Abstract
Natural killer (NK) cells are a component of the innate immunity against viral infections through their rapid cytotoxic activity and cytokine production. Although the synthetic double-stranded (ds) RNA polyinosinic-polycytidylic acid (poly I:C), a mimic of a common product of viral infections, is known to rapidly up-regulate their in vivo functions, NK cell ability to directly respond to dsRNA is still mostly unknown. Our results show that treatment with poly I:C significantly up-regulates both natural and CD16-mediated cytotoxicity of highly purified human NK cells. Poly I:C also induces the novel capability of producing CXCL10 chemokine in human NK cells and synergistically enhances interferon-γ (IFN-γ) production induced by either adaptive or innate cytokines. In accordance with the expression of Toll-like receptor-3 (TLR3) and of TRIF/TICAM-1 adaptor, poly I:C stimulation induces the activation of interferon regulatory factor-3 (IRF-3) transcription factor and of p38 mitogen-activated protein kinase (MAPK) in human NK cells. Finally, we demonstrate that p38 MAPK activity is required for the dsRNA-dependent enhancement of cytotoxicity and CXCL10 production. The occurrence of dsRNA-induced signaling and functional events closely correlates with the TLR3 mRNAprofile in different NK cell populations. Taken together, these data identify p38 as a central component of NK cell ability to directly respond to dsRNA pathogen-associated molecular pattern (PAMP).
Introduction
Natural killer (NK) cells are a small lymphocyte subpopulation resident in peripheral blood and in some lymphoid and nonlymphoid organs, capable of rapidly migrating to peripheral sites in response to infections or to neoplastic transformation.1 They represent an important component of innate immunity by exerting both a constitutive cytotoxic activity, directed against infected or transformed cells and immature hematopoietic precursors, and the antibody-dependent cytolytic activity (ADCC), thanks to the presence of CD16, the low-affinity Fc receptor for immunoglobulin G (IgG) (FcγRIII), on the vast majority of them.2,3 Besides their cytolytic function, NK cells also rapidly secrete a variety of cytokines and chemokines in response to stimulation, by means of which they amplify the recruitment and activation of other effector cell populations.1,4,5
NK cell activation is regulated by the fine balance of positive and negative signaling pathways initiated by multiple receptors displaying either activating, costimulatory, or inhibitory activity, whose expression and/or functional capability can be modulated during NK cell activation/differentiation.6-8 NK cell functions are rapidly augmented by a vast array of both innate and adaptive cytokines and a number of other biologic response modifiers.1,5
NK cells play a crucial role in the natural resistance against viral infections, both by exerting an effector function in the early containment of the infection and by participating in the instructive phase of the adaptive immune response.9-11 NK cells rely on a variety of cues to precociously sense and respond to the presence of viral infections, such as the up-regulation of viral or virus-induced peptidic ligands for different activating receptors, the virus-induced down-modulation of major histocompatibility complex (MHC) class I ligands for inhibitory receptors, and the presence of proinflammatory cytokines, such as type I interferon (IFN), which promptly up-regulate NK cytolytic activity and cytokine production.5,9-13 In particular, the synthetic copolymer polyinosinic-polycytidylic acid (poly I:C), which mimics double-stranded (ds) RNA viral products, has been previously shown to rapidly promote NK cell responses in vivo through its capacity to induce type I IFN production.1,9-11,14
Innate immunity effector cells recognize the presence of different pathogens mainly through a recently identified family of 10 genetically invariant receptors, the Toll-like receptors (TLR1-10), capable of recognizing distinct molecular components of microbes.15,16 In particular, TLR3 is the specific receptor for dsRNA, a common intermediate in the reproductive cycle of many viruses; indeed, responsiveness to viral dsRNA or poly I:C is severely compromised in TLR3 knock-out mice.17 TLR3 expression in the human hematopoietic compartment has been initially reported to be restricted mainly to myeloid dendritic cells, where it induces cell maturation, and the production of inflammatory and antimicrobial cytokines, such as type I IFN, interleukin-12 (IL-12), IL-6, and tumor necrosis factor-α (TNF-α)15-18 ; only few reports have analyzed TLR3 expression in human lymphocyte subpopulations,19-23 where its functional role remains mostly unexplored.
The signaling pathways initiated by TLR3 have been clarified only in part and crucially depend on the recently identified Toll-interleukin 1 receptor domain (TIR)–containing adapter inducing IFN-β (TRIF)/TIR-containing adapter molecule-1 (TICAM-1), which associates to the receptor and, by nucleating the formation of enzymatic complexes, initiates multiple signaling pathways leading to the activation of extracellular signal-regulated kinase (ERK), Janus kinase (JNK), and p38 members of the mitogen-activated protein kinase (MAPK) family, and nuclear factor–κB (NFκB) and interferon regulatory factor-3 (IRF-3) transcription factors.15,16,24-27 These are then responsible for the activation of cytokine and chemokine genes and for the expression of costimulatory molecules.28,29 The crucial role of TRIF/TICAM-1 adaptor in the TLR3-mediated cell activation is underscored by the observation that responsivity to dsRNA is almost completely abrogated in TRIF/TICAM-1 knock-out mice.30,31 Recently, TLR3 expression on fresh human NK cells and the ability of dsRNA to augment NK cell functions has been reported,32 but the mechanisms enabling NK cells to functionally respond to this product of viral infections are mostly unknown.
In this report we characterize the ability of different NK cell populations to respond to dsRNA by demonstrating that poly I:C stimulation up-regulates both natural and CD16-dependent cytotoxic functions and synergizes with both innate and adaptive cytokines in the production of IFN-γ; moreover, we provide evidence that poly I:C stimulation rapidly induces CXCL10 production, a previously unknown function of NK cells. Here we show for the first time that dsRNA stimulation induces the activation of p38 MAPK and IRF-3 transcription factor in human NK cells, and we demonstrate the central role of p38 MAPK activation in the dsRNA-induced up-regulation of NK cell cytotoxic activity and chemokine production. Interestingly, the ability of dsRNA to modulate NK cell functions and signaling closely correlates with TLR3 receptor expression in different NK cell populations, thus emphasizing the essential role of this receptor in enabling the functional response to dsRNA.
Materials and methods
Cell lines
The following human NK cell lines were used: NK92,33 NKL (provided by Dr M. J. Robertson34 ), YTCl2 and YTS (2 sublines of YT kindly provided by Dr L. L Lanier35 ). NK92 was maintained in α-minimal essential medium (α-MEM) containing 200 U/mL human recombinant IL-2 (rIL-2) (EuroCetus BV, Amsterdam, The Netherlands) supplemented with 20% fetal calf serum (FCS), 2 mM l-glutamine, 1% nonessential amino acids, and 1% sodium pyruvate. The other cell lines were grown in RPMI 1640 containing 10% FCS with 2 mM l-glutamine (complete medium [CM]). NKL was maintained in CM plus 200 U/mL human rIL-2.
NK92 and NKL cells were deprived of IL-2 for 24 hours before each experiment.
Cell lines used as targets in cytotoxicity assays were K562 (human erythroleukemia), 721.221 (human B-lymphoblastoid cell line [B-LCL]), and P815 (murine FcγR-positive mastocytoma), all cultured in CM.
Antibodies and reagents
Poly I:C was purchased from Sigma-Aldrich (Milan, Italy) and Amersham (Amersham-Pharmacia Biotech Italia, Milan, Italy); ionomycin, phorbol myristate acetate (PMA) and cycloheximide were from Sigma-Aldrich; SB203580 was from Alexis (Lausanne, Switzerland). Recombinant human IL-12 was from the Genetics Institute (Cambridge, MA). The following mouse monoclonal antibodies (mAbs) were used: anti-CD3 (Leu4), anti-CD16 (Leu11c), anti-CD56 (Leu19), anti-CD5 (Leu1), anti-CD14, all from BD Biosciences (San Jose, CA); anti–β-tubulin mAb was from Sigma-Aldrich. Anti–USF-2, anti–IRF-3, and anti-MKK3 rabbit antisera were obtained from Santa Cruz Biotechnology (Santa Cruz, CA); antiphospho-p38, anti-p38, and anti-phosphoMKK3/MKK6 rabbit antisera were purchased from Cell Signaling Technology (Beverly, MA). Anti-CD16 mAb (B73.1) used for cytotoxicity experiments was a kind gift of Dr G. Trinchieri (Schering Plough, Dardilly, France).
NK cell preparations
Human polyclonal NK cell populations were obtained by coculturing nylon nonadherent peripheral blood mononuclear cells (PBMCs) with irradiated RPMI 8866 (an Epstein-Barr virus [EBV]–positive lymphoblastoid B cell line) for 9 to 10 days at 37°C in a humidified 5% CO2 atmosphere as previously described.36 For each experiment, polyclonal fresh or cultured NK cells were highly purified using a magnetic-activated cell separation (MACS) NK cell isolation kit (Miltenyi Biotec, Bologna, Italy) or anti-CD3–coated Dynabeads (Dynal, Lake Success, NY), respectively. The purity of fresh and cultured NK cell populations ranged between 96% to 99% and 98% to 99%, respectively, as evaluated by anti-CD16, anti-CD56, anti-CD3, anti-CD5, and anti-CD14 immunostaining and cytofluorimetric analysis.
RNA isolation and RT-PCR
Total RNA was isolated from purified NK cells and cell lines using the TRIzol solution according to the manufacturer (Invitrogen, Carlsbad, Germany). When required, isolated RNA was treated with RNase-free DNase I (Roche, Mannheim, Germany) for 30 minutes at 37°C. Reverse transcription (RT) was performed using the First Strand cDNA synthesis kit (Roche) following the manufacturer's instructions. Polymerase chain reaction (PCR) amplification was performed with AmpliTaq Gold polymerase (Applied Biosystems, Foster City, CA). The primer pairs used for PCR analyses were as follows: TLR3, forward GATCTGTCTCATAATGGCTTG and reverse GACAGATTCCGAATGCTTGTG37 ; TLR3 second primer pair, forward AAATTGGGCAAGAACTCACAGG and reverse GTGTTTCCAGAGCCGTGCTAA38 ; TRIF/TICAM-1, forward CCAGATGCAACCTCCACTGG and reverse CTGTTCCGATGATGATTCC24 ; CXCL10, forward GGAACCTCCAGTCTCAGCACC and reverse CAGCCTCTGTGTGGTCCAATCC39 ; β-actin, forward GGGTCAGAAGGATTCCTATG and reverse GGTCTCAAACATGATCTGGG.
Cytotoxicity assay
The 51chromium release assay was used to measure cytotoxic activity, as previously described.36 Anti-CD16 mAb was employed at 0.5 μg/mL in the reverse ADCC (rADCC) assay. The percent specific 51Cr release was determined by the equation ([experimental cpm - spontaneous cpm]/total cpm incorporated) × 100. All determinations were performed in triplicate, and radioactivity was measured with TopCount (PerkinElmer Life and Analytical Sciences, Monza, Italy).
Cytokine production assay
IFN-γ and CXCL10 production in cell-free supernatants was quantitated with an enzyme-linked immunosorbent assay (ELISA) kit (Biosource, Camarillo, CA, and R&D Systems, Lille, France, respectively).
Cell stimulation, lysis, and immunoblotting
Cells were stimulated with 100 μg/mL poly I:C for different time periods at 37°C. Total cell lysates were obtained by using a lysis buffer containing 1% Triton X-100, 1 mM CaCl2, 1 mM MgCl2, 150 mM NaCl, 10 mM NaF, 1 mM sodium orthovanadate, 10 mM iodoacetamide, and protease inhibitor cocktail tablet (Roche) in phosphate-buffered saline (PBS). In some experiments, cells were pretreated for 1 hour with SB203580 or cycloheximide prior to stimulation with poly I:C.
For cytosolic and nuclear extracts, cells were lysed in a hypotonic buffer (50 mM KCl, 0.5% Nonidet P-40 [NP-40], 25 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] pH 7.8, 50 mM NaF, 1 mM sodium orthovanadate, and protease inhibitor cocktail) for 5 minutes, followed by centrifugation at 5000g for 5 minutes; supernatants were collected as the cytosolic fraction. Nuclei-containing pellets were resuspended in 20 mM HEPES, pH 7.9, 0.4 M NaCl, 1 mM EDTA (ethylenedia-minetetraacetic acid), 1 mM EGTA (ethylene glycol tetraacetic acid), 50 mM NaF, 1 mM sodium orthovanadate, and protease inhibitor cocktail, vigorously rocked at 4°C for 15 minutes, and centrifuged at maximum speed for 5 minutes; supernatants were collected as the nuclear fraction.40 Cell extracts were normalized, and equal protein amounts were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred on Immobilon-P nitrocellulose membranes (Millipore, Bedford MA). After blocking nonspecific reactivity, filters were probed with specific Abs diluted in PBS plus 0.05% Tween 20. After extensive washing, immunoreactivity was detected using an enhanced chemiluminescence (ECL) kit (Amersham-Pharmacia Biotech Italia).
Results
Expression of TLR3 and TRIF/TICAM-1 in normal and tumor NK cell lines
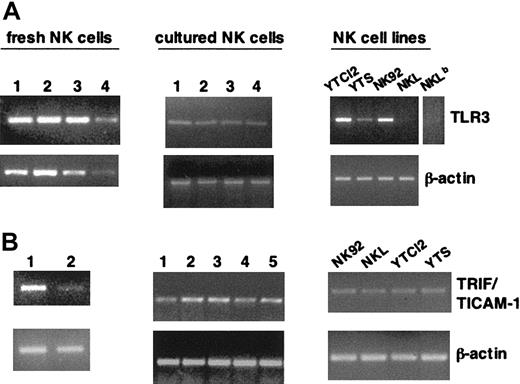
TLR3 receptor and its specific adaptor, TRIF/TICAM-1, have been shown to be crucially involved in the ability to sense and respond to the presence of dsRNA.15-17,24,25,30,31 Here we show that a panel of highly purified (96% to 99%) fresh and short-term cultured human NK cell samples, obtained from different healthy donors, consistently express TLR3 mRNA, as evaluated by RT-PCR analysis (Figure 1A, upper panels). TLR3 is also present in some NK cell lines, with the exception of NKL, where it is only barely detectable. These results were confirmed with a second primer pair, encompassing a different region of the TLR3 gene (not shown), and validate and extend recent observations.32 Our analysis also shows the expression of TRIF/TICAM-1 mRNA on highly purified fresh (in accord with previous evidence24 ) and cultured human NK cells and on all the NK cell lines analyzed (Figure 1B, upper panels). RT-PCR analysis of β-actin expression was used as control (Figure 1A-B, lower panels). Together, these data demonstrate that human NK cells express the molecular machinery enabling them to respond to dsRNA.
Human NK cells and NK cell lines express TLR3 and TRIF/TICAM-1 mRNA. Total RNA from highly purified fresh (both panels, left) and short-term cultured (A-B, middle) NK cells from different donors and a panel of NK cell lines (both panels, right) was subjected to RT-PCR with specific primers for TLR3 (A) and TRIF/TICAM-1 (B); β-actin amplification, as control, is shown in the lower lanes. A longer exposure for the TLR3 sample in NKL is shown in panel A (NKLb). The data shown are representative of 1 out of 4 independent experiments.
Human NK cells and NK cell lines express TLR3 and TRIF/TICAM-1 mRNA. Total RNA from highly purified fresh (both panels, left) and short-term cultured (A-B, middle) NK cells from different donors and a panel of NK cell lines (both panels, right) was subjected to RT-PCR with specific primers for TLR3 (A) and TRIF/TICAM-1 (B); β-actin amplification, as control, is shown in the lower lanes. A longer exposure for the TLR3 sample in NKL is shown in panel A (NKLb). The data shown are representative of 1 out of 4 independent experiments.
Poly I:C stimulation enhances the lytic ability of TLR3-expressing NK cell populations in a time- and protein synthesis–dependent manner
NK cells are important cytotoxic effectors in the immune response against viral infections,9,11 and their cytolytic ability is potentiated upon in vivo exposure to the synthetic dsRNA poly I:C.14 Very recent evidence has shown the up-regulation of cytotoxic activity of fresh human NK cells by stimulation with poly I:C.32 Data reported in Figure 2 show that poly I:C treatment enhances the lytic ability of highly purified polyclonal short-term cultured NK cells. Both natural killing of K562 and 721.221 prototypical NK target cell lines and the CD16-dependent lysis of FcγR-positive P815 target cells are up-regulated by dsRNA stimulation (Figure 2A); interestingly, no significant enhancing effect is observed on the lysis of P815 (in the absence of antibody) and of other NK cell–resistant targets (data not shown).
Poly I:C–induced augmentation of NK cytotoxic activity is time- and protein synthesis–dependent. (A-C) Highly purified short-term cultured NK cells (3 × 106/mL [A]) and NK92 (0.2 × 106/mL [B]) and NKL (0.4 × 106/mL [C]) cell lines were left untreated (NT, dashed line) or stimulated with 100 μg/mL poly I:C (solid line) for 24 hours and then subjected to cytotoxicity assay against K562 and 721.221 target cells; P815 target cell lysis was assayed in the presence (•) or absence of anti-CD16 (B73.1) mAb (A). The data shown are representative of at least 5 independent experiments. (D-E) Cytotoxic activity against K562 target cells was tested in a 51Cr release assay. Highly purified polyclonal NK cells (left) and the NK92 cell line (right) were left untreated (dashed line) or stimulated with poly I:C (100 μg/mL) (open symbols) for different times (D); highly purified human polyclonal short-term cultured NK cells were stimulated with poly I:C (closed symbols) or left untreated (open symbols) for 24 hours in the presence (triangles) or not (circles) of cycloheximide (CHX, 10 μg/mL) (E). These results are representative of 1 out of 2 independent experiments.
Poly I:C–induced augmentation of NK cytotoxic activity is time- and protein synthesis–dependent. (A-C) Highly purified short-term cultured NK cells (3 × 106/mL [A]) and NK92 (0.2 × 106/mL [B]) and NKL (0.4 × 106/mL [C]) cell lines were left untreated (NT, dashed line) or stimulated with 100 μg/mL poly I:C (solid line) for 24 hours and then subjected to cytotoxicity assay against K562 and 721.221 target cells; P815 target cell lysis was assayed in the presence (•) or absence of anti-CD16 (B73.1) mAb (A). The data shown are representative of at least 5 independent experiments. (D-E) Cytotoxic activity against K562 target cells was tested in a 51Cr release assay. Highly purified polyclonal NK cells (left) and the NK92 cell line (right) were left untreated (dashed line) or stimulated with poly I:C (100 μg/mL) (open symbols) for different times (D); highly purified human polyclonal short-term cultured NK cells were stimulated with poly I:C (closed symbols) or left untreated (open symbols) for 24 hours in the presence (triangles) or not (circles) of cycloheximide (CHX, 10 μg/mL) (E). These results are representative of 1 out of 2 independent experiments.
We also assayed the effect of poly I:C on the cytotoxic ability of different NK tumor cell lines and found that NK92 cells display an enhanced killing ability when pretreated with poly I:C (Figure 2B). By contrast, no augmentation of cytotoxicity is observed in NKL under the same experimental conditions (Figure 2C), in accordance with the almost complete absence of TLR3 mRNA in this cell line.
Poly I:C–induced enhancement of NK cell cytotoxic activity begins to be evident after few hours (Figure 2D), with a slightly different kinetics in the normal NK cell populations (left) compared with the NK92 cell line (right); moreover, pretreatment of NK cells with cycloheximide, while partially affecting basal killing, completely abrogates the dsRNA-induced enhancement of cytotoxic activity (Figure 2E), suggesting the requirement for de novo protein synthesis in the poly I:C–mediated augmentation of NK cell cytotoxicity.
Collectively taken, this set of data demonstrates that the direct stimulation with dsRNA up-regulates, in a time- and protein synthesis–dependent manner, the cytotoxic activity of TLR3-high–but not of TLR3-low–expressing NK cells against different susceptible, but not resistant, target cells.
Poly I:C treatment enhances the cytokine-induced production of IFN-γ in human NK cells
Different stimuli, and particularly cytokines of both innate and adaptive immunity, induce IFN-γ production by NK cells.1,5,9
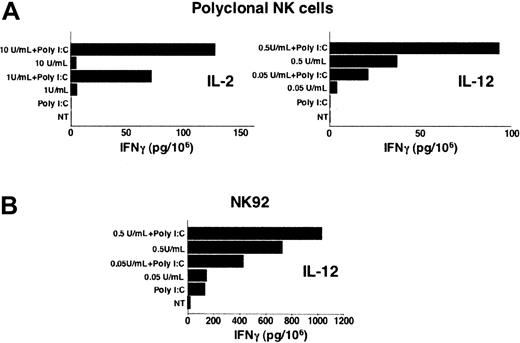
We analyzed whether dsRNA treatment could affect NK cell ability to produce IFN-γ. Highly purified polyclonal short-term cultured NK cells and NK92 were stimulated with poly I:C in the presence or not of different cytokines, and IFN-γ production was measured in cell culture supernatants. As shown in Figure 3A, although poly I:C stimulation of polyclonal short-term cultured NK cells does not induce cytokine production by itself, it synergistically enhances IFN-γ secretion induced by suboptimal doses of either IL-2 or IL-12. Interestingly, poly I:C treatment is by itself sufficient to mediate IFN-γ release by the IL-2–dependent cell line NK92 and, similarly to what is observed in polyclonal NK cells, the production is strongly increased by IL-12 costimulation (Figure 3B). These results demonstrate that dsRNA synergizes with both adaptive and innate cytokines in stimulating IFN-γ production by human NK cells.
Poly I:C stimulation up-regulates IFN-γ production in human NK cells. Highly purified polyclonal NK cells (A) and the NK92 cell line (B) were left untreated (NT), or stimulated with poly I:C (100 μg/mL) or different doses of IL-2 (A, left) or IL-12 (A, right, and B), in the presence or absence of poly I:C for 24 hours. IFN-γ production was measured in cell culture supernatants. Data presented are representative of 1 out of 3 independent experiments.
Poly I:C stimulation up-regulates IFN-γ production in human NK cells. Highly purified polyclonal NK cells (A) and the NK92 cell line (B) were left untreated (NT), or stimulated with poly I:C (100 μg/mL) or different doses of IL-2 (A, left) or IL-12 (A, right, and B), in the presence or absence of poly I:C for 24 hours. IFN-γ production was measured in cell culture supernatants. Data presented are representative of 1 out of 3 independent experiments.
Poly I:C treatment directly and rapidly induces CXCL10 mRNA and protein production in human NK cells
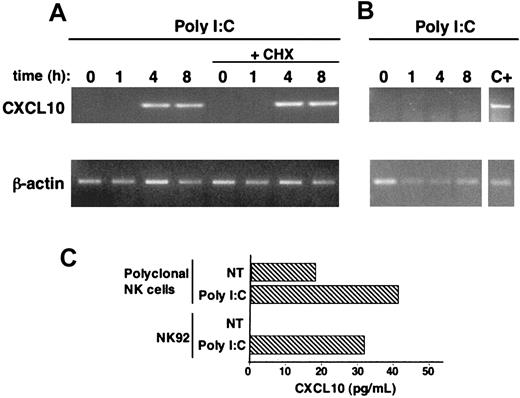
The ability of NK cells to produce chemokines plays a crucial role in their antiviral function.9,10,13,41 CXCL10 chemokine is involved in the immune responses against a variety of infections42 and specifically attracts activated T cells and NK cells.4,42 CXCL10 gene activation has been reported to be controlled by TLR3- and TRIF/TICAM-1–dependent signaling pathways.28,29 Here we show that both human polyclonal NK cells and the NK92 cell line produce CXCL10 upon stimulation with poly I:C (Figure 4C); this production most probably depends on gene activation, because a short-term exposure to poly I:C (4 hours) promptly induces the appearance of CXCL10 mRNA, as shown by RT-PCR analysis (Figure 4A). Strikingly, CXCL10 mRNA induction is directly dependent on dsRNA-mediated signaling, because it is not affected by the inhibition of protein synthesis, as shown by pretreatment with cycloheximide (Figure 4A). Interestingly, no production of CXCL10 is observed in the NKL cell line under the same experimental conditions (Figure 4B). Taken together, these data firstly show the ability of dsRNA to directly induce the production of CXCL10 chemokine in high but not low TLR3-expressing NK cells and strongly suggest that dsRNA stimulation directly induces CXCL10 gene activation in human NK cells.
Poly I:C treatment stimulates CXCL10 chemokine production in human NK cells. (A-B) NK92 (A) or NKL (B) cell lines were stimulated with poly I:C for the indicated times, in the presence or absence of cycloheximide (CHX, 10 μg/mL); RT-PCR with specific primers for CXCL10 and for β-actin (as control) was then performed on total RNA. Positive control (C+) is represented by an RNA sample of poly I:C–stimulated NK92 cells processed in the same experiment. (C) CXCL10 secretion in culture supernatants of poly I:C–stimulated highly purified polyclonal NK cells (3 out of 4 donors) and NK92 cells was quantitated by specific ELISA. These data are representative of 1 out of at least 3 independent experiments.
Poly I:C treatment stimulates CXCL10 chemokine production in human NK cells. (A-B) NK92 (A) or NKL (B) cell lines were stimulated with poly I:C for the indicated times, in the presence or absence of cycloheximide (CHX, 10 μg/mL); RT-PCR with specific primers for CXCL10 and for β-actin (as control) was then performed on total RNA. Positive control (C+) is represented by an RNA sample of poly I:C–stimulated NK92 cells processed in the same experiment. (C) CXCL10 secretion in culture supernatants of poly I:C–stimulated highly purified polyclonal NK cells (3 out of 4 donors) and NK92 cells was quantitated by specific ELISA. These data are representative of 1 out of at least 3 independent experiments.
Poly I:C stimulation induces the activation of IRF-3 transcription factor and of p38 MAPK in human NK cells
TLR3 signaling in response to dsRNA ligand has been shown to lead to the activation of MAPK family members and of several transcription factors.15-17 In particular, IRF-3 is a key transcription factor responsible for TLR3-mediated gene activation and is crucially involved in the synthesis of cytokines and chemokines in response to dsRNA stimulation; the activation of IRF-3 has been specifically ascribed to TRIF/TICAM-1–coupled TLRs (TLR3 and TLR4).24-26,28-30 Recently, the dsRNA-stimulated degradation of IκB in fresh human NK cells has been shown32 ; nevertheless, the molecular pathways initiated by TLR3 ligand in human NK cells remain mostly unknown.
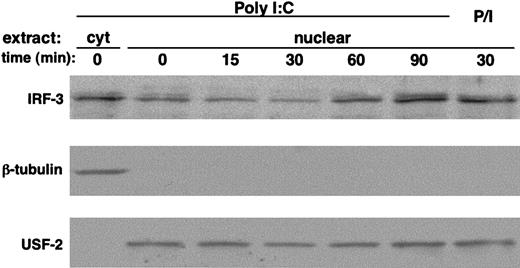
Here we report that poly I:C stimulation of polyclonal short-term cultured NK cells triggers the time-dependent activation of IRF-3, as shown by the nuclear translocation of the transcription factor (Figure 5, upper panel). Stimulation of NK cells with PMA/ionomycin was used as positive control. The same membrane was reprobed with anti–USF-2 and anti–β-tubulin Abs to verify equal loading and to exclude cytosolic protein contamination (Figure 5, lower and middle panels, respectively).
Poly I:C triggers IRF-3 activation in human NK cells. Polyclonal highly purified cultured NK cells (10 × 106 per sample) were stimulated with poly I:C (100 μg/mL) or PMA (50 ng/mL)/ionomycin (0.5 μM) (P/I) at 37°C for the indicated times. Cytosolic and nuclear extracts were separated on SDS-PAGE and immunoblotted with anti–IRF-3 Ab (top). Subcellular fractionation was checked by blotting with anti–β-tubulin (middle) and anti–USF-2 transcription factor (bottom) Abs. These data are representative of 1 out of 3 independent experiments.
Poly I:C triggers IRF-3 activation in human NK cells. Polyclonal highly purified cultured NK cells (10 × 106 per sample) were stimulated with poly I:C (100 μg/mL) or PMA (50 ng/mL)/ionomycin (0.5 μM) (P/I) at 37°C for the indicated times. Cytosolic and nuclear extracts were separated on SDS-PAGE and immunoblotted with anti–IRF-3 Ab (top). Subcellular fractionation was checked by blotting with anti–β-tubulin (middle) and anti–USF-2 transcription factor (bottom) Abs. These data are representative of 1 out of 3 independent experiments.
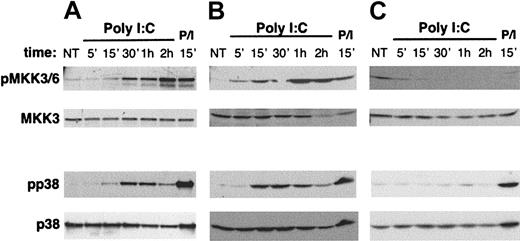
p38 members of the MAPK family are important mediators of the inflammatory TLR-mediated response and are activated through an enzymatic cascade whose upstream components are represented by MAPK kinase-3 (MKK3) and MKK6.15,16,43 We investigated whether poly I:C stimulation could initiate the signaling pathway leading to the activation of p38 MAPK. Polyclonal short-term cultured human NK cells and the NK92 and NKL cell lines were stimulated with poly I:C for different time periods, and the activation state of p38 and of MKK3/MKK6 was evaluated by Western blotting with Abs directed against their phosphorylated forms. The results reported in Figure 6 (lower half) show that poly I:C treatment leads to the time-dependent phosphorylation of p38 in both polyclonal NK cells (Figure 6A) and NK92 (Figure 6B); in the same experimental conditions, the activation of MKK3 and MKK6 upstream enzymes was also observed (Figure 6, upper half); interestingly, activation of neither p38 nor MKK3/MKK6 was observed in the NKL cell line, barely expressing TLR3 mRNA, under the same experimental conditions (Figure 6C). Stimulation with PMA/ionomycin was used as positive control. Western blots with anti-MKK3 and anti-p38 Abs (Figure 6, lower panels in both top and bottom halves) confirmed the comparable loading of all lanes; the activation state of both MKK3/MKK6 and p38 did not vary significantly with time in untreated samples (not shown).
Poly I:C induces MKK3/6 and p38 MAPK activation in human NK cells. (A) Highly purified cultured NK (10 × 106 per sample), (B) NK92 (4 × 106 per sample), and (C) NKL (4 × 106 per sample) cells were stimulated with poly I:C at 37°C for the indicated times. Cell lysates were electrophoresed under reducing condition and blotted with antiphospho-MKK3/6 and anti-MKK3 (top panels) or antiphospho-p38 and anti-p38 Ab (bottom panels). PMA (50 ng/mL)/ionomycin (0.5 μM) (P/I) stimulation was used as positive control. These data are representative of 1 out of at least 4 independent experiments.
Poly I:C induces MKK3/6 and p38 MAPK activation in human NK cells. (A) Highly purified cultured NK (10 × 106 per sample), (B) NK92 (4 × 106 per sample), and (C) NKL (4 × 106 per sample) cells were stimulated with poly I:C at 37°C for the indicated times. Cell lysates were electrophoresed under reducing condition and blotted with antiphospho-MKK3/6 and anti-MKK3 (top panels) or antiphospho-p38 and anti-p38 Ab (bottom panels). PMA (50 ng/mL)/ionomycin (0.5 μM) (P/I) stimulation was used as positive control. These data are representative of 1 out of at least 4 independent experiments.
Taken together, these data demonstrate that dsRNA stimulation directly induces the activation of TRIF/TICAM-1–dependent transcription factor IRF-3 and of p38 MAPK in TLR3-expressing human NK cell populations.
Poly I:C–triggered activation of p38 MAPK is involved in the augmentation of cytotoxic activity and in the production of CXCL10 chemokine
p38-dependent pathways control the synthesis of proinflammatory cytokines and chemokines and are also required for the development of cytotoxic activity in human NK cells.43-47 We thus addressed the role of p38 in poly I:C–induced modulation of NK cell functions by using SB203580, a specific pharmacologic inhibitor that reversibly blocks p38 MAPK enzymatic activity.48 Here we show that the presence of SB203580 during the stimulation period with poly I:C significantly reduces, in a dose-dependent manner, the augmentation of cytotoxic activity (Figure 7A). Notably, because the inhibitor is only used in pretreatment and is not present throughout the cytotoxicity assay, it does not appreciably affect basal cytolytic activity.
p38 MAPK activity controls poly I:C–triggered up-regulation of cytotoxic activity and CXCL10 production in human NK cells. (A) The NK92 cell line was stimulated with poly I:C (closed symbols) or left untreated (open symbols) in the presence (squares, triangles) or absence (circles) of SB203580 pharmacologic inhibitor for 24 hours; cytotoxic activity was then tested against K562 target in a 4-hour 51Cr release assay. These data are representative of 1 of 2 independent experiments. (B) The NK92 cell line was stimulated with poly I:C in the presence or absence of SB203580. RT-PCR with specific primers for CXCL10 and for β-actin (as control) was then performed on total RNA. These data are representative of 1 out of 4 independent experiments. (C) CXCL10 secretion in culture supernatants of untreated (empty bars) or poly I:C–stimulated highly purified polyclonal NK cells and NK92 cells (solid bars), in either the presence or absence of SB203580, was quantitated by specific ELISA. These data are representative of 1 out of at least 4 independent experiments.
p38 MAPK activity controls poly I:C–triggered up-regulation of cytotoxic activity and CXCL10 production in human NK cells. (A) The NK92 cell line was stimulated with poly I:C (closed symbols) or left untreated (open symbols) in the presence (squares, triangles) or absence (circles) of SB203580 pharmacologic inhibitor for 24 hours; cytotoxic activity was then tested against K562 target in a 4-hour 51Cr release assay. These data are representative of 1 of 2 independent experiments. (B) The NK92 cell line was stimulated with poly I:C in the presence or absence of SB203580. RT-PCR with specific primers for CXCL10 and for β-actin (as control) was then performed on total RNA. These data are representative of 1 out of 4 independent experiments. (C) CXCL10 secretion in culture supernatants of untreated (empty bars) or poly I:C–stimulated highly purified polyclonal NK cells and NK92 cells (solid bars), in either the presence or absence of SB203580, was quantitated by specific ELISA. These data are representative of 1 out of at least 4 independent experiments.
Our results also indicate that the inhibition of p38 activity significantly reduces poly I:C–triggered production of CXCL10 in both polyclonal NK cells and the NK92 cell line (Figure 7C) and suggest that this event is dependent on CXCL10 mRNA induction and/or accumulation, as shown by RT-PCR analysis (Figure 7B).
Collectively, these results demonstrate that the dsRNA-triggered activation of p38 MAPK controls the enhancement of cytotoxicity and the production of CXCL10 chemokine in human NK cells.
Discussion
Here we show that different human NK cell populations express the molecular machinery (ie, TLR3 and TRIF/TICAM-1) enabling them to directly respond to dsRNA; accordingly, our results demonstrate that stimulation with poly I:C leads to the activation of IRF-3 transcription factor and of the MKK3/MKK6-p38 MAPK pathway as a link between TLR3 receptor and the functional response. Here we show that p38 MAPK activity is crucially involved in the functional activation of NK cells induced by dsRNA stimulation by controlling the poly I:C–mediated up-regulation of cytotoxic activity and cytokine production; in particular, we report the dsRNA-stimulated production of CXCL10 chemokine, a novel ability of NK cells. Interestingly, the poly I:C–triggered enhancement of NK cell functions and signaling events strictly correlates with the expression profile of TLR3 in different NK cell lines, thus underscoring the essential role of the receptor in the capacity of NK cells to respond to dsRNA.
Our results on the presence of the essential molecular machinery that confers the capability to respond to dsRNA15-17,30,31 are in agreement with recent data reporting TLR3 and TRIF/TICAM-1 expression in fresh human NK cells24,32 and extend the analysis on short-term activated NK cells and on a panel of NK cell lines. Among the cell lines tested, NKL only was found to express TLR3 mRNA at barely detectable levels.
Here we show that poly I:C stimulation of NK cells leads to the activation of IRF-3. This event is considered a specific signature of TRIF/TICAM-1–coupled TLRs15,16,26 and plays a crucial role in the TLR3- and virus-induced synthesis of a vast array of cytokines and chemokines. In particular, CXCL10 chemokine has been identified among the primary target genes of IRF-3.28,29,49 The dsRNA-triggered activation of IRF-3 depends on TRIF/TICAM-1–mediated formation of a molecular complex containing TANK-binding kinase-1 (TBK1) and IκB kinase-ϵ (IKKϵ), essential components of the IRF-3 signaling pathway.26,50-53 Whether these mediators play a role also in the poly I:C–induced activation of IRF-3 in human NK cells is still unknown.
TLR3 shares with the other members of the family the ability to induce the activation of MAPK, central transduction elements mediating cell activation and proliferation upon a variety of extracellular stimuli.54,55 Among them, p38 primarily mediates a proinflammatory response in many immune cells. p38 activation generally depends on MKK3 and MKK6, whose activity can be controlled by different upstream kinases, acting as MAPK kinase kinase (MKKK), depending on the extracellular stimulus or receptor involved.43,44,48 We show that poly I:C treatment triggers the time-dependent activation of MKK3/MKK6 and p38 in both polyclonal human NK cells and in the TLR3-positive NK92 cell line. The coincident activation of MKK3/MKK6 and p38 strongly suggests that MKK3/MKK6 are the upstream elements in the dsRNA-mediated activation of p38 in human NK cells. The receptor-proximal molecular intermediates regulating the p38 cascade have not been identified yet, even if transforming growth factor β-activated kinase (TAK) 1 has been proposed to act as MKKK in the TLR3-induced activation of p38.27
TLR3-mediated recognition of dsRNA modulates functional activities in different cell types.15-17,26 Here we show that poly I:C stimulation up-regulates the cytotoxic functions of both polyclonal short-term activated human NK cells and NK92.
NK cell cytotoxic capability may be modulated by mechanisms qualitatively and/or quantitatively affecting either the binding/activation step, which is controlled by a vast array of activating, costimulatory, and adhesion receptors, or the late execution step, which mainly depends on the release of perforin-containing cytoplasmic granules and on Fas-FasL interaction.1,6-8,56 Interestingly, we have found that poly I:C treatment enhances both the natural cytotoxicity and the CD16-mediated cell lysis, whose recognition mechanisms supposedly rely on distinct activating receptors, while it does not significantly affect the lysis of NK-resistant target, such as the P815 cell line in the absence of anti-CD16 antibody, or a panel of both hematopoietic and nonhematopoietic NK-resistant targets (data not shown), thus suggesting that this effect does not depend either on the expression of a specific activating receptor or on the induction of new recognition capabilities. Supporting this notion, poly I:C stimulation does not significantly affect the expression of either 2B4 and NKG2D activating receptors or that of β1 and β2 integrin adhesion and costimulatory receptors in the NK-target interaction6,7,57 ; nor does it up-regulate NKp44 inducible activation receptor6,7 (data not shown). Moreover, no changes in the perforin cell content were detected upon poly I:C stimulation (S.P., G. Pisegna, and G. Palmieri, unpublished results, 2003). The poly I:C–induced up-regulation of natural killing in fresh human NK cells has been recently shown, consistent with our observations.32
The molecular mechanisms underlying the poly I:C–induced augmentation of NK cell cytotoxicity are still unknown. We provide evidence that this event is time- and protein synthesis–dependent and, by using a selective pharmacologic inhibitor, we found that p38 MAPK activity is involved in the dsRNA-mediated augmentation of NK cell cytotoxicity. While a role for p38 in the development of NK cell cytotoxicity has been previously noted,45,46 the molecular effectors involved are still unknown. p38 MAPK plays an important role in the regulation of mRNA expression, affecting either gene activation, posttranscriptional stabilization, or translation accessibility of specific target genes, in different cell systems.43,44,54,55
Our results also demonstrate that dsRNA stimulation modulates the cytokine and chemokine production by human NK cells, which underlie their immunoregulatory function.
While poly I:C stimulation of the NK92 cell line (this work) and fresh NK cells32 is capable of inducing IFN-γ production by itself, this is not observed in highly purified polyclonal short-term–cultured NK cells. Interestingly, poly I:C stimulation synergizes with either IL-2 and IL-12 in inducing the production of IFN-γ in both polyclonal NK and NK92 cells, underlining that responsiveness to dsRNA may be affected by NK cell activation/differentiation state and modulated by effector cell populations of both innate and adaptive immune response at the infection site, as also suggested by similar data.58 The molecular mechanisms responsible for the functional cooperation between poly I:C and IL-2 or IL-12 are still unknown; whether poly I:C–dependent signals may modulate cytokine receptor expression and/or transduction ability is still open to investigation.
NK cell production of chemokines plays an important role in the organization of local antiviral immune responses.5,9,10,41 Interestingly, CXCL8 chemokine production in fresh human NK cells has been shown upon poly I:C stimulation.32 CXCL10 is produced during the course of a number of inflammatory or infectious conditions and is involved in the antimicrobial immune responses by selectively attracting activated Th1 lymphocytes and NK cells and thus contributing to their infiltration of and persistence at the infectious site; moreover, it has been reported to enhance the cytotoxic activity of fresh NK cells.4,42,59 Here we found that poly I:C treatment of NK cells induces CXCL10 chemokine secretion. Interestingly, NK cell ability to produce CXCL10 has not been described previously and could be relevant for the early NK-dependent recruitment of other effector cell populations of the antiviral response at the infection site, as previously shown for a functionally related chemokine.41
Different TLRs, including TLR3, have been reported to induce CXCL10 production, whose gene is a primary target of IRF-3 transcription factor in the antiviral, dsRNA-dependent response.28,29,60 Poly I:C–triggered CXCL10 production by human NK cells is possibly regulated at the transcriptional or posttranscriptional level, because protein production correlates with induction of mRNA appearance and is controlled by p38 activity. The rapid dsRNA-triggered induction of CXCL10 mRNA does not depend on protein neosynthesis, because it is not affected by cycloheximide treatment and thus seems to be a direct consequence of TLR3-mediated signaling pathways. Whether TLR3-induced CXCL10 production can be up-regulated by exposure to cytokines, as shown in other cell systems,60 is the object of ongoing work.
Collectively, the results presented in this paper show that dsRNA directly up-regulates both cytotoxic and immunomodulatory functions of human NK cells; the essential role of TLR3 receptor is underscored by the evidence that the presence of signaling events and functional up-regulation closely correlates with the TLR3 mRNA expression profile in different NK cell populations.
The dsRNA-mediated effect is probably due to multiple mechanisms, as it requires or not de novo protein synthesis, depending on the specific function involved. Our data indicate a central role for p38 activation in the dsRNA-mediated augmentation of killing activity and chemokine production, thus providing novel evidence about the downstream events controlled by TLR signaling pathways; in this context, the role of TLR-mediated p38 activation in the development of the phagocytic function has been recently shown.61
The boosting of NK activity by in vivo exposure to poly I:C or during viral infections has been shown to depend on type I IFN production.1,5,9-11,13,14 Although no production of either IFN-α or -β in poly I:C–stimulated NK cell samples was observed (data not shown), our results underscore the ability of the dsRNA microbial component to directly augment NK cell functions, thus providing the basis for a contribution of this mechanism to the potentiation of NK effector and immunoregulatory functions during viral infections.
Moreover, they also highlight the importance of the crosstalk with cells of both the innate and adaptive immune response in the dsRNA-mediated regulation of NK cell functions during viral infections1,5,9-11,62,63 and could represent the rational basis for the immunotherapeutic exploitation of this immunostimulatory pathway.
Prepublished online as Blood First Edition Paper, August 17, 2004; DOI 10.1182/blood-2004-05-1860.
Partially supported by grants from the Italian Association for Cancer Research (AIRC), the Italian Ministry for University and Research (MIUR), and the Centre of Excellence (BEMM).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank A. M. Bressan, B. Milana, P. Birarelli, and A. Procaccini for expert technical assistance and Drs. M. J. Robertson, L. L. Lanier, and G. Trinchieri for sharing reagents and cell lines.

![Figure 2. Poly I:C–induced augmentation of NK cytotoxic activity is time- and protein synthesis–dependent. (A-C) Highly purified short-term cultured NK cells (3 × 106/mL [A]) and NK92 (0.2 × 106/mL [B]) and NKL (0.4 × 106/mL [C]) cell lines were left untreated (NT, dashed line) or stimulated with 100 μg/mL poly I:C (solid line) for 24 hours and then subjected to cytotoxicity assay against K562 and 721.221 target cells; P815 target cell lysis was assayed in the presence (•) or absence of anti-CD16 (B73.1) mAb (A). The data shown are representative of at least 5 independent experiments. (D-E) Cytotoxic activity against K562 target cells was tested in a 51Cr release assay. Highly purified polyclonal NK cells (left) and the NK92 cell line (right) were left untreated (dashed line) or stimulated with poly I:C (100 μg/mL) (open symbols) for different times (D); highly purified human polyclonal short-term cultured NK cells were stimulated with poly I:C (closed symbols) or left untreated (open symbols) for 24 hours in the presence (triangles) or not (circles) of cycloheximide (CHX, 10 μg/mL) (E). These results are representative of 1 out of 2 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/104/13/10.1182_blood-2004-05-1860/6/m_zh80240470760002.jpeg?Expires=1770460540&Signature=0qAOdz0aQP9qoyaaFgBZ53Q5w3rGhGvk-W2iwaYSUcF2qehCDZ-lLhUpzrhxWO~3SWke-kFoOFNUOLo2IhzZJ~78NgBwSuWk~WGoIYZEi8QOLofmuKyZyBunsMb3k5sW~LZtgMhm1-8SJC35~Yy8DIFVd~thxyaVD3rv7KgGTAVJWJZbTSkTpX5B3ZuZCRi68kLhwg6jBo3JmRwcuVuEinFYo64PqZoC6~TDO8idaL6qVog02mObg6qKyY0wrdbpNyFzfWL8H4ZkylNPLfdqrvWfvPlsGQjbgoKo9vT-m5yY7NdlcO1~3NgsYbB159NzRqqTaZjxwGEZfPvLEvh5ig__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal