Abstract
Dendritic cells (DCs), the mononuclear cells that initiate immune response, and osteoclasts, the multinucleated bone-resorbing cells, are derived from monocyte/macrophage precursor cells. Granulocyte-macrophage colony-stimulating factor and macrophage colony-stimulating factor (M-CSF) reciprocally regulate the differentiation of both lineages in mice. Using human monocyte-derived DCs generated in vitro, we show that immature DCs transdifferentiate into functional osteoclasts (OCs) in the presence of M-CSF and receptor activator of nuclear factor-κB ligand (RANKL). Transdifferentiation operates through fusion of intermediate adherent bipolar fusiform mononuclear cells expressing CD14, CD1a, and RANKL and able to induce RANKL+ T-cell proliferation. Surprisingly, DC fusion in vitro is faster and more efficient than monocyte fusion to form multinucleated giant cells. The transdifferentiation process reported here supports the existence of a high cellular plasticity within differentiated myeloid phagocytes. Importantly, this process is greatly enhanced by rheumatoid arthritis synovial fluid and involves proinflammatory cytokines such as interleukin 1 or tumor necrosis factor α, as well as components of the extracellular matrix such as hyaluronic acid. Our data therefore suggest that DC-derived OCs may be directly involved in the osteolytic lesions observed in human inflammatory bone diseases such as rheumatoid arthritis or in particular forms of Langerhans cell histiocytosis, characterized by accumulation of immature skin DCs and chronic lytic bone lesions. (Blood. 2004;104:4029-4037)
Introduction
Cells of the monocyte-macrophage lineage derive from hematopoietic progenitors. They are able to differentiate into dendritic cells (DCs) or osteoclasts (OCs) depending on the extracellular environment. Both DCs and OCs are characterized by phagocytic activity but they also have markedly distinct morphologic and functional properties.
DCs are specialized mononucleated antigen-presenting cells (APCs), able to both initiate immune responses and to induce tolerance.1 DCs arise from myeloid or lymphoid progenitors. Their functions are determined by their origin as well as by their maturation stage, which depends on the signals received from pathogens and T cells. Myeloid DCs are distributed as sentinels throughout the entire body and classified into different subsets, according to their phenotype and localization. The relationships between those different myeloid DC subtypes are still not clearly understood, but the current paradigm is that most human DCs are derived from blood monocytes or monocytic precursors under the control of granulocyte-macrophage colony-stimulating factor (GM-CSF) associated with either interleukin 4 (IL-4) or IL-13 or tumor necrosis factor α (TNF-α).1-3
In the mouse, it was recently shown that myeloid DCs share a common Flt3+ progenitor with OCs, the specialized multinucleated bone-resorbing cells.4,5 OCs are located in the vicinity of bones and play an essential role in bone development and remodeling and in calcium homeostasis.6,7 They originate from fusion of mononucleated cells belonging to the myeloid lineage in the presence of stromal-derived factors. In humans, OCs can be generated in vitro either from bone marrow common myeloid progenitors8 or from blood monocytes9 under the control of 2 osteoclastogenic cytokines, macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB (NF-κB) ligand (RANKL). The healthy skeleton is an ever-changing organ, whose mass and shape are dictated by a delicate balance between the activity of bone-forming osteoblasts and bone-resorbing OCs.6,7 Most adult skeletal diseases, such as rheumatoid arthritis (RA), Paget disease, and multiple myeloma, are due to excessive formation or excessive activity of these cells, leading to an exacerbated bone resorption and to a phenotype called osteoporosis.
A new emerging field of research, termed osteoimmunology by Arron and Choi,10 deals with the role of the immune system in bone remodeling. In this context, many DC subtypes can be found in the joints of patients with RA and may contribute to the exacerbated osteoclastogenesis.11-13 Santiago-Schwarz et al have recently suggested that the inflamed RA joint controls DC growth and may be a reservoir for DC amplification and the perpetuation of DC-driven inflammatory responses.11 They proposed that DC contribution to osteoclastogenesis is only indirect and linked to their ability to activate naive T cells, which then produce RANKL and stimulate OC differentiation.14,15
Although monocyte/macrophage lineage has hitherto been assumed to differentiate either in DCs or in OCs following 2 separate pathways,4 this report describes how immature human monocyte-derived DCs undergo differentiation into OCs when cultured in vitro in the presence of M-CSF and RANKL. This process, termed transdifferentiation, demonstrates the existence of a high cellular plasticity of the differentiated myeloid phagocytes. Relevant to the physiopathology, transdifferentiation is enhanced by proinflammatory cytokines and especially RA synovial fluid (RASF). These data suggest that immature DCs may be directly involved in osteoclastogenesis and that DC-derived OCs may participate, in vivo, in osteolytic lesions associated with RA or in Langerhans cell histiocytosis, a pathology characterized by the accumulation of immature skin DCs and chronic lytic bone lesions.
Materials and methods
Cell purification, culture, and reagents
Monocytes and T cells were purified16 from the blood of healthy adult volunteer donors (Etablissement français du sang, Lyon Gerland, France). Monocyte-derived DCs were generated in vitro, as previously described.16 Briefly, monocytes were seeded at 106 cells/mL and maintained in RPMI 1640 (Life Technologies, Paisley, United Kingdom) supplemented with 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 2 mM l-glutamine, 40 μg/mL gentamicin (Life Technologies), 10% heat-inactivated fetal calf serum (FCS; Boehringer Mannheim, Meylan, France), 50 ng/mL human recombinant GM-CSF (hrGM-CSF), and 500 U/mL hrIL-4. After 6 days of culture, more than 95% of the cells were immature DCs as assessed by CD1a labeling. Macrophages were derived in vitro from monocytes, plated in 24-well plates at a density of 1600 cells/mm2, and cultured during 5 days in the presence of 50 ng/mL M-CSF. T-cell activation was performed at 106 cells/mL with 1 μg/mL anti-CD3 (HIT3a murine monoclonal antibody [mAb]) and 10 μg/mL anti-CD28 (CD28.2 murine mAb) from PharMingen (San Diego, CA). In the T-cell cocultures, 105/mL monocytes or DCs or 3-day M-CSF plus RANKL-stimulated DCs (intermediate CD14+CD1a+ cells) were cultured together with 106/mL T cells. Recombinant human M-CSF, GM-CSF, RANKL, IL-4, TNF-α, and IL-1α were purchased from PeproTech (Rocky Hill, NJ). IL-1 receptor antagonist (IL1-RA; anakinra) and TNF receptor-Fc (TNFR-Fc; etanercept) were purchased from Amgen (Thousand Oaks, CA). Bovine testes hyaluronidase was purchased from Sigma-Aldrich (H-3506; Saint Quentin Fallavier, France).
Osteoclast differentiation and TRAP assay
Monocytes or DCs were seeded at 1600 cells/mm2 in 24-or 96-well plates in α-minimum essential medium (α-MEM; Life Technologies) supplemented with 10% fetal calf serum (FCS; Boehringer Mannheim, Mannheim, Germany), 2 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Life Technologies) in the presence of 25 ng/mL M-CSF and 100 ng/mL RANKL. Cytokines (M-CSF, RANKL, IL-1α and TNF-α), inhibitors (IL-1RA, TNFR-Fc), or enzyme (hyaluronidase) were added at the beginning of the culture and then replenished every 3 days. When used, RASF was added only at day 0. In the experiments illustrated in Figure 5A-C, a pool of RASF from 8 patients was used; in Figure 5D, RASF from 5 patients was cultured separately. RASF was first centrifuged at 430 g for 15 minutes and filtered on 0.22-μm filters to totally exclude cells (cell free-RASF). Tartrate-resistant acid phosphatase (TRAP) activity in adherent OC cultures was assessed using the leukocyte acid phosphatase kit (Sigma-Aldrich). Culture and TRAP pictures were analyzed using a Leica DMiRB microscope equipped with ×40/0.30 NA or ×40/0.55 NA objective lenses (Leica, Wetzlar, Germany) a Leica DC300F camera and the Leica FW400 software.
Effects of RASFs on DC-derived and monocyte-derived osteoclast formation. (A-B) Counts of TRAP+ MGCs derived from DCs cultured with increasing amounts of RASF alone (A) or in combination with M-CSF and RANKL (B) and their associated dentine resorptions. Mean ± SD from 3 experiments. Percentages of total dentine slice surface resorbed are indicated on each picture. (C) Quantification of CTX release (dentine resorption) in culture supernatants of DC-derived (▪) and monocyte-derived (▦) OCs after 20 days of culture in the presence of M-CSF and RANKL, in combination with increasing amounts of RASF or with OASF control. Means ± SD of 2 triplicate experiments. (D) Quantification of CTX release in culture supernatants of DC-derived OCs after 20 days of culture in the presence of M-CSF and RANKL either alone, with a combination of 20% RASF with or without TNFR-Fc (2 μg/mL) or IL-1RA (2 μg/mL) or both, or with hyaluronidase (10 U/mL and 50 U/mL). Results are means ± SD from triplicate experiments and are expressed as resorption index normalized on M-CSF plus RANKL-induced resorption.
Effects of RASFs on DC-derived and monocyte-derived osteoclast formation. (A-B) Counts of TRAP+ MGCs derived from DCs cultured with increasing amounts of RASF alone (A) or in combination with M-CSF and RANKL (B) and their associated dentine resorptions. Mean ± SD from 3 experiments. Percentages of total dentine slice surface resorbed are indicated on each picture. (C) Quantification of CTX release (dentine resorption) in culture supernatants of DC-derived (▪) and monocyte-derived (▦) OCs after 20 days of culture in the presence of M-CSF and RANKL, in combination with increasing amounts of RASF or with OASF control. Means ± SD of 2 triplicate experiments. (D) Quantification of CTX release in culture supernatants of DC-derived OCs after 20 days of culture in the presence of M-CSF and RANKL either alone, with a combination of 20% RASF with or without TNFR-Fc (2 μg/mL) or IL-1RA (2 μg/mL) or both, or with hyaluronidase (10 U/mL and 50 U/mL). Results are means ± SD from triplicate experiments and are expressed as resorption index normalized on M-CSF plus RANKL-induced resorption.
Bone resorption
To assess resorption activity, cells were cultured on dentine slices (a generous gift from Dr T. Takahashi, Biomedical Research Laboratories, Sankyo, Tokyo, Japan) for 14 days in a 5% CO2 incubator. Following complete cell removal by immersion in water and scraping, dentine slices were stained with toluidine blue to detect resorption pits under a light microscope. Resorbed dentine areas were evaluated using a Leica DMiRB microscope and Analysis software (Olympus, Rungis, France). Results are expressed as resorbed dentine area in percent of total dentine slice surface. Because depth of resorption pits could not be quantified by this technique, the release of C-terminal type I collagen fragments (CTX), consecutive to resorption of dentine slices, was also quantified in the culture supernatants using the CrossLaps enzyme-linked immunosorbent assay (ELISA) kit (Nordic Bioscience Diagnostics, Herlev, Denmark).17
Flow cytometry
Cell suspensions were labeled according to standard procedures using the following mAbs: CD1a-phycoerythrin (PE), CD11b-fluorescein isothiocyanate (FITC), CD11c-FITC, CD14-PE, CD16-FITC, CD18-PE, CD25-PE, CD32-PE, CD36-FITC, CD40-PE, CD80-FITC, CD83-PE, CD86-PE, major histocompatibility complex (MHC) I (HLA-ABC-FITC), MHC II (HLA-DR-FITC), or an isotype control (Beckman Coulter, Villepinte, France). Immunostaining was performed in 1% bovine serum albumin (BSA) and 3% human serum-phosphate-buffered saline (PBS) and then quantified on a FACSCalibur (Becton Dickinson, Pont de Claix, France).
Syngeneic and allogeneic T-cell stimulation
Monocyte-derived DCs were cultured in various numbers (10-105), for 5 to 6 days, in the presence of a constant number of T cells (105 cells/well) purified either from the blood of the same donor (syngeneic) or from the blood of another donor (allogeneic), as previously described.16 [3H]dThd incorporation was measured after a 12-hour pulse with 1 μCi (0.037 MBq) [3H]TdR/well, using a Top Count NXT counter (Packard Bioscience, Perkin Elmer Life Sciences, Courtaboeuf, France).
CFSE/CD14-PE labeling
DCs were suspended at 107 cells/mL in α-MEM containing 2% FCS. After 13 minutes of incubation in the presence of 10 μM carboxyfluorescein diacetate, succinimidyl ester (CFSE), the CFSE incorporation was blocked by the addition of a large excess of α-MEM, containing 2% FCS. DCs were then washed twice by centrifugation at 1500 rpm for 10 minutes at 4°C in α-MEM containing 2% FCS and seeded in α-MEM containing 10% FCS. Cells were then harvested at different time points during their differentiation process into OCs, by a 45-minute trypsin treatment (Sigma-Aldrich) and scraping, and finally immunostained with a CD14-PE antibody as previously described (see “Flow cytometry”). The expression of CD14-PE and CFSE was followed with a FACSCalibur quantification.
Immunofluorescence staining
Cells cultured on glass coverslips were first fixed for 10 minutes with 3.7% formaldehyde in PBS and permeabilized with 0.1% Triton X-100 in PBS for 7 minutes. After preincubation for 20 minutes in normal human serum with 10% PBS, cells were incubated with anti-αvβ3 (23C6 mouse mAb PharMingen) or anticathepsin K (goat polyclonal; Santa Cruz Biotechnology, Santa Cruz, CA) or anti-RANKL (sc-9073 rabbit polyclonal; Santa Cruz Biotechnology) or anti-CD3 (UCHT1 mAb; Beckman Coulter) antibodies. Coverslips were then treated with the appropriate conjugated secondary antibodies (donkey anti-mouse, donkey anti-goat, or donkey anti-rabbit antibodies; Jackson ImmunoResearch, Villepinte, France). Actin was stained by 10 μg/mL rhodamine-labeled phalloidin (Sigma-Aldrich). DNA nuclei were stained by adding 10 μg/mL Hoechst (33342, Sigma) in the last PBS washing buffer. Primary and secondary antibodies were applied for 60 minutes, in a humidified chamber. Between each step, coverslips were washed 3 times for 5 minutes in PBS buffer. Observations were performed by epifluorescence using a Zeiss axioplan microscope (Carl Zeiss, Le Pecq, France).
May-Grünwald-Giemsa staining and cell counts
Cells were fixed for 5 minutes in methanol and stained with May-Grünwald-Giemsa stain. Image analysis was performed using “Analysis” software (Olympus). Counts were made of (1) the total number of nuclei, (2) the number of multinucleated giant cells (MGCs)-included nuclei, and (3) the number of MGCs at the same time in each condition. Cells were considered as MGCs when containing strictly more than 2 nuclei. These 3 counts allowed the calculation of the number of mononucleated cells (total number of nuclei - number of MGC-included nuclei), the mean number of nuclei per MGCs (number of MGCs-included nuclei/number of MGCs at the same time in each condition), and the percentage of MGC-included nuclei (number of MGC-included nuclei/total number of nuclei × 100).
Results
M-CSF and RANKL induce the transdifferentiation of immature DCs into OCs
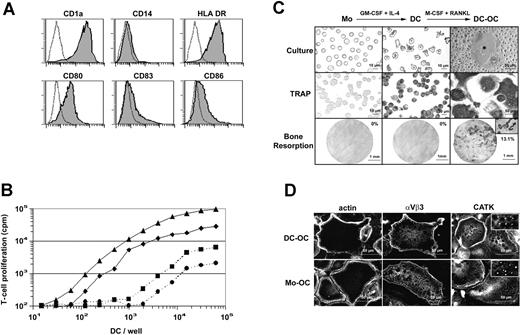
Nonadherent DCs were generated by culturing purified human blood monocytes in the presence of GM-CSF and IL-4 for 6 days.18 These cells were classically CD1a+ and CD14- and their immature phenotype was shown by their intermediate expression level of MHC class II molecules HLA-DR and their low or negative expression of CD83 and of the costimulatory molecules CD80 and CD86 (Figure 1A). Immature monocyte-derived DCs displayed a typical dendritic morphology and elicited greatly enhanced allogeneic T-cell responses compared with syngeneic stimulation (Figure 1B). Surprisingly, and contrary to purified human blood monocytes,19 monocyte-derived DCs expressed TRAP activity, an enzymatic marker commonly used by bone specialists to identify OCs or OC precursors. A combination of M-CSF and RANKL was then applied to these immature DCs. After 12 days of treatment, MGCs exhibiting intense TRAP activity were formed (Figure 1C). Whereas monocytes and DCs did not resorb dentine, DC-derived MGCs formed typical resorption pits and tracks on dentine slices (13.1% of surface) indicating that they were functional mobile OCs. After differentiation, DC-derived OCs neither expressed the DC marker CD1a nor the monocytic marker CD14 (Figure 2C). To date, OCs were classically derived from monocytes. The intracellular distributions of actin, of the integrin αvβ3, and of the cysteine protease cathepsin K (CATK), 3 characteristic markers of OCs, were then compared, using immunofluorescence staining in the DC-derived OCs and in monocyte-derived OCs (Figure 1D). DC-derived OCs exhibited characteristic osteoclastic belts of actin podosomes, identical to those observed in monocyte-derived OCs. The αvβ3 integrin delineated the cell periphery and colocalized with the belt of podosomes in OCs. At higher magnification (not shown), αvβ3 was found in rosettelike structures surrounding the actin core of podosomes as previously described.20,21 CATK was detected in small intracytoplasmic granules of both DC-derived and monocyte-derived OCs. This punctiform labeling (Figure 1D inserts), already described for human OCs from giant cell tumors,22 may reflect the processing of pro-CATK in CATK, which occurs in the lysosomal compartment.23 In addition, both monocyte-derived OCs and DC-derived OCs were CD1a-, CD14-, αvβ5-, CD11c+ (data not shown). In conclusion, M-CSF and RANKL induce in vitro transdifferentiation of human immature DCs into OCs that express the same morphology, phenotype, and function as monocyte-derived OCs. However, we were unable to transdifferentiate lipopolysaccharide (LPS)-stimulated or CD40-activated mature DCs into OCs (data not shown).
M-CSF and RANKL induce transdifferentiation of immature DCs into OCs. (A) Surface phenotype of human immature monocyte-derived DCs. Dotted lines represent isotypic control. (B) Allostimulatory and synstimulatory properties of immature monocyte-derived DCs cultured for 12 hours in the presence of T cells purified from 2 allogeneic donors (bold lines, ▴ or ♦) or 2 syngeneic donors (dashed lines, • or ▪), respectively. (C) Monocytes (Mo), dendritic cells (DC), and DC-derived OC (DC-OC) are shown in culture (top row) and after TRAP activity detection (middle row). Microscopic pictures of dentine slices show resorptive capacities of these 3 cell types (bottom row). Percentages of total dentine slice surface resorbed are indicated on each picture in the bottom row. The insert shows the resorption tracks at higher magnification (×320). (D) F-actin, αvβ3 integrin and cathepsin K staining on permeabilized DCs-OCs versus monocyte-derived OCs (Mo-OC). Results are from more than 10 experiments.
M-CSF and RANKL induce transdifferentiation of immature DCs into OCs. (A) Surface phenotype of human immature monocyte-derived DCs. Dotted lines represent isotypic control. (B) Allostimulatory and synstimulatory properties of immature monocyte-derived DCs cultured for 12 hours in the presence of T cells purified from 2 allogeneic donors (bold lines, ▴ or ♦) or 2 syngeneic donors (dashed lines, • or ▪), respectively. (C) Monocytes (Mo), dendritic cells (DC), and DC-derived OC (DC-OC) are shown in culture (top row) and after TRAP activity detection (middle row). Microscopic pictures of dentine slices show resorptive capacities of these 3 cell types (bottom row). Percentages of total dentine slice surface resorbed are indicated on each picture in the bottom row. The insert shows the resorption tracks at higher magnification (×320). (D) F-actin, αvβ3 integrin and cathepsin K staining on permeabilized DCs-OCs versus monocyte-derived OCs (Mo-OC). Results are from more than 10 experiments.
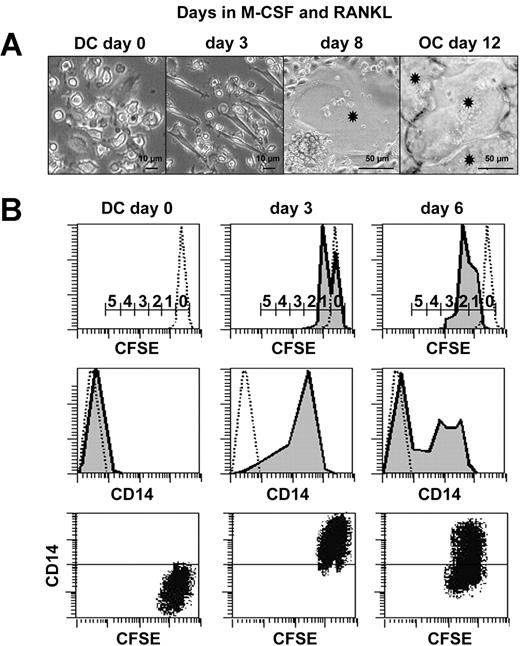
Characterization of intermediate CD14+CD1a+-proliferating cells during transdifferentiation. (A) Kinetic microscopic photographs of cultures during DC to OC transdifferentiation. Asterisks indicate OCs. (B) Analysis of cell proliferation during DC-to-OC transdifferentiation by kinetic FACS analyses performed on CFSE-labeled DCs cultured in the presence of M-CSF and RANKL. Numbers on scale bars indicate numbers of cell divisions (top row). Concomitant surface CD14 expression on dotted lines of isotypic controls (middle row). Dot plot shows simultaneous CD14 and CFSE fluorescence during transdifferentiation (bottom row). Negative control on CD14 axis is positioned by horizontal line. Results are representative of 3 independent experiments.
Characterization of intermediate CD14+CD1a+-proliferating cells during transdifferentiation. (A) Kinetic microscopic photographs of cultures during DC to OC transdifferentiation. Asterisks indicate OCs. (B) Analysis of cell proliferation during DC-to-OC transdifferentiation by kinetic FACS analyses performed on CFSE-labeled DCs cultured in the presence of M-CSF and RANKL. Numbers on scale bars indicate numbers of cell divisions (top row). Concomitant surface CD14 expression on dotted lines of isotypic controls (middle row). Dot plot shows simultaneous CD14 and CFSE fluorescence during transdifferentiation (bottom row). Negative control on CD14 axis is positioned by horizontal line. Results are representative of 3 independent experiments.
Characterization of an intermediate CD14+-proliferating stage during transdifferentiation of immature DCs
Three cell morphologies could be distinguished during transdifferentiation of immature DCs to OCs (Figure 2A). Initially, DCs (day 0) rapidly attached to the polystyrene and acquired a bipolar fusiform morphology (day 3). The first fusion events (first MGCs containing more than 2 nuclei) could be detected at day 8. Finally, a nearly confluent mono-layer of strongly adherent OCs could be observed (day 12). To determine whether this transdifferentiation process was accompanied by cell divisions or apoptosis, in vitro monocyte-derived DCs were loaded with CFSE and seeded in the presence of M-CSF and RANKL. CFSE is a green fluorescent molecule that labels all cellular proteins and enables an estimation of the number of cell divisions. Indeed, during the culture, each division was accompanied by a 50% decrease in mean intensity of green fluorescence, a consequence of CFSE- protein neosynthesis. Mononucleated cells were harvested at day 0, 3, and 6 and labeled with anti-CD14 antibodies. CFSE/CD14 double-staining revealed that cells acquired CD14 expression and entered into the cell cycle between days 0 and 3 (Figure 2B). The majority (61%) of the mononucleated cells underwent 2 divisions between days 0 and 6 (data not shown). CD14 expression was maintained during cell divisions and then was down-regulated. DNA fragmentation, quantified by ELISA, revealed that in situ apoptosis was less than 10% (data not shown). To our knowledge, this is the first time that human DCs have been shown to enter the cell cycle and fuse together in the absence of virus infection.
To further characterize these intermediate CD14+-proliferating cells, extensive phenotypic analyses were performed by flow cytometry (Table 1). After 3 days in the presence of M-CSF and RANKL, these cells accumulated DC-specific markers (CD1a+, CD80+, CD86+, HLA-DRhigh), monocyte/macrophage-specific markers (CD11b+, CD14+, CD16+, CD18low, CD32+), and markers shared by DCs, monocytes, and macrophages (CD11c+, CD36+, CD40+, and HLA-ABC+). After cell division and concomitantly with cell fusion, monocyte/macrophage and DC markers were down-regulated. Such adherent cells were also observed during differentiation of monocyte-derived OCs, but they did not express CD1a. Thus, transdifferentiation of DCs to OCs may be divided into 2 steps: (1) before day 6, cells proliferate and give rise to an intermediate stage, exhibiting a bipolar fusiform morphology with a biphenotypic macrophage-DC phenotype; and (2) from day 6 to day 12, macrophage and DC markers are down-regulated, cell division stops, and cell fusion occurs to differentiate MGCs, which have the capacity to resorb dentine.
Phenotypes of cells
. | Monocytes . | Macrophages . | Immature DCs . | Intermediate CD14+ CD1a+ cells . | OCs . |
|---|---|---|---|---|---|
| CD1a | − | − | ++ | + | − |
| CD80 | − | + | + | + | − |
| CD86 | + | + | + | + | + |
| HLA DR | + | + | ++ | + | + |
| CD11b | + | + | + | + | + |
| CD14 | + | + | − | + | − |
| CD16 | +/− | + | − | + | − |
| CD18 | + | + | + | + | + |
| CD32 | + | + | +/− | + | +/− |
| CD11c | + | + | + | + | + |
| CD34 | − | − | − | − | − |
| CD36 | + | + | + | + | +/− |
| CD40 | + | + | ++ | + | + |
| HLA ABC | ++ | + | ++ | ++ | ++ |
. | Monocytes . | Macrophages . | Immature DCs . | Intermediate CD14+ CD1a+ cells . | OCs . |
|---|---|---|---|---|---|
| CD1a | − | − | ++ | + | − |
| CD80 | − | + | + | + | − |
| CD86 | + | + | + | + | + |
| HLA DR | + | + | ++ | + | + |
| CD11b | + | + | + | + | + |
| CD14 | + | + | − | + | − |
| CD16 | +/− | + | − | + | − |
| CD18 | + | + | + | + | + |
| CD32 | + | + | +/− | + | +/− |
| CD11c | + | + | + | + | + |
| CD34 | − | − | − | − | − |
| CD36 | + | + | + | + | +/− |
| CD40 | + | + | ++ | + | + |
| HLA ABC | ++ | + | ++ | ++ | ++ |
Table summarizes the phenotypes of intermediate CD14+ CD1a+-proliferating cells compared with those of purified monocytes, monocyte-derived macrophages (macrophages), monocyte-derived DCs (immature DCs), and DC-derived OCs (OCs). Arbitrary units: −, absent; +, positive low; ++, positive high; +/−, 2 subpopulations positive low and negative. Results from at least 3 experiments.
Immature DCs are more potent for cell fusion than monocytes
To compare the efficiency of MGC differentiation from either monocytes or DCs, cells and their nuclei were precisely quantified. Immature DCs or monocytes were cultured in the presence of M-CSF plus RANKL. At days 0, 8, 12, and 15, cultures were fixed and stained with May-Grünwald-Giemsa and then MGCs (=3 nuclei), mononucleated cells, and nuclei were counted. Formation of MGCs was faster in DC-derived than in monocyte-derived cultures (Figure 3A). Indeed, MGC numbers had already reached 2000/well by day 8 in DC-derived cultures, whereas this occurred around day 15 in monocyte-derived cultures. MGCs were bigger in DC-derived than in monocyte-derived cultures as demonstrated by the mean number of nuclei in MGCs: 7 and 4 nuclei/MGC (Figure 3B), respectively. Consecutively, at day 8, 40% to 50% of nuclei were included in MGCs in DC-derived cultures, whereas less than 10% of the cells were multinucleated in monocyte-derived cultures (Figure 3C). Thus, DC-derived MGC formation is faster and more efficient in terms of fusion than monocyte-derived MGC formation.
Comparative kinetic studies of DC-derived osteoclast and monocyte-derived osteoclast formation. (A) Kinetic studies of monocytes or DCs cultured in the presence of M-CSF and RANKL. The number of mononucleated cells (bold lines and ▴, read on left y-axis) and the number of DC-derived MGCs and monocyte-derived MGCs (dashed lines and ▪, read on right y-axis) were counted. (B) Estimate of the average number of nuclei in DC-derived MGCs (▪) versus monocyte-derived MGCs (▦). (C) Percentage of nuclei included in DC-derived MGCs (▪) or monocyte-derived MGCs (▦) in comparison with the total number of nuclei in the culture. Results are means ± SD of 3 independent experiments.
Comparative kinetic studies of DC-derived osteoclast and monocyte-derived osteoclast formation. (A) Kinetic studies of monocytes or DCs cultured in the presence of M-CSF and RANKL. The number of mononucleated cells (bold lines and ▴, read on left y-axis) and the number of DC-derived MGCs and monocyte-derived MGCs (dashed lines and ▪, read on right y-axis) were counted. (B) Estimate of the average number of nuclei in DC-derived MGCs (▪) versus monocyte-derived MGCs (▦). (C) Percentage of nuclei included in DC-derived MGCs (▪) or monocyte-derived MGCs (▦) in comparison with the total number of nuclei in the culture. Results are means ± SD of 3 independent experiments.
Proinflammatory cytokines enhance DC-derived OC formation
Because proinflammatory cytokines such as TNF-α and IL-1α modulate osteoclastogenesis,24 we studied the impact of these cytokines on transdifferentiation of immature DCs to OCs. DCs were cultured in the presence of M-CSF and RANKL plus TNF-α or IL-1α. MGCs were counted following May-Grünwald-Giemsa staining. Kinetic studies, performed at days 6, 9, and 13, showed that TNF-α as well as IL-1α enhanced transdifferentiation (Figure 4A). Addition of IL-1α or TNF-α to M-CSF plus RANKL induced earlier fusion, as demonstrated by 2.4 and 3.8 increases in MGC number at day 6, respectively. Potentiation of MGC formation was dependent on dose (data not shown) and maximal at 20 ng/mL. At day 6, the average number of nuclei per MGC was also increased, from 4 to 10 in the presence of TNF-α or to 20 in the presence of IL-1α (Figure 4B). Furthermore, IL-1α was a potent activator of dentine resorption (Figure 4C), as previously described,24 because ranges of bone resorption for M-CSF plus RANKL versus M-CSF plus RANKL plus TNF-α or M-CSF plus RANKL plus IL-1α were 8% to 15% versus 10% to 14% or 25% to 29%, respectively (not shown). TNF-α or IL-1α did not enhance bone resorption performed by pure human monocytes cultured with M-CSF and RANKL (data not shown). Thus, TNF-α and IL-1α promote cell fusion during DC transdifferentiation and lead to larger MGCs than M-CSF plus RANKL alone. However, only IL-1α increases dentine resorption.
Effects of proinflammatory cytokines on DC-derived osteoclast formation. (A) Counts of TRAP+ DC-derived MGCs in different culture conditions. DCs treated with M-CSF and RANKL alone as control (□) or in combination with TNF-α (▦) or IL-1α (▪). Means ± SD of 3 experiments. (B) MGCs derived from DCs, after 13 days in the same culture conditions (original magnification × 100). (C) Dentine resorption by DC-derived MGCs obtained in the same culture conditions. Percentages of total dentine slice surface resorbed are indicated on each photograph.
Effects of proinflammatory cytokines on DC-derived osteoclast formation. (A) Counts of TRAP+ DC-derived MGCs in different culture conditions. DCs treated with M-CSF and RANKL alone as control (□) or in combination with TNF-α (▦) or IL-1α (▪). Means ± SD of 3 experiments. (B) MGCs derived from DCs, after 13 days in the same culture conditions (original magnification × 100). (C) Dentine resorption by DC-derived MGCs obtained in the same culture conditions. Percentages of total dentine slice surface resorbed are indicated on each photograph.
Cell-free RASF increases bone resorption by DC-derived OCs
Because OCs have a crucial role in the pathology of RA, the influence of cell-free RASF on DC-derived OC formation was examined. Immature DCs were grown in the presence of different amounts of cell-free RASF, with or without M-CSF and RANKL. After 12 days, the number of MGCs per well and dentine resorption were assessed. Strikingly, in the absence of M-CSF plus RANKL, cell-free RASF alone was already able to transdifferentiate immature DCs into MGCs, in a dose-dependent manner (Figure 5A). Although these MGCs were TRAP+ (data not shown), they were unable to resorb dentine (Figure 5A). In the presence of M-CSF plus RANKL, the addition of cell-free RASF had no effect on MGC number, but greatly enhanced dentine resorption (Figure 5B); dentine resorption induced by M-CSF and RANKL was increased 4.6- and 6.7-fold by 2% and 5% RASF, respectively. To evaluate the reciprocal contributions of DC-derived OCs and monocyte-derived OCs in RA, monocytes and immature DCs from the same blood donors were grown on dentine slices with M-CSF and RANKL, in the presence of control osteoarthrosis synovial fluid (OASF) or different amounts of RASF. The release of CTX subsequent to resorption of dentine slices was quantified after 20 days of resorption. Cell-free RASF dose dependently increased dentine resorption by DC-derived OCs (Figure 5C) but had no effect on monocyte-derived OC resorption. Cell-free OASF, used as noninflammatory joint disease control, neither modified DC-derived OC resorption nor monocyte-derived OC resorption. Thus, cell-free RASF stimulates transdifferentiation of immature DCs, but not monocytes, into nonresorbing MGCs and acts synergistically with M-CSF or RANKL (or both) to activate their resorptive function.
RASF-induced resorption enhancement depends on IL-1α, TNF-α, and other uncharacterized soluble factors
To verify the putative contribution of IL-1α or TNF-α to RASF effects, immature DCs were cultured on dentine slices with M-CSF, RANKL, and 20% of RASF from 5 different patients. TNFR-Fc or IL-1RA or both were added to inhibit TNF-α or IL-1α or both, respectively. The release of CTX was quantified after 20 days of resorption. Among these patients, 4 were undergoing an anti-TNF or an anti-IL-1 treatment, and the fifth was not undergoing any treatment. TNR-Fc or IL-1RA inhibited DC-derived OC dentine resorption induced by the RASF from the untreated patient and their effects were additive (Figure 5D). In contrast, TNFR-Fc and IL-1RA had no or very low effects on DC-derived OC dentine resorption induced by the RASF from the 4 patients undergoing treatment (data not shown). To test whether other components of the joint fluid could play a role in modulating the response of DCs to M-CSF and RANKL, transdifferentiation cultures were performed in the presence of hyaluronidase, which cleaves glycosidic bonds in hyaluronic acid, chondroitin, and chondroitin sulfates. Addition of hyaluronidase dose-dependently increased bone resorption (Figure 5D). Thus, the enhancement of resorption induced by RASF depends on TNF-α, IL-1α, and on other unknown soluble factors, which may include fragments of hyaluronic acid or proteoglycans.
DCs and intermediate CD14+CD1a+ cells induce T-cell proliferation and RANKL expression by T cells
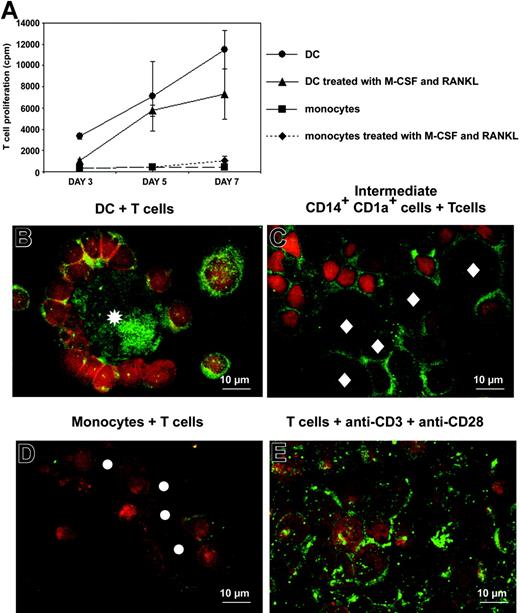
Proliferation of allogeneic T cells cultured with monocytes or immature DCs that have been either untreated or treated for 3 days with M-CSF plus RANKL was quantified by thymidine incorporation. A total of 103 APCs/well were cocultured with 105 T cells/well. Phenotypes of these APCs are shown in Figure 2C. After 3 days of culture with M-CSF plus RANKL, monocytes exhibit pure macrophage phenotype. DCs and, to a lesser extent, intermediate CD14+CD1a+-proliferating cells stimulated allogeneic T-cell proliferation, contrary to monocytes and M-CSF/RANKL-treated monocytes (Figure 6A). Thus, after 3 days of treatment with M-CSF/RANKL, intermediate cells between DCs and OCs and intermediate cells between monocytes and OCs were different in their phenotypes (CD14+CD1a+ versus CD14+CD1a-, respectively) and in their functions (sustained allogeneic T-cell proliferation or not, respectively).
DCs and intermediate CD14+CD1a+ cells induce proliferation and RANKL expression by T cells. (A) Allostimulatory properties of monocyte-derived DCs (•), intermediate CD14+CD1a+ cells (immature DCs treated for 3 days with M-CSF and RANKL; ▴), purified monocytes (▪), and purified monocytes treated for 3 days with M-CSF and RANKL (♦), cultured with allogeneic T cells purified from 3 different donors. A total of 103 APCs were cocultured with 105 T cells. (B-E) RANKL (green) and CD3 (red) stainings of T cells, after 5 days of culture with (B) immature DCs, (C) intermediate CD14+CD1a+ cells, (D) monocytes, and (E) anti-CD3 plus anti-CD28 antibodies. Asterisks indicate immature DCs, diamonds indicate intermediate CD14+CD1a+ cells, and circles indicate monocytes. T cells were distinguished from other cells based on CD3+ expression and Hoechst nuclei staining (not shown).
DCs and intermediate CD14+CD1a+ cells induce proliferation and RANKL expression by T cells. (A) Allostimulatory properties of monocyte-derived DCs (•), intermediate CD14+CD1a+ cells (immature DCs treated for 3 days with M-CSF and RANKL; ▴), purified monocytes (▪), and purified monocytes treated for 3 days with M-CSF and RANKL (♦), cultured with allogeneic T cells purified from 3 different donors. A total of 103 APCs were cocultured with 105 T cells. (B-E) RANKL (green) and CD3 (red) stainings of T cells, after 5 days of culture with (B) immature DCs, (C) intermediate CD14+CD1a+ cells, (D) monocytes, and (E) anti-CD3 plus anti-CD28 antibodies. Asterisks indicate immature DCs, diamonds indicate intermediate CD14+CD1a+ cells, and circles indicate monocytes. T cells were distinguished from other cells based on CD3+ expression and Hoechst nuclei staining (not shown).
RANKL expression has been reported on activated T cells from RA synovial tissues and also on T cells activated by anti-CD3 plus anti-CD28 antibodies (Figure 6E).15,25 Immunofluorescent staining was used to study the expression of RANKL on T cells cocultured with different APCs: DCs, intermediate CD14+CD1a+ cells, or monocytes. T cells cocultured for 5 days with DCs or with intermediate CD14+CD1a+ cells strongly expressed RANKL on their surface (Figure 6B-C). DCs also displayed surface and intracellular RANKL expression. Intermediate CD14+CD1a+ cells expressed RANKL only at their cell surface. In contrast, T cells cocultured with monocytes weakly expressed RANKL and monocytes did not express RANKL themselves (Figure 6D). These results suggest that in RASF, RANKL may be produced not only by activated T cells but also by DCs. They also further support a crucial role for the DC/T-cell couple in bone resorption associated with RA.
Discussion
In this report, we demonstrate that human immature monocyte-derived DCs transdifferentiate into OCs in the presence of M-CSF and RANKL, in vitro. To our surprise, DC-derived OC formation is faster and more efficient in terms of fusion rate than the monocyte-derived OC formation pathway classically described.26 This transdifferentiation process generates intermediate CD14+CD1a+ cells, which proliferate moderately. Their fusion gives rise to OCs able to acquire a resorptive function on dentine slices. In addition, we show that the inflammatory environment provided by cell-free RASF, and notably by IL-1 or TNF-α as well as by extracellular matrix components, strongly increases dentine resorption by DC-derived OCs, but not by monocyte-derived OCs.
Transdifferentiation, evidence for high plasticity of myelomonocytic cells
Culture of monocytes in the presence of GM-CSF and IL-4 has become the essential methodologic approach for DC production to be used in DC-mediated cancer immunotherapy protocols, but the physiologic counterparts of in vitro monocyte-derived DCs is still a matter of debate in humans. They may correspond to resident and inflammatory-induced interstitial DCs27 or resident lymphoid organ DCs.28 Monocytes belong to the myeloid lineage and represent essential pivotal precursors, distributed in large amounts through the whole body, via the bloodstream. They are known to be the precursors of many cell types inside and outside of the myeloid system, such as macrophages, DCs, OCs, vascular endothelial cells, epithelial cells, T lymphocytes, neuronal cells, and liver cells.9,18,29 GM-CSF and M-CSF reciprocally regulate the differentiation of DC and OC pathways from monocytes, respectively. c-Fos is a key transcription factor in the lineage commitment between DCs and OCs, favoring OCs when it is expressed.4 These studies assumed that DC and OC development proceeds from monocyte/macrophage precursor cells and may be exclusive.4 Monocytes cultured with M-CSF plus RANKL differentiate into OCs via an intermediate stage that corresponds to macrophages. It is also well-known that DCs have the ability to convert into macrophages, either spontaneously in culture in the absence of cytokines or in response to M-CSF (C.S.D., unpublished results, 1999, and Palucka et al30 ). Our data show that rather than exclusive pathways of differentiation, cellular plasticity seems to be the law within the myeloid lineage. Indeed, as shown in this report, immature DCs have the ability to differentiate into OCs. Thus, like macrophages, DCs may represent a cell type further down than monocytes in the OC differentiation pathway. DCs may express unknown transcription factors that favor OC differentiation. These potential transcription factors would be different from c-Fos and not expressed in monocytes. This will be a very interesting point for further research. Thus, it appears that the myeloid lineage exhibits a high cellular plasticity either through differentiation processes from precursors cells such as monocytes or through transdifferentiation processes from differentiated cells such as immature DCs. However, regarding the weak number of resident DCs in steady state, we propose that the transdifferentiation process may occur at a very low level in healthy individuals and may become predominant in chronic inflammatory states, when monocytes are recruited to inflamed tissues and neodifferentiate into DCs.
RA and transdifferentiation
RA is a chronic inflammatory bone disease characterized by the progressive destruction of articular cartilage and bone at all sites of inflammation.31 OCs are supposed to be the key mediators of all forms of bone loss in RA, but it is unclear whether the increased bone resorption is due to an increased number of newly formed OCs or to an increased activity of preexisting OCs. The inflammatory lesions of joints in patients with RA are characterized by blood-derived cell infiltrates in the synovial membrane, particularly lymphocytes and cells of the monocyte/macrophage lineage and by synovial fibroblast proliferation. Some studies have also reported that at least 2 DC subpopulations are represented in the joint, both in the RASF and in the RA synovial tissue.11,13 In the synovial tissue, fully differentiated perivascular DCs are found tightly associated to T cells and to B-cell follicles. These DCs are supposed to migrate from the blood and may play 2 essential roles in the pathology of RA. First, they may drive an autoimmune response by presenting unknown self-antigens to autoreactive T cells,13 which would then release proteolytic enzymes that degrade connective tissues. Second, they may induce RANKL expression by activated T cells, thus indirectly inducing OC differentiation.32 Another DC subpopulation has been identified within the joint, located in the RASF, but their relationships with perivascular DCs are unknown. However, these DCs exhibit an immature phenotype, similar to that of monocyte-derived DCs in vitro.13 Our present results demonstrate that when immature DCs are cultured in the presence of different amounts of cell-free RASF in combination with M-CSF and RANKL, they do not mature, but transdifferentiate into bone-resorbing OCs. This synergy could result from the enhancement of osteoclast number or activity or both. In contrast, RASF does not potentiate bone resorption by monocyte-derived OCs. Furthermore, DCs treated with M-CSF and RANKL for 3 days, contrary to monocytes, expressed RANKL and sustained proliferation of the RANKL+ T cells they had activated. Altogether, these data further support the crucial role of the DC/T-cell couple in bone resorption associated with RA. Therefore, the chronic inflammatory environment of RA may both induce a failure in DC maturation and migration and their transdifferentiation into bone-resorbing OCs. Thus, DCs may play a direct role in the pathogenesis of RA in vivo.
Potentiation of resorption by cell-free RASF
RASF is a true “cytokine soup” and several cytokines have been found in abnormal amounts in this fluid: RANKL, TNF-α, IL-1α, transforming growth factor β (TGF-β), GM-CSF, IL-18, and IL-6.11,31,33-35 We have demonstrated that TNF-α and IL-1 increase DC-derived OC formation and activate their function, respectively. In vivo, TNF-α induces IL-1 release by synovial fibroblasts and macrophages. Moreover, in the presence of permissive levels of RANKL, TNF-α is a potent stimulator of OC differentiation, whereas IL-1 is a major survival and activation signal for nascent OCs.24,31 The effect of RASF from an untreated patient was blocked with TNF-α and IL-1 inhibitors. Consequently, TNF-α and IL-1, acting in concert with RANKL, can powerfully promote OC recruitment, activation, and osteolysis in RA. However, other cytokines such as TGF-β are probably involved in RASF-mediated resorption.36-38 The inefficiency of anti-TNF-α and anti-IL-1 treatment in certain patients supports this conclusion. As well as cytokines, DC transdifferentiation was modulated by other components of the joint fluid. Using hyaluronidase, we showed that degradation of proteoglycans strongly enhances DC transdifferentiation into OCs, leading to exacerbated dentine resorption. Proteolytic degradation of glycosaminoglycans (GAGs) and GAG-rich proteoglycans may lead to the release of the cytokines they trap in the extracellular matrix and may enhance cytokine receptor accessibility at the cell surface. Finally, soluble fragments of hyaluronic acid (sHA) have been shown to increase the production of IL-1 and TNF-α by human monocyte-derived DCs.39 These effects are highly specific for sHA because they are not induced by other GAGs such as chondroitin sulfate or heparan sulfate or their fragmentation products. These observations may explain why the delicate balance between the whole HA molecule and sHA is highly correlated with the evolution of RA disease. It would be very interesting to exhaustively study the modulation of DC transdifferentiation by proteoglycans and their derivatives.
RASF alone induces DC fusion, but addition of recombinant RANKL is required to induce the terminal differentiation of these MGCs into functional bone-resorbing OCs. In response to M-CSF and RANKL, DCs are able to synthesize RANKL. DCs also induce RANKL expression on T cells they activate. In the context of RA, we propose that the immune system may contribute to bone resorption, in 2 different ways: directly through transdifferentiation of immune cells (DCs or monocytes) into OCs and indirectly through the secretion of proinflammatory cytokines, such as TNF-α or IL-1. These cytokines then stimulate RANKL synthesis by various cell types, including DCs and activated T cells, and would be responsible for the installation of an autocrine/paracrine loop of RANK-RANKL, which could sustain OC formation.
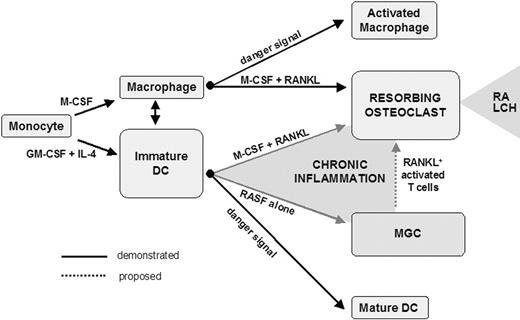
In conclusion, the present report provides further evidence for the high cellular plasticity potential of differentiated myeloid phagocytes. In healthy individuals, monocytes give rise to mononucleated phagocytes, either macrophages or DCs. Palucka et al demonstrated that these cell types have the ability to convert into each other.30 Macrophages are also considered to be a reservoir of OC precursors in normal physiologic conditions. Consequently, the exacerbated bone resorption associated with chronic inflammation has been considered, until now, to be the result of an enhancement of constitutive osteoclastogenesis from bone marrow monocyte/macrophage precursors. We suggest that it also results from transdifferentiation of DCs or activation of DC-derived MGCs by RANKL+ activated T cells in the bone microenvironment (Figure 7). The chronic inflammatory context of RA may exploit the cellular plasticity of DCs and skew their final fate. This may also be the case in Langerhans cell histiocytosis, another chronic inflammatory pathology due to accumulation of proliferative immature DCs associated with chronic lytic bone lesions in 50% to 80% of patients.40 DC-derived OCs may thus represent key therapeutic targets in inflammation-induced bone resorption, raising the question of the in vivo fate of injected DCs during immunotherapy protocols.
Skewing of differentiated myeloid phagocyte plasticity by chronic inflammatory bone microenvironment. In the chronic inflammatory bone microenvironment, OCs derived from either DCs or DC-derived MGCs may participate in the formation of osteolytic lesions in response to RANKL provided by activated T cells. LCH indicates Langerhans cell histiocytosis.
Skewing of differentiated myeloid phagocyte plasticity by chronic inflammatory bone microenvironment. In the chronic inflammatory bone microenvironment, OCs derived from either DCs or DC-derived MGCs may participate in the formation of osteolytic lesions in response to RANKL provided by activated T cells. LCH indicates Langerhans cell histiocytosis.
Prepublished online as Blood First Edition Paper, August 12, 2004; DOI 10.1182/blood-2004-01-0041.
Supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Université Claude Bernard Lyon I, the Centre National de la Recherche Scientifique, the emergence project of Rhone-Alpes Region, the Association pour la Recherche sur le Cancer (ARC 4800), the Ministère de l'Education Nationale et de la Recherche Technologique (Action Concertée d'Initiative, ACI8BC05H), and the Association de Recherche sur la Polyarthrite.
A.R. and M.M. contributed equally to the work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Drs R. Buckland, Y. Zaffran, and K. Veitch for critical review of the manuscript and C. Bella for technical assistance on IFR128 flow cytometry platform.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal