Abstract
CD1d-restricted Vα24-invariant natural killer T cells (iNKTs) are important in immunoregulation. CD4+ and CD4- iNKTs develop with similar frequencies in murine thymus and depend on interleukin-15 (IL-15) in periphery. However, homeostatic requirements of iNKTs have not been analyzed in humans. We evaluated thymic production, peripheral dynamics, and functional maturation of human iNKTs. CD4+ subset comprises 90% of iNKTs in mature thymocytes and cord blood (CB) but only 40% in adult blood. Using T-cell receptor excision circle (TREC) analysis, we directly measured in vivo replicative history of CD4+ and CD4- iNKT cells. Compared to CD4+, CD4- iNKTs contain fewer TRECs, express higher levels of IL-2Rβ, and proliferate with higher rate in response to IL-15. In contrast, CD4+ cells express higher levels of IL-7Rα and better respond to IL-7. Neither thymic nor CB iNKTs are able to produce cytokines unless they are induced to proliferate. Therefore, unlike in the mouse, human CD4+ iNKTs are mainly supported by thymic output and limited peripheral expansion, whereas CD4- cells undergo extensive peripheral expansion, and both subsets develop their functions in periphery. These findings reveal important differences in homeostatic requirements and functional maturation between murine and human iNKTs that are to be considered for clinical purposes.
Introduction
CD1d-restricted Vα24-invariant natural killer T (iNKT) cells are an evolutionary conserved sublineage of T cells with effector-memory phenotype that often express NK cell surface antigen CD161 (NKR-P1A) and is characterized by reactivity to self-glycolipids and α-galactosylceramide that are presented by monomorphic HLA class I–like molecule CD1d.1,2 The hallmark of iNKT cell functional responses to specific stimulation is rapid production of a wide spectrum of cytokines including those of type 2 (interleukin 13 [IL-13], IL-4) and type 1 (interferon-γ [IFN-γ]).3,4 The potential importance of iNKT cells has been demonstrated in several animal models of autoimmunity, infectious diseases, and cancer5-8 as well as in patients with the respective pathologies.9-12
Murine analog of iNKT cells expresses Vα14i canonical T-cell receptor (TCR)-α chain and has been demonstrated to be a distinct sublineage of T cells since its development depends on a number of genes, which are dispensable for conventional T cells.1,13-15 Although early reports suggested that extrathymic developmental pathway may exist for murine iNKT cells,16-20 recent studies have excluded such a possibility.21-24 Current models suggest that Vα14i cells appear as a result of spontaneous TCR-α rearrangement at the stage of CD4 CD8 double-positive (DP) thymocyte followed by CD1d-directed positive and negative selection and maturation of CD4+ single-positive (SP) and CD4 CD8 double-negative (DN) Vα14i T cells.23 The ratio of CD4+ to CD4- mature iNKT thymocytes is approximately 6:4 in murine thymus, which is close to that in peripheral blood and other tissues.25,26 This suggests that thymic output provides a balanced supply of both CD4+ and DN iNKT cells in the mouse. However, CD4+ subset prevails in murine perinatal26 and human fetal27 thymus. The postnatal phase of human iNKT thymocyte development has not yet been analyzed.
Both CD4+ and DN Vα14i T cells are functionally identical3,28 and mainly depend on IL-15 for their survival and homeostatic expansion in periphery.29 In contrast to the murine system, human CD4+ and CD4- (mainly, DN and CD8αα SP) iNKT cells comprise 2 functionally distinct subsets that have Th0-like (IL-4, IL-13, IFN-γ) and Th1-like (IFN-γ) cytokine profiles, respectively.30,31 The balance between these 2 subpopulations may be important for the initiation and regulation of Th2 and Th1 immune responses. However, the homeostatic requirements of human CD4+ and CD4- iNKT cell subsets have not been investigated.
The contribution of peripheral expansion to the maintenance of cell number and function has been critical to the understanding of T-cell homeostasis.32,33 However, the extent of iNKT cell post-thymic proliferation has not been directly evaluated either in mice or in humans. T-cell receptor excision circle (TREC) analysis recently has been applied to analyze thymic origin and peripheral dynamics of human T cells.34-36 TRECs are episomal DNA by-products of TCR-α chain rearrangement in thymus that are stable, not replicated, and therefore diluted during cell division.36 Cellular proliferation is the main mechanism of TREC loss, especially in neonates.37,38 Here we used TREC analysis to directly measure the in vivo replicative history of freshly isolated peripheral blood CD4+ and CD4- iNKT cell subsets. We also compared subset distribution and responsiveness to homeostatic cytokines, and cytokine production in iNKT cells that were freshly isolated from human thymus, cord, and adult peripheral blood. We demonstrate that in contrast to uniform murine iNKT cells, there are major differences in the rates of thymic output and peripheral expansion between human CD4+ and CD4- iNKT subsets. We also demonstrate that peripheral expansion is required for functional maturation of iNKT cells in humans.
Materials and methods
Isolation of iNKT cells from CB, adult peripheral blood, and thymus
Human umbilical CB (CB) and adult peripheral blood were obtained from normal deliveries and donations from healthy adults. Thymuses were obtained from children 0 to 5 years of age undergoing corrective cardiac surgery. The use of all human tissues was approved by the Committee on Clinical Investigations of Childrens Hospital Los Angeles institutional review board. Blood samples were collected in heparinized tubes for isolation of peripheral blood mononuclear cells (PBMCs). After Ficoll-Histopaque density centrifugation, iNKT cells were isolated using magnetic separation with phycoerythrin (PE)-6B11 anti–Vα24-Jα18 CDR3 mAb (BD Biosciences, San Diego, CA) and anti-PE microbeads (Miltenyi Biotec, Auburn, CA) followed by fluorescence-activated cell-sorter (FACS) sorting with Diva module of FACSVantage (BD Immunocytometry Systems, San Jose, CA) or Coulter EPICS Elite ESP (Beckman-Coulter, Miami, FL). When indicated, CD4+ and CD4- cells were first separated from PBMCs using CD4-Multisort microbeads (Miltenyi Biotech) followed by iNKT isolation from CD4+ and CD4- subsets. We also used FACS to sort T cells from CB and PBMCs into naive (CD45RA+CD45RO-) and memory (CD45RA-CD45RO+) subsets using 3-color staining with mAbs against CD3, CD45RA, and CD45RO (BD Biosciences). Thymuses were harvested and dissected free from surrounding tissue. Single cell suspensions were obtained by gentle disruption of the dissected tissue in phosphate-buffered saline (PBS). When indicated, thymocytes were magnetically depleted from CD8+ using anti-CD8 G42-8 mAb (BD Biosciences) and rat antimouse microbeads (Miltenyi Biotech) followed by immunophenotypic analysis. Purity of all isolated subpopulation was examined by flow cytometry and found to be from 96% to 99.9%.
TREC analysis
Quantification of TRECs in sorted cells was performed by real-time quantitative polymerase chain reaction (PCR) by means of the 5′ nuclease (TaqMan) assay as previously described39,40 with minor modifications for optimal performance with Smart Cycler system (Cepheid, Sunnyvale, CA). Cells were counted and lysed with 100 μg/mL PCR-grade proteinase K (Boehringer, Indianapolis, IN), 10 mM Tris [tris(hydroxymethyl)aminomethane]-HCl, 0.1 mM EDTA (ethylenediaminetetraacetic acid), and 0.1% Tween-20 (Sigma-Aldrich, St Louis, MO) for 2 hours at 56°C, and then 10 minutes at 95°C at 107 cells per milliliter or less. Real-time quantitative PCR was performed with the primers 5′-CACATCCCTTTCAACCATGCT (forward), 5′-GCCAGCTGCAGGGTTTAGG (reverse), and probe FAM-5′-ACACCTCTGGTTTTTGTAAAGGTGCCCACT-TAMRA (Integrated DNA Technologies, Coralville, IA). In addition to 5 μL cell lysate, PCR reaction contained 0.5 U Platinum Taq polymerase, 12.5 μL 2× universal PCR Master mix (Roche Molecular Systems, Branchbury, NJ), 500 nM of each primer, 150 nM probe, and 5 μLH2O. TREC DNA plasmid was kindly provided by Dr Douek (Vaccine Research Center, NIAID, National Institutes of Health, Bethesda, MD) and used as an internal standard. We also used sorted DP thymocytes as a biologic standard. Conditions were 95°C for 10 minutes, 95°C for 15 seconds, and 60°C for 1 minute for 40 cycles. A standard curve was plotted, and TREC values for samples were calculated from CT values using SigmaPlot 8.0 software. Samples were analyzed in duplicates.
Flow cytometry analysis of cell surface antigens
Cell surface phenotype was analyzed in direct multicolor immunofluorescence using the following mAbs: fluorescein isothiocyanate (FITC)– and PE–anti-Va24 C15, FITC, and PE–anti-Vb11 C21, energy-coupled dye (ECD)–anti-CD8 (Beckman-Coulter); FITC– and CyChrome–anti-CD3 UCHT1, FITC– and CyChrome–anti-CD4 RPA-T4, FITC–anti-CD8 HIT8a, CyChrome–anti-CD161 DX12, CyChrome–anti-CD56 B159, PE– and FITC–anti-Vα24-Jα18 6B11, and PE–anti–IL-7Rα (BD PharMingen). Surface expression of cytokine receptors was analyzed using biotinylated anti-CD122 Mik-β3 (BD Pharmingen) and anti-IL15Rα antibodies (R&D Systems) and CyChrome-streptavidin (BD Biosciences). Isotype matching control mAbs (BD PharMingen) have been used to set up regions.
Flow cytometry analysis of intracellular cytokines
Nonadherent PBMCs, CB cells, or CD8-depleted thymocytes (106/mL) in 6-well tissue culture plates were stimulated with plate-bound anti-CD3 mAb and anti-CD28 mAbs. After 3 hours of activation, 1 μL/mL GolgiPlug (BD Biosciences) was added, and the cells were cultured further. After a total of 6 hours of activation, cells were stained with PE-6B11 mAb, fixed in 2% formaldehyde for 30 minutes at 4°C, washed twice, permeabilized in PBS containing 0.1% saponin and 1% BSA for 15 minutes at 4°C (all reagents from Sigma-Aldrich), followed by 45 minutes of incubation (RT) with APC–anti–IL-4 and FITC–anti–IFN-γ mAbs (BD Bioscience). Cells were analyzed using Coulter EPICS Elite ESP flow cytometer.
CFSE cell proliferation assay
Lymphoid cells derived from thymus, CB, and adult peripheral blood were suspended in PBS containing 0.5% FCS (Gemini-Bio-Products, Woodland, CA), incubated with 5 μg/mL carboxyfluorescein diacetate succinimidyl ester (CFSE) (Molecular Probes) (30 minutes, 37°C), washed one time in PBS, and resuspended in RPMI 1640 medium containing 10% FCS. Cells were seeded into 6-well plates with or without coating with 1 μg/mL purified 6B11 mAb (kindly provided by Dr B. Wilson, Massachusetts General Hospital, Cambridge, MA) and cultured with indicated concentrations of IL-2, IL7, or IL15 (R&D Systems) for 7 days. Anti-CD1d 42.1 mAb, 10 μg/mL (kindly provided by Dr S. Porcelli, Albert Einstein College of Medicine, Bronx, NY) was added to the culture medium when indicated. After staining with PE-, Cy-Chrome-, or APC-labeled mAbs, cells were analyzed for CFSE fluorescence in relationship to cell surface phenotype and cytokine profile by flow cytometry.
Data analyses
Flow-cytometry data were analyzed with EXPO 32 Analysis software (Beckman-Coulter). Excel (Microsoft, Redmond, WA) and SigmaPlot 8.0 (Jandell Scientific, San Rafael, CA) were used to create graphs. GraphPad Prism 4.0 (GraphPad Software, San Diego, CA) was used to perform t test, Mann Whitney test, or one-way ANOVA with the Tukey-Kramer post-test comparison of group means. Significance was accepted when P was less than .05.
Results
High CD4+/CD4- iNKT cell ratio in human thymus
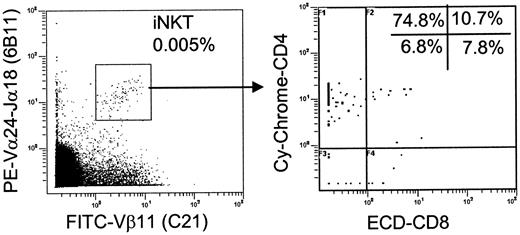
Although iNKT cells are relatively frequent in murine thymus,25,26 the frequency of their human counterparts is progressively decreasing during gestation,27 and this has not yet been analyzed in postnatal period. To identify iNKT TCR in freshly isolated thymocytes, we used a combination of 6B11 mAb that recognizes invariant Vα24-Jα18 CDR3-α loop41 and C21 mAb that recognizes Vβ11, which pairs with the invariant TCR-α-chain in human iNKT cells.42,43 iNKT cells comprised only 0.0045% ± 0.0006% (n = 10) of all thymocytes (Figure 1, left), and there were 0.017% ± 0.008% of mature thymocytes after CD8- depletion (removal of DP and CD8 SP cells). In comparison, iNKT cell frequency in CB and adult PBMCs was 0.083% ± 0.04% (n = 12) and 0.14% ± 0.13% (n = 18), respectively. Four-color flow cytometry analysis revealed CD4 and CD8 distribution in iNKT thymocytes (Figure 1, right). Like in the mouse,23 a small subset of human iNKT thymocytes had DP phenotype. However, about 90% of more mature cells were CD4 SP, which is in sharp contrast to the approximately 6:4 ratio of CD4+ to CD4- iNKT subsets in murine thymus.25 These data demonstrate that, unlike in the mouse, thymic output mostly delivers CD4+ iNKT cells and may not be sufficient to support number of peripheral CD4- iNKT cells in humans.
High CD4+/CD4- iNKT cell ratio in human thymus. iNKT cells were identified in freshly isolated thymocytes as 6B11/Vβ11-double–positive events after collecting 2 to 3 × 106 events (left), and CD4/CD8 distribution was analyzed within iNKT subset (right). Representative diagrams from 10 independent experiments are shown.
High CD4+/CD4- iNKT cell ratio in human thymus. iNKT cells were identified in freshly isolated thymocytes as 6B11/Vβ11-double–positive events after collecting 2 to 3 × 106 events (left), and CD4/CD8 distribution was analyzed within iNKT subset (right). Representative diagrams from 10 independent experiments are shown.
Inversion of CD4+/CD4- ratio in human iNKT cell ontogenesis
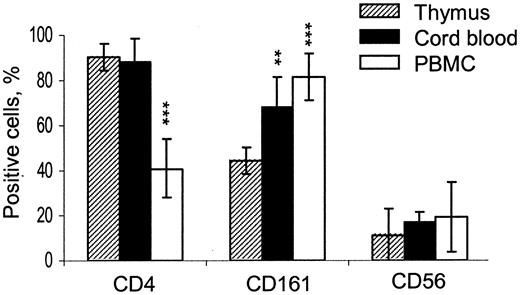
Since the great majority of neonatal T cells are recent thymic emigrants,37,38 we hypothesized that high CD4+/CD4- ratio would remain in CB iNKT cells if they were mainly supported by the thymic output. It is also not known whether NK cell markers first appear on iNKT cells in thymus or in periphery. Therefore, we compared the distribution of CD4 and NK-associated surface antigens (CD161 and CD56) in iNKT cells from mature thymocytes, CB cells, and adult PBMCs (Figure 2). CD4+ cells comprised 90.5% ± 5.8%, 87.9% ± 8.2%, and 40.9% ± 13.1% of iNKT cells in thymus, CB, and adult PBMCs, respectively (P < .001 for PBMC vs thymus). Although the expression of CD161 was lower in thymus compared to cord and adult peripheral blood (P < .01), both CD161 and CD56 were frequent in thymic iNKT cells relative to expression of these markers in the rest of thymocytes (< 2%, P < .001, not shown). These data demonstrate that high thymic CD4+/CD4- iNKT cell ratio remains unchanged in neonate blood when the thymic output is maximal, and it is reversed in adult blood when peripheral expansion is largely responsible for T-cell homeostasis. In addition, our data indicate that NK-like phenotype is acquired by human iNKT cells in thymus and does not require peripheral expansion, although it does not exclude that CD161- iNKT thymic emigrants may acquire CD161 in periphery as well.
Inversion of CD4+/CD4- ratio in human iNKT cell ontogenesis. Histogram shows frequency of CD4, CD161, and CD56 expression in iNKT cells from CD8-depleted thymocytes compared to their counterparts from CB and adult PBMCs. Data are mean ± SD from thymuses, CB, and PBMCs obtained from 10, 8, and 12 individuals, respectively. Expression levels in CB and PB compared to that in thymus using one-way ANOVA; ** and *** indicate that the P value is less than .01 and .001, respectively.
Inversion of CD4+/CD4- ratio in human iNKT cell ontogenesis. Histogram shows frequency of CD4, CD161, and CD56 expression in iNKT cells from CD8-depleted thymocytes compared to their counterparts from CB and adult PBMCs. Data are mean ± SD from thymuses, CB, and PBMCs obtained from 10, 8, and 12 individuals, respectively. Expression levels in CB and PB compared to that in thymus using one-way ANOVA; ** and *** indicate that the P value is less than .01 and .001, respectively.
CD4- iNKT cells make more divisions in periphery than CD4+ counterparts
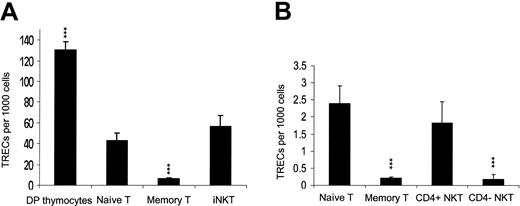
Previous reports have demonstrated that human neonatal iNKT cells have effector-memory phenotype that is associated with the loss of CD45RA and CCR7 and expression of CD45RO and NK cell markers CD161 and CD56.44,45 Since such phenotype in conventional T cells is associated with extensive peripheral expansion both in adults and neonates,46 it has been suggested that peripheral expansion contributes substantially to iNKT cell number even before birth.44,45 However, there is no direct evidence to support such model of iNKT cell homeostasis. On the contrary, data presented in Figure 2 demonstrate that peripheral expansion is not required for expression of NK markers on iNKT cells. To directly assess the in vivo replicative history of iNKT cells, we measured TREC copy number in iNKT cells compared to that in naive and memory T cells in CB and adult PBMCs. Figure 3A demonstrates that TREC number in CB iNKT (mostly CD4+) cells was 56.1 ± 11.05, (n = 8), similar to that in naive (CD45RA+CD45RO-) T cells (42.8 ± 7.5, P > .05) that was more than 10-fold higher than in memory (CD45RA-CD45RO+) T cells (P < .001). This indicates that unlike in conventional T cells, effector-memory phenotype of neonatal iNKT cells is not associated with increased peripheral expansion, suggesting that a unique pathway may exist for iNKT cell development in thymus.
TREC analysis of iNKT cells. Histogram shows mean ± SD of TREC content in indicated subpopulations isolated from (A) thymus (n = 5), CB (n = 8), or (B) PBMCs of healthy adults (n = 7). Comparison is made to naive T cells. Purity of sorted subpopulations was 96% to 99.9%. *** indicates that P < .001.
TREC analysis of iNKT cells. Histogram shows mean ± SD of TREC content in indicated subpopulations isolated from (A) thymus (n = 5), CB (n = 8), or (B) PBMCs of healthy adults (n = 7). Comparison is made to naive T cells. Purity of sorted subpopulations was 96% to 99.9%. *** indicates that P < .001.
Sufficient numbers of both CD4+ and CD4- iNKT cells could be isolated from PBMCs of some adult donors so that we compared TREC number in CD4+ and CD4- iNKT subsets. Figure 3B demonstrates that despite a greater individual variability, the TREC number in CD4+ iNKT cells (1.84 ± 0.61) (n = 7) remained (as in CB) close to that in naive T cells (2.39 ± 0.52), whereas the TREC number in CD4- iNKT cells (0.19 ± 0.13) was similar to that in memory T cells (0.22 ± 0.08), which was 10-fold lower than that in naive T cells (P < .001). We have to note, however, that CD4+ iNKT cells as well as naive T cells do cycle in periphery as evidenced by a 50- to 200-fold decrease of their TRECs compared to DP thymocytes (Figure 3A-B) Therefore, TREC analysis and immunophenotyping of human iNKT cells in thymus, CB, and adult PBMCs are consistent and provide strong evidence that homeostasis of CD4+ iNKT cells is mainly supported by thymic output and slow cycling in periphery, whereas CD4- iNKT cells accumulate with age, which is associated with accelerated rate of their peripheral expansion.
Differential expression of receptors to IL-15 and IL-7 in CD4- and CD4+ iNKT cells
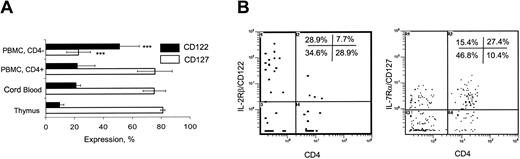
To explain the difference in the rate of peripheral expansion between CD4- and CD4+ iNKT subsets, we examined expression of receptors for IL-15 and IL-7, the major cytokines responsible for homeostasis of murine iNKT cells29 and proliferation of human iNKT thymocytes,27 respectively. Figure 4A demonstrates that common β-chain of IL-2/IL-15 receptor (CD122) was expressed only in 9.5% ± 3% and 21% ± 3.1% of CD4+ iNKT cells from thymus and CB, respectively. Due to low frequency, CD4- iNKT cells could not be analyzed in thymus and CB. In adult PBMCs, CD122 was expressed in 51.1% ± 13.8% of CD4- compared to 22% ± 12% of CD4+ iNKT subsets (P < .01) (Figure 4A-B). IL-15Rα could not be accurately assessed due to very low level of its expression in human T cells and low frequency of iNKT cells (data not shown). In contrast to CD122, IL-7Rα (CD127) was expressed in about 80% of thymic and CB iNKT cells. Opposite to CD122 distribution, CD127 was expressed in 75.7% ± 12.1% and 22.9% ± 13.8% of CD4+ and CD4- of adult peripheral blood iNKT cells, respectively (P < .001). Thus, CD122 (IL-2/IL-15 receptor) is preferentially expressed in CD4- iNKT subset, whereas CD127 (IL-7 receptor) is more frequently expressed in CD4+ iNKT subset.
Expression of IL-2Rβ (CD122) and IL-7Rα (CD127) in iNKT cells. (A) Histogram shows mean ± SD frequency of CD122 and CD127 expression in iNKT cells from CD8-depleted thymus (n = 8), CB (n = 6), and adult PBMCs (n = 10). Data compared to that in thymus. (B) Representative diagrams show distribution of CD122 (left) and CD127 (right) expression in CD4+ versus CD4- cells after gating on 6B11+ events in adult PBMCs.
Expression of IL-2Rβ (CD122) and IL-7Rα (CD127) in iNKT cells. (A) Histogram shows mean ± SD frequency of CD122 and CD127 expression in iNKT cells from CD8-depleted thymus (n = 8), CB (n = 6), and adult PBMCs (n = 10). Data compared to that in thymus. (B) Representative diagrams show distribution of CD122 (left) and CD127 (right) expression in CD4+ versus CD4- cells after gating on 6B11+ events in adult PBMCs.
CD4- iNKT cells predominantly respond to IL-15
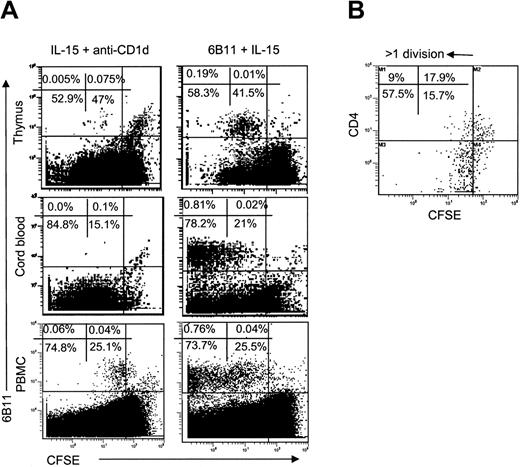
In the next step, we evaluated the proliferative response of iNKT cell subsets to IL-15 with anti-CD1d blocking mAb (to exclude TCR stimulation with an endogenous ligand) or with iNKT-specific TCR stimulation (plate-bound 6B11 mAb). IL-15 alone induced little or no proliferation of thymic or neonatal iNKT cells, which are mostly CD4+ (Figure 5A, left). In contrast, adult blood iNKT cells underwent low-rate expansion in response to IL-15. However, analysis of CD4 distribution in proliferated adult blood iNKT cells demonstrated that about 74% ± 13.5% CD4- cells made more than 1 division, whereas only 31.5% ± 15.1% of CD4+ cells divided to such extent (P < .001, Figure 5B). In general, however, even in excess of cytokines in culture, iNKT cells proliferated much less than other lymphocytes, especially in CB and thymus. In contrast, iNKT cells from all sources vigorously proliferated after specific stimulation (Figure 5A, right), and no difference was observed between CD4+ and CD4- iNKT subsets (data not shown). Thus, in the absence of TCR stimulation, CD4- subset proliferates better in response to IL-15 than does CD4+ subset of iNKT cells that can be explained by the higher frequency of IL-15 receptor expression in the former.
Proliferative response of iNKT cells to IL-15. (A) CD8-depleted thymocytes, CB cells, or PBMCs were stained with CFSE and cultured for 7 days in the presence of IL-15 and anti-CD1d blocking mAb (left) or 6B11-coated plates plus IL-15 (right). iNKT cells were identified with PE-6B11 and plotted against CFSE-fluorescence. (B) Distribution of CFSE-fluorescence in CD4+ versus CD4- subsets of iNKT cells from adult PBMCs cultured with IL-15 + anti-CD1d mAb. Representative diagrams from 8 independent experiments are shown.
Proliferative response of iNKT cells to IL-15. (A) CD8-depleted thymocytes, CB cells, or PBMCs were stained with CFSE and cultured for 7 days in the presence of IL-15 and anti-CD1d blocking mAb (left) or 6B11-coated plates plus IL-15 (right). iNKT cells were identified with PE-6B11 and plotted against CFSE-fluorescence. (B) Distribution of CFSE-fluorescence in CD4+ versus CD4- subsets of iNKT cells from adult PBMCs cultured with IL-15 + anti-CD1d mAb. Representative diagrams from 8 independent experiments are shown.
CD4+ iNKT cells are more responsive to IL-7
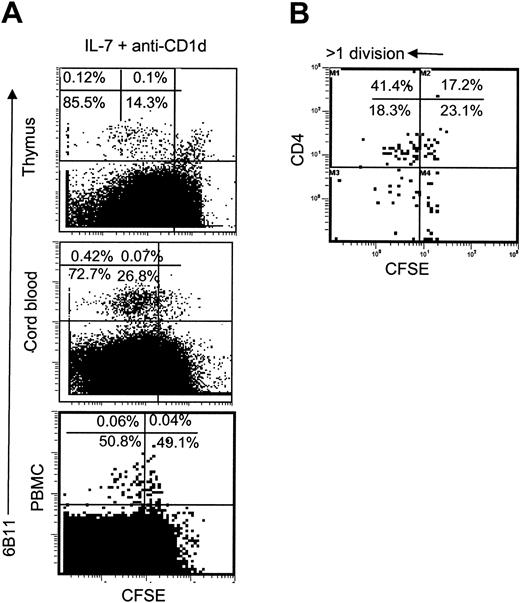
While this manuscript was in review, Sandberg et al reported that similar to conventional T cells, CD4+ iNKT precursors in human fetal thymus were responsive to IL-7. It was not known, however, whether post-thymic iNKT cells preserved responsiveness to IL-7. Furthermore, in the light of higher frequency of IL-7Rα expression (Figure 4) in CD4+ compared to CD4- peripheral blood iNKT cells, it was important to examine whether the former subset was more responsive to IL-7. Figure 6A demonstrates that most iNKT cells from thymus, CB, or adult PBMC proliferated in response to IL-7 even in the presence of anti–CD1d mAb, which was similar to conventional thymocytes and neonatal lymphocytes. Adult blood iNKT cells were less responsive to IL-7, although 68.2% ± 8.6% and 40.5% ± 5.1% CD4+ and CD4-iNKT cells proliferated, respectively (P < .01, Figure 6B). We have to note, however, at very high concentrations of IL-7 (> 100 ng/mL) proliferation of CD4+ and CD4- iNKT cells was equal (data not shown). Therefore, CD4+ subset is more sensitive to IL-7 compared to CD4- subset of iNKT cells that can be explained by the higher level (higher frequency of detectable level) of IL-7 receptor expression in the former.
Proliferative response of iNKT cells to IL-7. (A) CD8-depleted thymocytes, CB cells, or PBMCs were stained with CFSE and cultured for 7 days in the presence of IL-7 and anti-CD1d blocking mAb. iNKT cells were identified with PE-6B11 and plotted against CFSE fluorescence. (B) Distribution of CFSE-fluorescence in CD4+ versus CD4- subsets of iNKT cells from adult PBMCs cultured with IL-7 + anti-CD1d mAb. Representative diagrams from 5 independent experiments are shown.
Proliferative response of iNKT cells to IL-7. (A) CD8-depleted thymocytes, CB cells, or PBMCs were stained with CFSE and cultured for 7 days in the presence of IL-7 and anti-CD1d blocking mAb. iNKT cells were identified with PE-6B11 and plotted against CFSE fluorescence. (B) Distribution of CFSE-fluorescence in CD4+ versus CD4- subsets of iNKT cells from adult PBMCs cultured with IL-7 + anti-CD1d mAb. Representative diagrams from 5 independent experiments are shown.
iNKT cells are functionally immature either in thymus or in CB
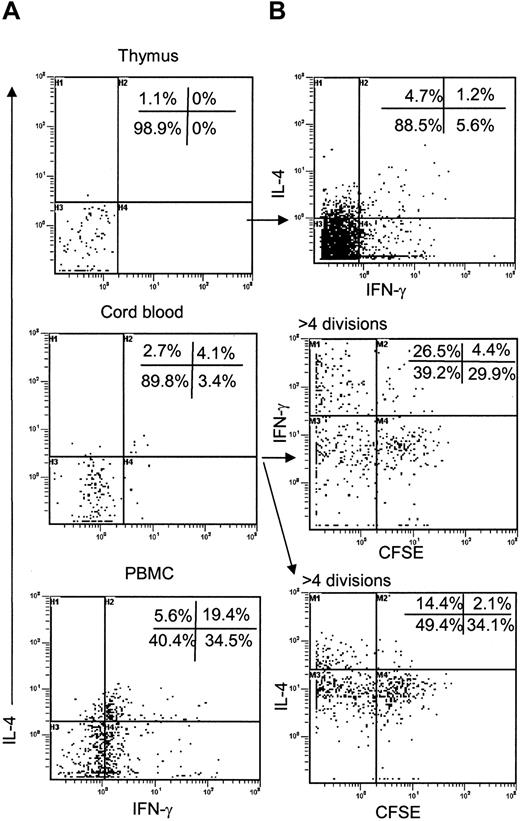
As thymic iNKT cells acquire NK markers that are associated with increased ability to produce cytokines in mature T cells,47,48 we measured accumulation of intracellular cytokines in thymic iNKT cells after TCR stimulation in comparison to iNKT cells derived from CB or adult PBMCs (positive control). Intracellular flow cytometry demonstrated that in contrast to adult blood iNKT cells, iNKT thymocytes and CB iNKT cells could produce neither IL-4 (or IL-13) nor IFN-γ (Figure 7A) unless they were in vitro expanded using 6B11 mAb-coated plates and IL-2 (Figure 7B). Thymic iNKT cells required up to 3 weeks in vitro expansion before a small subset became able to produce IL-4 and IFN-γ (Figure 7B, top). CB-derived iNKT cells acquired functional competence much quicker than their thymic precursors. After 7 days of TCR-mediated in vitro expansion, about 40% of CB iNKT cell were able to produce IFN-γ and about 20% produced IL-4. The majority of cytokine+ cells were among the cells with the highest division number (Figure 7B, middle, bottom). Similar results were obtained when proliferation of CB iNKT cell was induced by IL-7 instead of TCR stimulation (data not shown). Therefore, unlike in the mouse, functional maturation of human iNKT cells is not complete either in thymus or in CB and may require repeated antigen encounter and/or cytokine-mediated signals in periphery.
Functional maturation of iNKT cells. (A) CD8-depleted thymocytes (n = 5), CB cells (n = 6), or PBMCs (n = 6) were stimulated with agonistic anti-CD3 mAb-coated plates followed by staining of iNKT cells with 6B11 and detection of intracellular IL-4 and IFN-γ by flow cytometry. Diagrams show expression of IL-4 and IFN-γ after gating on 6B11-positive events. (B) Staining for intracellular cytokines is repeated in iNKT cells from thymus (top) and CB (middle, bottom) after in vitro expansion with 6B11 and IL-2. Expression of cytokines in expanded CB iNKT cells is plotted against CFSE fluorescence. Representative results from 5 independent experiments are shown.
Functional maturation of iNKT cells. (A) CD8-depleted thymocytes (n = 5), CB cells (n = 6), or PBMCs (n = 6) were stimulated with agonistic anti-CD3 mAb-coated plates followed by staining of iNKT cells with 6B11 and detection of intracellular IL-4 and IFN-γ by flow cytometry. Diagrams show expression of IL-4 and IFN-γ after gating on 6B11-positive events. (B) Staining for intracellular cytokines is repeated in iNKT cells from thymus (top) and CB (middle, bottom) after in vitro expansion with 6B11 and IL-2. Expression of cytokines in expanded CB iNKT cells is plotted against CFSE fluorescence. Representative results from 5 independent experiments are shown.
Discussion
This is the first study that has evaluated homeostatic requirements of iNKT cells in humans. We demonstrate that human iNKT cells are produced in postnatal thymus with high CD4+/CD4- ratio and number of CD4+ iNKT cells is mainly supported by thymic output and survival in periphery with limited number of cell divisions. In contrast, number of CD4- iNKT cells mostly depends on peripheral expansion. Accordingly, CD4- iNKT cells more frequently express IL-15R and better respond to IL-15 compared to CD4+ iNKT cells, although both subsets respond equally well to TCR stimulation. However, CD4+ subset expresses higher level of IL-7R and is more responsive to IL-7 that may contribute to a low-rate homeostatic cycling of these cells after age-related decrease of thymic output. Finally, we demonstrate that unlike in the mouse, human iNKT cells do not become functionally mature in thymus and may require additional stimuli in periphery to acquire ability to rapidly produce cytokines upon antigen encounter.
In this report we have identified and characterized iNKT cell precursors in postnatal human thymus. In the mouse, iNKT cells comprise 0.3% to 0.5% thymocytes and can be clearly identified using α-GalCer/CD1d-tetramers.49 However, frequency of iNKT thymocytes in humans does not exceed tetramer background staining even after CD8- depletion (10-fold enrichment). A recently generated mAb 6B11 against the conserved CDR3 region of the canonical Vα24-Jα18 TCR provides an excellent new tool for analysis of iNKT cells in humans.41 Using this mAb in combination with anti–Vβ11 C21 mAb, we have found that iNKT cells comprise about 0.005% of human thymocytes, which is 100-fold less frequent than in the mouse. Despite such low frequency, we could clearly and reproducibly detect DP iNKT cell precursors among freshly isolated thymocytes after collecting 2 to 3 × 106 events. Surprisingly, 90% of remaining iNKT thymocytes were CD4 SP regardless of patient age (up to 5 years old). Such high CD4/CD4- ratio was observed only in perinatal mice followed by decline to a 6:4 ratio several weeks after birth.25,26 DP phenotype and high CD4 SP/CD4- ratio also were reported in a recent study that used a combination of anti–Vα24 C15 and anti–Vβ11 C21 mAbs to identify iNKT cells in human fetal thymuses.27 We have to note, however, that this mAb combination identifies a cell population that is enriched by iNKT cells but may contain up to 50% of noninvariant Vα24/Vβ11 T cells.31,50
We found that iNKT cells contain TRECs like the majority of αβ-T cells. Since dilution during cell division is the main or the only (in neonates) mechanism of TREC loss, TREC analysis allowed us for the first time to measure the in vivo replicative history of iNKT cells. We found that both in neonates and adults, CD4+ iNKT cells maintain TREC content close to that in naive T cells. Since most naive T cells in CB are recent thymic emigrants,37,38 thymic output is the main mechanism that can support iNKT cell number in neonates. This is consistent with the fact that, like in the thymus, CD4+ cells comprise 90% of CB iNKT cells. In contrast, CD4- iNKT cells increase later in life (60% of iNKT cells in adult PBMCs) and contain on average 10-fold fewer TRECs than their CD4+ counterparts in the same individuals. This indicates that CD4- iNKT cells undergo more extensive peripheral expansion than CD4+ ones that may explain the reversal of CD4+/CD4- ratio in adult compared to thymic and neonatal iNKT cells. Alternatively, TRECs could be diluted in CD4- iNKT cells if some of them developed at extrathymic sites where TREC generation was inefficient.39,51 Additionally, the accumulation of CD4- iNKT cells with age could be explained by the loss of CD4 expression as a result of linear differentiation of less mature CD4+ cells, as it was suggested in a recent report.27 However, the latter possibility is not supported by the existence of stable clones of human CD4+ iNKT cells41,52,53 that maintain CD4 expression even after 8 months of continuous culture (Dr Brian Wilson, Massachusetts General Hospital, Cambridge, MA, personal communication via e-mail on June 11, 2004). Although the extent of iNKT cell peripheral expansion in mice has not been directly analyzed, small sizes of individual iNKT cell clones in periphery54 and high frequency of Vα14i cells in thymus25 suggest that both CD4+ and CD4- mouse iNKT cells are more dependent upon thymic output as opposed to peripheral expansion. Therefore, in contrast to the mouse iNKT system, the numbers of CD4+ and CD4- iNKT cells are differentially regulated in humans.
Our data demonstrate that like in the mouse,25 human thymic iNKT cells acquire NK cell markers, which conventional T cells express only when they develop into memory type in periphery after extensive cell division.48 Furthermore, TREC numbers in CB iNKT cells is equal to that in naive T cells despite the effector-memory phenotype of the former. This suggests that even if iNKT cells exit from thymus in relatively immature CD161- stage like in the mouse,26 acquisition of their mature NK-like/memory phenotype in the thymus and in peripheral organs does not require cell division. Therefore, our data do not support a previous notion that effector-memory phenotype of iNKT cells in neonates is indicative of their extensive peripheral expansion in response to an unknown endogenous antigen before birth.45 Instead, iNKT cell precursors undergo a developmental shortcut in thymus and possibly in other tissues. In support of this concept, a recent report demonstrated that T-cell–specific ablation of IKK2 leads to a complete abrogation of iNKT cell generation in murine thymus,15 whereas development of conventional T cells in this system is blocked only at their transition from naive to memory type in periphery.55 Therefore, TCR- and/or cytokine-mediated NF-κB activation may be a part of a common signaling pathway that is required for iNKT and memory T-cell development in thymus and in periphery, respectively. In addition, another report demonstrated that RelB expression in thymic stromal cells is required for iNKT transition from NK-1.1–negative to mature NK-1.1–positive iNKT cells.14 Therefore, our data in human system and murine models are consistent that proliferation-independent iNKT cell differentiation in thymus and elsewhere is sufficient for generation of memory- and NK-like phenotype.
A higher frequency of IL-15 receptor expression in CD4- than CD4+ iNKT cells is consistent with the observed better proliferative response of the former to IL-15 stimulation in culture. This suggests that IL-15 may have been conserved in the mammalian evolution as a homeostatic cytokine for at least CD4- subset of iNKT cells. IL-15 is produced by a variety of cells in extralymphoid tissues,56 where iNKT cells tend to localize.57 In contrast, CD4+ iNKT cells more frequently express detectable levels of IL-7Rα and are more sensitive to IL-7. This suggests that IL-7 that is produced in secondary lymphoid organs (in addition to thymus and bone marrow)58 may support post-thymic cycling of CD4+ iNKT cells similar to that of naive T cells.59,60 Furthermore, both CD4+ and CD4- iNKT cells can proliferate equally well in response to specific stimulation. This suggests that iNKT cell number may depend not only on homeostatic factors but also on the frequency, strength, and duration of antigenic stimulation. This may explain why despite little individual variations in thymus and CB, iNKT cell frequency ranges from less than 0.001% to 0.6% of peripheral blood T cells in healthy adults. High proliferative potential of iNKT cells in response to antigenic stimulation recently has been confirmed in a phase 1 clinical trial of α-GalCer–pulsed dendritic cells in cancer patients61 as well as in mice that were treated with this ligand.62 This is important for iNKT cell use in immunotherapy. However, normal immune function might require a limitation in the size of iNKT cell population. Indeed, it has been demonstrated that despite short-term spikes in iNKT cell number, their frequency remained at remarkably constant levels in adult PBMCs within individuals for years,50 which is probably due to tight competition for IL-15 and IL-7 with other lymphocytes.63
We demonstrate that human iNKT thymocytes are not able to produce either IL-4 or IFNγ. Consistent with being recent thymic emigrants and despite the effector-memory phenotype, freshly isolated CB iNKT cells also are functionally immature. Like in naive T cells, the ability to produce IL-4 and IFN-γ is acquired by these cells after a number of cell divisions. These data suggest that in contrast to proliferation-independent acquisition of effector-memory phenotype, development of iNKT cell functional competence require peripheral expansion. Since CD4+ and CD4- iNKT cells have substantial differences in their chemokine receptor profiles,57,64 the nature of microenvironment where they localize and proliferate, may determine the functional differences between these iNKT subsets.
In conclusion, our findings reveal important differences in thymic output and peripheral homeostatic requirements between human CD4+ and CD4- iNKT cells. Since disease and therapy may differentially target thymic function and microenvironment of peripheral tissues, the balance between these functionally distinct subsets could be affected accordingly. Thus, revealing homeostatic mechanisms of iNKT cells is important for a better understanding of their potential contribution to a variety of pathologic processes and for a rational design of iNKT-based immunotherapeutic strategies.
Supported in part by grants from Stop Cancer, M. Hoefflin, and T. J. Martell Foundations (L.S.M.) NIH 2R01 HL54729-07 (K.I.W.) and RO1 HL077912 (G.M.C.). G.M.C. is a Scholar of the Leukemia and Lymphoma Society.
Prepublished online as Blood First Edition Paper, August 24, 2004; DOI 10.1182/blood-2004-04-1629.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal