Abstract
The effects of interleukin 16 (IL-16) on dendritic cell (DC) generation from human CD34+ progenitor cells are not known. Here, we show that IL-16 added to a basal cocktail comprised of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-4, Flt-3 ligand (Flt3L), and tumor necrosis factor α (TNF-α) does induce the CD34+ hematopoietic cells to proliferate in vitro and to differentiate into phenotypically and functionally mature DCs. IL-16 exerts this function more efficiently than stem cell factor (SCF) as a control, thrombopoietin (TPO), or IL-16 plus TPO. Moreover, we show that the combination of IL-16 plus TPO induces the generation of tolerogenic DCs, able to induce an anergic state in T cells that persists when T cells are rechallenged with immunogenic DCs. An altered pattern of cytokine production, a reduced expression of the C-type lectin DC-SIGN, and an increased surface expression of the inhibitory molecules immunoglobulin-like transcript 2 (ILT-2), ILT-3, and ILT-4 may all contribute to confer the tolerogenic properties of these DCs. Generation of tolerogenic DCs may aid the exploration of new therapeutic strategies to promote tolerance to autoantigens and prevent disease development. (Blood. 2004;104:4020-4028)
Introduction
Dendritic cells (DCs) are professional antigen-presenting cells (APCs), able to induce primary T-cell activation, polarization, and, in certain circumstances, tolerance.1-3 Immature DCs in the periphery capture antigens and then, through inflammatory stimuli, migrate to draining lymph nodes where they present processed antigens to naive T cells.3,4 At this more mature stage of their life, DCs up-regulate expression of major histocompatibility (MHC) class II as well as costimulatory molecules, such as CD40, CD80, and CD86.5 The ability of DCs to initiate immune responses has focused much attention on the potential use of DCs for immunotherapy. Progress in the understanding of DC biology has been made slowly and with difficulty because of the paucity of these cells in the peripheral blood and in other tissues. In recent years, several investigators successfully generated in vitro human DCs from human peripheral blood monocytes and from human CD34+ cells obtained from various sources such as peripheral blood, cord blood, or bone marrow, by culture with multiple cytokine combinations. Standard monocyte-derived DCs are obtained by culture of monocytes in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) for 5 to 7 days. DCs with immune properties similar to those of mature monocyte-derived DCs can be generated from CD34+ progenitors using combinations of the cytokines stem cell factor (SCF), Flt-3 ligand (Flt3L), GM-CSF, IL-4, and tumor necrosis factor-α (TNF-α), in cultures lasting 14 days.6 In this model system, SCF and Flt3L support the development of hematopoietic progenitors,7,8 whereas GM-CSF, IL-4, and TNF-α promote the differentiation into DCs.9,10 The use of cytokines other than SCF and Flt3L to expand hematopoietic progenitors could represent a good strategy to improve the system, aimed to obtain larger numbers of cultured DCs that can be used to better define DC biology and for immunotherapeutic purposes.
IL-16 in its biologically active form is a cationic homotetramer of 14-kDa chains, which is unrelated to previously described chemokines or other chemotactic or growth factor cytokines.10,11 IL-16 is a chemotactic and activating factor for CD4+ T cells, monocytes, eosinophils, and DCs. The biologic activities of IL-16 depend on the cell surface expression of CD4,12-14 through which IL-16 conveys its functional signal.13,14 It has been reported that a subpopulation of CD34+ hematopoietic cells expresses CD4 on its surface; it has been suggested that this population may be represented by very early cells.15 Therefore, we were interested in exploring whether IL-16 may be a possible candidate cytokine able to induce CD34+ cells to proliferate or differentiate versus DC lineage.
Human stem cells also express the receptor for thrombopoietin (TPO) on their surface.16 TPO is a factor endowed with the ability to induce the proliferation of primitive hematopoietic progenitor cells17 ; when combined with Flt3L and SCF, TPO not only induces the generation of long-lasting DC precursors from CD34+ cells, but also seems to condition the irreversible commitment of CD14+ cells into monocytic lineage.18,19
In the present report we provide evidence, for the first time to our knowledge, that IL-16 in combination with Flt3L, GM-CSF, IL-4, and TNF-α can induce CD34+ hematopoietic cells to proliferate in vitro and to differentiate into phenotypically and functionally mature DCs. IL-16 exerts this function more efficiently than SCF or TPO or IL-16 plus TPO. Moreover, the combination of IL-16 plus TPO induces the generation of tolerogenic DCs, able to induce an anergic state in T cells that persists when T cells are rechallenged with immunogenic DCs. An altered pattern of cytokine production, a reduced expression of the C-type lectin DC-SIGN, and an increased surface expression of the inhibitory molecules immunoglobulin-like transcript-2 (ILT-2), ILT-3, and ILT-4 may all contribute to confer the tolerogenic properties of these DCs.
Materials and methods
Isolation of human CD34+ cell progenitors
Leukapheresis products were obtained from healthy donors and from patients with solid tumors undergoing peripheral blood stem cell (PBSC) mobilization with recombinant human granulocyte-colony stimulating factor (G-CSF) after informed consent was provided. Approval for these studies was obtained from the Instituto Clinical Humanitas institutional review board. Leukapheresis samples contained 0.5% to 9.1% CD34+ cells. Peripheral blood mononuclear cells (PBMCs) were obtained by Ficoll density gradient centrifugation (Ficoll-Hypaque, Pharmacia Biotech, Uppsala, Sweden). CD34+ cells were purified using immunomagnetic selection with mini-magnetic-activated cell sorter (MACS) cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions.
Generation of DCs from CD34+ progenitor cell cultures
CD34+-enriched cells (mean purity 90.0% ± 8.4%) were cultured in 25-cm2 flasks at 1 × 105/mL for 14 days in RPMI 1640 (Gibco Laboratories, Milan, Italy), supplemented with 10% heat-inactivated fetal calf serum (FCS; Euroclone, Wetherby, United Kingdom), in the presence of a basal cocktail, comprised of human recombinant GM-CSF (50 ng/mL), IL-4 (10 ng/mL), Flt3L (50 ng/mL), TNF-α (2.5 ng/mL), plus either SCF (10 ng/mL; SCF-DCs), or TPO (10 ng/mL; TPO-DCs), or IL-16 (10 ng/mL; IL-16-DCs), or IL-16 plus TPO (IL-16/TPO-DCs). All the cytokines were from PeproTech (London, United Kingdom). Each 3 days, half of the culture medium was replaced by fresh medium and growth factors. At the end of the culture, cells were collected and used for subsequent analysis. The cell morphology was assessed on cytospin preparations after May-Grünwald-Giemsa or acridine orange (AO) staining. The peak of DC production occurred at day 14.
Cell sorting
Cell sorting of the CD34+CD4+ and CD34+CD4- cells was performed starting from magnetic bead-isolated CD34+ cells. Purified CD34+ cells (1 × 106 cells) were isolated simultaneously with fluorescein isothiocyanate (FITC; CD34) and phycoerythrin (PE; CD4) using a modified dual laser fluorescence-activated cell sorter (FACS) Vantage (Becton Dickinson, San Jose, CA). Dead cells were excluded from cytometric analysis by propidium iodide (PI) staining. The mean purity of the sorted cells was always more than 99% for the indicated surface marker phenotype.
Immunophenotypic analysis
DCs were incubated with different FITC- or PE-conjugated monoclonal antibodies (mAbs) for 30 minutes at 4°C in the dark. The following mAbs were used: anti-CD1a, -CD3, -CD4, -CD14, -CD15, -CD19, -CD34, -CD40, -CD41, -CD80, -CD83, -CD86, -HLA-ABC, -HLA-DR (Becton Dickinson); -CD11c (Caltag, Burlingame, CA); -CD123 (PharMingen, San Diego, CA); -CD209 (DC-SIGN; R&D Systems, Minneapolis, MN); and -ILT-2, -ILT-3, -ILT-4 (a generous gift of Prof E. Berti, University of Milano-Bicocca, Italy). Negative controls were isotype-matched irrelevant mAbs. Cells were collected and analyzed using a FACScan (Becton Dickinson) flow cytometer. Data analysis was performed by CellQuest software (Becton Dickinson). Cells were electronically gated according to light scatter properties to exclude cell debris.
Mannose receptor-mediated endocytosis
FITC-dextran (Molecular Probes, Eugene, OR) was used to assess cell endocytosis, as described elsewere.20 Briefly, 1 × 105 cells were incubated with 1 mg/mL FITC-dextran at 37°C or 0°C for 60 minutes. Samples were extensively washed prior to flow cytometry analysis. The level of antigen uptake by DCs was expressed as the difference in mean fluorescence intensity (ΔMFI) between the test (37°C) and control (0°C) tubes for each sample.
Phagocytosis of apoptotic cells
The ability of DCs to internalize apoptotic cells was assessed as described elsewere.20 Briefly, monocyte-depleted PBMCs were labeled with 0. 5 μM chloro-methyl-fluorescein-diacetate (CMFDA; Molecular Probes) for 30 minutes at 37°C. Cell apoptosis was induced by 24-hour treatment with 10 μM H2O2 and assessed by morphology and staining with PI and AO. The labeled apoptotic cells were subsequently cocultured with CD1a-labeled allogeneic DCs at a 1:1 ratio. After 2 hours, the cells were washed and treated with 0.05% trypsin/0.02% EDTA (ethylenediaminetetraacetic acid) for 5 minutes to disrupt cell-to-cell binding. Phagocytosis was quantified by flow cytometry as percentage of double-positive cells (CMFDA+/CD1a+). Negative controls were performed at 0°C.
Allogeneic and autologous T-cell proliferation assay
To test their allostimulatory activity, DCs (15 × 103) were cocultured in 96-well plates with 3 × 105 allogeneic, monocyte-depleted PBMCs in triplicate for 5 days.20 To test their ability to present soluble antigens to autologous lymphocytes, DCs were incubated with or without influenza virus vaccine (FLU) for 24 hours and then cocultured with autologous, monocyte-depleted PBMCs for 7 days. In all cases, 5-bromo-2′-deoxyuridine (BrdU, 20 μM; Sigma Chemicals, St. Louis, MO) was added in each well during the last 6 hours of culture, and lymphocyte proliferation was assessed by flow cytometry as BrdU incorporation by CD4+ lymphocytes, as described by Toba et al.21 Results were expressed as percentage of proliferating (BrdU+) lymphocytes.
Tolerance assay
CD45RA+CD4+ naive T lymphocytes, purified as previously described,20 were cocultured with allogeneic SCF-DCs or IL-16/TPO-DCs as for primary allogeneic cultures. After 5 days, the T cells were harvested, washed, and rested for an additional 2 days in culture medium, as described by Nouri-Shirazi and Guinet.22 The rested T cells from primary cultures were rechallenged in a second proliferation assay with 1.5 × 104 SCF-DCs, and proliferation assessed as percentage of BrdU-incorporating lymphocytes.
Cytokine measurements
The release of cytokines in the supernatants of unstimulated DCs was determined by a specific enzyme-linked immunosorbent assay (ELISA). To this aim, CD34+ cells were plated at 2.5 × 105/mL in 24-well plates and cultured as described (see “Generation of DCs from CD34+ progenitor cell cultures”). Supernatants were harvested at the indicated time points, and the concentration of IL-10, IL-12p70, and interferon-γ (IFN-γ) was measured by use of commercially available pairs of mAbs (Endogen, Woburn, MA). Transforming growth factor-β (TGF-β) and IFN-α were measured with TGF-β1 Quantikine (R&D Systems) and IFN-α ModuleSet (Bender MedSystems, Vienna, Austria), respectively, used according to the manufacturers' instructions.
The production of cytokines by T cells cocultured with allogeneic DCs was determined by flow cytometry as intracellular cytokine expression, as previously described.20 Briefly, after the indicated days of DC-T coculture, T cells were reactivated with 12-myristate-13-acetate (PMA; 25 ng/mL) plus ionomycin (1 μg/mL) for 5 hours. Brefeldin-A (BFA; 10 μg/mL; Sigma) was added during the last 4 hours to accumulate most of the cytokine in the Golgi complex. Cells were labeled with CD4 mAb, fixed, and permeabilized using Fix&Perm (Caltag), and then labeled with mAb against cytokine IL-2, IFN-γ, or IL-10.
Statistical analysis
Statistical analysis was performed with Openstat 3 software (open source statistics package by Bill Miller, Iowa State University).
Results
Cell proliferation and differentiation in cultures of CD34+ progenitor cells
CD34+ fractions ranging in purity from 80% to 92% were cultured for 2 weeks in the presence of Flt3L, GM-CSF, IL-4, TNF-α (basal cocktail) plus either SCF as a control, IL-16, TPO, or IL-16 plus TPO. In the presence of IL-16 the total number of in vitro expanded cells was higher (fold increase, mean ± SEM, 19.04 ± 3.24) than in the presence of the other growth factors, namely, SCF (10.84 ± 2.01; P = .016), TPO (11.86 ± 2.79; P = .051), or IL-16 plus TPO (14.89 ± 3.03; P not significant; Figure 1A). No differences were observed between cultures of samples collected from healthy donors or patients. Exposure of CD34+ cells to the 4 cytokine combinations led to progressive appearance of typical DC morphology. After 14 days of culture, the majority of the cells showed lobulated nuclei and numerous fine cytoplasmic projections, as assessed by May-Grünwald-Giemsa staining, and confirmed by fluorescence microscopy after AO staining (Figure 1B). IL-16 appeared to be more efficient than the other factors in inducing the differentiation of CD34+ cells into DCs because the cells grown in the presence of IL-16 showed a more rapid and efficient down-regulation of CD34 and surface expression of the DC marker CD1a (Figure 1C; Table 1). After 7 days of culture, the percentage of cells still expressing CD34 was significantly lower in the presence of IL-16 (mean ± SEM, 5.2% ± 0.4%) than in the presence of the basal cocktail alone (25.4% ± 1.8%; P < .001) or with SCF (13.3% ± 2.0%; P = .004), TPO (15.6% ± 0.9%; P < .001), or IL-16 plus TPO (24.0% ± 0.8%; P < .001). Moreover, after 7 days of culture the percentage of cells already expressing CD1a was significantly higher in the presence of IL-16 (mean ± SEM, 81.4% ± 1.2%) than in the presence of the basal cocktail alone (76.1% ± 6.4%), or with SCF (66.3% ± 2.7%; P < .001), TPO (71.4% ± 1.6%; P = .001), or IL-16 plus TPO (74.4% ± 1.6%; P = .005). After 14 days of culture, the superior efficiency of IL-16 in the differentiation process of DCs was still more evident when the yield of DCs generated in vitro, obtained by multiplying the percentage of CD1a+ cells for the absolute number of viable cells, expressed as fold increase, was considered (Figure 1D).
Cell proliferation and differentiation of CD34+ progenitor cells in different culture conditions. (A) After 14 days, the total number of cells expanded in vitro in the presence of IL-16 added to the basal cocktail was higher than in the presence of the other cytokine combinations. The mean fold increase over cell number plated on day 0 is shown. Mean ± SEM from 16 independent experiments. Comparison between treatments performed with the 2-tailed t test. (B) Morphology of the cells after 14 days of culture. The majority of the cells showed lobulated nuclei and numerous fine cytoplasmic projections, as assessed by AO staining. Photographs were taken under a Leitz-DIALUX22 fluorescence microscope (Leica, Milan, Italy) equipped with a Kodak DX7590 camera (Kodak-italia, Milan, Italy). Original magnification × 400. No differences were observed among the different culture conditions. (C) Down-regulation of CD34 and up-regulation of CD1a expression after 7 and 14 days of culture in the presence of the different cytokine combinations. IL-16 (♦) added to the basal cocktail (▵) appeared to be more efficient than SCF (▴), TPO (•), and IL-16 plus TPO (▪) in inducing the differentiation of CD34+ cells into DCs. The mean of 9 independent experiments is shown. (D) After 14 days, the number of DCs expanded in vitro in the presence of IL-16 added to the basal cocktail was higher than in the presence of the other cytokine combinations. The mean fold increase, obtained by multiplying the percentage of CD1a+ cells for the absolute number of viable cells, is shown. Mean ± SEM from 16 independent experiments. Comparison between treatments performed with the 2-tailed t test.
Cell proliferation and differentiation of CD34+ progenitor cells in different culture conditions. (A) After 14 days, the total number of cells expanded in vitro in the presence of IL-16 added to the basal cocktail was higher than in the presence of the other cytokine combinations. The mean fold increase over cell number plated on day 0 is shown. Mean ± SEM from 16 independent experiments. Comparison between treatments performed with the 2-tailed t test. (B) Morphology of the cells after 14 days of culture. The majority of the cells showed lobulated nuclei and numerous fine cytoplasmic projections, as assessed by AO staining. Photographs were taken under a Leitz-DIALUX22 fluorescence microscope (Leica, Milan, Italy) equipped with a Kodak DX7590 camera (Kodak-italia, Milan, Italy). Original magnification × 400. No differences were observed among the different culture conditions. (C) Down-regulation of CD34 and up-regulation of CD1a expression after 7 and 14 days of culture in the presence of the different cytokine combinations. IL-16 (♦) added to the basal cocktail (▵) appeared to be more efficient than SCF (▴), TPO (•), and IL-16 plus TPO (▪) in inducing the differentiation of CD34+ cells into DCs. The mean of 9 independent experiments is shown. (D) After 14 days, the number of DCs expanded in vitro in the presence of IL-16 added to the basal cocktail was higher than in the presence of the other cytokine combinations. The mean fold increase, obtained by multiplying the percentage of CD1a+ cells for the absolute number of viable cells, is shown. Mean ± SEM from 16 independent experiments. Comparison between treatments performed with the 2-tailed t test.
Immunophenotypic pattern of CD34-derived DCs obtained under different culture conditions
Marker . | BC, % . | BC+SCF,*% . | BC+IL-16,†% . | BC+TPO,†% . | BC+IL-16+TPO,†% . | BC, MFI . | BC+SCF,*MFI . | BC+IL-16,†MFI . | BC+TPO,†MFI . | BC+IL-16+TPO,†MFI . |
|---|---|---|---|---|---|---|---|---|---|---|
| CD34 | 6.3 ± 1.2 | 1.0 ± 0.6‡ | 0.8 ± 0.3 | 2.5 ± 0.6‡ | 1.4 ± 0.8 | 17.6 ± 2.9 | 6.8 ± 1.8‡ | 4.5 ± 1.2§ | 9.4 ± 2.3§ | 8.3 ± 2.1 |
| CD14 | 0.2 ± 0.1 | 0.5 ± 0.4 | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.1 | 2.9 ± 0.4 | 3.4 ± 0.6 | 2.8 ± 0.3 | 2.7 ± 0.5 | 3.7 ± 0.4 |
| CD1a | 78.5 ± 11.2 | 79.1 ± 7.4 | 93.0 ± 3.1‡ | 87.1 ± 5.7‡ | 80.0 ± 6.5 | 134.6 ± 11.3 | 287.1 ± 124.5 | 342.8 ± 182.1 | 294.2 ± 86.9 | 210.7 ± 78.8 |
| CD80 | 79.3 ± 8.1 | 82.9 ± 3.5 | 96.3 ± 2.2‡ | 87.7 ± 5.8§ | 89.7 ± 5.4‡ | 814.3 ± 51.6 | 844.5 ± 115.3 | 1004.0 ± 70.0§ | 970.1 ± 110.2§ | 982.3 ± 75.7§ |
| CD86 | 52.9 ± 2.3 | 58.0 ± 8.7 | 66.6 ± 7.5§ | 68.0 ± 7.7‡ | 66.3 ± 7.3§ | 273.6 ± 43.3 | 402.1 ± 103.4§ | 795.4 ± 38.1‡ | 567.3 ± 119.1‡ | 570.6 ± 29.3‡ |
| CD40 | 8.2 ± 0.2 | 8.4 ± 1.7 | 19.5 ± 1.1‡ | 8.6 ± 1.0 | 10.7 ± 1.6§ | 35.6 ± 3.7 | 37.0 ± 3.1 | 71.2 ± 3.2‡ | 47.3 ± 4.6‡ | 44.5 ± 9.4§ |
| CD83 | 3.1 ± 1.0 | 2.5 ± 0.7 | 12.3 ± 0.6‡ | 6.1 ± 1.8‡ | 6.2 ± 1.9‡ | 21.6 ± 6.7 | 23.0 ± 7.2 | 57.7 ± 4.5‡ | 39.4 ± 4.4‡ | 47.1 ± 6.4‡ |
| HLA-ABC | 97.2 ± 0.4 | 96.8 ± 1.9 | 96.1 ± 3.4 | 98.2 ± 0.6 | 98.5 ± 0.8 | 1702.0 ± 87.4 | 2146.3 ± 183.7§ | 2293.7 ± 165.2 | 2195.0 ± 163.8 | 2246.3 ± 131.8 |
| HLA-DR | 97.0 ± 1.5 | 93.8 ± 3.9 | 94.6 ± 4.6 | 90.2 ± 3.3 | 95.9 ± 2.5 | 1660.0 ± 148.9 | 1843.5 ± 86.0§ | 1927.5 ± 93.8 | 1906.6 ± 173.1 | 2172.6 ± 200.3‡ |
| CD4 | 91.7 ± 7.1 | 88.5 ± 5.8 | 92.4 ± 3.3 | 89.3 ± 3.5 | 87.9 ± 2.7 | 538.3 ± 72.9 | 566.8 ± 94.4 | 802.4 ± 58.7‡ | 819.6 ± 59.5‡ | 815.8 ± 44.8‡ |
| CD11c | 96.5 ± 3.3 | 94.9 ± 3.2 | 96.6 ± 3.5 | 94.2 ± 3.4 | 99.4 ± 0.2 | 605.7 ± 13.6 | 609.3 ± 19.1 | 606.9 ± 16.6 | 606.9 ± 20.9 | 611.7 ± 8.2 |
| CD123 | 43.4 ± 5.6 | 38.7 ± 13.9 | 48.3 ± 19.9 | 65.4 ± 12.1§ | 68.1 ± 2.9§ | 70.8 ± 9.3 | 58.4 ± 19.2 | 61.8 ± 24.0 | 95.6 ± 26.1§ | 111.5 ± 11.1§ |
Marker . | BC, % . | BC+SCF,*% . | BC+IL-16,†% . | BC+TPO,†% . | BC+IL-16+TPO,†% . | BC, MFI . | BC+SCF,*MFI . | BC+IL-16,†MFI . | BC+TPO,†MFI . | BC+IL-16+TPO,†MFI . |
|---|---|---|---|---|---|---|---|---|---|---|
| CD34 | 6.3 ± 1.2 | 1.0 ± 0.6‡ | 0.8 ± 0.3 | 2.5 ± 0.6‡ | 1.4 ± 0.8 | 17.6 ± 2.9 | 6.8 ± 1.8‡ | 4.5 ± 1.2§ | 9.4 ± 2.3§ | 8.3 ± 2.1 |
| CD14 | 0.2 ± 0.1 | 0.5 ± 0.4 | 0.3 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.1 | 2.9 ± 0.4 | 3.4 ± 0.6 | 2.8 ± 0.3 | 2.7 ± 0.5 | 3.7 ± 0.4 |
| CD1a | 78.5 ± 11.2 | 79.1 ± 7.4 | 93.0 ± 3.1‡ | 87.1 ± 5.7‡ | 80.0 ± 6.5 | 134.6 ± 11.3 | 287.1 ± 124.5 | 342.8 ± 182.1 | 294.2 ± 86.9 | 210.7 ± 78.8 |
| CD80 | 79.3 ± 8.1 | 82.9 ± 3.5 | 96.3 ± 2.2‡ | 87.7 ± 5.8§ | 89.7 ± 5.4‡ | 814.3 ± 51.6 | 844.5 ± 115.3 | 1004.0 ± 70.0§ | 970.1 ± 110.2§ | 982.3 ± 75.7§ |
| CD86 | 52.9 ± 2.3 | 58.0 ± 8.7 | 66.6 ± 7.5§ | 68.0 ± 7.7‡ | 66.3 ± 7.3§ | 273.6 ± 43.3 | 402.1 ± 103.4§ | 795.4 ± 38.1‡ | 567.3 ± 119.1‡ | 570.6 ± 29.3‡ |
| CD40 | 8.2 ± 0.2 | 8.4 ± 1.7 | 19.5 ± 1.1‡ | 8.6 ± 1.0 | 10.7 ± 1.6§ | 35.6 ± 3.7 | 37.0 ± 3.1 | 71.2 ± 3.2‡ | 47.3 ± 4.6‡ | 44.5 ± 9.4§ |
| CD83 | 3.1 ± 1.0 | 2.5 ± 0.7 | 12.3 ± 0.6‡ | 6.1 ± 1.8‡ | 6.2 ± 1.9‡ | 21.6 ± 6.7 | 23.0 ± 7.2 | 57.7 ± 4.5‡ | 39.4 ± 4.4‡ | 47.1 ± 6.4‡ |
| HLA-ABC | 97.2 ± 0.4 | 96.8 ± 1.9 | 96.1 ± 3.4 | 98.2 ± 0.6 | 98.5 ± 0.8 | 1702.0 ± 87.4 | 2146.3 ± 183.7§ | 2293.7 ± 165.2 | 2195.0 ± 163.8 | 2246.3 ± 131.8 |
| HLA-DR | 97.0 ± 1.5 | 93.8 ± 3.9 | 94.6 ± 4.6 | 90.2 ± 3.3 | 95.9 ± 2.5 | 1660.0 ± 148.9 | 1843.5 ± 86.0§ | 1927.5 ± 93.8 | 1906.6 ± 173.1 | 2172.6 ± 200.3‡ |
| CD4 | 91.7 ± 7.1 | 88.5 ± 5.8 | 92.4 ± 3.3 | 89.3 ± 3.5 | 87.9 ± 2.7 | 538.3 ± 72.9 | 566.8 ± 94.4 | 802.4 ± 58.7‡ | 819.6 ± 59.5‡ | 815.8 ± 44.8‡ |
| CD11c | 96.5 ± 3.3 | 94.9 ± 3.2 | 96.6 ± 3.5 | 94.2 ± 3.4 | 99.4 ± 0.2 | 605.7 ± 13.6 | 609.3 ± 19.1 | 606.9 ± 16.6 | 606.9 ± 20.9 | 611.7 ± 8.2 |
| CD123 | 43.4 ± 5.6 | 38.7 ± 13.9 | 48.3 ± 19.9 | 65.4 ± 12.1§ | 68.1 ± 2.9§ | 70.8 ± 9.3 | 58.4 ± 19.2 | 61.8 ± 24.0 | 95.6 ± 26.1§ | 111.5 ± 11.1§ |
DCs generated in vitro by culture of CD34+ cells with GM-CSF + IL-4 + Flt3L + TNF-β (basal cocktail) plus either SCF or IL-16 or TPO or IL-16 + TPO were analyzed after 14 days as described in “Materials and methods.” Results report the mean ± SD of 16 independent experiments. CD11c and CD123 were performed on 5 experiments.
BC indicates basal cocktail.
Compared with BC.
Compared with SCF.
P ≤ .005 (t test for independent samples).
P < .05.
Next, we investigated whether the effects of IL-16 were mainly exerted on the CD34+ subpopulation that expresses CD4.15 To this purpose, we cultured CD34+CD4+ and CD34+CD4- sorted cells with the basal cocktail in the presence or absence of IL-16. As shown in Figure 2A, IL-16 induced a cell expansion of the CD34+CD4+ cells significantly higher than the basal cocktail alone (fold increase, mean ± SEM, 46.77 ± 5.80 versus 33.28 ± 5.22; paired Wilcoxon signed rank test; P = .006). The effects of IL-16 on cell expansion of CD34+CD4- cells were only marginal (30.03 ± 4.40 versus 25.96 ± 4.02; P not significant). Similar results were observed when the yield of DCs was considered (Figure 2B).
Cell proliferation and differentiation of CD34+/CD4+ and CD34+/CD4- sorted cells culture with or without IL-16. (A) After 14 days, the total number of cells expanded in vitro from CD34+/CD4+ cells, but not from CD34+/CD4- cells, in the presence of IL-16 added to the basal cocktail was higher than in the presence of the cocktail alone. The mean fold increase over cell number plated on day 0 is shown. Mean ± SEM from 10 independent experiments. (B) After 14 days, the number of DCs expanded in vitro from CD34+/CD4+ cells, but not from CD34+/CD4- cells, in the presence of IL-16 added to the basal cocktail was higher than in the presence of the cocktail alone. The mean fold increase is shown. Mean ± SEM from 10 independent experiments. Comparison between treatments performed with the paired Wilcoxon signed rank test. (C) CD34+/CD4- sorted cells grown in the presence of basal cocktail alone (broken line) or with IL-16 (solid line) showed a progressive expression of CD4 molecule on their surface. One representative of 2 independent experiments is shown.
Cell proliferation and differentiation of CD34+/CD4+ and CD34+/CD4- sorted cells culture with or without IL-16. (A) After 14 days, the total number of cells expanded in vitro from CD34+/CD4+ cells, but not from CD34+/CD4- cells, in the presence of IL-16 added to the basal cocktail was higher than in the presence of the cocktail alone. The mean fold increase over cell number plated on day 0 is shown. Mean ± SEM from 10 independent experiments. (B) After 14 days, the number of DCs expanded in vitro from CD34+/CD4+ cells, but not from CD34+/CD4- cells, in the presence of IL-16 added to the basal cocktail was higher than in the presence of the cocktail alone. The mean fold increase is shown. Mean ± SEM from 10 independent experiments. Comparison between treatments performed with the paired Wilcoxon signed rank test. (C) CD34+/CD4- sorted cells grown in the presence of basal cocktail alone (broken line) or with IL-16 (solid line) showed a progressive expression of CD4 molecule on their surface. One representative of 2 independent experiments is shown.
Immunophenotype of cultured cells
The immunophenotype of the cells cultured in the presence of the different cytokine combinations was analyzed after 7 and 14 days of culture. As shown in Table 1, the percentage of cells still expressing CD34 or CD14 at the end of the culture was negligible in all the culture conditions, except for culture with the basal cocktail alone. The contamination of lymphocytes (CD3+ or CD19+) and megakaryocytes (CD41+) was also negligible (< 1%) in all the conditions tested and granulocytes (CD15+) did not exceed 10%, without differences among treatments.
Compared with SCF-DCs, IL-16-DCs, TPO-DCs, and IL-16/TPO-DCs appeared more activated because they showed increased expression of the costimulatory molecules CD80, CD86, and CD40 (Table 1). IL-16-DCs had the most activated profile, with expression of CD80 as well CD40 significantly higher than TPO-DCs (P < .003 and P < .001, respectively) and IL-16/TPO-DCs (P < .001 in both cases). As shown in Table 1, IL-16-DCs, TPO-DCs, and IL-16/TPO-DCs appeared also more mature than SCF-DCs, as assessed by expression of the maturation marker CD83. Again, IL-16-DCs were the most mature, with CD83 expression significantly higher than TPO-DCs (P < .001) and IL-16/TPO-DCs (P < .001). In 5 cases we analyzed the expression of the plasmacytoid marker CD123 and myeloid marker CD11c. TPO-DCs and IL-16/TPO-DCs had higher expression of CD123 than DCs grown in the absence of TPO (compared with SCF-DCs: P = .018 and P = .012, respectively). CD123 was coexpressed with CD11c, which was present on the majority of DCs in all the culture conditions (Table 1).
The immunophenotypic characterization of the cells obtained by culture of CD34+CD4+ and CD34+CD4- sorted cells documented a progressive expression of CD4 molecule on the surface of the CD34+CD4- subpopulation (CD4+ cells, 75.9% ± 9.18% at day 7; 85.4% ± 4% at day 14), both in the absence and presence of IL-16. A representative time-course experiment is shown in Figure 2C. As in the case of unfractioned cells, also in the sorted cell preparations IL-16 induced a higher expression of the activation and maturation markers than the basal cocktail alone, with similar patterns observed in the CD34+CD4+ and CD34+CD4- cultures (data not shown).
Antigen capture capacity of DCs
The capacity of DCs to take up antigens was measured in 2 systems using FITC-dextran, as an indicator of mannose-receptor (MR)-mediated endocytosis, and CMFDA-labeled apoptotic cells, as an indicator of phagocytosis. DCs obtained by culture with IL-16 or TPO were as efficient as SCF-DCs, as a control, in capturing antigens. In fact, similar levels of FITC-dextran uptake were presented by SCF-DCs (ΔMFI, 41.2 ± 1.7, mean ± SEM of 5 independent experiments), IL-16-DCs (39.3 ± 1.7), TPO-DCs (35.9 ± 2.1), and IL-16/TPO-DCs (37.0 ± 3.0). Similarly, phagocytosis of CMFDA-labeled apoptotic cells (> 70% dead cells as determined by PI and AO staining), assessed by flow cytometry as the percentage of CD1a-labeled DCs that expressed the green dye CMFDA and confirmed by the increased granularity of the double-positive population at forward scatter/side scatter analysis, did not differ among DCs obtained with different cytokine combinations (data not shown).
Lymphocyte proliferation induced by DCs
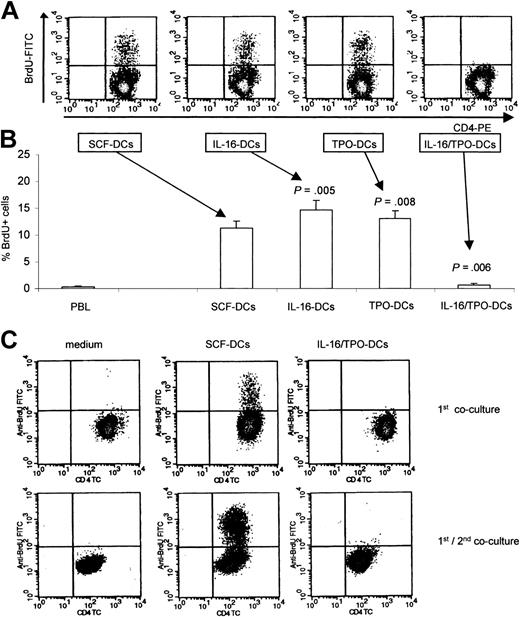
Next, we evaluated the allostimulatory proprieties of the DCs obtained by culture in the different cytokine combinations. To this purpose, the direct pathway of alloantigen presentation was assessed in mixed leukocyte reaction as BrdU incorporation by allogeneic, monocyte-depleted PBMCs. As shown in Figure 3A-B, IL-16-DCs and TPO-DCs showed excellent allostimulatory activity, even superior to that of the control SCF-DCs (percent BrdU+ lymphocytes, mean ± SEM of 9 independent experiments: SCF-DCs, 11.22 ± 1.40; IL-16-DCs, 14.61 ± 1.87, paired Wilcoxon signed rank test compared with SCF-DCs, P = .005; TPO-DCs, 13.00 ± 1.55, P = .008). Surprisingly, IL-16/TPO-DCs were unable to stimulate the proliferation of allogeneic T-cells (0.68 ± 0.22, P = .006). In a few experiments, we analyzed the surface expression of the activation marker CD25 by T cells that had been cocultured in primary allogeneic culture either with IL-16/TPO-DCs or with standard immunogenic SCF-DCs. We found that the reduced T-cell proliferative response induced by IL-16/TPO-DCs was paralleled by a reduced activation of T cells (percent CD25+ lymphocytes, mean ± SEM of 3 independent experiments: SCF-DCs, 25.99 ± 2.84; IL-16/TPO-DCs, 2.18 ± 0.49). To further determine whether IL-16/TPO-DCs induced tolerance of naive alloreactive T cells, we tested the proliferative response of T cells in a 2-step culture system. As shown in Figure 3C, T cells cocultured with SCF-DCs in the first coculture responded robustly to rechallenge with the same stimulators. In contrast, the T cells cocultured with IL-16/TPO-DCs in the first culture were hyporesponsive to further stimulation with SCF-DCs. To better define the role of IL-16 and TPO in conditioning the tolerogenic properties of IL-16/TPO-DCs, we performed kinetic experiments in which IL-16 and TPO were added to or removed from the cultures at different times during the differentiation process of DCs. As shown in Figure 4A, the removal of IL-16 plus TPO early or late during the first week of culture of IL-16/TPO-DCs was enough to generate DCs endowed with excellent allostimulatory capacity. Figure 4B shows that the single removal of either IL-16 or TPO early or late during the first week of culture was enough to restore the allostimulatory capacity of IL-16/TPO-DCs. Therefore, the concomitant presence of IL-16 and TPO during the first 7 days of coculture was required to induce the differentiation of IL-16/TPO-DCs lacking T-stimulatory activity. We next investigated the effects of IL-16/TPO combination on sorted CD34+/CD4+ and CD34+/CD4- cell subpopulations. As shown in Figure 4C, we observed that IL-16 plus TPO induced from both cell subsets the generation of DCs unable to stimulate the proliferation of allogeneic T cells.
Induction of lymphocyte proliferation in primary and secondary allogeneic cultures. (A) The allostimulatory activity of DCs obtained in the different cytokine combinations was tested in primary cultures, using monocyte-depleted PBMCs. Proliferating lymphocytes were identified in density plots as CD4+/BrdU+ double-positive cells. (B) In primary cultures, IL-16-DCs and TPO-DCs showed higher allostimulatory activity than control SCF-DCs, whereas IL-16/TPO-DCs were unable to stimulate the proliferation of allogeneic T lymphocytes. The mean ± SEM from 9 independent experiments is shown. IL-16-DCs, TPO-DCs, and IL-16/TPO-DCs compared with SCF-DCs with the paired Wilcoxon signed rank test. (C) Tested in a 2-step culture system, IL-16/TPO-DCs induced tolerance of cocultured T cells. Purified naive T cells were cultured alone (left column) or cocultured with either SCF-DCs (center column) or IL-16/TPO-DCs (right column). After 5 days, the T cells were harvested, washed, and rested for 2 days. The rested T cells from primary cultures were subsequently rechallenged with SCF-DCs. Proliferation of T lymphocytes was assessed as BrdU incorporation after 4 days. One representative of 3 independent experiments is shown.
Induction of lymphocyte proliferation in primary and secondary allogeneic cultures. (A) The allostimulatory activity of DCs obtained in the different cytokine combinations was tested in primary cultures, using monocyte-depleted PBMCs. Proliferating lymphocytes were identified in density plots as CD4+/BrdU+ double-positive cells. (B) In primary cultures, IL-16-DCs and TPO-DCs showed higher allostimulatory activity than control SCF-DCs, whereas IL-16/TPO-DCs were unable to stimulate the proliferation of allogeneic T lymphocytes. The mean ± SEM from 9 independent experiments is shown. IL-16-DCs, TPO-DCs, and IL-16/TPO-DCs compared with SCF-DCs with the paired Wilcoxon signed rank test. (C) Tested in a 2-step culture system, IL-16/TPO-DCs induced tolerance of cocultured T cells. Purified naive T cells were cultured alone (left column) or cocultured with either SCF-DCs (center column) or IL-16/TPO-DCs (right column). After 5 days, the T cells were harvested, washed, and rested for 2 days. The rested T cells from primary cultures were subsequently rechallenged with SCF-DCs. Proliferation of T lymphocytes was assessed as BrdU incorporation after 4 days. One representative of 3 independent experiments is shown.
Effects of IL-16/TPO combination on the generation of tolerogenic DCs. The allostimulatory activity of DCs was tested in primary cultures, using monocyte-depleted PBMCs. (A-B) Effects of the presence of IL-16 and TPO at different times of culture. The concomitant presence of IL-16 and TPO during the first 7 days of culture was required to induce the differentiation of tolerogenic DCs. The mean ± SEM from 3 independent experiments is shown. (C) Effects of IL-16/TPO combination on sorted cell subpopulations. The presence of IL-16 plus TPO induced the generation of tolerogenic DCs, which were unable to stimulate the proliferation of allogeneic T lymphocytes, from both CD34+/CD4+ (top row) and CD34+/CD4- (bottom row) cells. Proliferating lymphocytes identified as CD4+/BrdU+ double-positive cells. One representative of 4 independent experiments is shown.
Effects of IL-16/TPO combination on the generation of tolerogenic DCs. The allostimulatory activity of DCs was tested in primary cultures, using monocyte-depleted PBMCs. (A-B) Effects of the presence of IL-16 and TPO at different times of culture. The concomitant presence of IL-16 and TPO during the first 7 days of culture was required to induce the differentiation of tolerogenic DCs. The mean ± SEM from 3 independent experiments is shown. (C) Effects of IL-16/TPO combination on sorted cell subpopulations. The presence of IL-16 plus TPO induced the generation of tolerogenic DCs, which were unable to stimulate the proliferation of allogeneic T lymphocytes, from both CD34+/CD4+ (top row) and CD34+/CD4- (bottom row) cells. Proliferating lymphocytes identified as CD4+/BrdU+ double-positive cells. One representative of 4 independent experiments is shown.
Last, we analyzed the ability of DCs to present influenza virus vaccine (FLU), as a soluble antigen, to autologous lymphocytes. We measured BrdU incorporation by autologous CD4+ lymphocytes cultured with FLU-pulsed DCs. As shown in Figure 5, the excellent APC capacity of IL-16-DCs and TPO-DCs observed in mixed lymphocyte reaction (MLR) was confirmed in this experimental model. On the other hand, IL-16/TPO-DCs were unable to induce lymphocyte proliferation on presentation of FLU, thus confirming the tolerogenic profile of these DCs.
Induction of proliferation of autologous lymphocytes on presentation of a soluble antigen, FLU. The lack of T-stimulatory activity of IL-16/TPO-DCs was confirmed when the proliferation of autologous T lymphocytes on presentation of FLU was tested. The capacity of DCs obtained in the different cytokine combinations to induce T-cell proliferation was compared. Proliferating lymphocytes were identified in density plots as CD4+/BrdU+ double-positive cells. Control cultures included monocyte-depleted PBMCs alone (left column), cocultured with unpulsed DCs (middle column), or antigen-specific proliferation assessed in cultures with FLU-pulsed DCs (right column). The numbers represent the percentage of cells in each quadrant. One representative of 3 independent experiments is shown.
Induction of proliferation of autologous lymphocytes on presentation of a soluble antigen, FLU. The lack of T-stimulatory activity of IL-16/TPO-DCs was confirmed when the proliferation of autologous T lymphocytes on presentation of FLU was tested. The capacity of DCs obtained in the different cytokine combinations to induce T-cell proliferation was compared. Proliferating lymphocytes were identified in density plots as CD4+/BrdU+ double-positive cells. Control cultures included monocyte-depleted PBMCs alone (left column), cocultured with unpulsed DCs (middle column), or antigen-specific proliferation assessed in cultures with FLU-pulsed DCs (right column). The numbers represent the percentage of cells in each quadrant. One representative of 3 independent experiments is shown.
Cytokine production by DCs and T cells
DC-derived cytokines were measured by ELISA in the supernatants of unstimulated DC cultures. Figure 6A shows kinetic experiments aimed at comparing cytokine production at different time points during the differentiation process of DCs grown in the different cytokine combinations. The analysis of cytokine production at the end of the differentiation process indicated that, as shown in Figure 6B, tolerogenic IL-16/TPO-DCs produced markedly higher levels of the immunosuppressive cytokines TGF-β and IL-10, and lower levels of the stimulatory cytokine IL-12p70, than standard immunogenic SCF-DCs. Also IL-16-DCs and TPO-DCs produced higher levels of IL-10 than SCF-DCs, but at lower levels than IL-16/TPO-DCs. Unlike the other types of DCs, IL-16/TPO-DCs also produced IFN-α. As shown in Figure 6B, the pattern of cytokine production of SCF-DCs and IL-16/TPO-DCs obtained from either CD34+/CD4+ or CD34+/CD4- sorted cells was similar to that of DCs obtained from unfractionated cells. The production of IFN-γ was negligible by DCs cultured in all the cytokine combinations (not shown).
Production of cytokines by DCs and T cells. (A) Production of cytokines at different time points during the differentiation process of DCs grown in different cytokine combinations. CD34+ cells were cultured in the presence of SCF (▴), IL-16 (♦), TPO (•), or IL-16 plus TPO (▪), and the released cytokines were measured in the supernatants by ELISA. The concomitant presence of IL-16 and TPO induced the production of higher levels of TGF-β, IL-10, and IFN-α, and lower levels of IL-12p70, which became increasingly evident during the second week of culture. The mean ± SEM from 2 independent experiments is shown. (B) Production of cytokines by DCs. Unfractionated CD34+ cells, CD34+/CD4+, and CD34+/CD4- sorted cells were grown in the presence of the indicated cytokine combinations for 14 days, and the released cytokines were measured in the supernatants by ELISA. IL-16/TPO-DCs obtained either from unfractionated CD34+ cells or from CD34+/CD4+ or CD34+/CD4- cells produced higher levels of TGF-β, IL-10, and IFN-α, and lower levels of IL-12p70, than the other types of DCs. Mean ± SEM from 4 independent experiments. IL-16-DCs, TPO-DCs, and IL-16/TPO-DCs compared with SCF-DCs with the paired Wilcoxon signed rank test; *P < .05. (C) Production of cytokines by T cells cocultured with IL-16/TPO-DCs compared with SCF-DCs. Monocyte-depleted PBMCs were cultures alone (left columns), or with SCF-DCs (central columns), or with IL-16/TPO-DCs (right columns). After the indicated days of DC/T coculture, T cells were reactivated with PMA and ionomycin for 5 hours, and their intracellular expression of cytokines detected by flow cytometry. Gated on CD4+ T cells, density plots show the expression of IFN-γ and IL-2 (upper row) or IL-10 (lower row). Quadrants were set according to the fluorescence intensities of isotype-matched controls. T cells cocultured with IL-16/TPO-DCs failed to express IL-2 and IFN-γ. IL-10 was not expressed in any condition. One representative of 3 independent experiments is shown.
Production of cytokines by DCs and T cells. (A) Production of cytokines at different time points during the differentiation process of DCs grown in different cytokine combinations. CD34+ cells were cultured in the presence of SCF (▴), IL-16 (♦), TPO (•), or IL-16 plus TPO (▪), and the released cytokines were measured in the supernatants by ELISA. The concomitant presence of IL-16 and TPO induced the production of higher levels of TGF-β, IL-10, and IFN-α, and lower levels of IL-12p70, which became increasingly evident during the second week of culture. The mean ± SEM from 2 independent experiments is shown. (B) Production of cytokines by DCs. Unfractionated CD34+ cells, CD34+/CD4+, and CD34+/CD4- sorted cells were grown in the presence of the indicated cytokine combinations for 14 days, and the released cytokines were measured in the supernatants by ELISA. IL-16/TPO-DCs obtained either from unfractionated CD34+ cells or from CD34+/CD4+ or CD34+/CD4- cells produced higher levels of TGF-β, IL-10, and IFN-α, and lower levels of IL-12p70, than the other types of DCs. Mean ± SEM from 4 independent experiments. IL-16-DCs, TPO-DCs, and IL-16/TPO-DCs compared with SCF-DCs with the paired Wilcoxon signed rank test; *P < .05. (C) Production of cytokines by T cells cocultured with IL-16/TPO-DCs compared with SCF-DCs. Monocyte-depleted PBMCs were cultures alone (left columns), or with SCF-DCs (central columns), or with IL-16/TPO-DCs (right columns). After the indicated days of DC/T coculture, T cells were reactivated with PMA and ionomycin for 5 hours, and their intracellular expression of cytokines detected by flow cytometry. Gated on CD4+ T cells, density plots show the expression of IFN-γ and IL-2 (upper row) or IL-10 (lower row). Quadrants were set according to the fluorescence intensities of isotype-matched controls. T cells cocultured with IL-16/TPO-DCs failed to express IL-2 and IFN-γ. IL-10 was not expressed in any condition. One representative of 3 independent experiments is shown.
To better characterize the effects of IL-16/TPO-DCs compared with standard SCF-DCs on T lymphocytes, we analyzed the production of cytokines involved in cell activation and proliferation, by T lymphocytes cocultured with DCs. Because we performed bidirectional allogeneic cultures, we analyzed by flow cytometry intracellular cytokine expression by T lymphocytes. In accordance with the proliferative responses, we observed that following culture with SCF-DCs T lymphocytes expressed IL-2 and IFN-γ, whereas following culture with IL-16/TPO-DCs they failed to express both cytokines (Figure 6C). T-cell expression of IL-10 was undetectable on culture with either stimulator.
Expression of DC-SIGN and of the inhibitory molecules ILT-2, ILT-3, and ILT-4 on DC surface
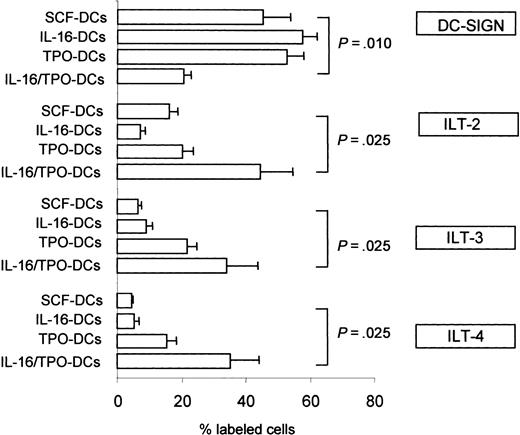
To investigate other mechanisms possibly contributing to the tolerogenic profile of IL-16/TPO-DCs, we next examined the expression on the DC surface of additional molecules involved in DC/T-cell interactions. Therefore, we analyzed the DC expression of the C-type lectin DC-SIGN and found that although the percentage of DCs expressing DC-SIGN in IL-16-DCs and TPO-DCs was similar to that observed in the control SCF-DCs (percent DC-SIGN+ DCs, mean ± SEM of 4 independent experiments: 57.6 ± 4.4, 52.6 ± 3.1, and 45.1 ± 8.8, respectively), the percentage of DC-SIGN+ in IL-16/TPO-DCs was markedly decreased (20.5 ± 1.1, Wilcoxon signed rank test, P = .010; Figure 7). DC-SIGN expression was directly correlated with lymphocyte proliferation induced in allogeneic cultures (Spearman rank correlation: Rho = 0.626, P = .016). Moreover, we examined DC expression of the inhibitory molecules ILT-2, ILT-3, and ILT-4. As shown in Figure 7, the expression of these molecules was markedly increased in IL-16/TPO-DCs compared with control SCF-DCs (percent ILT-2+ DCs, 44.3 ± 10.4 versus 15.9 ± 2.8, P = .025; percent ILT-3+ DCs, 33.9 ± 8.1 versus 6.2 ± 0.6, P = .025; percent ILT-4+ DCs, 35.3 ± 7.6 versus 4.6 ± 0.2, P = .025). The expression of ILT-2, ILT-3, and ILT-4 by DCs was inversely correlated with the proliferative response induced in allogeneic cultures (ILT-2, Rho = -0.798, P = .003; ILT-3, Rho = -0.560, P = .073; ILT-4, Rho = -0.661, P = .027).
Expression of DC-SIGN and of ILT-2, ILT-3, and ILT-4 by DCs. The frequency of DCs expressing DC-SIGN was markedly lower in IL-16/TPO-DCs compared with the DCs grown in the other cytokine combinations. In the same cells the frequency of DCs expressing ILT-2, ILT-3, and ILT-4 was markedly higher. The mean ± SEM of 4 independent experiments is shown. Comparison between treatments performed with the paired Wilcoxon signed rank test.
Expression of DC-SIGN and of ILT-2, ILT-3, and ILT-4 by DCs. The frequency of DCs expressing DC-SIGN was markedly lower in IL-16/TPO-DCs compared with the DCs grown in the other cytokine combinations. In the same cells the frequency of DCs expressing ILT-2, ILT-3, and ILT-4 was markedly higher. The mean ± SEM of 4 independent experiments is shown. Comparison between treatments performed with the paired Wilcoxon signed rank test.
Discussion
Our data demonstrate for the first time that IL-16, added to a basal cocktail of cytokines composed of Flt3L, GM-CSF, IL-4, and TNF-α, can induce human CD34+ cells obtained from G-CSF-mobilized blood of adult individuals, to proliferate and differentiate into fully mature DCs. Compared with other growth factors, namely, SCF (used as a control), TPO, or IL-16 plus TPO, IL-16 exerted this function more efficiently, as indicated by the observation that after 14 days of culture the total number of cells expanded in vitro in the presence of IL-16 was higher than in the presence of the other growth factors. IL-16 also induced the differentiation of higher numbers of CD1a+ DCs than did the other growth factors. The physiologic in vivo relevance of these results is possibly supported by the notion that CD34+ cells are able to secrete IL-16 themselves, thus suggesting that IL-16 could play a key role, with other endogenously secreted factors, in the biology of early hematopoietic cells.23 A better understanding of the role of IL-16 in regulating the biology of human hematopoietic cells will rely on a fine characterization of the structure and the function of this molecule, both of which are currently under intense investigation.
Next, we proceeded to the functional characterization of the DCs obtained with the different cytokine combinations. We found that IL-16-DCs had a more activated and mature immunophenotype than the other DCs, as assessed by a higher expression of the costimulatory molecules CD80, CD86, CD40, and the maturation marker CD83. Notably, this higher stage of maturation did not affect the ability of these cells to internalize antigens. In fact, the DCs obtained with all the cytokine combinations showed similar good capacities to capture antigens either by MR-mediated endocytosis or by phagocytosis. Moreover, IL-16-DCs were endowed with excellent APC function, measured as allostimulatory activity and ability to induce T-cell proliferation on presentation of FLU. In this respect, IL-16-DCs were quite similar to SCF-DCs, used as a control, and to TPO-DCs. All together, these results seem to suggest that the substitution of standard SCF with either IL-16 or, to a lesser extent, TPO may represent alternative tools to improve the efficiency of in vitro generation of immunogenic DCs.
IL-16/TPO-DCs were tolerogenic. In fact, in primary cultures they were unable to stimulate the proliferation of either allogeneic T cells or autologous T cells on presentation of influenza virus vaccine. This lack of proliferation resulted from the ability of IL-16/TPO-DCs to induce an anergic state in T cells, documented by low expression of CD25, IL-2, and IFN-γ in cocultured T lymphocytes, which persisted when IL-16/TPO-DC-conditioned T cells were rechallenged with immunogenic SCF-DCs. The concomitant presence of IL-16 and TPO during the first 7 days of culture was required to induce tolerogenic DCs, as indicated by kinetic experiments. Because treatment of developing DCs with TGF-β or IL-10 or both promotes the generation of tolerogenic DCs,24-26 we asked whether induction of these cytokines might represent a possible mechanism used by IL-16/TPO combination to influence DC differentiation. We found indeed that the levels of both TGF-β and IL-10 were higher in the milieu of developing IL-16/TPO-DCs than in the milieu of the other DCs. Next, we considered the possible mechanisms used by IL-16/TPO-DCs to induce tolerance of cocultured T-cells. The tolerogenic properties of IL-16/TPO-DCs could not be ascribed to an immature state because their surface expression of HLA and costimulatory molecules was not lower than expression on immunogenic DCs grown in the other cytokine combinations. However, “semimature DCs” have been described that induce T-cell tolerance despite their mature phenotype if they produce low levels of IL-12p70.24,27,28 Indeed, our results indicated that IL-16/TPO-DCs produced markedly lower levels of IL-12p70 than the other types of DCs. This finding, together with the observation that IL-16/TPO-DCs produce high levels of the immunosuppressive cytokines TGF-β and IL-10, may suggest that an altered pattern of cytokine production may contribute to the tolerogenic properties of IL-16/TPO-DCs.
To further investigate other possible mechanisms used by IL-16/TPO-DCs to induce T-cell tolerance, we evaluated their surface expression of additional molecules involved in DC/T-cell interactions. DC-SIGN (CD209) is a recently identified DC-specific adhesion receptor belonging to the C-type lectin family, involved in the control of many functions of DCs including initiation and regulation of interactions between DCs and T cells.29,30 Our results indicated that the expression of DC-SIGN was markedly decreased on IL-16/TPO-DCs and was directly correlated with the intensity of the induced T-cell proliferation. ILT-2, ILT-3, and ILT-4 belong to a family of inhibitory receptors that can be expressed on many cell types; it has been recently demonstrated that the expression of these molecules on DCs, induced by regulatory T cells, renders DCs tolerogenic.31,32 We analyzed the expression of ILT molecules on our DCs and observed indeed that the expression of ILT-2, ILT-3, and ILT-4 was increased on IL-16/TPO-DCs and was inversely correlated with the induced proliferation of allogeneic T cells. Therefore, our results may suggest that low surface expression of DC-SIGN and high expression of ILT inhibitory molecules may represent additional mechanisms possibly involved in the tolerogenic properties of IL-16/TPO-DCs.
Our results seem to indicate that the IL-16/TPO combination does condition the development of a distinct tolerogenic DC subtype that shares some features, but not others, with other defined DC subsets. In fact, similarly to Langerhans-type DCs, which are developed by culture of CD34+ cells in the presence of exogenous TGF-β combined with GM-CSF, TNF-α, Flt3L, and SCF,33,34 IL-16/TPO-DCs express the Langerhans cell-restricted molecule CD1a and lack DC-SIGN expression. But, unlike Langerhans-type DCs, which are immature DCs, they have a mature phenotype. Similarly to plasmacytoid DCs that can be developed by culture of CD34+ cells in the concomitant presence of Flt3L and TPO,35 IL-16/TPO-DCs express CD123 and produce IFN-α. But, unlike these plasmacytoid DCs, they coexpress the myeloid marker CD11c. Notably, the possibility that these plasmacytoid features may contribute to the tolerogenic properties of IL-16/TPO-DCs appears unlikely because DCs coexpressing CD123 and CD11c and producing IFN-α, obtained by culture of monocytes in GM-CSF plus IFN-α, are endowed with excellent APC function, as we recently reported elsewhere.20
Last, we considered the effects of the cytokine combinations on the sorted subpopulations enriched in CD34+CD4+ and CD34+CD4- cells. Our results clearly indicate that IL-16 is more effective on the CD34+CD4+ subpopulation. This finding is not surprising because secreted IL-16 is known to be the natural soluble ligand of CD4 molecule.36 Indeed, we observed that culture of CD34+CD4- cells in basal cocktail with or without IL-16 induced a progressive surface expression of CD4. Because CD4 expression was observed on a relevant percentage of cells after 3 to 4 days of culture, it may be argued that the proliferative effect of IL-16 on CD4+ cells is mainly exerted during the first days of culture. Interestingly, the effects of IL-16 compared with basal cocktail were more marked on unfractionated cells, containing both CD4+ and CD4- cells, than on sorted CD34+CD4+ cells. We suggest that synergistic cooperation between different cell populations, with the possible involvement of other soluble factors, may be promoted by IL-16 in unfractionated cells and may be lost when the cells are sorted. On the other hand, IL-16/TPO combination was similarly effective on CD34+CD4+ and CD34+CD4- subpopulations in inducing the generation of tolerogenic DCs. Because IL-16 plus TPO exert their effects during the first days of culture, the possibility that some of the effects of IL-16 may be mediated not only by CD4 receptor but also by other coactivators, as already reported elsewhere,37-39 may be considered. Further experiments will be required to clarify this point.
In conclusion, in this study we provide evidence that IL-16 and TPO may represent new tools for the in vitro generation of DCs from CD34+ progenitors. In different combinations with other cytokines they can improve the efficiency of generation of immunogenic DCs, or induce the generation of tolerogenic DCs that may aid the exploration of new therapeutic strategies to promote tolerance to autoantigens and prevent disease development.
Prepublished online as Blood First Edition Paper, August 10, 2004; DOI 10.1182/blood-2004-03-0885.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr M. Saresella and I. Marventano at IRCCS Fondazione Don Gnocchi, Milan, Italy, and I. Zaccaria at Ospedale S. Raffaele, Milan, Italy, for cell sorting.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal