Abstract
Nuclear receptors are ligand-modulated transcription factors regulated by interactions with corepressors and coactivators, whose functions are not fully understood. Acute promyelocytic leukemia (APL) is characterized by a translocation, t(15;17), that produces a PML/RARα fusion oncoprotein, whose abnormal transcriptional function is successfully targeted by pharmacologic levels of all-trans-retinoic acid (ATRA). Mutations in the ligand-binding domain of PML/RARα that confer resistance to ATRA have been studied by expression in nonhematopoietic cells, such as Cos-1. Here, we show that ATRA binding and transcriptional activation by the same PML/RARα mutant differ markedly between nonhematopoietic and leukemic cell lines. Differential expression of the corepressor isoform silencing mediator for retinoid and thyroid receptors β (SMRTβ) correlates with increased ligand binding and transcription by the mutant PML/RARα. Transient and stable overexpression of SMRTβ in hematopoietic cells that only express SMRTα increased ATRA binding, ligand-induced transcription, and ATRA-induced cell differentiation. This effect may not be limited to abnormal nuclear receptors, because overexpression of SMRTβ increased ATRA-induced binding and transcriptional activation of wild-type receptors PML/RARα and RARα. Our results suggest a novel role for the SMRTβ isoform whereby its cell-specific expression may influence the binding and transcriptional capacities of nuclear receptors, thus providing new evidence of distinct functions of corepressor isoforms and adding complexity to transcriptional regulation.
Introduction
Many key aspects of vertebrate physiology, reproduction, and development are regulated through the actions of small, lipophilic hormones. These hormones include the steroids, retinoids, and thyroid hormones and mediate their effects through the actions of a corresponding family of nuclear hormone receptors. The nuclear receptors function as hormone-regulated transcription factors, binding to specific DNA sequences, denoted hormone response elements, and regulating the transcription of adjacent target genes.1
Many nuclear hormone receptors can either repress or activate expression of a given target gene depending on the presence of hormones, nature of the promoter, and the cell context. These regulatory properties are closely linked to the ability of these receptors to recruit corepressors and coactivators, which help mediate the transcriptional response.2 Thus, in absence of all-trans-retinoic acid (ATRA), retinoic acid receptor-α (RARα) binds to corepressors silencing mediator for retinoid and thyroid receptors (SMRT) and nuclear receptor corepressor (NCoR), which in turn recruit a larger protein complex containing histone deacetylase (HDAC). The best characterized is the NCoR/SMRT-HDAC3-TBL1 complex first identified in HeLa cells that contains HDAC3 and TBL1 (transducin β–like protein 1). Subsequently, GPS2 (G protein pathway suppression 2) was also shown to be a component of this TBL1 or TBLR1 (TBL1-related protein 1) complex.3-6 This complex hypoacetylates chromatin and restricts accessibility of the DNA template to the transcriptional machinery.7-9 Conversely, binding of ATRA leads to a conformational change in the receptor that releases the corepressor complex and recruits instead coactivator complexes, many of which possess histone acetyltransferase activity. Histone acetylation is believed to create a more accessible and therefore more readily transcribed chromatin structure.2,10,11
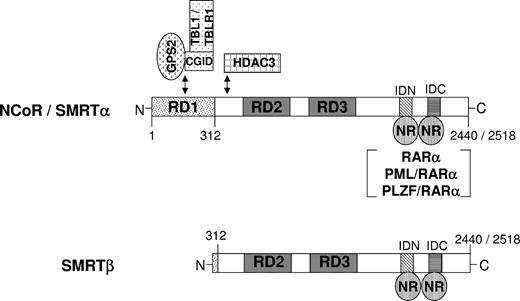
SMRT and NCoR are modular proteins that contain N-terminal repression domains (RDs) and C-terminal nuclear receptor interaction domains (RIDs). Two variant cDNA clones have been identified in the screening for the N-terminal region of the m-SMRT, which potentially represent products of an alternative splicing event. The longer 10-kb isoform, designated SMRTα, refers to full-length SMRT. The shorter 8.5-kb isoform is designated SMRTβ and harbors a natural deletion of amino acids 36 to 254.12 Based on sequence similarity to NCoR, this deletion removes most of the repressor domain 1 (RD1), including a portion of the TBL1/GPS2-binding region. The region of SMRT corresponding to NCoR RD1 has the most potent repressor activity, which is presumably caused by the presence of the TBL1/GPS2 interaction domain. However, no cell-specific differential function of these isoforms has been reported to date.
A model for the interaction of nuclear receptors and coregulators in cancer cells has been provided by acute promyelocytic leukemia (APL), in which a translocation invariably involving the retinoic acid receptor-α (RARα) leads to the uncontrolled growth and failure of normal differentiation of promyelocytes. In most cases of APL, a translocation of PML and RARα genes produces a fusion protein that acts as a dominant negative inhibitor of retinoid-mediated transcription.13,14 PML/RARα is found exclusively in APL patients15,16 and causes APL in transgenic mice.17,18
The PML/RARα protein retains most functional domains of RARα and behaves as an abnormal receptor with altered transactivation functions. The physiological importance of an active transcriptional repression is demonstrated by the role of corepressors in APL. In APL, the fusion protein can bind ATRA, but higher concentrations are required to activate transcription.19,20 This may be due to a more tightly associated PML/RARα corepressor complex, where the corepressor is not released at physiological ATRA concentrations.21-23 The release of corepressor induced by higher ATRA concentrations allows recruitment of coactivators and may underlie the cytodifferentiation of APL cells.21-24
Similar concentrations of ATRA can be achieved in APL patients, leading to dramatic remissions associated with granulocytic differentiation of leukemic cells.15,25 Nevertheless, APL cells develop resistance to ATRA in vitro and in vivo. Although combined cytotoxic chemotherapy with ATRA cures a high percentage of patients with APL, relapse with ATRA-resistant cells still occurs.26-31
Although altered pharmacokinetics may play a role, we and others have reported NB4 subclones that are rendered highly resistant to retinoid-induced cytodifferentiation by expression of dominant negative mutations of the PML/RARα fusion protein.32-37 Similar mutations of PML/RARα have been reported in APL cells from a growing number of patients who developed ATRA resistance, demonstrating the clinical relevance of these genetic alterations as a mechanism of resistance.31,38-41
PML/RARα mutations found in ATRA-resistant APL patients have been studied by expression of the cloned mutated receptor in established cell lines, such as Cos-1 or CV-1 cells, which do not express the PML/RARα protein. We and others have found that these amino acid changes cause a variety of abnormalities in ligand binding, transactivation of retinoic acid response elements (RAREs), and ligand-dependent interactions with the transcriptional coregulators SMRT and activator of thyroid and retinoic acid receptors (ACTR).42,43 Interestingly, a recent report found no correlation between in vitro response in Cos-1 cells and the clinical response to ATRA combined with HDAC inhibitors in 5 patients with resistant APL involving different PML/RARα mutations.40 However, these in vitro analyses did not examine whether the cell line in which PML/RARα mutations are studied affects the results obtained. In this report, we evaluate the use of Cos-1 cells as a model for the characterization of patient-derived PML/RARα mutations and find that ligand binding and transcriptional activation by a PML/RARα mutant overexpressed in Cos-1 cells differ markedly from those observed in APL cells. We report a difference in the expression of transcriptional coregulators in APL cells and Cos-1 cells that may be responsible for the differences we observed. Specifically, we show that expression of the isoform β of the transcriptional corepressor SMRT positively modulates the ability of PML/RARα mutant I410T to bind ligand, activate transcription, and induce cell differentiation. Our results therefore demonstrate an isoform-specific ability of transcriptional corepressor SMRT to coactivate a response to ligand, suggesting a novel mechanism for transcriptional regulation of nuclear receptors.
Materials and methods
Cell culture
The establishment and conditions for cell culture of the ATRA-resistant APL cell line NB4-MRA1 have been published previously.43 NB4, NB4-MRA1, Jurkat, U937, and Cos-1 cells are routinely cultured in ATRA-free RPMI 1640 medium (Life Technologies [GIBCO], Burlington, ON, Canada) supplemented with 10% fetal calf serum (FCS) (Wisent, St-Bruno, QC, Canada) at 37°C with 5% CO2. ATRA was obtained from Sigma (St Louis, MO), dissolved in ethanol, and kept at -80°C in the dark.
Stable transfection
The SMRT cDNA was subcloned in the PINCO retroviral vector. Virus production, cell infection, and purification of green fluorescent protein (GFP)–positive cells were performed as described previously.44
Plasmid constructs
PML/RARα with the I410T mutation was cloned into the pSG5 mammalian expression vector harboring wild-type PML/RARα (Long) cDNA using a QuikChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA). The construct was verified by sequencing analysis using the dsDNA Cycle Sequencing System (Life Technologies).
Northern blot analysis
Total RNA was extracted from different cells with guanidine thiocyanate, and Northern blotting was performed as previously described.45 Blots were probed to an 885-bp pGEX2T-NCoR-IDN/BamHI fragment46 and a 285-bp pGEX-KG-SMRT-IDII/BamHI-HindIII fragment.21 The filters subsequently were stripped and rehybridized with a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe for RNA loading normalization.
Western blot analysis
Nuclear extracts were diluted 1:1 with 2 × sodium dodecyl sulfate (SDS) sample buffer. Proteins were then fractionated by electrophoresis on 4% to 12% precast SDS polyacrylamide gels (Invitrogen, Carlsbad, CA), electroblotted on nitrocellulose membrane (Protran; Schleicher & Schuell, Dassel, Germany), and detected by the enhanced chemiluminescence (ECL) Western blotting detection kit (Amersham, Piscataway, NJ). Proteins that reacted with either the anti-RARα RPα (Ab directed against F region of RARα) antibody47 or the anti-RARα (C-20; Santa Cruz Biotechnology, Santa Cruz, CA) antibody were used at a 1:1000 dilution. Proteins that reacted with either the anti-SMRT (S93920; Transduction Laboratories, Lexington, KY) antibody or anti-SMRT (PA1-842; Affinity BioReagents, Golden, CO) antibody were used at a 1:200 dilution and a 1:250 dilution, respectively.
Assay for ligand-binding activity
Nuclear extracts were prepared from 1 to 5 × 107 cells and incubated for 18 hours at 4°C with 10 nmol/L [3H]-ATRA (50.7 Ci/mmol [1.88 TBq/mmol]; DuPont-NEN, Boston, MA) or with [3H]-ATRA in the presence of 200-fold excess of unlabeled ATRA. The extracts were subsequently fractionated at 4°C by high-performance liquid chromatography (HPLC) using a Superose 6 HR 10/30 size exclusion column (Pharmacia, Uppsala, Sweden) as previously described.19
Transient transfection experiments for transcriptional activity
Suspension cells (NB4, NB4-MRA1, A1-GFP, A1-GFP-SMRTβ, Jurkat, U937) were grown in RPMI 1640 with 10% FCS, and 5 × 106 cells per transfection were used. Cells were rinsed with serum-free OPTI-MEM (Life Technologies) and transfected by electroporation with 5 μg per transfection of the reporter plasmids DR5-tk-CAT,48 1 to 5 μg receptor (pSG5-RARα, pSG5-PML/RARα, or pSG5-PML/RARα-I410T) plasmid DNA, and 1 to 5 μg pCMX-SMRTβ or pCMX-SMRTα plasmid DNA.12 In all transfection experiments, the amount of transfected DNA was equalized with empty vector DNA (pSG5, pCMX). A1-GFP and A1-GFP-SMRTβ were transfected with 10 μg per transfection of the reporter plasmid DR5-tk-CAT. Cells were electroporated and replenished with 5 mL RPMI 1640 with 10% FCS and grown for 48 hours in the absence or presence of different concentrations of ATRA. The chloramphenicol acetyltransferase (CAT) activity was measured using a modified protocol of the organic diffusion method as described previously.42 After reporter gene activity had been measured, the values were normalized with respect to protein content of the cell lysates to obtain the relative CAT activity. Each transfection was performed in triplicate and repeated 3 times.
Adherent cells (Cos-1) were grown in RPMI 1640 with 10% FCS and were seeded at 120 000 cells per well in 6-well plates 1 day before transfection. Cells were rinsed with serum-free OPTI-MEM (Life Technologies) and transfected by the lipofectamine method (Life Technologies) with 0.7 μg wild-type or mutant fusion protein plasmid, 1 μg reporter plasmid DR5-tk-CAT, and 0.3 μg pCMV-βgalactosidase (βgal) as an internal control for the transfection efficiency. Cells were transfected for 5 hours, replenished with 2 mL RPMI 1640 with 10% FCS, and grown for 24 hours in the absence or presence of different concentrations of ATRA. CAT activity was measured using a modified protocol of the organic diffusion method as described previously.42 After reporter gene activity had been measured, the values were normalized with respect to βgal to obtain the relative CAT activity. Each transfection was performed in triplicate and repeated 3 times. The Student t test was used to analyze the significance of the results obtained in transfection experiments for transcriptional activity.
Cell differentiation
Cell differentiation was evaluated by direct immunofluorescence staining of CD11b and CD11c cell-surface myeloid-specific antigens (nos. 555388 and 555392, PharMingen, Mississauga, ON, Canada) (FACSCalibur flow cytometer, BD BioSciences, Mississauga, ON, Canada) and by nitrobluetetrazolium (NBT) dye reduction assays as previously described.49 The cells were also analyzed for the isotype control phycoerythrin (PE)–conjugated mouse immunoglobulin G1κ (IgG1κ) (no. 555749; PharMingen). In each sample viable cells were gated, and expression of CD11b and CD11c surface markers of 5 × 103 cells was evaluated.
Results
PML/RARα mutant I410T differs in ATRA binding and transcriptional activity when endogenously expressed in the ATRA-resistant NB4-MRA1 APL cells than when expressed in Cos-1 cells
The resistant subclone NB4-MRA1 harbors a missense mutation in the ligand-binding domain (LBD) of PML/RARα in the ATRA-resistant NB4-MRA1 APL cells.43 This mutation results in amino acid substitution of isoleucine (Ile, I) (ATC) for threonine (Thr, T) (ACC) in the long form (L, Bcr1) of PML/RARα protein, which corresponds to codon 410 (I410T) in wild-type RARα. The mutation is centrally located in the highly conserved core of the activator function-2 (AF-2) activation domain within the helix 12 (H12).
To evaluate how this point mutation of PML/RARα alters the function of the LBD, we examined the binding of [3H]-ATRA to nuclear extracts from the ATRA-sensitive NB4 and the ATRA-resistant NB4-MRA1 cells. The size exclusion HPLC profile of extracts from NB4 cells expressing the wild-type form of PML/RARα is characterized by the 50-kDa peak representing the binding of ATRA to endogenous RARs and the approximately 670-kDa peak representing macromolecular complexes formed by the interaction of PML/RARα with itself and/or other nuclear proteins.19,50 Nuclear extracts from NB4-MRA1 cells expressing the PML/RARα mutation I410T showed an HPLC profile consistent with ATRA binding to the mutated chimeric protein but at a much decreased level (Figure 1A). PML/RARα mutations found in ATRA-resistant APL patients have been studied by expression of the cloned mutated receptor in established cells, such as Cos-1, Cos-7, or CV-1, which do not express the PML/RARα protein. To validate the use of Cos-1 cells as a model for the evaluation of these patient-derived PML/RARα mutations, we compared ATRA-binding data of PML/RARα mutant I410T expressed in Cos-1 cells with binding of the same mutation in its APL cells of origin, NB4-MRA1. In contrast to the PML/RARα mutant I410T expressed in its cells of origin, the I410T mutant transfected into Cos-1 cells presents a specific ATRA-binding profile similar to that of the wild-type PML/RARα (Figure 1B).
ATRA binding and transcriptional activation by a PML/RARα mutant depend on the cell line in which the receptor is expressed. (A) Comparison of ATRA-binding activity of PML/RARα expressed by NB4 cells (○) with the PML/RARα mutant I410T expressed by NB4-MRA1 (•) cells. Nuclear extracts of NB4 and NB4-MRA1 cells were subjected to Western blot analysis. The left-pointing arrow indicates expression of the 110-kDa PML/RARα wild-type or mutant I410T. (B) ATRA-binding activity of PML/RARα (•) and PML/RARα mutant I410T (○) overexpressed in Cos-1 cells. Nuclear extracts were incubated with 10 nM [3H]-ATRA or with the addition of 200-fold excess unlabeled ATRA (▪) and subjected to HPLC analysis using a 6 HR 10/30 size exclusion column. Down-pointing arrows indicate approximate retention times of void volume, PML/RARα multimers (670 kDa), and RARα monomers (50 kDa). Nuclear extracts of Cos-1–transfected cells were subjected to Western blot analysis for PML/RARα wild-type or mutant I410T (left-pointing arrow). (C) Comparison of the ligand-dependent transcriptional activity, on a DR5-tk-CAT, of PML/RARα wild-type and mutant I410T endogenously expressed in APL cells and overexpressed in Cos-1 cells. Assays were performed in triplicate and repeated at least 3 times. Bars represent standard deviation of the mean.
ATRA binding and transcriptional activation by a PML/RARα mutant depend on the cell line in which the receptor is expressed. (A) Comparison of ATRA-binding activity of PML/RARα expressed by NB4 cells (○) with the PML/RARα mutant I410T expressed by NB4-MRA1 (•) cells. Nuclear extracts of NB4 and NB4-MRA1 cells were subjected to Western blot analysis. The left-pointing arrow indicates expression of the 110-kDa PML/RARα wild-type or mutant I410T. (B) ATRA-binding activity of PML/RARα (•) and PML/RARα mutant I410T (○) overexpressed in Cos-1 cells. Nuclear extracts were incubated with 10 nM [3H]-ATRA or with the addition of 200-fold excess unlabeled ATRA (▪) and subjected to HPLC analysis using a 6 HR 10/30 size exclusion column. Down-pointing arrows indicate approximate retention times of void volume, PML/RARα multimers (670 kDa), and RARα monomers (50 kDa). Nuclear extracts of Cos-1–transfected cells were subjected to Western blot analysis for PML/RARα wild-type or mutant I410T (left-pointing arrow). (C) Comparison of the ligand-dependent transcriptional activity, on a DR5-tk-CAT, of PML/RARα wild-type and mutant I410T endogenously expressed in APL cells and overexpressed in Cos-1 cells. Assays were performed in triplicate and repeated at least 3 times. Bars represent standard deviation of the mean.
We then compared transcriptional activation by the mutant PML/RARα protein in its cell of origin, NB4-MRA1, with transcription when overexpressed in Cos-1 cells. As shown in Figure 1C, significant ligand-dependent transcriptional activity of the wild-type PML/RARα chimeric protein was observed in ATRA-sensitive NB4 cells and in Cos-1 cells at concentrations of ATRA from 0.01 to 1 μM. Induction of transcriptional activity was substantially impaired in the ATRA-resistant NB4-MRA1 cells at all concentrations of ATRA. However, when transfected into Cos-1 cells, consistent with the differences in binding, the PML/RARα mutant I410T showed a significant increase in transcriptional activity in the presence of 0.1 to 1.0 μM ATRA (Figure 1C). Thus, ATRA binding and transcriptional activation by the same PML/RARα mutant are different in these 2 cell lines. Right panels of Figure 1A-B show that the NB4 and NB4-MRA1 cell lines, as well as Cos-1 cells transfected with wild-type or mutated PML/RARα, expressed similar levels of the PML/RARα fusion proteins. Thus, the cell-specific differences we observed in ATRA-binding capacity and transcriptional activation of PML/RARα were not due to a variation in protein expression.
U937 leukemic cells expressing PML/RARα I410T show ATRA binding and transcriptional activity similar to that of NB4 APL cells
We sought to determine whether the differences in binding and transcriptional activity of mutant PML/RARα we observed in NB4-MRA1 and Cos-1 cells are specific to those cells or reflect more general differences between cell types. We analyzed the HPLC ATRA-binding capacity and the transcriptional activity of the mutant I410T expressed in a non-APL leukemic cell line, U937. In these cells, as in the NB4 subclone, the mutant I410T has a minimal ATRA-binding capacity (Figure 2A) and transcriptional activity (Figure 2B). When expressed in additional nonhematopoietic adherent cell lines (HeLa and CV-1), ATRA binding and transcriptional activity of PML/RARα I410T were similar to those observed when expressed in Cos-1 cells (data not shown).
U937 leukemic cells expressing PML/RARα I410T show ATRA binding and transcriptional activity similar to that of NB4 APL cells. (A) Comparison of specific ATRA-binding profiles of the PML/RARα mutant I410T overexpressed in U937 cells. Nuclear extracts of U937-transfected cells were subjected to Western blot analysis for the 110-kDa PML/RARα wild-type or mutant I410T (left-pointing arrow). (B) Comparison of the ligand-dependent transcriptional activity, on a DR5-tk-CAT, of overexpressed PML/RARα wild-type and mutant I410T into U937 cells. Assays were performed in triplicate. Bars represent standard deviation of the mean.
U937 leukemic cells expressing PML/RARα I410T show ATRA binding and transcriptional activity similar to that of NB4 APL cells. (A) Comparison of specific ATRA-binding profiles of the PML/RARα mutant I410T overexpressed in U937 cells. Nuclear extracts of U937-transfected cells were subjected to Western blot analysis for the 110-kDa PML/RARα wild-type or mutant I410T (left-pointing arrow). (B) Comparison of the ligand-dependent transcriptional activity, on a DR5-tk-CAT, of overexpressed PML/RARα wild-type and mutant I410T into U937 cells. Assays were performed in triplicate. Bars represent standard deviation of the mean.
The isoform β of the corepressor SMRT is expressed in nonhematopoietic cells but is not expressed in hematopoietic cells
Previous work emphasizing the importance of coactivators and corepressors of transcription in the response to altered nuclear receptors led us to hypothesize that differential expression of transcriptional coregulators may be responsible for the discrepancy we observed between cell lines in HPLC ATRA binding and transcriptional analyses. To test this hypothesis, we compared the RNA expression levels of the transcriptional corepressors NCoR and SMRT in our APL clones with those of Cos-1, U937, and a variety of other cell lines by Northern blot analysis. Figure 3A shows that the expression level of the corepressor NCoR is similar in all the cell lines tested. However, analysis of the expression of the corepressor SMRT shows marked differences among the cell lines. ATRA-sensitive and ATRA-resistant APL cells as well as other hematopoietic cells, U937 and Jurkat, express only the SMRTα 10-kb transcript, whereas the nonhematopoietic cells, Cos-1, Cos-7, CV-1, and HeLa, express both SMRTα and SMRTβ isoforms as transcripts of 10 kb and 8.5 kb, respectively. Figure 3B also shows marked differences among those cell lines at the level of protein expression as analyzed by Western blotting for SMRT. ATRA-sensitive NB4, as well as another hematopoietic cell line, Jurkat, express only the SMRTα isoform (Figure 3B, top band), whereas the nonhematopoietic cells, Cos-1, HeLa, and T47-D, express both SMRTα and SMRTβ isoforms (Figure 3B, bottom band). These results suggest that the presence of the SMRTβ isoform in nonhematopoietic cell lines may modulate the capacity of the PML/RARα mutant I410T to bind ligand and subsequently activate transcription.
Differential expression of transcriptional corepressors NCoR and SMRT in leukemic cells and nonhematopoietic adherent cells. (A) Fifty micrograms of total RNA were analyzed by Northern blot. A 10-kb major transcript, SMRTα isoform, was detected in all cell lines examined. Note the presence of an 8.5-kb transcript, SMRTβ isoform, only in the nonhematopoietic adherent cells and its absence in all the hematopoietic cell lines tested. (B) Nuclear extracts of Jurkat, NB4, Cos-1, HeLa, and T47-D were subjected to Western blot analysis for SMRTα (top band) and SMRTβ (bottom band). Note the presence of a SMRTβ isoform only in the nonhematopoietic adherent cells and its absence in all the hematopoietic cell lines tested. (C) Overexpression of SMRTβ, but not SMRTα, induces ATRA binding to PML/RARα I410T in NB4-MRA1 cells. Nuclear extracts of cells transfected with SMRTβ (▾) or SMRTα (○) were incubated with 10 nM [3H]-ATRA and subjected to HPLC analysis using a 6 HR 10/30 size exclusion column. Nuclear extracts of NB4-MRA1 transfected cells with SMRTβ or SMRTα were subjected to Western blot analysis. The arrow indicates expression of the 110-kDa PML/RARα wild-type or mutant I410T.
Differential expression of transcriptional corepressors NCoR and SMRT in leukemic cells and nonhematopoietic adherent cells. (A) Fifty micrograms of total RNA were analyzed by Northern blot. A 10-kb major transcript, SMRTα isoform, was detected in all cell lines examined. Note the presence of an 8.5-kb transcript, SMRTβ isoform, only in the nonhematopoietic adherent cells and its absence in all the hematopoietic cell lines tested. (B) Nuclear extracts of Jurkat, NB4, Cos-1, HeLa, and T47-D were subjected to Western blot analysis for SMRTα (top band) and SMRTβ (bottom band). Note the presence of a SMRTβ isoform only in the nonhematopoietic adherent cells and its absence in all the hematopoietic cell lines tested. (C) Overexpression of SMRTβ, but not SMRTα, induces ATRA binding to PML/RARα I410T in NB4-MRA1 cells. Nuclear extracts of cells transfected with SMRTβ (▾) or SMRTα (○) were incubated with 10 nM [3H]-ATRA and subjected to HPLC analysis using a 6 HR 10/30 size exclusion column. Nuclear extracts of NB4-MRA1 transfected cells with SMRTβ or SMRTα were subjected to Western blot analysis. The arrow indicates expression of the 110-kDa PML/RARα wild-type or mutant I410T.
Overexpressed SMRTβ isoform increases ATRA-binding capacity of the PML/RARα mutant I410T in ATRA-resistant NB4-MRA1 APL cells
To directly test whether SMRTβ isoform modulates the ability of the PML/RARα mutant I410T to bind ligand, we transfected the SMRTβ or SMRTα isoforms into ATRA-resistant NB4-MRA1 APL cells and performed an HPLC ATRA-binding analysis (Figure 3C). In the absence of the transfected corepressor SMRT isoforms (Figure 3C, “mock”), the mutant I410T fusion protein exhibited minimal binding capacity to ATRA as represented by the absence of the 670-kDa peak. The presence of the 670-kDa peak in NB4-MRA1 transfected with SMRTβ indicates that expression of the SMRTβ isoform increased the ATRA-binding capacity of the PML/RARα mutant I410T, while increased expression of SMRTα isoform in those cells has no significant effect on ligand binding. The right panel of Figure 3C shows a similar PML/RARα protein level in NB4-MRA1 compared with those cells transfected with SMRTα or SMRTβ, confirming that expression of SMRT isoforms does not alter the levels of PML/RARα protein. These results are in agreement and support our hypothesis that difference in expression of SMRT isoforms might be responsible for cell-specific responsiveness to hormone.
Overexpressed SMRTβ isoform promotes ligand-induced transactivation of PML/RARα mutant I410T in ATRA-resistant NB4-MRA1 APL cells and other cell lines
The activity of SMRTβ ATRA-binding analysis prompted us to address the role of SMRTβ in regulating the transcriptional activity of the PML/RARα mutant I410T in cell lines that varied in expression of this isoform. We conducted transient cotransfection assays to determine the effects of SMRTβ and SMRTα overexpression on I410T activity using a DR5-tk-CAT reporter gene in 4 cell lines (Figure 4). We found that expression of SMRTβ resulted in an activation of transcription by PML/RARα I410T in ATRA-resistant NB4-MRA1 APL cells in response to 1 μM ATRA treatment, producing a 24.1-fold induction compared with a 10.6-fold induction with the empty vector pCMX (Figure 4A, “control”). We next analyzed the effect of overexpression of SMRT isoforms in the hematopoietic Jurkat cells. Jurkat cells were cotransfected either with an empty expression vector or with expression vectors for PML/RARα I410T and specific vectors for SMRTβ or SMRTα. As shown in Figure 4B, the mutant PML/RARα activated DR5-tk-CAT expression 26.7-fold in the presence of ATRA. Interestingly, expression of SMRTβ in Jurkat increases this activation to 72.6-fold, while additional expression of SMRTα had no significant effect. We also analyzed the effect of overexpression of SMRT isoforms in the hematopoietic U937 cells. As shown in Figure 4C, the mutant PML/RARα activated DR5-tk-CAT expression 16.2-fold in the presence of ATRA. Expression of SMRTβ in U937 cells increases this activation to 32.1-fold, while SMRTα isoform decreases the activation to 9.9-fold. Finally, we analyzed the effect of overexpression of SMRT isoforms in the nonhematopoietic Cos-1 cells. As shown in Figure 4D, the mutant PML/RARα activated DR5-tk-CAT expression 16.9-fold in the presence of ATRA. Interestingly, overexpression of SMRTα decreases the induction to 6.1-fold. Expression of SMRT isoforms without PML/RARα I410T had no significant effect on transcriptional activity (data not shown). In general, expression of either SMRT isoform was associated with decreased basal transcription, consistent with its ability to repress transcription, but this repression was relieved by ligand with the expression of SMRTβ. Taken together these results indicate that SMRTβ induces a ligand-dependent transcriptional activation by PML/RARα I410T in all the cell lines tested. This suggests an antirepression role for the SMRTβ isoform in the presence of ligand and that its cell-specific presence may influence the binding and ligand-dependent transcriptional capacities of nuclear receptors.
Overexpressed SMRTβ isoform promotes ligand-induced transactivation of PML/RARα mutant I410T in ATRA-resistant NB4-MRA1 APL cells and other cell lines. Transient cotransfection assays to determine the effects of SMRTβ and SMRTα overexpression on I410T activity using a DR5-tk-CAT reporter gene in (A) ATRA-resistant APL NB4-MRA1, (B) hematopoietic Jurkat, (C) hematopoietic U937, and (D) nonhematopoietic Cos-1 cell lines. Cells were cotransfected either with empty expression vectors pCMX or pSG5 (control) or with expression vectors for PML/RARα I410T and specific vectors for SMRTβ or SMRTα. Assays were performed in triplicate and repeated at least 3 times. Bars represent standard deviation of the mean. Numbers in parentheses indicate the fold induction produced by ATRA. There was a significant difference (**P < .01) for fold induction of transcription in cells transfected with SMRTβ compared with control transfection (A-C), and there is no significant difference between cells transfected with SMRTα. In Cos-1 cells (D), SMRTα-transfected cells showed a significant decrease (*P < .01) in induction relative to control- or SMRTβ-transfected I410T.
Overexpressed SMRTβ isoform promotes ligand-induced transactivation of PML/RARα mutant I410T in ATRA-resistant NB4-MRA1 APL cells and other cell lines. Transient cotransfection assays to determine the effects of SMRTβ and SMRTα overexpression on I410T activity using a DR5-tk-CAT reporter gene in (A) ATRA-resistant APL NB4-MRA1, (B) hematopoietic Jurkat, (C) hematopoietic U937, and (D) nonhematopoietic Cos-1 cell lines. Cells were cotransfected either with empty expression vectors pCMX or pSG5 (control) or with expression vectors for PML/RARα I410T and specific vectors for SMRTβ or SMRTα. Assays were performed in triplicate and repeated at least 3 times. Bars represent standard deviation of the mean. Numbers in parentheses indicate the fold induction produced by ATRA. There was a significant difference (**P < .01) for fold induction of transcription in cells transfected with SMRTβ compared with control transfection (A-C), and there is no significant difference between cells transfected with SMRTα. In Cos-1 cells (D), SMRTα-transfected cells showed a significant decrease (*P < .01) in induction relative to control- or SMRTβ-transfected I410T.
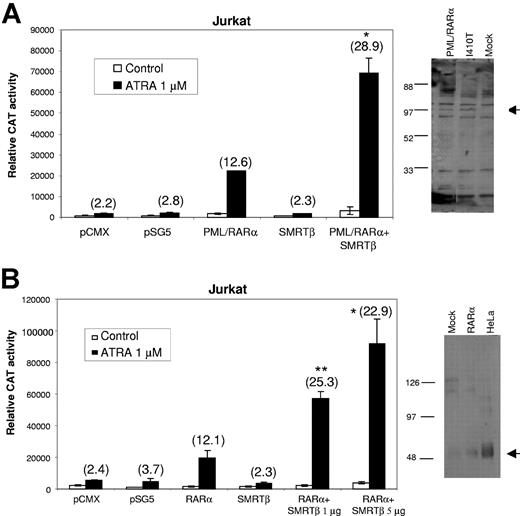
Expression of SMRTβ activates transcription by wild-type nuclear receptors PML/RARα and RARα
We next sought to determine whether the ligand-induced activation by SMRTβ we observed with the PML/RARα mutant I410T is limited to this mutated receptor or reflects a more general action of SMRTβ on activation of retinoid receptors. We tested the effects of coexpression of SMRTβ with wild-type retinoid receptors PML/RARα and RARα in Jurkat cells, which do not endogenously express SMRTβ and show low levels of endogenous RAR activity.51 pCMX-SMRTβ was cotransfected with a DR5-tk-CAT reporter plasmid and a pSG5-PML/RARα into Jurkat cells and tested for ligand response. As shown in Figure 5A, in the absence of cotransfected corepressor, PML/RARα exhibited a 12.6-fold ligand-mediated induction, while coexpression of SMRTβ increased induction to about 28.9-fold. Expression of empty vectors or SMRTβ alone had no significant effect on the ATRA-induced transcription. To investigate the effects of overexpressing SMRTβ on RARα-mediated reporter gene expression, increasing amounts of pCMX-SMRTβ were cotransfected with a DR5-tk-CAT reporter plasmid and a pSG5-RARα into Jurkat cells. As shown in Figure 5B, increasing coexpression of SMRTβ resulted in an increased transcriptional activation. Expression of empty vectors or SMRTβ has no significant effect on the ATRA-induced transcription. In addition, coexpression of SMRTα did not enhance transcription at any concentration of ligand tested (data not shown). The right panels in Figure 5 show PML/RARα and RARα protein levels in mock and transfected Jurkat. In Figure 5B, note the very low level of endogenous expression of RARα in mock Jurkat as compared with the positive control HeLa, confirming the results reported by Ballow et al.51
Expression of SMRTβ promotes ligand-induced transactivation of wild-type nuclear receptors PML/RARα and RARα. Coexpression of SMRTβ with wild-type retinoid receptors (A) PML/RARα and (B) RARα in Jurkat cells, which do not endogenously express SMRTβ. Specific SMRT vectors were cotransfected with a DR5-tk-CAT reporter plasmid and expression plasmids for PML/RARα or RARα into Jurkat cells and tested for ligand response. Nuclear extracts of transfected Jurkat cells with PML/RARα or RARα were subjected to Western blot analysis. The arrow indicates expression of the 110-kDa PML/RARα (A) or the 50-kDa RARα (B). Assays were performed in triplicate and repeated at least 3 times. Bars represent standard deviation of the mean. Numbers in parentheses indicate the fold induction produced by ATRA. There was a significant difference between Jurkat cells transfected with SMRTβ compared with transfected PML/RARα (A) or RARα (B); *P < .05; **P < .01.
Expression of SMRTβ promotes ligand-induced transactivation of wild-type nuclear receptors PML/RARα and RARα. Coexpression of SMRTβ with wild-type retinoid receptors (A) PML/RARα and (B) RARα in Jurkat cells, which do not endogenously express SMRTβ. Specific SMRT vectors were cotransfected with a DR5-tk-CAT reporter plasmid and expression plasmids for PML/RARα or RARα into Jurkat cells and tested for ligand response. Nuclear extracts of transfected Jurkat cells with PML/RARα or RARα were subjected to Western blot analysis. The arrow indicates expression of the 110-kDa PML/RARα (A) or the 50-kDa RARα (B). Assays were performed in triplicate and repeated at least 3 times. Bars represent standard deviation of the mean. Numbers in parentheses indicate the fold induction produced by ATRA. There was a significant difference between Jurkat cells transfected with SMRTβ compared with transfected PML/RARα (A) or RARα (B); *P < .05; **P < .01.
Stable expression of SMRTβ in the ATRA-resistant NB4-MRA1 cells promotes ligand binding, ligand-induced transcriptional activation, and ATRA-induced cell differentiation
The activity of transiently expressed SMRTβ observed in ATRA binding and transcriptional activity analyses in various cell lines prompted us to stably express the SMRTβ isoform in ATRA-resistant NB4-MRA1 APL cells (A1-GFP-SMRTβ). The right panel of Figure 6A confirms that the ATRA-resistant NB4-MRA1 cells stably transfected with the empty GFP vector (A1-GFP) expressed only SMRTα isoform (Figure 6A, top band), whereas NB4-MRA1 stably transfected with GFP-SMRTβ (A1-GFP-SMRTβ) expressed SMRTα and SMRTβ (Figure 6A, bottom band), as analyzed by Western blotting.
Stable expression of SMRTβ in the ATRA-resistant NB4-MRA1 APL cells promotes ligand binding, ligand-induced transcriptional activation, and ATRA-induced cell differentiation. (A) Effects of stable expression of SMRTβ isoform on the ATRA-binding capacity of PML/RARα mutant I410T in A1-GFP-SMRTβ APL cells. SMRTβ induces ATRA binding to PML/RARα I410T in A1-GFP-SMRTβ cells (▾) compared with A1-GFP cells (•). Nuclear extracts were incubated with 10 nM [3H]-ATRA or with the addition of 200-fold excess unlabeled ATRA and subjected to HPLC analysis usinga6HR 10/30 size exclusion column. Nuclear extracts of stably transfected cells A1-GFP and A1-GFP-SMRTβ were subjected to Western blot analysis for the SMRTα (top band) and SMRTβ (bottom band). (B) Effects of stable SMRTβ expression on transcriptional activation. Cells stably expressing the empty GFP vector (A1-GFP) or the GFP vector harboring SMRTβ (A1-GFP-SMRTβ) were transiently transfected with the RARE reporter gene DR5-tk-CAT. Assays were performed in triplicate and repeated at least 3 times. Bars represent standard deviation of the mean. There was a significant difference between cells stably transfected with SMRTβ compared with transfected cells with the empty vector: *P < .05, **P < .0005. Nuclear extracts of stably transfected cells A1-GFP and A1-GFP-SMRTβ were subjected to Western blot analysis for the 110-kDa PML/RARα mutant I410T (arrow). (C-D) Expression of the cell-surface myeloid-specific differentiation markers CD11b (C) and CD11c (D) by flow cytometry analysis. Cells stably expressing the empty GFP vector (A1-GFP) or the GFP vector harboring SMRTβ (A1-GFP-SMRTβ) were treated with 0.01 and 1 μM ATRA for 5 days, stained with an antibody specific for CD11b or CD11c, and subjected to flow cytometry analysis. The cells were also analyzed for the isotype control PE-conjugated mouse IgG1κ for CD11b-PE and CD11c-PE. In each sample, viable cells were gated, and expression of CD11b or CD11c surface markers of 5 × 103 cells was evaluated. Numbers in parentheses indicate the percentage of positive CD11b or CD11c cells. This experiment is representative of 3 that gave comparable results.
Stable expression of SMRTβ in the ATRA-resistant NB4-MRA1 APL cells promotes ligand binding, ligand-induced transcriptional activation, and ATRA-induced cell differentiation. (A) Effects of stable expression of SMRTβ isoform on the ATRA-binding capacity of PML/RARα mutant I410T in A1-GFP-SMRTβ APL cells. SMRTβ induces ATRA binding to PML/RARα I410T in A1-GFP-SMRTβ cells (▾) compared with A1-GFP cells (•). Nuclear extracts were incubated with 10 nM [3H]-ATRA or with the addition of 200-fold excess unlabeled ATRA and subjected to HPLC analysis usinga6HR 10/30 size exclusion column. Nuclear extracts of stably transfected cells A1-GFP and A1-GFP-SMRTβ were subjected to Western blot analysis for the SMRTα (top band) and SMRTβ (bottom band). (B) Effects of stable SMRTβ expression on transcriptional activation. Cells stably expressing the empty GFP vector (A1-GFP) or the GFP vector harboring SMRTβ (A1-GFP-SMRTβ) were transiently transfected with the RARE reporter gene DR5-tk-CAT. Assays were performed in triplicate and repeated at least 3 times. Bars represent standard deviation of the mean. There was a significant difference between cells stably transfected with SMRTβ compared with transfected cells with the empty vector: *P < .05, **P < .0005. Nuclear extracts of stably transfected cells A1-GFP and A1-GFP-SMRTβ were subjected to Western blot analysis for the 110-kDa PML/RARα mutant I410T (arrow). (C-D) Expression of the cell-surface myeloid-specific differentiation markers CD11b (C) and CD11c (D) by flow cytometry analysis. Cells stably expressing the empty GFP vector (A1-GFP) or the GFP vector harboring SMRTβ (A1-GFP-SMRTβ) were treated with 0.01 and 1 μM ATRA for 5 days, stained with an antibody specific for CD11b or CD11c, and subjected to flow cytometry analysis. The cells were also analyzed for the isotype control PE-conjugated mouse IgG1κ for CD11b-PE and CD11c-PE. In each sample, viable cells were gated, and expression of CD11b or CD11c surface markers of 5 × 103 cells was evaluated. Numbers in parentheses indicate the percentage of positive CD11b or CD11c cells. This experiment is representative of 3 that gave comparable results.
In an HPLC binding analysis of NB4-MRA1 stably expressing the GFP empty vector (A1-GFP), the mutant I410T fusion protein exhibited minimal binding capacity to ATRA, as represented by a decreased 670-kDa peak (Figure 6A). The increased 670-kDa peak in A1-GFP-SMRTβ cells indicates increased ATRA-binding capacity of the PML/RARα mutant I410T, confirming the results we obtained in transient transfections. Interestingly, there is also an increased 50-kDa peak, suggesting that expression of SMRTβ promotes ATRA binding to the wild-type RARα receptor as well. This is consistent with the results we obtained in Figure 5 showing a more general action of SMRTβ on transcriptional activation of wild-type retinoid receptors PML/RARα and RARα.
We determined the effects of stable SMRTβ overexpression on I410T transcriptional activity using a transiently transfected DR5-tk-CAT reporter gene (Figure 6B). We found increased transcription by PML/RARα I410T in A1-GFP-SMRTβ cells in response to 0.01 and 1 μM ATRA treatment, compared with A1-GFP stably transfected with a GFP empty vector. The right panel of Figure 6B demonstrates similar levels of PML/RARα protein in A1-GFP and A1-GFP-SMRTβ, showing that stable expression of SMRTβ into NB4-MRA1 cells does not affect the expression or stability of the PML/RARα protein. These results again confirm those we obtained in transient transfections of SMRTβ.
Finally, we evaluated the biologic response to ATRA of A1-GFP-SMRTβ cells stably expressing the SMRTβ corepressor. We tested whether the presence of SMRTβ increases sensitivity of A1-GFP-SMRTβ cells to ATRA-induced differentiation by evaluating the expression of 2 cell-surface markers of myeloid differentiation by flow cytometry. NB4-MRA1 cells stably expressing a GFP empty vector or a GFP-SMRTβ vector were treated for 5 days with 0.01 μM and 1 μM ATRA. We observed no evidence of differentiation in NB4-MRA1 expressing the GFP empty vector, whereas stable expression of SMRTβ in A1-GFP-SMRTβ cells led to increases in expression of CD11b (Figure 6C) and CD11c (Figure 6D) in response to ATRA treatment. We obtained similar results using the same ATRA concentrations for a 3-day treatment (data not shown). To confirm these data with an additional assay of myeloid differentiation, a nitrobluetetrazolium (NBT) assay was performed on cells treated for 9 days with 10 μM ATRA. In control A1-GFP cells, ATRA treatment yielded 11% NBT reduction, while in SMRTβ-expressing A1-GFP-SMRTβ cells, NBT increased to 41% with ATRA treatment.
Discussion
Leukemic oncoproteins that alter transcription, including wild-type and mutated PML/RARα, have been studied by expression of the cloned oncoprotein in established cell lines, such as Cos-1, Cos-7, or CV-1 cells. In this report, we utilized an ATRA-resistant NB4 subclone, NB4-MRA1, to evaluate the use of Cos-1 cells as a model for the characterization of patient-derived PML/RARα mutations.
In NB4-MRA1 cells, the replacement of isoleucine at position 410 by threonine abolished almost completely the capacity of the fusion protein PML/RARα to bind ATRA and significantly impaired ligand-dependent transcriptional activity on a RARE. In contrast, when transiently expressed in Cos-1 cells, the same I410T mutant presents an ATRA-binding profile and an increased transcriptional activity similar to that of the wild-type PML/RARα. Expression in additional nonleukemic cell lines gave results similar to those seen in Cos-1, while ATRA binding and transcriptional activation by PML/RARα I410T expressed in other leukemic cell lines resemble that of NB4.
Our evaluation of the levels of expression of the transcriptional corepressors NCoR and SMRT suggested a potential mechanism for these differences. All the cell lines tested express a similar RNA level of NCoR and the longer 10 kb transcript of SMRTα. Interestingly, only the nonhematopoietic adherent cell lines express the shorter SMRTβ transcript of 8.5 kb. The tested hematopoietic cell lines, including ATRA-sensitive and ATRA-resistant cells, express only the longer SMRTα transcript and do not express the shorter transcript. This tissue-specific difference in expression was confirmed by Western blotting.
Figure 7 shows a schematic representation of NCoR and SMRT. The full-length form of either corepressor harbors 3 adjacent domains, denoted repressor domain (RD1-RD3), which play important roles in corepressor-mediated transcriptional repression. The SMRTβ isoform harbors a natural deletion that removes most of the RD1 domain. The most extensively characterized interaction is the ability of RD1 to bind to GPS2, TBL1, and related proteins, which in turn facilitate recruitment of histone deacetylase and transcriptional repression of unliganded nuclear receptors.3-6,52 A recent report has shown that this NCoR/SMRT-TBLR1-HDAC corepressor complex is recruited by unliganded RARα, PML/RARα, and PLZF/RARα, leading to histone deacetylation and transcriptional repression in vivo of ATRA-responsive promoters.53 The ability to assemble this SMRT/TBL1/HDAC complex is closely linked to the ability of SMRT to repress, so that the absence of RD1 can impair or prevent transcriptional repression. The effect of this deletion on SMRT function has been confirmed by cotransfection experiments comparing repression by SMRTα and SMRTβ.12
Schematic representation of nuclear transcriptional corepressors NCoR and SMRT. Model for NCoR/SMRT-mediated transcriptional repression by nuclear receptors. NCoR and SMRTα isoform can interact with GPS2/TBL1/TBLR1 via the repressor domain 1 (RD1). The shorter isoform, SMRTβ, harbors a natural deletion of amino acids 36 to 254. Based on sequence similarity to NCoR, this deletion removes most RD1, including the GPS2/TBL1/TBLR1-binding regions. IDN and IDC represent the receptor interacting domains N- and C-terminal, respectively. RD indicates repressor domain; ID, nuclear receptor interacting domain; NR, nuclear receptor; HDAC, histone deacetylase; GPS2, G protein pathway suppression 2; TBL1, transducin β–like protein 1; TBLR1, TBL1-related protein 1; and CGID, GPS2-interacting domain of TBL1/TBLR1.
Schematic representation of nuclear transcriptional corepressors NCoR and SMRT. Model for NCoR/SMRT-mediated transcriptional repression by nuclear receptors. NCoR and SMRTα isoform can interact with GPS2/TBL1/TBLR1 via the repressor domain 1 (RD1). The shorter isoform, SMRTβ, harbors a natural deletion of amino acids 36 to 254. Based on sequence similarity to NCoR, this deletion removes most RD1, including the GPS2/TBL1/TBLR1-binding regions. IDN and IDC represent the receptor interacting domains N- and C-terminal, respectively. RD indicates repressor domain; ID, nuclear receptor interacting domain; NR, nuclear receptor; HDAC, histone deacetylase; GPS2, G protein pathway suppression 2; TBL1, transducin β–like protein 1; TBLR1, TBL1-related protein 1; and CGID, GPS2-interacting domain of TBL1/TBLR1.
We find cell-specific differences in ligand binding and transcriptional activity of a mutated nuclear receptor that reflect differences in SMRT isoform expression. Expression of the SMRTβ into hematopoietic cells that express only SMRTα confirmed that SMRTβ modulates ligand binding and transcription. Expression of SMRTβ, but not SMRTα, increased ATRA binding and transcriptional activation by the mutant PML/RARα, while increased expression of SMRTα in Cos-1 cells decreased transcription. The results from transient transfection of SMRT isoforms have been confirmed in cells stably transfected with SMRTβ. Furthermore, the SMRTβ-induced increase of ligand binding and transcriptional activity of PML/RARα I410T is associated with evidence of ATRA-induced differentiation of NB4-MRA1 cells stably expressing SMRTβ. Taken together, these data suggest that specific corepressors can play a novel role as mediators of cell-specific responsiveness to ATRA.
Indeed, we have evidence of a more general role in activation of retinoid receptors by SMRTβ. Expression of SMRTβ in Jurkat cells enhanced the transcriptional activity of nonmutated PML/RARα as well as wild-type nuclear receptor RARα (Figure 5), consistent with the increased binding of ligand to wild-type RARα seen in the stably transfected A1-GFP-SMRTβ cells (Figure 6). These results suggest that the role of coactivator that we identified for SMRTβ is not restricted to a specific PML/RARα mutation but may apply to the large family of nuclear receptors. We are currently evaluating the role of SMRTβ as a positive modulator of ligand-induced activation on various nuclear receptors like vitamin D receptor (VDR), thyroid hormone receptor (TR), progesterone receptor (PR), and androgen receptor (AR).
There is evidence in the literature for cell-specific stimulatory actions of corepressors.54-58 Consistent with our data, Baniahmad et al54 report that RARα displays cell-specific transcriptional properties. In the absence of hormone, RARα is a transcriptional activator in CV-1 cells but acts as a silencer in L cells. Addition of hormone leads to a further activation in CV-1 cells. Interestingly, they show that coexpression of SMRT, but not NCoR, in CV-1 cells strongly enhances the hormonal response. The authors of the report, published in 1998, used the first SMRT cloned,59 which lacks a 1000 amino acid (aa) N-terminal portion that contains the RD1 repressor domain12,60 and so may be similar to the SMRTβ isoform. They conclude that the level of certain cellular corepressors is an important factor in regulation of target genes and the hormonal response of the cell, and the expression of a specific corepressor can lead to a dramatic increase of hormonal response in a cell-specific manner. Transient cotransfection of mammalian cells with SMRT, again using the first SMRT cloned,59 and a TCDD-inducible luciferase reporter containing the dioxin-responsive domain from the mouse CYP1A1 regulatory region revealed enhancement, not repression, of AhR signaling.56 Interestingly, one report suggested that a putative splicing variant of NCoR, NCoR-I,61 which lacks the N-terminal repressor domain, can act as an activator for the mouse preprothyrotropin-releasing hormone gene.62 NCoR-I also inhibits the ligand-independent repression by T3R at pHREs, presumably through competition with the endogenous NCoR and SMRT that are involved in this process.
Our data demonstrate that SMRTβ acts as a positive modulator of ligand-induced activation of mutated PML/RARα and suggest a similar action on unmutated PML/RARα and wild-type nuclear receptor RARα. It has been shown that coactivators of the nuclear receptor superfamily recruit histone acetyltransferases; conversely, corepressors recruit histone deacetylases. It has therefore been suggested that changes in histone acetylation and subsequent effects on chromatin structure may be the mechanism through which cofactors mediate regulation of transcription. It will now be important to investigate the complexes formed by nuclear receptors and specific corepressor isoforms. Future work will be needed to define components of these complexes that may facilitate enhanced ligand binding and transcription by SMRTβ and possibly other corepressors.
The growing list of mutations identified in ATRA-resistant APL patients strongly supports the hypothesis that resistance to ATRA can be mediated by genetic alterations of critical residues of the LBD of the PML/RARα gene. However, our data suggest that the pattern of SMRT isoform expression in Cos-1 cells may lead to results that do not accurately reflect the activity of PML/RARα mutations in the APL cells in which they arise. Indeed, this provides one possible explanation for the recent report that found no correlation between in vitro response and the clinical response to ATRA combined with HDAC inhibitors in 5 patients with resistant APL involving different PML/RARα mutations.40 Thus, we suggest that further characterization of PML/RARα mutations arising in ATRA-resistant APL patients be performed using cellular models that best reflect the nuclear milieu of APL cells. Reevaluation of the previously characterized PML/RARα mutations may be more appropriately performed in cells, possibly such as Jurkat or U937, that do not express SMRTβ. Other tissue-specific differences in transcriptional regulation might further complicate interpretation of data obtained from the study of leukemic oncoproteins in nonhematopoietic cells. Thus, increased caution may be needed in the choice of in vitro models to study hematopoietic malignancy.
More generally, this is the first report to our knowledge in which a specific corepressor isoform is shown to promote ligand binding to a nuclear hormone receptor and its ligand-induced transactivation. We have shown that the expression of the corepressor SMRTβ can lead to a dramatic increase of hormonal response in a cell-specific manner. This paradoxical finding may be explained by the fact that SMRTβ lacks its RD1 domain and its interaction with HDAC. Another possibility is the deletion of the RD1 domain might change the 3-dimensional conformation of the corepressor and the way it interacts with nuclear receptors and other members of the corepressor-coactivator complex. The fact that a cofactor previously identified as a corepressor can also function as a coactivator in another context increases the repertoire of responses that the cell can display to various signals.
Prepublished online as Blood First Edition Paper, August 19, 2004; DOI 10.1182/blood-2003-10-3583.
Supported by the Canadian Institutes of Health Research, the Leukemia and Lymphoma Society, Italian Association for Cancer Research, Italian Ministry for Instruction, and University and Research and Ministry of Health. S.C. is supported by Fonds de la Recherche en Santé du Québec and Israel Cancer Research Fund fellowships. W.H.M. is a Scientist of the Canadian Institutes of Health Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Ronald M. Evans for his generous gift of the plasmid vectors pCMX-SMRTα and pCMX-SMRTβ and Dr Sylvie Mader for the DR5-tk-CAT response element. We also thank Angelika Rosenauer and Sandra Chaker for technical assistance.

![Figure 1. ATRA binding and transcriptional activation by a PML/RARα mutant depend on the cell line in which the receptor is expressed. (A) Comparison of ATRA-binding activity of PML/RARα expressed by NB4 cells (○) with the PML/RARα mutant I410T expressed by NB4-MRA1 (•) cells. Nuclear extracts of NB4 and NB4-MRA1 cells were subjected to Western blot analysis. The left-pointing arrow indicates expression of the 110-kDa PML/RARα wild-type or mutant I410T. (B) ATRA-binding activity of PML/RARα (•) and PML/RARα mutant I410T (○) overexpressed in Cos-1 cells. Nuclear extracts were incubated with 10 nM [3H]-ATRA or with the addition of 200-fold excess unlabeled ATRA (▪) and subjected to HPLC analysis using a 6 HR 10/30 size exclusion column. Down-pointing arrows indicate approximate retention times of void volume, PML/RARα multimers (670 kDa), and RARα monomers (50 kDa). Nuclear extracts of Cos-1–transfected cells were subjected to Western blot analysis for PML/RARα wild-type or mutant I410T (left-pointing arrow). (C) Comparison of the ligand-dependent transcriptional activity, on a DR5-tk-CAT, of PML/RARα wild-type and mutant I410T endogenously expressed in APL cells and overexpressed in Cos-1 cells. Assays were performed in triplicate and repeated at least 3 times. Bars represent standard deviation of the mean.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2003-10-3583/6/m_zh80240470750001.jpeg?Expires=1767727746&Signature=Es46P-VKOB0gQv4EKmowrrO4JuwgXscRQ2oqL0jqd72doRyxWFJK7F~3kDq3nNd6DBkg6Rpyg0Xl6PdLp6zLIyjp0~T12De2gh8W66XLqpfokon4uANBqzChNikztgO2ZXo1~J0x0WLKvNeXTc8qaWOltzVYT5vGz8C7g6n2y8-UH-r6PVoAoJh9glG92ysUz3kWSFlj2j6vVk1-oDrnlYwsCk513AYgysM5QoBViAqTPOvqtQ~fGmBMkZms9bEB8XXAE4fhvLuyb~jc-SYuvJjoprSFRMKrcA4C-XxgkvI-L2lRZUlPHFElQ2ss6gpcxrvfYDxpYAPr0GAyJwQIdw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 3. Differential expression of transcriptional corepressors NCoR and SMRT in leukemic cells and nonhematopoietic adherent cells. (A) Fifty micrograms of total RNA were analyzed by Northern blot. A 10-kb major transcript, SMRTα isoform, was detected in all cell lines examined. Note the presence of an 8.5-kb transcript, SMRTβ isoform, only in the nonhematopoietic adherent cells and its absence in all the hematopoietic cell lines tested. (B) Nuclear extracts of Jurkat, NB4, Cos-1, HeLa, and T47-D were subjected to Western blot analysis for SMRTα (top band) and SMRTβ (bottom band). Note the presence of a SMRTβ isoform only in the nonhematopoietic adherent cells and its absence in all the hematopoietic cell lines tested. (C) Overexpression of SMRTβ, but not SMRTα, induces ATRA binding to PML/RARα I410T in NB4-MRA1 cells. Nuclear extracts of cells transfected with SMRTβ (▾) or SMRTα (○) were incubated with 10 nM [3H]-ATRA and subjected to HPLC analysis using a 6 HR 10/30 size exclusion column. Nuclear extracts of NB4-MRA1 transfected cells with SMRTβ or SMRTα were subjected to Western blot analysis. The arrow indicates expression of the 110-kDa PML/RARα wild-type or mutant I410T.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2003-10-3583/6/m_zh80240470750003.jpeg?Expires=1767727746&Signature=bQaDO23gVYEz~9elCwiZmVI8bWVDT8LAyro0Z986phV3XotFlbXhr2337L2s5b-pjG4X1ckIWz-FtpVFLAl4EJynhwwEWrfla9eBmcD17vanKGHAandu9Jx~zby2SmqeiO554AJeBgnAe-ebD5Jh-cPr0WthOdnTJnsfO6C7UvJ2-4izUjcsvb1sv-eghFZonQG2n8H~IV~lb-VJGnvS4aIXCF8fpOTM4mhRX0HO-~kh0nGjei2wGiVxg-xkhriiw5FGLHTwbCHKE4JDCjhbA27X82cuO~rh-P~9Ery-ui~2usVat5ybw9O6WoRgv1WFAOOjf804iqL~XPzeo41GYw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Figure 6. Stable expression of SMRTβ in the ATRA-resistant NB4-MRA1 APL cells promotes ligand binding, ligand-induced transcriptional activation, and ATRA-induced cell differentiation. (A) Effects of stable expression of SMRTβ isoform on the ATRA-binding capacity of PML/RARα mutant I410T in A1-GFP-SMRTβ APL cells. SMRTβ induces ATRA binding to PML/RARα I410T in A1-GFP-SMRTβ cells (▾) compared with A1-GFP cells (•). Nuclear extracts were incubated with 10 nM [3H]-ATRA or with the addition of 200-fold excess unlabeled ATRA and subjected to HPLC analysis usinga6HR 10/30 size exclusion column. Nuclear extracts of stably transfected cells A1-GFP and A1-GFP-SMRTβ were subjected to Western blot analysis for the SMRTα (top band) and SMRTβ (bottom band). (B) Effects of stable SMRTβ expression on transcriptional activation. Cells stably expressing the empty GFP vector (A1-GFP) or the GFP vector harboring SMRTβ (A1-GFP-SMRTβ) were transiently transfected with the RARE reporter gene DR5-tk-CAT. Assays were performed in triplicate and repeated at least 3 times. Bars represent standard deviation of the mean. There was a significant difference between cells stably transfected with SMRTβ compared with transfected cells with the empty vector: *P < .05, **P < .0005. Nuclear extracts of stably transfected cells A1-GFP and A1-GFP-SMRTβ were subjected to Western blot analysis for the 110-kDa PML/RARα mutant I410T (arrow). (C-D) Expression of the cell-surface myeloid-specific differentiation markers CD11b (C) and CD11c (D) by flow cytometry analysis. Cells stably expressing the empty GFP vector (A1-GFP) or the GFP vector harboring SMRTβ (A1-GFP-SMRTβ) were treated with 0.01 and 1 μM ATRA for 5 days, stained with an antibody specific for CD11b or CD11c, and subjected to flow cytometry analysis. The cells were also analyzed for the isotype control PE-conjugated mouse IgG1κ for CD11b-PE and CD11c-PE. In each sample, viable cells were gated, and expression of CD11b or CD11c surface markers of 5 × 103 cells was evaluated. Numbers in parentheses indicate the percentage of positive CD11b or CD11c cells. This experiment is representative of 3 that gave comparable results.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2003-10-3583/6/m_zh80240470750006.jpeg?Expires=1767727746&Signature=YuJgjMEwxS12gpfjpbc2QcgixOyGzAL15B~I9RMtyMdMVEWO9A-ZQwFZJLOCRZxJEzq9rXZExVghVsAUKrI~UuSLZvYoXUI9gKkZugYtItvq3wDGeiQLcKkglhWb6K4saEvygBWUYyg1hXitxhi7iqjNZgDcSthGP-b3TWO~HTOxUAYri3Ss73xbfELXEsLjlUrEXwgdnP8213vgBvNsNsD3WKUl89vY-MWgD~FlBp7vSvoWysVVaWfW0lqmqm8PKFwdmJgLlouQvARJrwU5s9mtexp6ttRHJKX-v72Gj5nOXJ80AfCEhSEuv-bAfuCqe92rKfgB6gQfb0OaG4-QXg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal