Adult and embryonic stem cells hold great promise for regenerative medicine. Expression profiling of stem cells revealed a characteristic imprint of genes, so-called “stemness” genes, providing resistance to stress. Circulating progenitor cells with an endothelial phenotype (EPCs) can be isolated from peripheral blood and contribute to neovascularization and endothelial regeneration. We investigated whether EPCs are equipped with an antioxidative defense to provide resistance against oxidative stress. EPCs exhibited a significantly lower basal reactive oxygen species (ROS) concentration as compared with mature umbilical vein endothelial cells (HUVECs). Incubation with H2O2 (500 μM) or the redox cycler LY-83583 (10 μM) profoundly increased the ROS concentration to 3- and 4-fold and induced apoptosis in HUVECs. In contrast, H2O2 and LY-83583 induced only a minor increase in intracellular ROS levels and apoptosis in EPCs. Consistently, the expression of the intracellular antioxidative enzymes catalase, glutathione peroxidase and manganese superoxide dismutase (MnSOD), was significantly higher in EPCs versus HUVECs and human microvascular endothelial cells. In accordance, combined inhibition of these antioxidative enzymes increased ROS levels in EPCs and impaired EPC survival and migration. Taken together, EPCs reveal a higher expression of antioxidative enzymes and, thus, are exquisitely equipped to be protected against oxidative stress consistent with their progenitor cell character.
Introduction
Stem cells have self-renewing capabilities and the capacity to differentiate into multiple cell lineages.1,2 Therefore, stem cells hold great promise for tissue repair and regenerative medicine.3 In contrast to embryonic stem cells, which are derived from mammalian embryos in the blastocyst stage and which have the ability to generate any terminally differentiated cell in the body, adult stem cells are part of the tissue-specific cells of the postnatal organism.4 Expression profiling of embryonic and adult stem cells revealed a characteristic expression of genes in stem cells, which provide resistance to stress with up-regulated DNA repair and detoxifier systems.5,6 These studies demonstrated that there is a subset of genes that is enriched in all 3 (hematopoietic, neural, and embryonic) stem cell lines. The enriched genes provide core stem cell properties (“stemness”) that underlie self-renewal and differentiation. It seems that at least some of these genes provide resistance against environmental stress.6
Vascular diseases, including hypertension, diabetes, and atherosclerosis, are characterized by impaired endothelium-derived nitric oxide (NO) bioactivity and elevated levels of reactive oxygen species (ROS).7,8 ROSs, including superoxide anions (
Stem cells, in particular the hematopoietic stem cells, can differentiate in several lineages, including the endothelial cell lines.4 Endothelial progenitor cells are the precursors of mature endothelial cells and were initially defined by the expression of CD34 or the more immature marker protein CD133 and the endothelial marker proteins such as vascular endothelial growth factor (VEGF) receptor 2 (KDR), von Willebrand factor (VWF), VE-cadherin, take-up of diacetylated low-density lipoprotein (LDL), and lectin binding.14,15 Endothelial progenitor cells can also be ex vivo–expanded out of isolated peripheral blood mononuclear cells (PBMCs) or obtained by cultivation of CD34+ or CD133+ hematopoietic progenitors.14-21
The endothelial progenitor cells (EPCs) used in the present study are ex vivo–expanded on fibronectin-coated dishes in the presence of VEGF and endothelial medium.15,17,22 EPCs were shown to exhibit an endothelial phenotype as demonstrated by the expression of KDR, endothelial nitric oxide synthase (eNOS), VWF, and VE-cadherin, and the response to shear stress.15,17,22 The progenitor cell characteristics of these EPCs is evidenced by their ability to generate differentiated progeny and their capacity to form endothelial colonies.18,23,24 Moreover, ex vivo–expanded EPCs were shown to integrate into blood vessels and improve neovascularization of ischemic tissue 16,17,21,25,26
In this study, we investigated the sensitivity of EPCs and mature endothelial cells toward oxidative stress and the differences in expression profile of oxidative stress–related genes.
Material and methods
Cell culture
Pooled human umbilical vein endothelial cells (HUVECs) were purchased from CellSystems (St Katharinen, Germany) and cultured in endothelial basal medium (EBM; CellSystems) supplemented with hydrocortisone (one μg/mL), bovine brain extract (2 μg/mL), gentamicin (50 μg/mL), amphotericin B (50 ng/mL), epidermal growth factor (10 ug/mL), and 10% fetal calf serum (FCS). After detachment with trypsin, cells (3.5 × 105 cells) were grown on 6-cm cell-culture dishes for at least 20 hours. Human dermal microvascular endothelial cells (HMVECs) were purchased from CellSystems and cultured according to the instructions of the manufacturer in endothelial basal medium-2 (EBM-2).
Freshly isolated HUVECs (passage 0) were isolated from human umbilical veins as previously described27 and cultured in MCDB 131 medium (Invitrogen, Karlsruhe, Germany) supplemented with 25 mL l-glutamine (200 mM), 8% FCS, and penicillin (50 μg/mL)/streptomycin (50 μg/mL) on 3.5-cm dishes for 2 days. Aminotriazole (AT; Sigma, Steinheim, Germany) and buthionine sulfoximine (BSO; Sigma) were incubated one hour before LY-83583 (LY; Alexis, Grünberg, Germany) and H2O2 (Merck, Darmstadt, Germany) stimulation.
EPC culture assay
Mononuclear cells (MNCs) were isolated by density gradient centrifugation with Biocoll (Biochrom KG, Berlin, Germany) from peripheral blood of healthy human volunteers as previously described.15 Immediately following isolation, total MNCs (8 × 106 cells/mL medium; cell density: 2.5 × 106 cells/cm2) were plated on 6-cm culture dishes coated with human fibronectin (Sigma) and maintained in EBM supplemented with EGM SingleQuots, VEGF (100 ng/mL), and 20% FCS. CD14+ monocytes were purified from mononuclear cells by positive selection with anti-CD14 microbeads (Miltenyi Biotec, Bergisch-Gladbach, Germany) using a magnetic cell sorter device (Miltenyi Biotec). Purity assessed by fluorescence-activated cell sorter (FACS) analysis was greater than 95% as previously described.25
FACS analysis
For measurement of the intracellular H2O2 and
For determination of apoptotic cells, HUVECs, HMVECs, and EPCs were washed with PBS and detached with trypsin for 3 minutes at 37°C, washed in PBS, and resuspended in binding buffer (BD Biosciences) according to the manufacturer's instructions. After centrifugation, the cell pellet was suspended in 50 μL binding buffer and incubated for 15 minutes at room temperature with 2.5 μL annexin–phycoerythrin (PE; BD Biosciences) and 2.5 μL 7AAD (BD Biosciences). Then, 200 μL binding buffer was added and the samples were analyzed by flow cytometry.
Microarray analysis
Gene expression profiling was performed using the gene chip expression assay. The protocol for sample preparation and microarray processing was carried out according to the instructions of Affymetrix. Data were analyzed with the software GeneSpring version 3.0 (Silicon Genetics, Redwood City, CA) as previously described.28
Western blot analysis
For determination of manganese superoxide dismutase (MnSOD), catalase, and glutathione-peroxidase expression, HUVECs, HMVECs and EPCs were lyzed with 200 μL lysis buffer (20 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA [ethylenediaminetetraacetic acid], 1 mM EGTA [ethylene glycol-bis(beta-aminoethyl ether)-N,N,N′,N′-tetraacetic acid], 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM β-glycerolphosphate, 1 mM Na3VO4,1 μg/mL leupeptin, and 1 mM phenylmethylsulfonyl fluoride) for 15 minutes on ice. After centrifugation for 15 minutes at 20 000g (4°C), the protein content of the samples was determined according to the Bradford method. Proteins (50 μg per lane) were loaded onto sodium dodecyl sulfate (SDS)–polyacrylamide gels and blotted onto polyvinylidine difluoride membranes. Western blots were performed by using antibodies directed against MnSOD (1:1000; BD Biosciences), catalase (1:2000; Calbiochem, Darmstadt, Germany) and glutathione-peroxidase (1:100; Oncogene, Schwalbach, Germany). Enhanced chemiluminescence was performed according to the instructions of the manufacturer (Amersham). Then, the blots were reprobed with ERK1/2 or tubulin (1:1000; Cell Signaling, Frankfurt, Germany, and Neomarkers, Hamburg, Germany, respectively). The autoradiographies were scanned and semiquantitatively analyzed.
RNA interference
MnSOD–RNA-interference (RNAi) (5′CCACGAUCGUUAUGCUGAG3′) and scrambled RNAi (5′AAUCUCGUGUCGUGACACG3′), both labeled with hexachlorofluorescein (HEX), were purchased from Eurogentec (Seraing, Belgium). For transfection 2.8 μg RNAi and 8.4 μL JetSI (Eurogentec) were both mixed with 100 μL medium without supplements. After 15 minutes at room temperature, the solutions were mixed and incubated for 30 minutes. Meanwhile, the medium was removed from the culture plates and EPCs (1 × 106 cells/3.5-cm well) were washed once in medium without FCS. One milliliter medium was added to the RNAi mixture and EPCs were incubated with this mixture for 4 hours at 37°C. Then, 2 mL culture medium was added and EPCs were incubated for 36 hours. HUVECs (3.5 × 105 cells/6-cm well) were transfected with Gene trans II solution (MoBiTec, Göttingen, Germany) and 6 μg RNAi.
Migration assay
After transfecting the cells with RNAi, migration of EPCs was determined in a modified Boyden chamber. Therefore, cells were harvested by centrifugation, resuspended in 500 μL EBM with supplements, counted and placed in the upper chamber of a modified Boyden chamber (2 × 104 cells/per chamber; BD Biosciences). The chamber was placed in a 24-well culture dish containing EBM with supplements and VEGF (50 ng/mL). After incubation at 37°C for 24 hours, the lower side of the filter was washed and fixed with 2% paraformaldehyde. For quantification, migrated transfected cells (hexachlorofluorescein-tagged cells) at the lower part of the filter were counted manually in 4 random microscopic fields.
Statistical analysis
Results from at least 3 independent experiments are expressed as means plus or minus the standard deviation (SD). Statistical analysis was performed by Mann Whitney U test. P values less than .05 were considered statistically significant. All analyses were performed with SPSS 11.5 (SPSS).
Results
EPC characterization
EPCs were generated from peripheral blood mononuclear cells as previously described.14,15 After 4 days of cultivation, the supernatant including the suspending cells was removed and the adherent cells were washed twice. Adherent cells grown from total MNCs stained positive for Dil-Ac-LDL and lectin as previously described.14,15 The stem/progenitor cell characteristics of EPCs are evidenced by their proliferative capacity to form endothelial colonies.25,29 Moreover, FACS analysis and reverse transcriptase–polymerase chain reaction (RT-PCR) revealed the expression of the endothelial marker proteins KDR and eNOS. Further cultivation up to 7 days leads to an additional increase in KDR and eNOS expression (Figure 1A-B).
Characterization of endothelial progenitor cells. (A) The surface expression of KDR on EPCs after 4 days and 7 days in culture was measured by FACS. The isotype control is shown as a thin line. (B) The mRNA expression of eNOS was analyzed by RT-PCR at day 4 and day 7 in culture. GAPDH is shown as loading control.
Characterization of endothelial progenitor cells. (A) The surface expression of KDR on EPCs after 4 days and 7 days in culture was measured by FACS. The isotype control is shown as a thin line. (B) The mRNA expression of eNOS was analyzed by RT-PCR at day 4 and day 7 in culture. GAPDH is shown as loading control.
EPCs exhibit a higher resistance to oxidative stress as compared with HUVECs or CD14+ monocytes
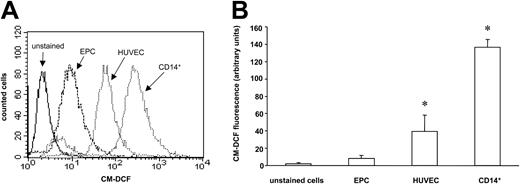
In order to investigate, whether EPCs are more resistant to oxidative stress, we analyzed ROS levels in EPCs and related cells using CM-H2DCF-DA, which is a cell-permeable indicator for ROS. EPCs exhibited a significantly lower basal ROS production as compared with HUVECs. Moreover, CD14+ monocytes also showed higher levels of ROS compared with EPCs (Figure 2A-B). Incubation with the redox-cycler LY-83583, which increases intracellular formation of
EPCs exhibit a higher resistance to oxidative stress as compared with HUVECs or CD14+ monocytes. (A) EPCs, HUVECs, and CD14+ monocytes were stained with the fluorescent dye CM-H2DCF-DA to measure ROS levels by FACS analysis. A histogram representative of 3 independent experiments is shown. (B) Data are mean ± SD, n = 3. *P < .05 versus EPCs.
EPCs exhibit a higher resistance to oxidative stress as compared with HUVECs or CD14+ monocytes. (A) EPCs, HUVECs, and CD14+ monocytes were stained with the fluorescent dye CM-H2DCF-DA to measure ROS levels by FACS analysis. A histogram representative of 3 independent experiments is shown. (B) Data are mean ± SD, n = 3. *P < .05 versus EPCs.
EPCs are more resistant to exogenous oxidative stress. (A) HUVECs and (B) EPCs were incubated with LY-83583 for 18 hours, then stained with CM-H2DCF-DA and measured by FACS analysis. Representative histograms out of 4 independent experiments are shown. Data are mean ± SD, n = 4, *P < .05 versus control. (C) HUVECs and (D) EPCs were incubated with LY-83583 (10 μM) for 18 hours, stained with DHE, and measured by FACS analysis. Data are mean ± SD, n = 3. (E) HUVECs and (F) EPCs were incubated with H2O2 for 18 hours, stained with CM-H2DCF-DA, and measured by FACS analysis. Representative histograms out of 4 independent experiments are shown (upper panels). Data in lower panels are mean ± SD, n = 4, *P < .05 versus control. (G) HUVECs and (H) EPCs were incubated with the redox cycler LY-83583 (10 μM) or H2O2 (500 μM) for the indicated time points, stained with CM-H2DCF-DA, and measured by FACS analysis. Data are mean ± SD, n = 4.
EPCs are more resistant to exogenous oxidative stress. (A) HUVECs and (B) EPCs were incubated with LY-83583 for 18 hours, then stained with CM-H2DCF-DA and measured by FACS analysis. Representative histograms out of 4 independent experiments are shown. Data are mean ± SD, n = 4, *P < .05 versus control. (C) HUVECs and (D) EPCs were incubated with LY-83583 (10 μM) for 18 hours, stained with DHE, and measured by FACS analysis. Data are mean ± SD, n = 3. (E) HUVECs and (F) EPCs were incubated with H2O2 for 18 hours, stained with CM-H2DCF-DA, and measured by FACS analysis. Representative histograms out of 4 independent experiments are shown (upper panels). Data in lower panels are mean ± SD, n = 4, *P < .05 versus control. (G) HUVECs and (H) EPCs were incubated with the redox cycler LY-83583 (10 μM) or H2O2 (500 μM) for the indicated time points, stained with CM-H2DCF-DA, and measured by FACS analysis. Data are mean ± SD, n = 4.
EPCs are less sensitive to oxidative stress–induced apoptosis as compared with HUVECs
Having shown that EPCs exhibited reduced intracellular ROS levels compared with HUVECs, we analyzed the susceptibility to oxidative stress–induced apoptosis. As shown in Figure 4A-B, H2O2 (500 μM) and LY-83583 (10 μM) increased apoptosis in HUVECs by 13- and 14-fold, respectively. In contrast, in EPCs a minor 2.5-fold (500 μM H2O2) and 3.5-fold (10 μM LY-83583) increase in apoptosis was detected, thus demonstrating that EPCs are less sensitive to ROS-induced apoptosis (Figure 4A-B). In addition, we compared the sensitivity of EPCs, HUVECs, and HMVECs toward growth factor depletion-induced apoptosis, which also involves the intracellular increase of ROS levels.31 Consistently, apoptosis induced by serum starvation for 18 hours was significantly enhanced in HUVECs and HMVECs compared with EPCs (Figure 4C).
EPCs are less sensitive to oxidative stress–induced apoptosis as compared with HUVECs or CD14+ monocytes. EPCs and HUVECs were incubated with (A) LY-83583 or (B) H2O2 for 18 hours. (C) EPCs, HUVECs, and HMVECs were incubated with serum-free medium for 18 hours. (A-C) Apoptosis was detected by annexin-V staining using FACS analysis. Data are mean ± SD, n = 3-6. *P < .05 versus control, #P < .05 versus EPCs with same treatment, **P = .07 versus control.
EPCs are less sensitive to oxidative stress–induced apoptosis as compared with HUVECs or CD14+ monocytes. EPCs and HUVECs were incubated with (A) LY-83583 or (B) H2O2 for 18 hours. (C) EPCs, HUVECs, and HMVECs were incubated with serum-free medium for 18 hours. (A-C) Apoptosis was detected by annexin-V staining using FACS analysis. Data are mean ± SD, n = 3-6. *P < .05 versus control, #P < .05 versus EPCs with same treatment, **P = .07 versus control.
Expression of antioxidative enzymes in EPCs
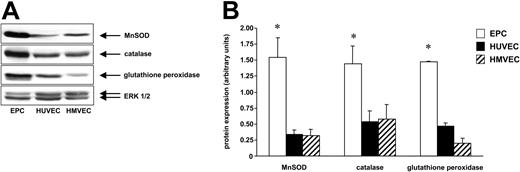
Since EPCs were more resistant to ROS, we studied the expression profile of ROS-metabolizing genes in human EPCs, HUVECs, HMVECs, and CD14+ monocytes using a microarray assay. A gene tree analysis of 115 oxidative stress–associated genes revealed that EPCs express significantly higher levels of the antioxidative enzymes MnSOD, catalase, and glutathione peroxidase (Figure 5A-D). The expression of MnSOD, catalase, and glutathione peroxidase was further elucidated by Western blot analysis. As shown in Figure 6, the protein expression of MnSOD, catalase, and glutathione peroxidase was significantly higher in EPCs compared with HUVECs and HMVECs.
EPCs are equipped with antioxidative enzymes. Total RNA of EPCs, HUVECs, HMVECs, and CD14+ monocytes (each n = 3) was isolated and the gene expression profile was assessed with the Affymetrix gene chip expression assay. (A) The mean of each group (EPCs, HUVECs, CD14+ cells) was calculated and used for a gene tree analysis. The color scale is shown on the right. The brightness indicates the trust. Blue color indicates low expression, red color indicates high expression. Expression of prominent clusters is marked on the right side. The mRNA expression (normalized data) of (B) SODs, (C) catalase, and (D) glutathione peroxidase mRNA expression is shown. Data are mean ± SD, n = 3. *P < .05 versus HUVECs, HMVECs, and CD14+ cells.
EPCs are equipped with antioxidative enzymes. Total RNA of EPCs, HUVECs, HMVECs, and CD14+ monocytes (each n = 3) was isolated and the gene expression profile was assessed with the Affymetrix gene chip expression assay. (A) The mean of each group (EPCs, HUVECs, CD14+ cells) was calculated and used for a gene tree analysis. The color scale is shown on the right. The brightness indicates the trust. Blue color indicates low expression, red color indicates high expression. Expression of prominent clusters is marked on the right side. The mRNA expression (normalized data) of (B) SODs, (C) catalase, and (D) glutathione peroxidase mRNA expression is shown. Data are mean ± SD, n = 3. *P < .05 versus HUVECs, HMVECs, and CD14+ cells.
EPCs express higher levels of MnSOD, catalase, and glutathione peroxidase. EPCs, HUVECs, and HMVECs were lyzed and expression of (A) MnSOD, catalase, and glutathione peroxidase was analyzed by Western blot. A representative ERK1/2 serves as loading control. Representative blots out of 3 independent experiments are shown. (B) Blots were scanned and expression of MnSOD, catalase, and glutathione peroxidase was quantified by densitometric analysis. The ratios for these enzymes are shown. Data are mean ± SD, n = 3. *P < .05 versus HUVECs and versus HMVECs.
EPCs express higher levels of MnSOD, catalase, and glutathione peroxidase. EPCs, HUVECs, and HMVECs were lyzed and expression of (A) MnSOD, catalase, and glutathione peroxidase was analyzed by Western blot. A representative ERK1/2 serves as loading control. Representative blots out of 3 independent experiments are shown. (B) Blots were scanned and expression of MnSOD, catalase, and glutathione peroxidase was quantified by densitometric analysis. The ratios for these enzymes are shown. Data are mean ± SD, n = 3. *P < .05 versus HUVECs and versus HMVECs.
Finally, we addressed the causal role of MnSOD, glutathione peroxidase, and catalase for the higher resistance to oxidative stress in EPCs. For that purpose, we transfected EPCs with MnSOD-RNAi or scrambled RNAi, which were both labeled with hexachlorofluorescein (HEX). FACS analysis revealed a transfection efficiency of about 60% with RNAi-oligonucleotides compared with untransfected EPCs (Figure 7A). In order to analyze the RNAi-mediated down-regulation of MnSOD, we sorted HEX-positive EPCs using FACS, lyzed these cells, and measured MnSOD expression by Western blot analysis. As shown in Figure 7A, the expression of MnSOD was abolished in EPCs transfected with MnSOD-RNAi compared with EPCs transfected with scrambled RNAi. To measure CM-DCF fluorescence in transfected cells, only the transfected, HEX-positive population of EPCs was subjected to further analysis by selective gating. As demonstrated in Figure 7B-D, transfection with MnSOD-RNAi alone or incubation with 10 μM l-buthionine-(S, R)-sulfoximine (BSO), an inhibitor of glutathione peroxidase, or 500 μM 3-amino-1,2,4-triazol (AT), an inhibitor for catalase, slightly elevated the ROS levels in EPCs. However, only the combined inhibition of all 3 enzymes (MnSOD-RNAi, AT, and BSO) was sufficient to induce ROS in EPCs to an extent observed in HUVECs (Figure 7C-D). To further analyze the functional consequences of elevated ROS levels, we measured the survival and migration of these cells. Survival of EPCs treated with H2O2 in the presence of MnSOD-RNAi, AT, and BSO was significantly lower as compared with cells transfected with scrambled RNAi (MnSOD-RNAi+AT+ BSO+H2O2: 41% ± 11%; scrambled-RNAi+H2O2: 94% ± 8% compared with scrambled without treatment (set as 100%; n = 3). Interestingly, increased ROS levels not only were associated with cell death but also with an impaired functional activity of EPCs in the remaining surviving cells. As shown in Figure 7E, the addition of H2O2 to EPCs after combined inhibition of all 3 antioxidative enzymes abolished the migratory response of EPCs toward VEGF.
ROS levels in MnSOD-RNAi–transfected and catalase- and glutathione peroxidase–inhibited cells. (A) EPCs were transfected with MnSOD-RNA-interference (RNAi) or scrambled RNAi, both labeled with hexachlorofluorescein (HEX). After 36 hours, HEX-positive EPCs (highly positive EPCs in gate R1) were sorted by FACS. Then HEX-positive EPCs were lysed and expression of MnSOD was measured by Western blot. Tubulin served as loading control. A representative blot is shown. (B) EPCs were incubated with AT (500 μM) and BSO (10 μM) 19 hours before FACS analysis or transfected with MnSOD-RNAi 36 hours before FACS analysis. To measure CM-DCF fluorescence in transfected cells, only the HEX-positive EPCs were subjected to further analysis of CM-DCF by selective gating. Data are mean ± SD, n = 3. *P < .05 versus MnSOD-RNAi and versus scrambled RNAi with AT and BSO. (C-D) Combination of MnSOD-RNAi transfection, AT, and BSO incubation. (E) EPCs were transfected with MnSOD-RNAi or scrambled RNAi, both labeled with hexachlorofluorescein (HEX). After 24 hours, cells were treated with H2O2 (500 μM) for another 24 hours. Then migration of surviving HEX-positive (trypan blue–negative) EPCs was analyzed using VEGF as stimulus in a modified Boyden chamber. Data are mean ± SD (% of scrambled), n = 3. *P < .05 versus scrambled.
ROS levels in MnSOD-RNAi–transfected and catalase- and glutathione peroxidase–inhibited cells. (A) EPCs were transfected with MnSOD-RNA-interference (RNAi) or scrambled RNAi, both labeled with hexachlorofluorescein (HEX). After 36 hours, HEX-positive EPCs (highly positive EPCs in gate R1) were sorted by FACS. Then HEX-positive EPCs were lysed and expression of MnSOD was measured by Western blot. Tubulin served as loading control. A representative blot is shown. (B) EPCs were incubated with AT (500 μM) and BSO (10 μM) 19 hours before FACS analysis or transfected with MnSOD-RNAi 36 hours before FACS analysis. To measure CM-DCF fluorescence in transfected cells, only the HEX-positive EPCs were subjected to further analysis of CM-DCF by selective gating. Data are mean ± SD, n = 3. *P < .05 versus MnSOD-RNAi and versus scrambled RNAi with AT and BSO. (C-D) Combination of MnSOD-RNAi transfection, AT, and BSO incubation. (E) EPCs were transfected with MnSOD-RNAi or scrambled RNAi, both labeled with hexachlorofluorescein (HEX). After 24 hours, cells were treated with H2O2 (500 μM) for another 24 hours. Then migration of surviving HEX-positive (trypan blue–negative) EPCs was analyzed using VEGF as stimulus in a modified Boyden chamber. Data are mean ± SD (% of scrambled), n = 3. *P < .05 versus scrambled.
Discussion
The results of the present study demonstrate that circulating, blood-derived EPCs are enriched for the expression of genes encoding for antioxidative proteins resulting in low baseline ROS levels and a reduced sensitivity toward ROS-induced cell death. EPCs were shown to home to sites of ischemia and augment neovascularization after hind limb ischemia or myocardial infarction.17,25,32 First clinical pilot data additionally indicate that EPCs might augment coronary flow reserve after acute myocardial infarction.33 Ischemic or reperfused tissue is characterized by high levels of inflammatory cytokines, which activate ROS production.34 One may speculate that the high expression of ROS-metabolizing enzymes in EPCs is essential to maintain survival of these cells in a hostile environment. Interestingly, stem cells in general are characterized by the expression of “stemness” genes, which provide resistance to stress with up-regulated DNA repair and detoxifier systems.5,6 The high expression of protective genes and the resistance of EPCs toward oxidative stress compared with related cells like HUVECs or HMVECs may be part of a “stemness” feature to allow for survival of the cells and regeneration of tissue under conditions of injury. Importantly, the low concentration of ROS in EPCs was not related to a potential monocytic phenotype since monocytes displayed the most pronounced endogenous ROS concentration compared with EPCs and HUVECs (Figure 2). Thus, although the blood-derived EPCs used in the present study likely consist of a heterogeneous cell population, 25,35 our data suggest that this cell population has unique features in comparison to closely related monocytic or endothelial cells. Interestingly, during revision of the present manuscript it has been reported that EPCs generated from cord blood CD34+ cells are also more resistant against cell death compared with HUVECs, 36 suggesting that EPCs from distinct sources are generally less sensitive for apoptosis. Moreover, since oxidative stress is also involved in telomere-shortening and regulation of cellular life span, 37,38 one may speculate that protection against oxidative stress can additionally prevent senescence and dysfunction of EPCs in vivo. Interestingly, the migratory capacity of EPCs toward VEGF was significantly impaired after down-regulation of antioxidative genes, suggesting that intracellular ROS levels indeed influence the functional activity of EPCs. The migratory capacity of EPCs was significantly impaired in patients with coronary artery disease22 and determined the improvement of patients after cell therapy.39 Therefore, one may speculate that a dysregulation of the ROS balance may contribute to a reduced migration and that influencing intracellular ROS levels might be a potential target for therapeutic improvement of EPCs.
The antioxidative capacity of EPCs appears to be mediated by the high expression of several potent antioxidative enzymes, including superoxide dismutase, catalase, and glutathione peroxidase, all of which physiologically reduce intracellular ROS levels.10 Superoxide dismutases, especially MnSOD, are major antioxidant enzymes, which catalyze the dismutation of the superoxide anion
Oxidative stress is associated with many diseases such as cancer and cardiovascular disorders. A role for ROS to mediate apoptosis of endothelial cells has been previously demonstrated, because the radical scavengers vitamin C and N-acetylcysteine inhibit apoptosis of endothelial cells.41,42 Likewise, apoptosis of other cell types was blocked by antioxidants.43-45 The equipment of EPCs with high levels of ROS-metabolizing proteins translates into a low sensitivity toward ROS-induced apoptosis. Furthermore, EPCs were more resistant against apoptosis induction by growth factor deprivation compared with HUVECs. This might be well explained by the finding that serum withdrawal–induced apoptosis involves ROS.31 Thus, the expression of antioxidative enzymes may be a general mechanism to protect against apoptosis.
Taken together, the data of the present study revealed that EPCs express high levels of antioxidative enzymes compared with related cell types. The exquisite protection of EPCs against ROS-induced cell death may be a prerequisite to maintain the survival of the cells in inflamed tissue, thereby allowing the neovascularization improvement of ischemic tissue.
Prepublished online as Blood First Edition Paper, May 25, 2004; DOI 10.1182/blood-2003-12-4103.
Supported by the Deutsche Forschungsgemeinschaft (FOR 501: Di 600/4-1 and BR 1839/2-1).
E.D. and C.U. contributed equally to this report.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank Melanie Näher and Andrea Knau for excellent technical help.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal