) by using IODO-GEN precoated iodination tubes (Pierce) according to the manufacturer's instructions. Reaction mixtures of washed platelets (3 × 104/μL), 125I-labeled mAb (12 μg/mL), and convulxin (40 ng/mL) or ERNES buffer (137 mM NaCl, 2.7 mM KCl, 3 mM NaH2PO4, 3.5 mM HEPES, 5.5 mM glucose, pH 7.3) were incubated for 30 minutes. Nonspecific binding was determined by adding 50-fold excess of unlabeled mAb to reaction mixtures. Four replicates of each binding condition were used. Each reaction mixture was layered onto a 20% sucrose solution and centrifuged at 5000g for 5 minutes. Top fluid was removed and radioactivity of the pellet-containing platelets and platelets with bound mAb was determined in a γ-counter.
Platelet adhesion under flow
Platelet interaction with immobilized Horm collagen (200 μg/mL) under flow conditions producing a wall shear rate of 1000 s-1 was studied using a parallel plate flow chamber essentially as described.17 Blood from healthy volunteers was collected on d-phenylalanyl-l-propyl-l-arginine chloromethyl ketone (PPACK; Calbiochem, La Jolla, CA) and IAC-1-FITC was added to whole blood at a final concentration of 10 μg/mL before perfusion. After 4 minutes of perfusion, the coverslips were rinsed with HEPES buffer (with 0.1% glucose, 0.1% bovine serum albumin [BSA], 2 mM CaCl2, and 1 U/mL heparin, pH 7.4) and adhered platelets were visualized by phase-contrast microscopy (Nikon Diaphot 200; Nikon, Tokyo, Japan) and fixed with 0.2% formyl saline. After permeabilization with 0.005% SDS, the actin skeleton of the platelets was stained with Texas-Red phalloidin (Molecular Probes, Leiden, The Netherlands). Coverslips were then sealed with 20 μLof 4′6-diamidino-2-phenylindole/1,4-diazabicyclo[2,2,2]octane (DAPI/DABCO; Sigma) and analyzed by confocal microscopy using an MRC 600 laser-scanning microscope system (Biorad, Hemel Hempstead, United Kingdom). Image processing was performed using Laserpix Software (Biorad). Alternatively, blood was taken either on PPACK or on 10% ACD and supplemented with 5 μM prostaglandin E1 (PGE1) to block platelet activation. After 3 minutes of perfusion, FITC-labeled mAbs MOPC-21 (10 μg/mL), IAC-1 (10 μg/mL), or Gi9 (5 μg/mL) were added for 15 minutes. Two-photon laser scanning microscopy was performed with a Biorad 2100 Multi-Photon System (Biorad), and image processing was performed using Laserpix Software (Biorad). Phase-contrast and fluorescence images were collected with a 40 × UV-transparent objective and 15 × to 20 × image magnification. Surface area coverage was analyzed exactly as described by Siljander et al.18
Statistical analysis
Student t test for paired data was used to test statistically significant differences. Data given in the text are means plus or minus standard error of the mean (SEM).
Results
mAb production and selection
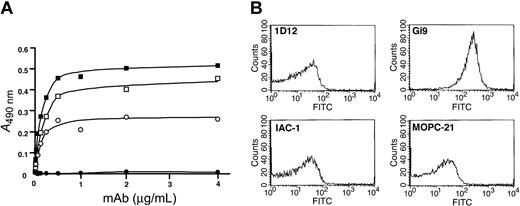
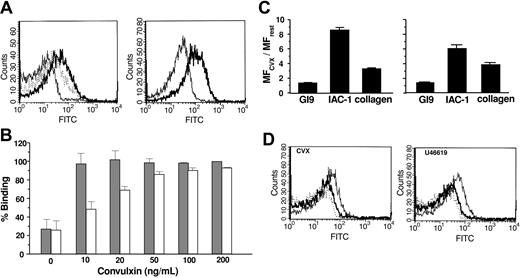
Immunization of BALB/c mice with recombinant human α2 I-MBP resulted in 88 anti-α2 I-MBP mAbs. Of these, 25 mAbs bound in a specific and dose-dependent manner to immobilized α2 I-domain and not to MBP. Figure 1A shows the saturation of 2 selected mAbs, IAC-1 and 1D12, compared with MOPC-21, a control IgG antibody, and Gi9, a well-characterized blocking anti-α2 I-domain mAb. Analysis of the binding of the mAbs to human platelets using flow cytometry, however, revealed that neither IAC-1 nor 1D12 recognized the α2β1 integrin on resting platelets, in contrast to Gi9 (Figure 1B).
Binding of anti-α2 I-domain mAbs to α2 I-His and to resting platelets. (A) Binding of control mAb MOPC-21 (•) or selected anti-α2 I-domain mAbs Gi9 (○), 1D12 (▪), and IAC-1 (□) to α2 I-domain-coated wells; bound mAbs were detected with GAM-HRP. (B) Staining of human platelets (PRP, 5 × 105 platelets/μL) with the indicated FITC-labeled anti-α2 I-domain mAbs (20 μg/mL) and analysis by flow cytometry.
Binding of anti-α2 I-domain mAbs to α2 I-His and to resting platelets. (A) Binding of control mAb MOPC-21 (•) or selected anti-α2 I-domain mAbs Gi9 (○), 1D12 (▪), and IAC-1 (□) to α2 I-domain-coated wells; bound mAbs were detected with GAM-HRP. (B) Staining of human platelets (PRP, 5 × 105 platelets/μL) with the indicated FITC-labeled anti-α2 I-domain mAbs (20 μg/mL) and analysis by flow cytometry.
Exposure of the neo-epitope for IAC-1 by convulxin
Given the different activation states of integrin α2β1, we tested whether the selected mAbs would recognize a neo-epitope that is exposed upon platelet activation. To this end, the snake venom convulxin (50 ng/mL) was used, which strongly activates platelets, mainly via GPVI. Interestingly, mAb IAC-1 did not bind to resting platelets but showed a pronounced and statistically significant increase in binding to platelets stimulated with convulxin (Figure 2). The MF increased 8-fold from 7.9 ± 3.4 (n = 11) for resting platelets to 61.9 ± 26.0 (n = 28, P < .001) for convulxin-stimulated platelets, suggesting that IAC-1 recognizes a neoepitope that is only exposed by platelet activation.
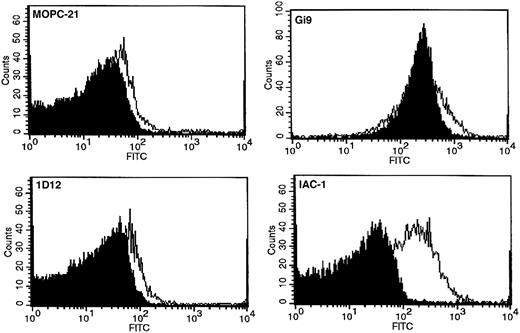
Binding of anti-α2 I-domain mAbs to resting and convulxin-stimulated platelets. Flow cytometric analysis on platelets (PRP, 5 × 105 platelets/μL) with 20 μg/mL mAb MOPC-21 (negative control), Gi9 (positive control), 1D12, or IAC-1. Platelets were either nonactivated (filled histogram) or convulxin stimulated (50 ng/mL; open histogram). Overlays are representatives of at least 3 experiments.
Binding of anti-α2 I-domain mAbs to resting and convulxin-stimulated platelets. Flow cytometric analysis on platelets (PRP, 5 × 105 platelets/μL) with 20 μg/mL mAb MOPC-21 (negative control), Gi9 (positive control), 1D12, or IAC-1. Platelets were either nonactivated (filled histogram) or convulxin stimulated (50 ng/mL; open histogram). Overlays are representatives of at least 3 experiments.
Gi9 binding was not altered in the presence of agonist (Figure 2). This suggests that the effect observed with IAC-1 is mediated by a conformational change in α2β1, rather than, for example, an increase in surface expression of the integrin. Because mAb 1D12 is unable to bind to platelets at all (Figure 2), its epitope appears to be encrypted in α2β1, regardless of whether the integrin is active or not. Platelet activation due to convulxin was confirmed by increased binding of anti-P-selectin mAb CD62P-PE in each sample (MF from 7.3 ± 4.3 to 1078.5 ± 260.7).
The increase in IAC-1 binding in activated platelets was confirmed using 125I-labeled IAC-1: specific binding to washed platelets increased from 392 ± 360 cpm (n = 16) to 2715 ± 881 cpm (n = 11) upon stimulation with convulxin (P = .01; not shown). Binding of a control anti-α2 I-domain mAb was not altered upon convulxin stimulation (P = .4; not shown).
Epitope mapping
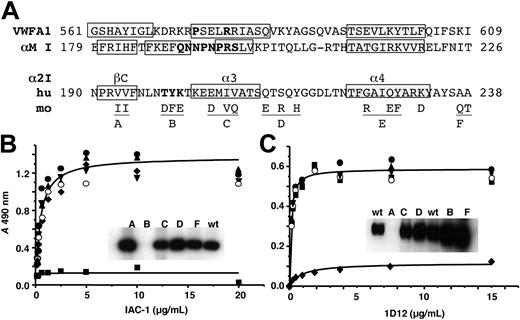
To localize the epitope of IAC-1 and 1D12, various chimeric α2 I-domains were expressed, purified, and tested both by ELISA and Western blot for their ability to support antibody binding. In these chimeras short stretches of human sequences were replaced by the corresponding mouse amino acids (Figure 3A). The IAC-1 epitope was mapped to T199Y200K201, located in the loop linking β-strand C with α-helix 3 (numbering as in Emsley et al8 ), as IAC-1 bound to all constructs except for mutant B in both ELISA and Western blot analysis (Figure 3B). This epitope is located at the opposite site of the collagen-binding site and is not involved in the I-domain shifts observed as a consequence of ligand binding. mAb 1D12 did not recognize construct A in either ELISA or Western blot, mapping its epitope to a different stretch at V193-V194 (Figure 3C).
Epitope mapping of mAbs IAC-1 and 1D12 with human/mouse chimeras of the α2 I-domain. (A) Sequence alignment of human (hu) VWF A1, αM I, α2 I, and mouse (mo) α2 I-domain with ClustalW.19 Only murine residues that differ from the human sequence are shown. β-strands (open boxes) and α-helices (gray shaded boxes) were derived from the crystal structures.7,20,21 Replacement of residues resulting in gain-of-function mutants are in bold. Underlined regions indicate the sequences substituted to give chimeras A to F. Dilution series of mAb IAC-1 (B) and 1D12 (C) were incubated with wells coated with wild-type α2 I-domain (•) or chimeras A (♦), B (▪), C (○), D (▾), or F (▴), and mAb binding was detected with GAM-HRP. Insets: Western blots of the chimeric α2 I-domains incubated with mAb IAC-1 (B) or 1D12 (C).
Epitope mapping of mAbs IAC-1 and 1D12 with human/mouse chimeras of the α2 I-domain. (A) Sequence alignment of human (hu) VWF A1, αM I, α2 I, and mouse (mo) α2 I-domain with ClustalW.19 Only murine residues that differ from the human sequence are shown. β-strands (open boxes) and α-helices (gray shaded boxes) were derived from the crystal structures.7,20,21 Replacement of residues resulting in gain-of-function mutants are in bold. Underlined regions indicate the sequences substituted to give chimeras A to F. Dilution series of mAb IAC-1 (B) and 1D12 (C) were incubated with wells coated with wild-type α2 I-domain (•) or chimeras A (♦), B (▪), C (○), D (▾), or F (▴), and mAb binding was detected with GAM-HRP. Insets: Western blots of the chimeric α2 I-domains incubated with mAb IAC-1 (B) or 1D12 (C).
Effect of IAC-1 on collagen interaction with α2β1
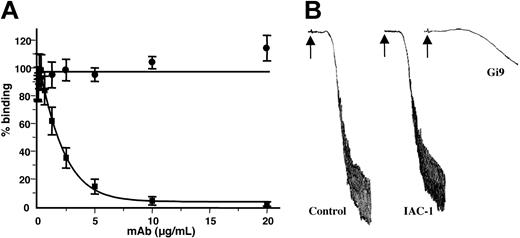
mAb IAC-1 has no effect on either static platelet-collagen adhesion or on collagen-induced platelet aggregation at a concentration of 20 μg/mL (Figure 4). In contrast, the same concentration of mAb Gi9 inhibited static platelet adhesion by more then 85% (Figure 4A) and almost completely blocked platelet aggregation induced by human collagen type I (Figure 4B). Thus, IAC-1 binding to α2β1 does not interfere with the binding of collagen to the α2 I-domain, which is compatible with its binding to a site distinct in the integrin.
IAC-1 has no effect on the platelet-collagen interaction. (A) Binding of washed platelets (3 × 105/μL) to collagen-coated wells in the presence of various concentrations of either IAC-1 (•) or Gi9 (▪) under static conditions. Error bars indicate the SEMs of 3 independent experiments. (B) Collagen-induced aggregation of washed platelets (3 × 105/μL), preincubated for 5 minutes with buffer, IAC-1 (10 μg/mL), or Gi9 (10 μg/mL). Aggregation was induced by 12 μg/mL human collagen type I (arrow). Data are representatives of 3 independent experiments.
IAC-1 has no effect on the platelet-collagen interaction. (A) Binding of washed platelets (3 × 105/μL) to collagen-coated wells in the presence of various concentrations of either IAC-1 (•) or Gi9 (▪) under static conditions. Error bars indicate the SEMs of 3 independent experiments. (B) Collagen-induced aggregation of washed platelets (3 × 105/μL), preincubated for 5 minutes with buffer, IAC-1 (10 μg/mL), or Gi9 (10 μg/mL). Aggregation was induced by 12 μg/mL human collagen type I (arrow). Data are representatives of 3 independent experiments.
Detection of different conformational states of α2β1
We investigated the binding of IAC-1 to platelets activated with various agonists in plasma and in washed platelets. Similar to convulxin, high concentrations of the GPVI-specific peptide CRP (not shown) and the thromboxane A2 analog U46619 stimulated a marked increase in binding of IAC-1 to platelets, reflected by a 6- to 8-fold increase in MF (Figure 5A, left panel). Stimulation with ADP, in contrast, resulted in a 3-fold increase in MF. Platelet activation was confirmed by simultaneous measurement of P-selectin exposure (MF increased from 4.1 to 1104 and to 547.4 for U46619- and ADP-activated platelets, respectively; not shown). Thrombin stimulation of IAC-1 binding was monitored in washed platelets to avoid activation of the coagulation cascade. Thrombin stimulated a 4-fold increase in MF (Figure 5A, right panel) together with an increase in P-selectin (MF increased from 22.4 to 1027.4; not shown).
Detection of the α2β1 conformation on platelets using flow cytometry. (A) Left panel: interaction of IAC-1-FITC (70 μg/mL) with resting PRP (thin line) or PRP stimulated with 50 μM ADP (dotted line) or 5 μM U46619 (bold line). Right panel: binding of IAC-1-FITC to resting washed platelets (thin line) or after stimulation with 1 U/mL thrombin (bold line). Tracings are representatives of at least 3 experiments. (B) Binding of IAC-1-FITC to platelets stimulated with indicated concentrations of convulxin in the absence (▦) or the presence (□) of 5 U/mL apyrase, expressed as the percentage of binding induced by 200 ng/mL convulxin. Error bars indicate the SEMs of 3 independent experiments. (C) Binding of either FITC-labeled Gi9 (20 μg/mL), IAC-1 (70 μg/mL), or soluble collagen (100 μg/mL) was measured to PRP with 50 ng/mL convulxin (left) or 5 μM U46619 (right). Data are represented as the ratio of MF for stimulated versus unstimulated platelets, and are means ± SEMs of at least 3 experiments. (D) Representative tracings of the binding of 100 μg/mL soluble collagen-FITC to unstimulated platelets in PRP (dotted line) and platelets stimulated (thin line) with 50 ng/mL CVX (left) or 5 μM U46619 (right); the enhanced collagen binding was reversed by 20 μg/mL blocking mAb Gi9 (bold line).
Detection of the α2β1 conformation on platelets using flow cytometry. (A) Left panel: interaction of IAC-1-FITC (70 μg/mL) with resting PRP (thin line) or PRP stimulated with 50 μM ADP (dotted line) or 5 μM U46619 (bold line). Right panel: binding of IAC-1-FITC to resting washed platelets (thin line) or after stimulation with 1 U/mL thrombin (bold line). Tracings are representatives of at least 3 experiments. (B) Binding of IAC-1-FITC to platelets stimulated with indicated concentrations of convulxin in the absence (▦) or the presence (□) of 5 U/mL apyrase, expressed as the percentage of binding induced by 200 ng/mL convulxin. Error bars indicate the SEMs of 3 independent experiments. (C) Binding of either FITC-labeled Gi9 (20 μg/mL), IAC-1 (70 μg/mL), or soluble collagen (100 μg/mL) was measured to PRP with 50 ng/mL convulxin (left) or 5 μM U46619 (right). Data are represented as the ratio of MF for stimulated versus unstimulated platelets, and are means ± SEMs of at least 3 experiments. (D) Representative tracings of the binding of 100 μg/mL soluble collagen-FITC to unstimulated platelets in PRP (dotted line) and platelets stimulated (thin line) with 50 ng/mL CVX (left) or 5 μM U46619 (right); the enhanced collagen binding was reversed by 20 μg/mL blocking mAb Gi9 (bold line).
The ability of convulxin to stimulate α2β1 activation was further investigated. IAC-1 binding induced by high concentrations of convulxin was maintained in the presence of apyrase, whereas it was reduced by up to 50% at lower concentrations (Figure 5B). This demonstrates that activation of α2β1 is potentiated by release of ADP but is not dependent on the release of the secondary agonist.
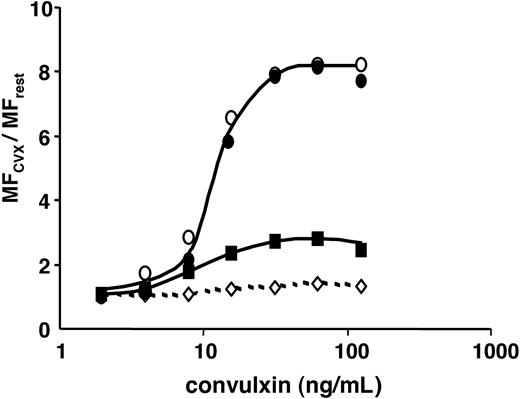
We compared the ability of IAC-1 to monitor α2β1 activation in convulxin-stimulated platelets with the increase in binding of collagen. As shown in Figure 5C, IAC-1 gave a more robust signal. Platelet activation with convulxin resulted in an 8-fold increase of IAC-1 binding compared with a 3-fold increase in collagen binding (n = 19, P < .001; Figure 5C, left panel). The increase in collagen binding was dependent on integrin α2β1 as it was largely blocked by the inhibitory mAb Gi9 (Figure 5D left). A similar set of observations was made with the thromboxane mimetic U46619 (n = 4, P = .008; Figure 5C-D right). The concentration response relationships for binding of IAC-1 and collagen in convulxin-stimulated platelets were compared with those for PAC-1 and P-selectin exposure, which monitor activation of integrin αIIbβ3 and α-granule secretion, respectively (Figure 6). The binding of all of the activation markers increased in parallel, reaching a plateau at a convulxin concentration of 60 ng/mL, with half-maximal concentration around 10 ng/mL. As expected, the binding of Gi9 was not altered.
Convulxin dose dependently raises several platelet activation markers. Flow cytometric detection of the binding of FITC-labeled IAC-1 (70 μg/mL; •), PAC-1 (1:10 vol/vol; ○), Gi9 (20 μg/mL; ⋄), or collagen (100 μg/mL; ▪) to platelets in PRP; stimulation was with indicated concentrations of convulxin. Data are represented as the ratio of MF of stimulated versus unstimulated platelets and are representative of 3 independent experiments.
Convulxin dose dependently raises several platelet activation markers. Flow cytometric detection of the binding of FITC-labeled IAC-1 (70 μg/mL; •), PAC-1 (1:10 vol/vol; ○), Gi9 (20 μg/mL; ⋄), or collagen (100 μg/mL; ▪) to platelets in PRP; stimulation was with indicated concentrations of convulxin. Data are represented as the ratio of MF of stimulated versus unstimulated platelets and are representative of 3 independent experiments.
Binding of IAC-1 to adhering platelets under flow
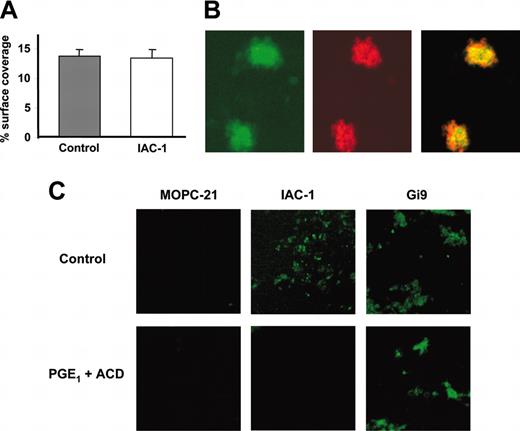
As IAC-1 is the first mAb specific for activated α2β1 and does not interfere with the platelet-collagen interaction, we evaluated its use for detecting integrin activation at arterial shear rates. To this end, platelet adhesion to collagen was analyzed in whole blood under flow conditions (1000 s-1) in a parallel flow chamber in the presence of 10 μg/mL IAC-1-FITC. The surface coverage measured by phase contrast microscopy was not significantly altered due to the presence of IAC-1 (Figure 7A), confirming that IAC-1 has no influence on platelet adhesion to collagen. However, a clear increase in fluorescence signal was obtained, reflecting activation of α2β1. Further, the surface coverage measured by phase contrast microscopy was comparable to the surface coverage measured by fluorescence microscopy (16.5% ± 1.1% and 14.2% ± 1.5%, respectively), indicating that most of the platelets in the aggregates had activated α2β1. To ensure that the fluorescence signal resulted from IAC-1 binding to platelets, their cytoskeleton was visualized with Texas-Red phalloidin and the signals compared by confocal microscopy. Phalloidin-positive platelet aggregates were also positive for IAC-1 binding (Figure 7B). Furthermore, IAC-1 binding was not observed under conditions that prevent platelet activation, namely in the presence of PGE1 and ACD. Fewer platelets adhered to the collagen surface under these conditions. These platelets showed no pseudopodia formation and were positive for mAb Gi9, as expected.
Interaction of IAC-1 to platelets deposited on collagen under flow. (A-B) Anticoagulated whole human blood supplemented with 10 μg/mL IAC-1-FITC was perfused over a collagen-coated surface at a wall shear rate of 1000 s-1. (A) Platelet adhesion was determined as the percent surface coverage in the presence or absence of IAC-1-FITC. Error bars indicate the SEMs of at least 3 independent experiments. (B) Confocal microscopy analysis of adhered platelet aggregates after 4 minutes of perfusion showed that the signal of IAC-1-FITC (green) colocalized with platelets as detected by actin staining with Texas-Red phalloidin (red). (C) Postperfusion binding of FITC-labeled MOPC-21 (10 μg/mL), IAC-1 (10 μg/mL), or Gi9 (5 μg/mL) to platelets in the presence or absence of 10% ACD and 10 μM PGE1.
Interaction of IAC-1 to platelets deposited on collagen under flow. (A-B) Anticoagulated whole human blood supplemented with 10 μg/mL IAC-1-FITC was perfused over a collagen-coated surface at a wall shear rate of 1000 s-1. (A) Platelet adhesion was determined as the percent surface coverage in the presence or absence of IAC-1-FITC. Error bars indicate the SEMs of at least 3 independent experiments. (B) Confocal microscopy analysis of adhered platelet aggregates after 4 minutes of perfusion showed that the signal of IAC-1-FITC (green) colocalized with platelets as detected by actin staining with Texas-Red phalloidin (red). (C) Postperfusion binding of FITC-labeled MOPC-21 (10 μg/mL), IAC-1 (10 μg/mL), or Gi9 (5 μg/mL) to platelets in the presence or absence of 10% ACD and 10 μM PGE1.
Discussion
Prior to this study, monoclonal antibodies were raised in our laboratory against the recombinant I-domain of the α2β1 integrin. Even though some of these mAbs recognized the α2 I-domain, they failed to react with resting platelets. As the recombinant α2 I-domain bound specifically to collagens,6 and these mAbs also recognized denatured α2 I-domain, it seemed highly unlikely that this was due to an ill folding of the recombinant protein. More likely, these mAbs recognized epitopes accessible in the isolated I-domain but hidden in the intact α2 chain or in the folded α2β1 complex. We therefore searched this panel of mAbs for candidates with hidden epitopes that become accessible upon α2β1 activation. mAb 1D12 failed in this regard, compatible with an internal epitope even in activated α2β1. On the other hand, IAC-1 binds to α2β1 on activated but not on resting platelets as observed by flow cytometry and binding studies with 125I-labeled antibodies. As platelet activation is not associated with enhanced surface exposure of α2β1, IAC-1 binding thus detects a different conformation of α2β1 following platelet activation. This is supported by binding studies with soluble collagen, which specifically binds to α2β1 as verified by the neutralizing anti-α2 mAb Gi9. The increase in collagen binding to activated platelets parallels the increase in IAC-1 binding. In this respect, IAC-1 binding to α2β1 resembles the binding of mAb PAC-1 which recognizes the activated form of αIIbβ3.
IAC-1 is only the second reported antibody that recognizes an activated I-domain within an integrin α subunit. In contrast to mAb CBRM1/5,22 which recognizes αM I-domain, IAC-1 does not prevent ligand binding (ie, it has no affect on the α2β1-dependent platelet-collagen adhesion or on collagen-induced platelet aggregation). This means that it can be used to measure integrin activation in stimulated platelets. IAC-1 binds to residues T199Y200K201 at the “bottom” of the I-domain in the loop between β-strand C and helix 3, an area not affected by ligand binding. Ligand binding mainly induces a shift in the position of the Mg2+ binding site and in the MIDAS loops 1 and 3, accompanied by a major downward shift of helix 7 (the “bell rope” movement23 ) and a reorganization of helix C and helix 6.8 The change detected by IAC-1 therefore is either a conformational change within the I-domain induced by inside-out signals that are different from the ligand-induced changes, or an unmasking of I-domain epitopes by movement of other α2β1 domains covering the I-domain, such as the α2 propeller or the β1 I-like domain.
In this respect it is interesting to note that substitutions of sequences located at the bottom side of human αM with murine segments24 or with corresponding sequences from αL25 result in gain-of-function mutants. Remarkably, some mutations in the homologous A1 domain of VWF that result in an increase in affinity for its platelet receptor are located also in the same bottom area of the domain, although the ligand binding site within both VWF-A1 and -A3 domains is not located at the “top.”26-28 These bottom sequence segments are believed to interact with the β propeller, thus keeping the I-domain in the inactive state. In this scenario, IAC-1 may detect the release of these constraining interactions during I-domain activation.
IAC-1 defines a new class of integrin neo-epitopes as it is distinct from the 2 known categories defined by Bazzoni and Hemler.29 Following their definition, anti-CLIBS (cation- and ligand-induced binding site) antibodies define ligand-bound conformations after they are stabilized naturally by ligand or artificially by manganese or by an activating antibody. In contrast to the IAC-1 epitope, CLIBSs do not appear when the integrin is activated by inside-out signaling in the absence of integrin ligand. IAC-1, in addition, does not belong to the ligand-mimetic group as it does not bind to the ligand-binding site and certainly does not block ligand binding.
Depending on the strength of the agonist, more I-domain becomes capable of binding IAC-1. This could be either due to more α2β1 being activated or to the presence of 3 different conformational states as proposed by Jung and Moroi and Moroi et al based on their analyses of binding of soluble collagen.10-13,30 Following their definition, our results show that IAC-1 recognizes the intermediate-affinity state as its binding to platelets could be induced by ADP or by low concentrations of various agonists. In addition, high concentrations of platelet agonists result in an increased degree of IAC-1 binding (ie, IAC-1 also recognizes the high-affinity state). Furthermore, IAC-1 binding induced by low convulxin concentrations was strongly inhibited by the ADP scavenger apyrase, whereas activation induced by a high concentration of this agonist was far less sensitive to this inhibitor. This confirms previous results10,11 suggesting that released ADP is the main stimulant of α2β1 activation induced by low agonist concentrations, whereas several signal transduction pathways are activated by high agonist concentrations. In addition, we showed that the threshold agonist concentration to induce IAC-1 or collagen binding is identical.
Similarly to αIIbβ3, IAC-1 binding to platelets could be induced by a variety of platelet agonists acting via different signal transduction mechanisms (eg, GPVI activates a tyrosine kinase cascade through the Fc-receptor γ-chain, and U46619 acts through a Gq-linked signal transduction pathway). In addition, we demonstrate that activation of both integrins occurs over similar time courses and with comparable dose-response relationships. This points to common mechanisms of activation in both integrins.
So far, α2β1 activation could only be detected by studying collagen binding. In this study, we describe for the first time a monoclonal antibody that detects an activation-dependent structural change in integrin α2β1. Use of IAC-1 has several advantages: IAC-1 can be readily used in flow cytometry and gives a more robust signal than that seen with soluble collagen. In addition, since IAC-1 does not interfere with collagen binding, it detects activated α2β1, even when bound to collagen, under static or dynamic conditions.
In the present study, we for the first time provide evidence that α2β1 indeed undergoes a conformational change under dynamic flow conditions. Further studies are required to determine the role of α2β1 clustering and the distribution of activated integrins in platelet aggregates.
In conclusion, IAC-1 is the first antibody that detects an activation-dependent structural change in integrin α2β1. It furthermore defines a new class of neo-epitopes, and therefore is a powerful and important tool to further study the role of α2β1 activation in hemostasis and beyond.
Prepublished online as Blood First Edition Paper, March 23, 2004; DOI 10.1182/blood-2003-12-4224.
Supported by grant Geconcerteerde Onderzoeksaltie GOA/03/009, a postdoctoral fellowship of the Fonds voor Wetenschappelijk Onderzoek Flanders (K.V.), and Vlaams Instituut voor de Bevordering Van Het Wetenschappelyk-Technologisch Onderzoek in de Industrie grant IWT 21394 (H.B.F.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr A. Goodall (University of Leicester, United Kingdom) and Dr S. Vanderschueren (AZ Groeninge, Kortrijk, Belgium) for helping with initial flow cytometric analysis and C. Lecut (University of Maastricht, The Netherlands) for assistance with the perfusion studies. Dr R. Farndale (University of Cambridge, United Kingdom) kindly provided us with CRP and Dr A. Tuckwell (University of Manchester, United Kingdom) with the human/mouse chimeras of the α2 I-domain used in this study. We are grateful to Dr S. Watson (University of Birmingham, United Kingdom) and Dr P. Siljander (University of Cambridge, United Kingdom) for critically reading the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal