Abstract
Donor leukocyte infusion (DLI) can induce graft-versus-leukemia (GvL) reactions in patients with chronic myeloid leukemia (CML) relapsing after allogeneic bone marrow transplantation (BMT), but the mechanisms of the antileukemic effect of DLI are unknown, and the procedure is complicated by graft-versus-host disease (GvHD) and graft failure. Here, we adapted a murine retroviral BMT model of Philadelphia+ leukemia by combining allogeneic bone marrow (BM) from C57Bl/6 (H-2b) mice with BCR-ABL–transduced Balb/c (H-2d) BM, inducing mixed chimerism and myeloproliferative disease in recipients resembling relapse of CML following allogeneic BMT. Infusions of allogeneic splenocytes eliminated BCR-ABL–induced CML-like disease in the majority of mixed chimeras, with significant GvL effects mediated by both CD4+ and CD4- cells. BCR-ABL–induced acute B-lymphoblastic leukemia was also eradicated by DLI in major histocompatibility complex (MHC)–mismatched chimeras. Most DLI-treated mice converted to full allogeneic chimerism but succumbed frequently to GvHD or graft failure. When MHC-matched B10.D2 (H-2d) mice were the allogeneic donors, CML-like disease was more resistant to DLI. These results suggest that depletion of CD8+ cells from DLI could impair GvL against CML, while increased MHC disparity between donor and recipient may improve the responsiveness of Philadelphia+ B-lymphoblastic leukemia to DLI.
Introduction
Chronic myeloid leukemia (CML) is caused by the product of the Philadelphia (Ph) chromosome, the Bcr-Abl tyrosine kinase. Despite the clinical success of the Abl kinase inhibitor imatinib mesylate, allogeneic stem cell transplantation (alloSCT) remains the only known curative treatment for CML. However, because of age restrictions and lack of suitable donors, SCT is only available to a minority of patients and can be complicated by recurrence of leukemia, the toxicity of the conditioning regimen, or potentially fatal graft-versus-host disease (GvHD).
A graft-versus-leukemia (GvL) effect of alloSCT is apparent from the higher risk of relapse with an identical twin donor compared with a human leukocyte antigen (HLA)–identical sibling, in recipients without clinical GvHD,1 and with T-cell depletion of the graft.2 For unknown reasons, CML is the most GvL sensitive of the leukemias.3 The potency of GvL in CML was illustrated directly by the demonstration that infusion of leukocytes from the allogeneic donor (donor leukocyte infusion or transfusion, DLI) could induce remissions in the majority of patients with CML who relapsed following alloSCT,4 but this came at the cost of significant GvHD and graft failure.5,6 Efforts to preserve the GvL effect of DLI while decreasing the severity of GvHD included modulating the number of total7 or CD8+8,9 T lymphocytes infused. Nonmyeloablative or reduced-intensity conditioning regimens can induce mixed hematopoietic chimerism in recipients,10,11 but DLI and conversion to full allogeneic chimerism is still complicated by frequent GvHD. Further improvements in adoptive immunotherapy for leukemia will require a better understanding of the basic immunologic mechanisms involved. For instance, it is not known to what extent GvL and GvHD are separate processes or what role minor histocompatibility or leukemia-specific antigens play in DLI responses.3
One approach to addressing these basic questions is to model adoptive immunotherapy of leukemia in mice. Most mouse models of DLI have used malignant hematopoietic cell lines such as BCL1 or EL4 as the source of leukemia.12-15 In these models, GvHD is mediated principally by CD4+ T cells, whereas GvL requires both CD4+ and CD8+ cells,16-18 but this varies with the mouse strain and type of leukemia cell line used. Significantly greater GvL effects are usually observed with major histocompatibility complex (MHC)–mismatched compared with MHC-matched allogeneic donors,12,19,20 but the mechanisms of GvL may differ.12 Mechanisms of GvHD and GvL21,22 and strategies to augment GvL and/or reduce GvHD with cytokines such as interleukin 11 (IL-11),23 IL-12,18 and IL-1824 have been explored in these models. However, the pathophysiology of the disease induced by immortalized cell lines differs significantly from human CML, which is a myeloproliferative disease originating from multipotential hematopoietic stem cells expressing the BCR-ABL oncogene.
We established previously BCR-ABL retroviral transduction/bone marrow transplantation (BMT) models of human Ph+ leukemia, which allow induction in mice of leukemias that resemble closely chronic phase CML25 and Ph+ B-cell acute lymphoblastic leukemia (B-ALL).26 The BCR-ABL–induced CML-like disease originates from multipotential stem/progenitor cells,25 is transplantable to secondary recipients,25,27 can progress to blast crisis,27 and is responsive to imatinib mesylate therapy.28 Here, we have adapted this model system to produce mixed allogeneic hematopoietic chimerism in recipients with BCR-ABL–induced CML-like myeloproliferative disease or B-ALL, similar to Ph+ leukemia that has relapsed following alloSCT. We further demonstrate significant antileukemic responses with adoptive immunotherapy.
Materials and methods
Bone marrow transduction and transplantation
For induction of CML-like leukemia, bone marrow (BM) was harvested from male Balb/c (H-2d) mice (all mice from Taconic Farms, Germantown, MD) 4 days after injection with 200 mg/kg 5-fluorouracil (5-FU) and transduced with p210 BCR-ABL MSCV-IRES/GFP retrovirus as described.25 T-cell depletion of BM was performed by using antibodies against CD4 and CD8 (Pharmingen, San Diego, CA) and magnetic microbeads (Miltenyi Biotec, Auburn, CA) according to the manufacturer's instructions. The ratio of syngeneic to allogeneic marrow necessary for induction of mixed hematopoietic chimerism in recipients reflects their relative stem cell content and the allogeneic engraftment barrier and was determined empirically in preliminary experiments (data not shown). The BM grafts were mixed together at a 1:150 ratio for the Balb/c:B6 strain combination (infusing 2 × 105 and 3 × 107 cells, respectively) and at a 1:30 ratio for the Balb/c:B10.D2 (H-2d) combination (2 × 105 and 6 × 106 BM cells, respectively), and injected intravenously into lethally irradiated (1200 cGy) female Balb/c recipients. For induction of B-ALL, BM from donors not treated with 5-FU was transduced once without cytokines as described,26 T-cell–depleted and 1 × 106 transduced cells were cotransplanted with 4 × 106 T-cell–depleted (TCD) B6 BM cells.
Donor leukocyte infusions
Splenocytes from 5- to 10-week-old B6 (H-2b) or B10.D2 (H-2d) mice were injected intravenously at a dose of 3.5 to 4.0 × 107 per recipient. In the early DLI protocol, splenocyte infusions were administered on days 14, 17, and 20 after BMT with the Balb/c:B6 strain combination or on days 15, 17, and 21 with the Balb/c:B10.D2 strain combination. In case of persistent leukemia, another dose of DLI was administered. One cohort of mice in each transplantation received the 2 BM grafts along with a single dose of 1.5 × 107 allogeneic splenocytes at the time of BMT. Positive selection (CD4+) and depletion (CD4-) of allogeneic CD4-expressing splenocytes was performed using antibody to CD4 (Pharmingen) and magnetic microbeads. The selected populations were more than 98% positive or negative by flow cytometric analysis of CD4 expression, as appropriate (data not shown). Flow cytometric analysis of normal B6 splenocytes demonstrated about 20% CD4+ cells (data not shown); hence, we injected about 8 × 106 CD4+ or 3.2 × 107 CD4- spleen cells for each DLI, equivalent to the absolute number of CD4+ and CD4- cells, respectively, in a total of 4 × 107 splenocytes.
Analysis of diseased mice
Premorbid animals were subjected to histopathologic analysis as described previously.25 Photomicrographs were obtained using an Olympus BH2 microscope (Olympus, Melville, NY) with 100 × Plan Achromat oil objective, and were acquired with an Olympus Qcolor5 digital camera and QCapture Suite software (Quantitative Imaging, Burnaby, BC, Canada). Hematopoietic chimerism was analyzed by costaining with biotinylated anti-H-2Dd (clone 34-2-12; Pharmingen) and Cy-Chrome–conjugated Streptavidin (Pharmingen), along with phycoerythrin (PE)–conjugated anti-H-2Db (clone CTDb; Cedarlane Laboratories, Hornby, ON, Canada). In the Balb/c:B10.D2 strain combination, allogeneic chimerism was determined by staining with a monoclonal antibody against the β2-microglobulinb polymorphism (clone S19.8; Pharmingen), which was biotinylated and secondarily stained with Streptavidin-Cy-Chrome. The presence of GFP+ leukemia was assessed by staining with PE-conjugated anti-H-2Dd or anti-Mac-1 (for CML-like leukemia), or with PE-conjugated anti-BP-1 (for B-ALL).
Southern blot analysis
Genomic DNA was digested with BglII, transferred to nylon membranes and hybridized with a radioactive probe from the GFP gene to detect distinct proviral integration events and subsequently with a human ABL probe to determine the total proviral content of each sample.25 A proviral copy number of less than 0.2 is consistent with the absence of provirus from a particular tissue. Finally, membranes were hybridized with a murine Cadherin-11 gene probe that detects a BglII restriction fragment length polymorphism between Balb/c and C57Bl/6, yielding a 7.2-kb fragment from Balb/c DNA and a 4.7-kb fragment from B6 DNA.29
Results
Induction of mixed hematopoietic chimerism and CML-like myeloproliferative disease in mice
We transduced BM from 5-FU–treated Balb/c (H-2d) donors with retrovirus coexpressing BCR-ABL and green fluorescent protein (GFP), and following T-cell depletion, transplanted the transduced syngeneic BM with TCD BM from fully MHC-mismatched C57Bl/6 mice (B6; H-2b) into lethally irradiated Balb/c recipients (Figure 1A). Without addition of allogeneic BM, all recipients developed CML-like myeloproliferative disease and succumbed within 30 days after transplantation because of overwhelming infiltration of spleen, liver, and lungs with maturing neutrophils,25 with pulmonary hemorrhage as the primary cause of morbidity or death (data not shown). When allogeneic splenocytes were included in the graft along with TCD allogeneic BM, all recipients engrafted by day 14 after transplantation as full allogeneic chimeras (Figure 1B). There was no evidence of H-2d+/GFP+ CML-like leukemia at any time (Figure 1B), and all recipients had normal or low PBL counts. All of these mice succumbed to severe GvHD with weight loss, alopecia, and diarrhea but no ascites. Because cotransplantation of allogeneic BM and splenocytes favors reconstitution by allogeneic stem cells,15,30 it is likely that this procedure prevents leukemia by blocking engraftment of syngeneic BCR-ABL–expressing hematopoietic stem cells but may not realistically model immunotherapy of established CML.
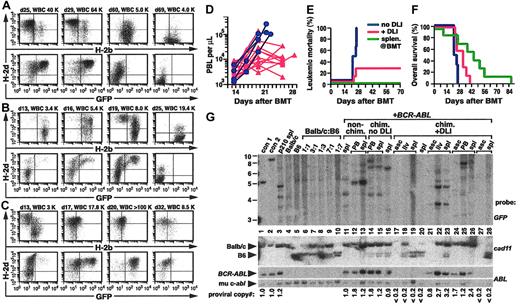
Establishment of mixed hematopoietic chimerism and CML-like leukemia in mice. (A) Schematic diagram of the adoptive immunotherapy model system. TCD BM from 5-FU–treated Balb/c mice is transduced with retrovirus coexpressing BCR-ABL and GFP (top), then mixed with TCD BM from allogeneic C57Bl/6 donors (middle) and transplanted into lethally irradiated Balb/c recipients (right). Allogeneic splenocytes from the B6 donor (DLI) are infused into mice with mixed hematopoietic chimerism and established CML-like leukemia (bottom). In some experiments, allogeneic splenocytes are included in the initial BM graft (dotted arrow). (B) Flow cytometric analysis of peripheral blood leukocytes (PBLs) from a representative mouse that received TCD BCR-ABL–transduced syngeneic (Balb/c) and TCD allogeneic (B6) BM along with 1.5 × 107 allogeneic B6 splenocytes as the initial graft, analyzed day 17 after transplantation (PBL count, 5.6 × 109/L [5600/μL]). Hematopoietic chimerism (top panel) was analyzed with antibodies against H-2b (x-axis) and H-2d (y-axis), whereas CML-like leukemia (bottom panel) is indicated by cells coexpressing H-2d (y-axis) and GFP (x-axis). The mouse was engrafted as a full allogeneic chimera with no signs of CML-like disease. (C) Flow cytometric analysis of PBLs, as in panel B, from a representative mouse that received TCD BCR-ABL–transduced syngeneic (Balb/c) and TCD allogeneic (B6) BM without splenocytes, analyzed day 19 after transplantation. The PBL count was 30 × 109/L (30 000/μL), and the allogeneic chimerism was 35% H-2b+; note the prominent population of H-2d+/GFP+ cells (bottom panel), indicative of CML-like leukemia. (D) Wright-Giemsa–stained peripheral blood (PB) from a chimeric mouse with CML-like myeloproliferative disease, demonstrating increased circulating maturing myeloid cells (magnification × 400). (E-G) Coexpression of H-2b and H-2d in PBLs from mixed chimeras. Flow cytometric analysis of chimerism in a recipient of untransduced TCD BM from 5-FU–treated Balb/c donors and TCD normal B6 BM. Note the significant population of double-positive cells (E) that are not observed when PBLs from Balb/c and B6 mice are either stained for both antigens and mixed prior to analysis (F) or mixed followed by staining (G). In leukemic chimeras, this population included Bcr-Abl–expressing myeloid cells, as assessed by GFP expression and the forward-side scatter distribution.
Establishment of mixed hematopoietic chimerism and CML-like leukemia in mice. (A) Schematic diagram of the adoptive immunotherapy model system. TCD BM from 5-FU–treated Balb/c mice is transduced with retrovirus coexpressing BCR-ABL and GFP (top), then mixed with TCD BM from allogeneic C57Bl/6 donors (middle) and transplanted into lethally irradiated Balb/c recipients (right). Allogeneic splenocytes from the B6 donor (DLI) are infused into mice with mixed hematopoietic chimerism and established CML-like leukemia (bottom). In some experiments, allogeneic splenocytes are included in the initial BM graft (dotted arrow). (B) Flow cytometric analysis of peripheral blood leukocytes (PBLs) from a representative mouse that received TCD BCR-ABL–transduced syngeneic (Balb/c) and TCD allogeneic (B6) BM along with 1.5 × 107 allogeneic B6 splenocytes as the initial graft, analyzed day 17 after transplantation (PBL count, 5.6 × 109/L [5600/μL]). Hematopoietic chimerism (top panel) was analyzed with antibodies against H-2b (x-axis) and H-2d (y-axis), whereas CML-like leukemia (bottom panel) is indicated by cells coexpressing H-2d (y-axis) and GFP (x-axis). The mouse was engrafted as a full allogeneic chimera with no signs of CML-like disease. (C) Flow cytometric analysis of PBLs, as in panel B, from a representative mouse that received TCD BCR-ABL–transduced syngeneic (Balb/c) and TCD allogeneic (B6) BM without splenocytes, analyzed day 19 after transplantation. The PBL count was 30 × 109/L (30 000/μL), and the allogeneic chimerism was 35% H-2b+; note the prominent population of H-2d+/GFP+ cells (bottom panel), indicative of CML-like leukemia. (D) Wright-Giemsa–stained peripheral blood (PB) from a chimeric mouse with CML-like myeloproliferative disease, demonstrating increased circulating maturing myeloid cells (magnification × 400). (E-G) Coexpression of H-2b and H-2d in PBLs from mixed chimeras. Flow cytometric analysis of chimerism in a recipient of untransduced TCD BM from 5-FU–treated Balb/c donors and TCD normal B6 BM. Note the significant population of double-positive cells (E) that are not observed when PBLs from Balb/c and B6 mice are either stained for both antigens and mixed prior to analysis (F) or mixed followed by staining (G). In leukemic chimeras, this population included Bcr-Abl–expressing myeloid cells, as assessed by GFP expression and the forward-side scatter distribution.
To develop a more physiologically relevant model, we cotransplanted TCD BCR-ABL–transduced syngeneic BM and TCD allogeneic BM in the appropriate ratio (see “Materials and methods”) into lethally irradiated recipients without including allogeneic splenocytes in the graft (Figure 1A). On day 13 after BMT, all recipients engrafted with PBL counts that were normal or slightly increased and demonstrated mixed allogeneic chimerism with 20% to 60% of cells expressing H-2b (Figure 1C). These chimeras also had clinical evidence of CML-like myeloproliferative disease, with splenomegaly and 10% to 50% circulating H-2d+/GFP+ cells (Figure 1C) that were predominantly maturing neutrophils (Figure 1D). These recipients therefore resembled patients with CML who relapse following alloSCT, and without treatment all succumbed to CML-like myeloproliferative disease (Figure 2E) that was clinicopathologically identical to that described previously.25 Interestingly, peripheral blood of both leukemic and, to a lesser extent, nonleukemic mixed chimeras frequently showed significant populations of cells staining for both H-2b and H-2d, which may represent cell adhesion or fusion occurring in vivo (Figure 1E-G).
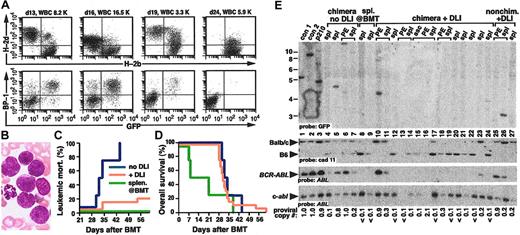
DLI in mixed chimeras leads to eradication of CML-like disease. (A) Serial flow cytometric analysis of PBL chimerism (top row) and CML-like leukemia (bottom row) from a representative mouse with mixed chimerism and CML-like leukemia treated with DLI beginning day 25 after transplantation. Blood sampling and analysis were performed on days 25, 29, 60, and 69 after BMT, with the PBL count indicated. DLI was administered on days 25, 30, and 40, with a total dose of 1.2 × 108 allogeneic splenocytes. Note the disappearance of GFP+ cells by day 60, accompanied by conversion to full allogeneic chimerism. (B) Earlier initiation of DLI improves the antileukemic response. Serial analysis of chimerism and leukemia were conducted as in panel A, from a representative mouse with mixed chimerism and CML-like myeloproliferative disease treated with early DLI. Blood sampling and analysis were performed on days 13, 16, 19, and 25 after BMT, and DLI was administered on days 14, 17, 20, and 23 with a total of 1.7 × 108 allogeneic splenocytes. (C) Persistent mixed chimerism in some recipients cured of CML-like leukemia by DLI. Serial analysis of chimerism and leukemia were conducted as in panel A, in a recipient treated with early DLI (days 14, 18, 23, and 26 with a total of 1.3 × 108 allogeneic splenocytes). Note the eradication of GFP+ leukemia cells but persistence of mixed chimerism with predominantly syngeneic (H-2d+) leukocytes. (D) Plot of PB counts (y-axis, logarithmic scale) as a function of time (x-axis) after BMT (blue circles indicate chimeras with CML-like disease but no DLI treatment; red triangles, chimeras with CML-like disease and early treatment with DLI). (E-F) Cumulative mortality as a result of leukemia (E) and overall survival (F) for recipients of TCD BCR-ABL–transduced and TCD B6 BM, either without DLI treatment (blue, n = 6), with administration of allogeneic splenocytes at the time of BMT (green, n = 7), or with early DLI treatment of established mixed chimeras with CML-like leukemia (red, n = 7), in a representative transplantation cohort (1 of 4 independent experiments). The frequency of fatal CML in the DLI-treated group was significantly lower than in untreated chimeras (P = .021, Fisher exact test) and the DLI-treated group's survival was significantly longer (P = .041, Mantel-Cox test). (G) DLI in chimeric mice leads to eradication of BCR-ABL proviral clones. Genomic DNA from the indicated tissues (spl indicates spleen; asc, ascites; liv, liver) was analyzed by Southern blot with a GFP gene probe to detect distinct bands from each provirus integration site (upper panel), a Cadherin-11 gene probe that distinguishes between Balb/c- or B6-derived DNA (middle panel), and an ABL probe that allows determination of the total proviral content of each sample (bottom panels). Con1 and con2 are control DNAs from cell lines that each contained a single BCR-ABL provirus, whereas p210 spl is spleen DNA from a nonchimeric mouse with BCR-ABL–induced CML-like disease. Lanes 6 to 10 contain DNA from normal Balb/c (lane 4) or B6 spleen (lane 5) or mixtures of Balb/c and B6 DNA at the indicated ratios. Lanes 11 to 13 are from mice that received BCR-ABL–transduced BM only and were not chimeras, lanes 14 to 16 are from mice that were chimeric but not treated with DLI, and lanes 17 to 28 are from chimeric mice treated with DLI. Brackets indicate samples from the same individual mouse. Note that in 3 mixed chimeric mice with CML-like leukemia, DLI led to the disappearance of BCR-ABL proviral clones and the representative tissues (liver or spleen) are either chimeric (lane 18) or completely donor-derived (lanes 20 and 28).
DLI in mixed chimeras leads to eradication of CML-like disease. (A) Serial flow cytometric analysis of PBL chimerism (top row) and CML-like leukemia (bottom row) from a representative mouse with mixed chimerism and CML-like leukemia treated with DLI beginning day 25 after transplantation. Blood sampling and analysis were performed on days 25, 29, 60, and 69 after BMT, with the PBL count indicated. DLI was administered on days 25, 30, and 40, with a total dose of 1.2 × 108 allogeneic splenocytes. Note the disappearance of GFP+ cells by day 60, accompanied by conversion to full allogeneic chimerism. (B) Earlier initiation of DLI improves the antileukemic response. Serial analysis of chimerism and leukemia were conducted as in panel A, from a representative mouse with mixed chimerism and CML-like myeloproliferative disease treated with early DLI. Blood sampling and analysis were performed on days 13, 16, 19, and 25 after BMT, and DLI was administered on days 14, 17, 20, and 23 with a total of 1.7 × 108 allogeneic splenocytes. (C) Persistent mixed chimerism in some recipients cured of CML-like leukemia by DLI. Serial analysis of chimerism and leukemia were conducted as in panel A, in a recipient treated with early DLI (days 14, 18, 23, and 26 with a total of 1.3 × 108 allogeneic splenocytes). Note the eradication of GFP+ leukemia cells but persistence of mixed chimerism with predominantly syngeneic (H-2d+) leukocytes. (D) Plot of PB counts (y-axis, logarithmic scale) as a function of time (x-axis) after BMT (blue circles indicate chimeras with CML-like disease but no DLI treatment; red triangles, chimeras with CML-like disease and early treatment with DLI). (E-F) Cumulative mortality as a result of leukemia (E) and overall survival (F) for recipients of TCD BCR-ABL–transduced and TCD B6 BM, either without DLI treatment (blue, n = 6), with administration of allogeneic splenocytes at the time of BMT (green, n = 7), or with early DLI treatment of established mixed chimeras with CML-like leukemia (red, n = 7), in a representative transplantation cohort (1 of 4 independent experiments). The frequency of fatal CML in the DLI-treated group was significantly lower than in untreated chimeras (P = .021, Fisher exact test) and the DLI-treated group's survival was significantly longer (P = .041, Mantel-Cox test). (G) DLI in chimeric mice leads to eradication of BCR-ABL proviral clones. Genomic DNA from the indicated tissues (spl indicates spleen; asc, ascites; liv, liver) was analyzed by Southern blot with a GFP gene probe to detect distinct bands from each provirus integration site (upper panel), a Cadherin-11 gene probe that distinguishes between Balb/c- or B6-derived DNA (middle panel), and an ABL probe that allows determination of the total proviral content of each sample (bottom panels). Con1 and con2 are control DNAs from cell lines that each contained a single BCR-ABL provirus, whereas p210 spl is spleen DNA from a nonchimeric mouse with BCR-ABL–induced CML-like disease. Lanes 6 to 10 contain DNA from normal Balb/c (lane 4) or B6 spleen (lane 5) or mixtures of Balb/c and B6 DNA at the indicated ratios. Lanes 11 to 13 are from mice that received BCR-ABL–transduced BM only and were not chimeras, lanes 14 to 16 are from mice that were chimeric but not treated with DLI, and lanes 17 to 28 are from chimeric mice treated with DLI. Brackets indicate samples from the same individual mouse. Note that in 3 mixed chimeric mice with CML-like leukemia, DLI led to the disappearance of BCR-ABL proviral clones and the representative tissues (liver or spleen) are either chimeric (lane 18) or completely donor-derived (lanes 20 and 28).
Eradication of CML-like leukemia by DLI
We used mixed chimeras with CML-like disease as recipients for DLI. In initial experiments, we started DLI beginning day 25 after transplantation with an intravenous dose of 4 × 107 allogeneic splenocytes from normal B6 mice. The recipients were followed by serial sampling of peripheral blood and flow cytometric analysis, and DLI was repeated on day 30 and once or twice thereafter, based on the clinical response. With this protocol, we were able to successfully eradicate the CML-like disease in some recipients, with elimination of the circulating leukemic (H-2d+/GFP+) cells and gradual conversion to full donor (H-2b+) chimerism (Figure 2A). However, in the majority of recipients, the CML-like leukemia was quite advanced by day 25 with circulating leukemic cells in excess of 105/μL, leading to loss of detectable mixed chimerism and a minimal response to DLI (data not shown). As clinical experience has shown that a response to adoptive immunotherapy requires donor chimerism and is most effective in the setting of minimal disease burden, we altered the schedule of DLI to include infusions beginning on day 14 after transplantation, with 2 or 3 subsequent treatments on days 17 to 18, 20 to 23, and 26 to 28, depending on the clinical response. With this protocol, CML-like leukemia was eradicated successfully in the majority of recipients (Table 1). Most recipients converted to full allogeneic chimerism with predominantly or exclusively H-2b+ leukocytes (Figure 2B), whereas a few maintained mixed hematopoietic chimerism despite being free of CML-like leukemia (Figure 2C). The latter mice demonstrate that nonleukemic syngeneic stem cells in the BM graft can reconstitute normal hematopoiesis following successful immunotherapy of leukemia.
Clinicopathological characteristics of mice with CML-like leukemia treated with MHC-mismatched DLI
. | . | . | CML* . | . | . | . | . | . | GvHD† . | . | . | . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | PBL, × 103/μL . | . | . | . | . | . | PBL, × 103/μL . | . | . | . | . | ||||||||||
| Experimental group . | No. . | Median survival, d . | Freq, % . | Day 17-22 . | Day 23-49 . | GFP+ %‡ . | Chim, %§ . | Spleen wt, mg . | Freq % . | Day 17-22 . | Day 23-49 . | GFP+ %‡ . | Chim, %§ . | Spleen wt, mg . | ||||||||||
| Non-chimeric, no DLI | 15 | 21.5 | 100 | 50 ± 11 | 92 ± 21 | 54 ± 4 | NA | 531 ± 60 | 0 | NA | NA | NA | NA | NA | ||||||||||
| Chimeric, no DLI | 12 | 22.5∥ | 100¶ | 42 ± 10 | 125 ± 38# | 60 ± 3 | 9 ± 2 | 600 ± 25# | 0 | NA | NA | NA | NA | NA | ||||||||||
| Chimeric, + DLI | 41 | 28.0∥ | 32¶ | 42 ± 11 | 91 ± 17 | 61 ± 4 | 4 ± 2 | 502 ± 27 | 68 | 14 ± 3 | 10 ± 1# | 2 ± 0 | 78 ± 3 | 108 ± 8# | ||||||||||
| Splenocytes at BMT | 6 | 53 | 0 | NA | NA | NA | NA | NA | 100 | 3 ± 0.8 | 2 ± 0.4 | 2 ± 1 | 67 ± 4 | 23 ± 6 | ||||||||||
. | . | . | CML* . | . | . | . | . | . | GvHD† . | . | . | . | . | . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | . | . | . | PBL, × 103/μL . | . | . | . | . | . | PBL, × 103/μL . | . | . | . | . | ||||||||||
| Experimental group . | No. . | Median survival, d . | Freq, % . | Day 17-22 . | Day 23-49 . | GFP+ %‡ . | Chim, %§ . | Spleen wt, mg . | Freq % . | Day 17-22 . | Day 23-49 . | GFP+ %‡ . | Chim, %§ . | Spleen wt, mg . | ||||||||||
| Non-chimeric, no DLI | 15 | 21.5 | 100 | 50 ± 11 | 92 ± 21 | 54 ± 4 | NA | 531 ± 60 | 0 | NA | NA | NA | NA | NA | ||||||||||
| Chimeric, no DLI | 12 | 22.5∥ | 100¶ | 42 ± 10 | 125 ± 38# | 60 ± 3 | 9 ± 2 | 600 ± 25# | 0 | NA | NA | NA | NA | NA | ||||||||||
| Chimeric, + DLI | 41 | 28.0∥ | 32¶ | 42 ± 11 | 91 ± 17 | 61 ± 4 | 4 ± 2 | 502 ± 27 | 68 | 14 ± 3 | 10 ± 1# | 2 ± 0 | 78 ± 3 | 108 ± 8# | ||||||||||
| Splenocytes at BMT | 6 | 53 | 0 | NA | NA | NA | NA | NA | 100 | 3 ± 0.8 | 2 ± 0.4 | 2 ± 1 | 67 ± 4 | 23 ± 6 | ||||||||||
All values are mean ± SE. Freq, indicates frequency; Chim, chimerism; wt, weight; NA, not applicable.
CML-like disease defined by leukocytosis with GFP+/Mac-1+ cells, splenomegaly, pulmonary myeloid infiltrates
GvHD defined by weight loss or diarrhea with mononuclear infiltration of dermis, hepatic portal tracts, and intestinal crypts
Percentage of GFP+ peripheral blood leukocytes at time of morbidity or death
Allogeneic chimerism, defined as percentage of H-2b+ peripheral blood leukocytes at time of morbidity or death
Significantly different (P < .0001, Mantel-Cox test)
Significantly different (P < .0001, Fisher Exact test)
Significantly different for Day 23-49 PBL counts and spleen weights (P < .0001, unpaired t test)
Early administration of DLI promptly reduced PBL counts (Figure 2D; Table 1) and resulted in elimination of CML-like leukemia in 70% of recipients (Figure 2E; Table 1), whereas all chimeric mice that did not receive DLI died of CML-like disease by day 25 with extreme leukocytosis (Figure 2D-E). DLI treatment significantly prolonged the survival of recipients in comparison to the untreated group (Table 1), but most DLI-treated mice became premorbid by day 40 (Figure 2F) because of development of GvHD or occasionally of graft failure (see next paragraph). Mice that received splenocytes at the time of transplantation neither developed nor died of CML-like leukemia (Figure 2D), but all succumbed to severe GvHD with a median survival of 53 days (Figure 2F).
It is important to demonstrate that the antileukemic responses observed in peripheral blood correlate with elimination of leukemia from other tissues. At necropsy, DLI-treated mice showed no pulmonary hemorrhage or splenomegaly, and histopathologic analysis demonstrated no infiltration of the lungs, liver, or spleen by myeloid cells (data not shown). We also assessed chimerism and the presence of leukemia in different tissues by Southern blot analysis of genomic DNA (Figure 2G), using a GFP probe to detect individual BCR-ABL proviral clones, an ABL probe to quantitate proviral copy number, and a Cadherin-11 probe29 to detect a restriction fragment length polymorphism between Balb/c and B6 DNA (see “Materials and methods”). As described previously,25 the CML-like disease induced in nonchimeric recipients was oligoclonal to polyclonal (Figure 2G, lanes 3, 11, and 12) and was similar to that induced in untreated mixed chimeras (Figure 2G, lanes 14-16), indicating that cotransplantation of TCD allogeneic BM does not interfere with engraftment of BCR-ABL–transduced stem cells. In a few recipients, circulating leukemic cells that persisted after DLI correlated with high levels (1-2 proviral copies per cell) of the BCR-ABL provirus in tissues and minimal or absent allogeneic chimerism (Figure 2G, lanes 21-23 and 24-26). In contrast, in DLI-treated mice with eradication of CML-like leukemia in peripheral blood, BCR-ABL provirus was reduced to undetectable levels (< 0.2 proviral copies per cell), and spleens were predominantly derived from cells of allogeneic B6 origin (Figure 2G, lanes 17-19, 20, and 28). Together, these results demonstrate that adoptive immunotherapy can completely eradicate CML-like leukemia in mixed chimeras.
Adoptive immunotherapy of CML-like leukemia is complicated by GvHD and graft failure
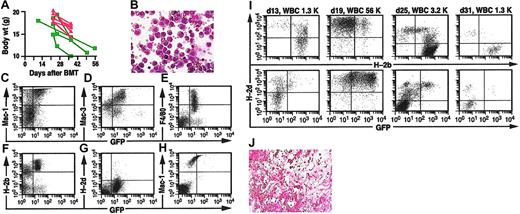
Acute graft-versus-host disease is a frequent and debilitating complication of alloSCT and DLI.31 In our experiments, the majority of the mice treated with DLI and cured of their CML-like disease became premorbid or died around day 35 to 40 after transplantation with hunched posture, ruffled fur, diarrhea, and weight loss that was more severe in mice that received allogeneic splenocytes at the time of BMT than in chimeric mice treated with DLI (Figure 3A). Histopathologic analysis (data not shown) revealed typical features of murine GvHD, with mononuclear cell infiltration of dermis, hepatic portal tracts, and intestinal submucosa accompanied by dyskeratosis and villous blunting. Interestingly, chimeric mice with GvHD following DLI often developed ascites or pleural effusions or both that was not observed in mice that developed GvHD from allogeneic splenocytes administered at the time of BMT. The ascites was predominantly composed of histiocyte-like cells (Figure 3B) expressing macrophage markers (Figure 3C-E) that were of allogeneic (B6) origin by flow cytometric analysis (H-2b+/H-2d-; Figure 3F-G) but also expressed GFP at low levels (Figure 3F). At necropsy, no GFP+ Mac-1+ leukocytes could be found in the peripheral blood of these mice (Figure 3H), and blood and BM showed complete allogeneic chimerism (Figure 2B). The low level of GFP expression in these histiocytes might reflect fusion with or phagocytosis of leukemic cells. Similar populations of allogeneic macrophages were also frequently observed in ascites or pleural effusions from nonleukemic mixed chimeras that received DLI (data not shown).
Successful immunotherapy of CML-like leukemia is complicated by GvHD with ascites and by graft failure. (A) Plot of body weight in grams (y-axis) as a function of time after BMT for the recipients in Figure 2E, showing chimeric mice treated with DLI (red) and mice that received allogeneic splenocytes at BMT (green). (B) Wright-Giemsa stain of histiocytic cells in ascites from a representative mixed chimeric mouse with CML-like leukemia that developed GvHD after DLI (magnification × 400). (C-G) Flow cytometric analysis of ascitic fluid from panel B. Analysis of GFP expression (x-axis) versus the macrophage markers Mac-1 (C), Mac-3 (D), and F4/80 (E), and MHC class I antigens H-2b (F) and H-2d (G). Note that H-2b+/H-2d- histiocytic cells express macrophage markers and low levels of GFP. (H) PB leukocytes from this mouse, analyzed for expression of Mac-1 (y-axis) and GFP (x-axis). Note that circulating GFP+ myeloid cells have been eradicated. (I) Analysis of chimerism (top row) and CML-like leukemia (bottom row) in a mixed chimera that developed pancytopenia following successful DLI. On day 31, the PB hemoglobin was 48 g/L (4.8 g/dL) and platelet count was 213 ×109/L (213 000/μL). (J) Hematoxylin and eosin (H&E)–stained bone marrow section from a representative mouse with pancytopenia after DLI, showing severe cellular hypoplasia (magnification × 150).
Successful immunotherapy of CML-like leukemia is complicated by GvHD with ascites and by graft failure. (A) Plot of body weight in grams (y-axis) as a function of time after BMT for the recipients in Figure 2E, showing chimeric mice treated with DLI (red) and mice that received allogeneic splenocytes at BMT (green). (B) Wright-Giemsa stain of histiocytic cells in ascites from a representative mixed chimeric mouse with CML-like leukemia that developed GvHD after DLI (magnification × 400). (C-G) Flow cytometric analysis of ascitic fluid from panel B. Analysis of GFP expression (x-axis) versus the macrophage markers Mac-1 (C), Mac-3 (D), and F4/80 (E), and MHC class I antigens H-2b (F) and H-2d (G). Note that H-2b+/H-2d- histiocytic cells express macrophage markers and low levels of GFP. (H) PB leukocytes from this mouse, analyzed for expression of Mac-1 (y-axis) and GFP (x-axis). Note that circulating GFP+ myeloid cells have been eradicated. (I) Analysis of chimerism (top row) and CML-like leukemia (bottom row) in a mixed chimera that developed pancytopenia following successful DLI. On day 31, the PB hemoglobin was 48 g/L (4.8 g/dL) and platelet count was 213 ×109/L (213 000/μL). (J) Hematoxylin and eosin (H&E)–stained bone marrow section from a representative mouse with pancytopenia after DLI, showing severe cellular hypoplasia (magnification × 150).
A second, less frequent complication of DLI was the development of graft failure (Figure 3I). This was characterized by a premorbid condition associated with pancytopenia (PBL count, < 2.0 ×109/L [< 2000/μL]; hemoglobin [Hgb], 30-50 g/L [3-5 g/dL]; platelet count 100-200 × 109/L [100-200 × 103/μL]) and bone marrow hypoplasia (Figure 3J) following successful eradication of CML-like leukemia by DLI. Most circulating leukocytes in these mice were derived from the allogeneic donor (Figure 3I), and the principal cause of morbidity or death appeared to be severe anemia. This condition resembles graft failure following DLI for CML, which is characterized by myelosuppression with leukopenia, thrombocytopenia, and anemia with reticulocytopenia.5
Both CD4+ and CD4- splenocytes can mediate GvL against CML-like leukemia
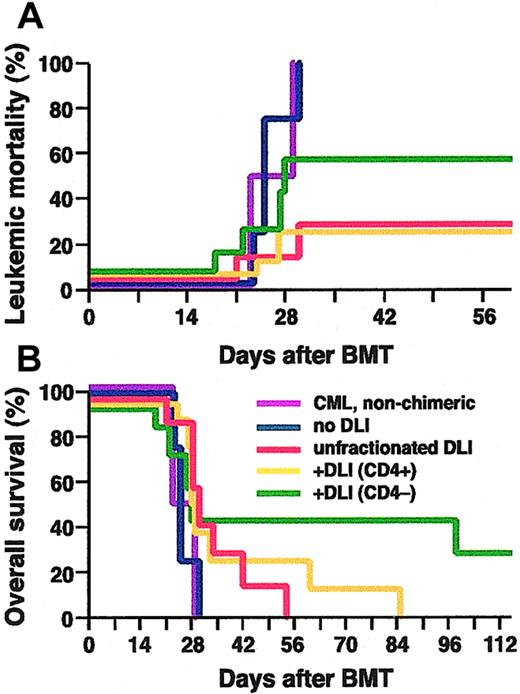
Several studies of DLI in CML have indicated that GvHD may be minimized with preservation of the antileukemic effect by reductions in the dose of CD8+ cells in the infusion,8,9 suggesting that CD4+ T cells might be most important for mediating GvL against CML. To investigate the lymphocyte subsets responsible for the GvL effect in our model system, we compared the response of chimeric mice with CML-like disease that received infusions of unfractionated allogeneic splenocytes to cohorts infused with splenocyte populations that were either depleted of (CD4-) or selected for (CD4+) CD4-expressing cells (Figure 4). Leukemic mice that were either nonchimeric or chimeric but untreated succumbed to CML-like disease within 30 days after transplantation. The GvL effect of infusion of equivalent doses of CD4+ splenocytes was about the same as infusion of unfractionated splenocytes, whereas CD4- splenocytes were somewhat less potent (Figure 4A). CML-like leukemia was eradicated in 71% (5 of 7) of chimeric mice infused with a total of 1.6 × 108 unfractionated splenocytes, in 75% (6 of 8) mice infused with 3.2 × 107 CD4+ splenocytes, but only in 43% (3 of 7) of mice infused with 9.6 × 107 CD4- splenocytes. However, the frequency of fatal GvHD differed significantly between the cohorts (Figure 4B), in which all chimeric mice that were cured of CML-like leukemia following DLI with CD4- splenocytes survived longer than 100 days. One of these long-term survivors succumbed to BCR-ABL–induced histiocytic sarcoma at 100 days after transplantation, which likely arose from BCR-ABL–transduced monocytic progenitors.25 This mouse had tumors of H-2d+/GFP+ histiocytes in liver and spleen despite being a complete allogeneic chimera in the peripheral blood (data not shown), suggesting that this malignancy is more resistant to DLI than the CML-like disease. These results argue that both CD4+ and CD4- immune cells can mediate an effective GvL response against chronic phase CML-like leukemia in mice, whereas CD4+ splenocytes mediate the majority of GvHD in this strain combination.
Both CD4+ and CD4- splenocytes can mediate GvL against CML-like leukemia. Cumulative mortality due to leukemia (A), and overall survival (B), of nonchimeric mice with BCR-ABL–induced CML-like leukemia (purple, n = 2), of leukemic mixed chimeras without DLI (blue, n = 4), or chimeras treated with DLI in form of unfractionated splenocytes (red, n = 7), CD4+ splenocytes (yellow, n = 8) or CD4- splenocytes (green, n = 7). The frequency of fatal CML in chimeras treated with CD4+ splenocytes was significantly lower than in untreated chimeras (P = .05, Fisher exact test). One mouse in the CD4- DLI cohort died at day 28 of a mixture of CML-like disease and GvHD, and another succumbed to histiocytic sarcoma at day 100 (see text).
Both CD4+ and CD4- splenocytes can mediate GvL against CML-like leukemia. Cumulative mortality due to leukemia (A), and overall survival (B), of nonchimeric mice with BCR-ABL–induced CML-like leukemia (purple, n = 2), of leukemic mixed chimeras without DLI (blue, n = 4), or chimeras treated with DLI in form of unfractionated splenocytes (red, n = 7), CD4+ splenocytes (yellow, n = 8) or CD4- splenocytes (green, n = 7). The frequency of fatal CML in chimeras treated with CD4+ splenocytes was significantly lower than in untreated chimeras (P = .05, Fisher exact test). One mouse in the CD4- DLI cohort died at day 28 of a mixture of CML-like disease and GvHD, and another succumbed to histiocytic sarcoma at day 100 (see text).
GvL effect of DLI against CML-like leukemia is proportional to the degree of MHC disparity
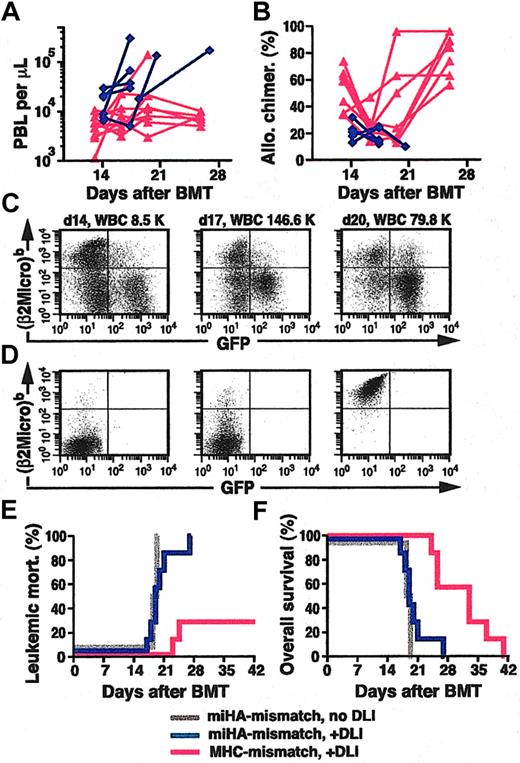
AlloSCT for human CML is most commonly done with MHC-matched sibling or unrelated donors. To model this clinical scenario in mice, we used donors (Balb/c) and recipients (B10.D2) that were matched for MHC antigens (H-2d) but differed in their minor histocompatibility antigens (miHA). To produce mixed hematopoietic chimerism in this model, we cotransplanted TCD p210 BCR-ABL–transduced BM from 5-FU–treated Balb/c donors and TCD B10.D2 BM at a ratio of 1:30 (see “Materials and methods”). On day 14 after BMT, PBL counts of recipients were normal or increased (Figure 5A), the mean percentage of allogeneic chimerism (Figure 5B), assessed using a polymorphism in β2-microglobulin (Figure 5C-D; see “Materials and methods”), was 21%, and an average of 23% of PBLs were GFP+ (Figure 5C and data not shown), with increased circulating maturing myeloid cells indicative of established myeloproliferative disease (data not shown). These mice therefore resemble CML patients relapsing in chronic phase following HLA-matched alloSCT. As before, we treated a cohort of these chimeric mice with early infusions of unfractionated allogeneic splenocytes, beginning at day 14 after transplantation. All chimeric but untreated mice died of CML-like leukemia by day 19 (Figure 5E). In contrast to MHC-mismatched chimeras, in which DLI eradicated CML-like disease in 70% of recipients, DLI slightly prolonged the survival of MHC-matched, miHA-mismatched chimeras, but all succumbed to leukemia by day 26 (Figure 5E-F). These recipients exhibited steadily increasing PBL counts (Figure 5A), decreasing mixed chimerism (Figure 5B-C), and progressive splenomegaly despite repeated DLI but had no clinical or pathologic evidence of GvHD. These results indicate that GvL effects against CML are most potent in the setting of MHC mismatch.11,19
GvL is more pronounced in MHC-mismatched than in MHC-matched, miHA-mismatched chimeras. (A-B) Plot of PBL counts (A) and percentage of allogeneic chimerism (B) as a function of time after BMT (x-axis) for MHC-mismatched (red triangles) and MHC-matched, miHA-mismatched (blue diamonds) chimeras with CML-like leukemia treated with DLI. Note that in the miHA-mismatched setting, because of the predominance of the syngeneic BCR-ABL–transduced BM, allogeneic chimerism never rose above 40%. (C) Flow cytometric analysis of allogeneic chimerism (y-axis, detecting the β2-microglobulinb allele expressed by B10.D2 leukocytes) and level of GFP+ leukemia cells (x-axis) in sequential peripheral blood samples from a representative mouse with MHC-matched, miHA-mismatched mixed chimerism and CML-like disease treated with DLI. The mouse was treated on days 15, 17, and 21 with a total dose of 1.1 × 108 B10.D2 splenocytes; note the increasing population of circulating GFP+ leukemia cells and diminishing allogeneic chimerism from 33% on day 14 to 15% on day 20. (D) Allotypic specificity of Cy-Chrome–labeled antibody against β2-microglobulinb (y-axis). Flow cytometric plots of PBLs from Balb/c mice stained with Cy-Chrome–labeled isotype control antibody (left panel) and PBLs from Balb/c (middle panel) or B10.D2 (right panel) mice stained with Cy-Chrome–labeled anti–β2-microglobulinb antibody. (E-F) Cumulative leukemic mortality (E) and overall survival (F) for DLI-treated recipients of TCD BCR-ABL–transduced Balb/c BM and TCD B6 BM (MHC-mismatch + DLI; red, n = 7) and for recipients of TCD BCR-ABL–transduced Balb/c BM and TCD B10.D2 BM (miHA-mismatch) with (blue, n = 7) or without (gray, n = 4) DLI treatment.
GvL is more pronounced in MHC-mismatched than in MHC-matched, miHA-mismatched chimeras. (A-B) Plot of PBL counts (A) and percentage of allogeneic chimerism (B) as a function of time after BMT (x-axis) for MHC-mismatched (red triangles) and MHC-matched, miHA-mismatched (blue diamonds) chimeras with CML-like leukemia treated with DLI. Note that in the miHA-mismatched setting, because of the predominance of the syngeneic BCR-ABL–transduced BM, allogeneic chimerism never rose above 40%. (C) Flow cytometric analysis of allogeneic chimerism (y-axis, detecting the β2-microglobulinb allele expressed by B10.D2 leukocytes) and level of GFP+ leukemia cells (x-axis) in sequential peripheral blood samples from a representative mouse with MHC-matched, miHA-mismatched mixed chimerism and CML-like disease treated with DLI. The mouse was treated on days 15, 17, and 21 with a total dose of 1.1 × 108 B10.D2 splenocytes; note the increasing population of circulating GFP+ leukemia cells and diminishing allogeneic chimerism from 33% on day 14 to 15% on day 20. (D) Allotypic specificity of Cy-Chrome–labeled antibody against β2-microglobulinb (y-axis). Flow cytometric plots of PBLs from Balb/c mice stained with Cy-Chrome–labeled isotype control antibody (left panel) and PBLs from Balb/c (middle panel) or B10.D2 (right panel) mice stained with Cy-Chrome–labeled anti–β2-microglobulinb antibody. (E-F) Cumulative leukemic mortality (E) and overall survival (F) for DLI-treated recipients of TCD BCR-ABL–transduced Balb/c BM and TCD B6 BM (MHC-mismatch + DLI; red, n = 7) and for recipients of TCD BCR-ABL–transduced Balb/c BM and TCD B10.D2 BM (miHA-mismatch) with (blue, n = 7) or without (gray, n = 4) DLI treatment.
DLI can cure Bcr-Abl–induced B-lymphoblastic leukemia in MHC-mismatched chimeras
Of all hematologic malignancies, CML is the most sensitive to adoptive immunotherapy,2,5 whereas acute myeloid and lymphoid leukemias that relapse after allogeneic BMT are less responsive to DLI.3 Ph+ B-ALL can be modeled in mice by modifying the retroviral transduction conditions. The leukemia is monoclonal to oligoclonal, originates from early BM B-lymphoid progenitors, and is characterized by lymphadenopathy, splenomegaly, BM infiltration, and a malignant hemorrhagic pleural effusion composed of immature pre–B-lymphoid blasts, leading to death by 40 to 50 days after transplantation.25,26
To investigate whether Bcr-Abl–induced B-ALL could be eradicated using the same immunotherapy protocol, we transduced BM from non–5-FU-treated Balb/c donors with p210 BCR-ABL retrovirus and transplanted lethally irradiated Balb/c recipients with a mixture of TCD BCR-ABL–transduced Balb/c BM and TCD B6 BM in a 1:4 ratio. This procedure reproducibly generated recipient mice with mixed hematopoietic chimerism (Figure 6A) and Bcr-Abl–induced acute B-lymphoblastic leukemia, with circulating immature BP-1+/GFP+ blasts at day 14 after transplantation (Figure 6A-B). All chimeric mice that were untreated died by day 45 after transplantation (Figure 6C) with characteristic clinicopathologic features of B-ALL, including lymphadenopathy and a hemorrhagic malignant pleural effusion.26 Interestingly, mice that received allogeneic splenocytes at the time of transplantation not only failed to develop B-ALL, but half of these recipients died around day 7 after transplantation with pancytopenia (Figure 6C-D), which is further evidence that cotransplantation of allogeneic splenocytes prevents engraftment of syngeneic Balb/c hematopoietic stem cells.
GvL against Bcr-Abl–induced B-lymphoblastic leukemia. (A) Flow cytometric analysis of chimerism (top row) and B-lymphoblastic leukemia (bottom row) in serial peripheral blood samples from a representative mouse with mixed chimerism and Bcr-Abl–induced B-ALL. Circulating leukemic cells were detected with antibody against the B-cell differentiation antigen BP-1/6C3.26 Blood sampling and analysis were performed on days 13, 16, 19, and 24 after BMT, and DLI was administered on days 14, 17, and 20 with a total of 1.1 × 108 allogeneic splenocytes. Note the disappearance of GFP+ cells by day 24, accompanied by conversion to full allogeneic chimerism. (B) Wright-Giemsa stain of day-16 peripheral blood from panel A, demonstrating circulating immature mononuclear blasts (magnification, 500 ×). (C-D) Cumulative leukemic mortality (C) and overall survival (D) for recipients of TCD p210 BCR-ABL–transduced BM from non–5-FU-treated Balb/c donors and TCD B6 BM, either without DLI treatment (blue, n = 4), with administration of allogeneic splenocytes at the time of BMT (green, n = 4), or with early DLI treatment of established mixed chimeras with B-ALL (yellow, n = 20). The frequency of fatal B-ALL in the DLI-treated group was significantly lower than in untreated chimeras (P = .007, Fisher exact test). Two mice in the cohort that received splenocytes at BMT died because of engraftment failure on day 7 and 8 after transplantation (see the second paragraph on this page). (E) Genomic DNA from the indicated tissues (PE indicates pleural effusion) was analyzed by Southern blot as in Figure 2G. Lanes 4 to 7 are DNAs from leukemic chimeric mice with B-ALL that did not receive DLI; note near single-copy levels of BCR-ABL provirus in lanes 5 and 6. Lanes 8 and 9 are the spleen DNAs from 2 different mice that received splenocytes at the time of BMT. No GFP+ clones or BCR-ABL provirus are detectable, and the spleen is composed mostly of cells of B6 origin. Lanes 10 to 24 represent DNAs from chimeric mice with B-ALL treated with DLI. Lanes 10 to 11 and 25 to 26 are from 2 mice that failed DLI because of poor or absent chimerism. Brackets indicate samples from the same individual mice.
GvL against Bcr-Abl–induced B-lymphoblastic leukemia. (A) Flow cytometric analysis of chimerism (top row) and B-lymphoblastic leukemia (bottom row) in serial peripheral blood samples from a representative mouse with mixed chimerism and Bcr-Abl–induced B-ALL. Circulating leukemic cells were detected with antibody against the B-cell differentiation antigen BP-1/6C3.26 Blood sampling and analysis were performed on days 13, 16, 19, and 24 after BMT, and DLI was administered on days 14, 17, and 20 with a total of 1.1 × 108 allogeneic splenocytes. Note the disappearance of GFP+ cells by day 24, accompanied by conversion to full allogeneic chimerism. (B) Wright-Giemsa stain of day-16 peripheral blood from panel A, demonstrating circulating immature mononuclear blasts (magnification, 500 ×). (C-D) Cumulative leukemic mortality (C) and overall survival (D) for recipients of TCD p210 BCR-ABL–transduced BM from non–5-FU-treated Balb/c donors and TCD B6 BM, either without DLI treatment (blue, n = 4), with administration of allogeneic splenocytes at the time of BMT (green, n = 4), or with early DLI treatment of established mixed chimeras with B-ALL (yellow, n = 20). The frequency of fatal B-ALL in the DLI-treated group was significantly lower than in untreated chimeras (P = .007, Fisher exact test). Two mice in the cohort that received splenocytes at BMT died because of engraftment failure on day 7 and 8 after transplantation (see the second paragraph on this page). (E) Genomic DNA from the indicated tissues (PE indicates pleural effusion) was analyzed by Southern blot as in Figure 2G. Lanes 4 to 7 are DNAs from leukemic chimeric mice with B-ALL that did not receive DLI; note near single-copy levels of BCR-ABL provirus in lanes 5 and 6. Lanes 8 and 9 are the spleen DNAs from 2 different mice that received splenocytes at the time of BMT. No GFP+ clones or BCR-ABL provirus are detectable, and the spleen is composed mostly of cells of B6 origin. Lanes 10 to 24 represent DNAs from chimeric mice with B-ALL treated with DLI. Lanes 10 to 11 and 25 to 26 are from 2 mice that failed DLI because of poor or absent chimerism. Brackets indicate samples from the same individual mice.
By contrast, early DLI in mixed chimeras with established B-ALL was remarkably effective in eradication of the leukemia (Figure 6C), as repeated infusions of unfractionated B6 splenocytes led to disappearance of circulating leukemic blasts and eventual conversion of recipients to full donor chimerism (Figure 6A). As with DLI for CML-like leukemia in MHC-mismatched chimeras, adoptive immunotherapy of Bcr-Abl–induced B-ALL was complicated by the development of GvHD in all recipients (Figure 6D), leading to morbidity or death with wasting, cachexia, and diarrhea with frequent pleural or peritoneal collections of histiocytes. At necropsy, the majority (80%) of DLI-treated mice had no evidence of B-ALL in BM, lymph nodes, spleen, or effusions (data not shown). Southern blot analysis demonstrated complete elimination of BCR-ABL provirus from spleen and pleural effusion of these mice, with conversion to predominantly or completely B6 origin (Figure 6E). In those mice in which DLI failed to eradicate B-ALL, there was minimal or absent chimerism detectable at day 13 after transplantation (data not shown), suggesting that a lack of allogeneic chimerism leads to rejection of the infused donor leukocytes. These results demonstrate that in an MHC-mismatched setting, Bcr-Abl–induced acute B-lymphoblastic leukemia can be eradicated completely by DLI.
Discussion
Allogeneic stem cell transplantation can cure a wide variety of hematologic malignancies, and we now know that most of the antileukemic effect of alloSCT results from transplantation of the donor immune system rather than the cytotoxic effects of the conditioning regimen. Adoptive immunotherapy in the form of donor leukocyte infusions can induce remissions in many patients that relapse after alloSCT, but different leukemias vary widely in their responsiveness to DLI, and DLI can be complicated by GvHD or severe myelosuppression. A better understanding of the immunologic mechanisms involved in the elimination of leukemic cells is necessary to develop strategies to maintain or increase GvL while reducing GvHD and graft failure. This is particularly important for chronic phase CML, which is the most responsive of all leukemias to DLI. Mouse models of immunotherapy have been used to address many of these questions, but nearly all use leukemia cell lines that do not accurately reproduce the pathophysiology of human CML; hence, there may be important differences in the GvL mechanisms involved. Here, we describe a mouse model of adoptive immunotherapy against 2 distinct Ph+ leukemias, chronic phase CML and Ph+ B-cell acute lymphoblastic leukemia. These leukemias arise from primary hematopoietic cells, express the BCR-ABL oncogene, and phenotypically and clinically resemble their human counterparts.
In some leukemia cell line models of immunotherapy, splenocytes are delivered at the same time as transplantation of the leukemia cells and allogeneic BM.18 We found that when allogeneic splenocytes were delivered at the time of transplantation in our models of CML and Ph+ B-ALL, no recipient died from either disease. However, previous studies have demonstrated that cotransplantation of a mixture of syngeneic and allogeneic stem cells and allogeneic lymphocytes drives preferential engraftment of the allogeneic stem cells.15,30 Murine CML-like leukemia originates from BCR-ABL–expressing hematopoietic stem/progenitor cells,25 whereas the B-ALL develops from early bone marrow B-lymphoid progenitors26 that require several additional events in addition to BCR-ABL transduction to become fully leukemic.32 In either instance, administration of allogeneic lymphocytes at the time of transplantation may interfere with the engraftment of BCR-ABL–transduced leukemia-initiating cells. Consistent with this, in our chronic phase CML model, recipients that were cotransplanted with allogeneic BM and splenocytes engrafted as full allogeneic chimeras with no BCR-ABL–expressing cells detectable after day 17 (Figure 1B), whereas in the B-ALL model, in which limiting numbers of stem cells are transplanted, several recipients cotransplanted with allogeneic BM and splenocytes failed to engraft at all (Figure 6D). A recent study also demonstrated that cotransplantation of allogeneic BM and lymphocytes with BCR-ABL–transduced BM can prevent or delay death from CML-like leukemia,33 but this could be explained by a decrease in the number of engrafting BCR-ABL clones, which was not assessed. Because of these concerns, we pursued a different model that more accurately represents patients with Ph+ leukemia who relapse following alloSCT.
To accomplish this, we cotransplanted BCR-ABL–transduced BM with TCD allogeneic BM alone. When mixed in the proper ratio to reflect the known allogeneic barriers to engraftment and differing stem cell contents, we reproducibly generated mice with BCR-ABL–induced CML-like disease or B-ALL and mixed hematopoietic chimerism, closely modeling the clinical scenario of relapse following alloSCT for Ph+ leukemia. We then treated these mice with adoptive immunotherapy in the form of DLI. Although immunotherapy of established leukemia is very challenging and is unsuccessful in many mouse models,18 we demonstrated that DLI could effectively eradicate both BCR-ABL–induced chronic phase CML-like myeloproliferative disease and B-ALL in MHC-mismatched chimeras. Although Bcr-Abl and GFP are foreign proteins, the immune responses were not directed primarily against either of these potential antigens, because infusions of syngeneic lymphocytes from Balb/c donors had no effect on the disease process (data not shown).
In the chronic phase CML model, we found CD4+ lymphocytes to have the most potent GvL activity, supporting clinical observations that selective depletion of CD8+ cells from the donor leukocyte population preserved the bulk of the antileukemic response.8,9 However, our studies suggest that CD4- cells, which may include both CD8+ cells and alloreactive natural killer (NK) cells, can also mediate GvL against chronic phase CML, and preservation or potentiation of this activity may be important for successful immunotherapy of CML. Eradication of CML-like disease was usually followed by conversion to complete donor chimerism but occasionally could be accompanied by stable mixed chimerism because of normal syngeneic stem cells that persist following DLI. DLI for CML-like leukemia was complicated by GvHD and, less frequently, by pancytopenia resembling graft failure. All these outcomes are observed in patients with CML treated with DLI and suggest that our model reproduces the entire range of clinical responses to DLI. A high incidence of GvHD is expected in the setting of lymphocyte infusion shortly after lethal irradiation, and future efforts will be directed at generating mice with CML-like disease and mixed chimerism through nonmyeloablative conditioning regimens.34 This should allow more time to elicit effective antileukemic responses with DLI while reducing the incidence of fatal GvHD.
In the MHC-matched, miHA-mismatched setting, DLI was ineffective in eradicating CML-like leukemia. This is in agreement with observations in patients and animal models that the magnitude of the GvL reaction is commensurate to the degree of MHC disparity between donor and host.11,12,19,20 Because adoptive immunotherapy of human CML is primarily carried out in recipients of allografts from fully or partially MHC-matched donors, extending GvL to miHA-mismatched chimeras would increase the clinical relevance of this model. The effectiveness of DLI in the MHC-matched setting is limited primarily by the rapid pace of the chronic phase CML-like disease in mice, as molecular monitoring studies in patients suggest that anti-CML responses to DLI require weeks to months.3 The aggressiveness of murine CML may be due to the polyclonal nature of the leukemia,25 and the disease might be attenuated by reducing the number of BCR-ABL–transduced stem cells that are transplanted or by treating mixed chimeras with imatinib mesylate28 to allow more time for GvL responses to develop. It may also be possible to increase immune responses in the miHA-mismatched setting by immunization of the donors or expansion of specific donor T-cell populations in vitro, followed by infusion. Although human B-ALL is less responsive to DLI3 and Ph+ B-ALL has a particularly ominous prognosis, we found that DLI could effectively eradicate Bcr-Abl–induced B-ALL in MHC-mismatched mixed chimeras. Together, these results imply that the majority of the GvL effect we observe against both the chronic phase CML-like and acute B-lymphoblastic leukemias in mice may be due to alloresponses driven by MHC disparity. Hence, GvL in this setting may in part represent a lymphohematopoietic graft-versus-host reaction.34 Our results suggest that MHC-mismatched alloSCT, such as haploidentical SCT, might be intentionally considered for patients with Ph+ B-ALL to derive a maximal GvL effect, particularly in a nonmyeloablative setting.
In conclusion, we have described here models of adoptive immunotherapy against Bcr-Abl–induced primary leukemias in mice. To increase the applicability of the model to immunotherapy of patients with CML, our current efforts focus on nonmyeloablative conditioning regimens and on enhancing GvL effects in MHC-matched chimeras. These model systems should be useful for understanding the mechanisms of GvL against chronic and acute phase CML, and to develop methods to increase GvL responses without concomitant GvHD.
Prepublished online as Blood First Edition Paper, August 24, 2004; DOI 10.1182/blood-2004-06-2229.
Supported by the National Institutes of Health (grant HL56949) and a Leukemia and Lymphoma Society Specialized Center of Research (SCOR) grant (R.A.V.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. Establishment of mixed hematopoietic chimerism and CML-like leukemia in mice. (A) Schematic diagram of the adoptive immunotherapy model system. TCD BM from 5-FU–treated Balb/c mice is transduced with retrovirus coexpressing BCR-ABL and GFP (top), then mixed with TCD BM from allogeneic C57Bl/6 donors (middle) and transplanted into lethally irradiated Balb/c recipients (right). Allogeneic splenocytes from the B6 donor (DLI) are infused into mice with mixed hematopoietic chimerism and established CML-like leukemia (bottom). In some experiments, allogeneic splenocytes are included in the initial BM graft (dotted arrow). (B) Flow cytometric analysis of peripheral blood leukocytes (PBLs) from a representative mouse that received TCD BCR-ABL–transduced syngeneic (Balb/c) and TCD allogeneic (B6) BM along with 1.5 × 107 allogeneic B6 splenocytes as the initial graft, analyzed day 17 after transplantation (PBL count, 5.6 × 109/L [5600/μL]). Hematopoietic chimerism (top panel) was analyzed with antibodies against H-2b (x-axis) and H-2d (y-axis), whereas CML-like leukemia (bottom panel) is indicated by cells coexpressing H-2d (y-axis) and GFP (x-axis). The mouse was engrafted as a full allogeneic chimera with no signs of CML-like disease. (C) Flow cytometric analysis of PBLs, as in panel B, from a representative mouse that received TCD BCR-ABL–transduced syngeneic (Balb/c) and TCD allogeneic (B6) BM without splenocytes, analyzed day 19 after transplantation. The PBL count was 30 × 109/L (30 000/μL), and the allogeneic chimerism was 35% H-2b+; note the prominent population of H-2d+/GFP+ cells (bottom panel), indicative of CML-like leukemia. (D) Wright-Giemsa–stained peripheral blood (PB) from a chimeric mouse with CML-like myeloproliferative disease, demonstrating increased circulating maturing myeloid cells (magnification × 400). (E-G) Coexpression of H-2b and H-2d in PBLs from mixed chimeras. Flow cytometric analysis of chimerism in a recipient of untransduced TCD BM from 5-FU–treated Balb/c donors and TCD normal B6 BM. Note the significant population of double-positive cells (E) that are not observed when PBLs from Balb/c and B6 mice are either stained for both antigens and mixed prior to analysis (F) or mixed followed by staining (G). In leukemic chimeras, this population included Bcr-Abl–expressing myeloid cells, as assessed by GFP expression and the forward-side scatter distribution.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-06-2229/6/m_zh80240471050001.jpeg?Expires=1769101504&Signature=fIm6r5250Hm9bVlp0TGMkzc0g187CETbA4KcWF595dtioKWyaMIjDa~ufwKCtCzpyRXRWhETkNaQdXC42di3ElwT8-TCTRn~SpRYRsb8rO--UbjoqbEq2uEFwUCrNFBRS9rVU9~yRJTnid1mzm2gTL4Yb9838cw8t0VbFafbluNAq6TvYG1nHiOwWgd~Q9hP6sD1K9xdMc4a761tbD3VwT~cH9lf-SsZxiOG5AdeiUjbIpusPqwuD3XJLSLiMVZHnB8DjJoe1smFjRXV8MXL1Wn9QkD1EpQi-0V7DW1tePrIt0rra~MlOBtM4b1gKeGH4OtUAGs9T~XIzEtTmPh1HQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal