Abstract
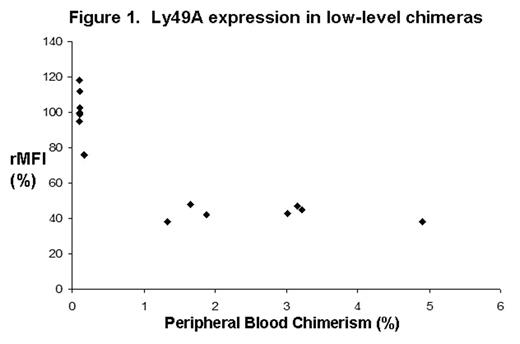
Despite clinical success with in utero hematopoietic stem cell transplantation in immunodeficient recipients, only microchimerism (<0.1%) has been observed in immunocompetent human or non-human primate hosts without conditioning. It has been postulated that an innate immune response to the low levels of chimerism caused the lack of engraftment. We have found that prenatal murine recipients of fetal allografts with low levels of engraftment (<2.0%) also lose their chimerism after a few months. To better understand fetal innate immunity as it relates to this problem, we studied the education of host NK cells in prenatal chimeras with low engraftment (<5.0%). We hypothesized that alloreactive NK receptor expression correlates with chimerism level. To test this hypothesis, prenatal chimeras were created by transplanting fetal liver light density cells from 14 dpc Balb/c (H2d) fetuses into 14 dpc B6 (H2b) fetuses by IP injection. Using flow cytometry, peripheral blood chimerism in the pups was first assessed at 3 weeks and followed for one year. At 12 weeks, following the acquisition of mature NK inhibitory receptors, the level of H-2d alloreactive Ly49A, C, F, G, and I receptor expression among host splenic NK cells was measured. The mean fluorescence intensity for each receptor relative to age-matched controls (rMFI) was plotted against chimerism and the Pearson correlation coefficient (r) calculated for comparison. Chimerism ranged from 0.1 – 5.0% without GVHD (n=13). As shown in figure 1, B6 recipient NK cells exhibited a dramatic negative correlation of Ly49A expression with increasing chimerism (rMFI = 38 –118%, r = −0.87) without change in the frequency of Ly49A+ NK cells (18–26%, r=0.37). No significant change in the expression of Ly49C, F, G, or I was seen within the range of chimerism analyzed. Five mice reached 12 months and were sacrificed for a repeat NK analysis to determine the stability of Ly49A expression, see table 1. In four mice, chimerism and Ly49A expression levels did not change significantly. However, chimerism was lost in one animal with a corresponding upregulation in Ly49A expression. The initial chimerism level was in a range where we find that very few mice maintain long-term chimerism in this system (<2.0%). The pattern of progressive Ly49A downregulation with increasing peripheral blood chimerism strongly supports a mechanism of NK education that is developmentally responsive to the composite MHC expression in the host chimera. This suggests that a threshold level of chimerism may be necessary to overcome the host innate immune response. Possible mechanisms by which this may occur include higher levels chimerism leading to further NK inhibition or possibly the expansion of a critical regulatory cell population in the donor cell fraction. An understanding of these potential mechanisms will facilitate the development of more effective transplantation regimens.
Table 1. Expression of Ly49A at 3 months and 1 year
| . | Chimerism Level . | . | Ly49A expression . | . |
|---|---|---|---|---|
| Animal . | PB-3 months (%) . | PB-12 months (%) . | rMFI-3 months (%) . | rMFI-12 months (%) . |
| 7831 | 0.2 | 0.1 | 76 | 74 |
| 7759* | 1.33 | 0.2 | 38 | 92 |
| 7830 | 3.22 | 1.81 | 45 | 29 |
| 7860 | 1.91 | 2.38 | 42 | 33 |
| 7838 | 4.9 | 5.2 | 38 | 32 |
| . | Chimerism Level . | . | Ly49A expression . | . |
|---|---|---|---|---|
| Animal . | PB-3 months (%) . | PB-12 months (%) . | rMFI-3 months (%) . | rMFI-12 months (%) . |
| 7831 | 0.2 | 0.1 | 76 | 74 |
| 7759* | 1.33 | 0.2 | 38 | 92 |
| 7830 | 3.22 | 1.81 | 45 | 29 |
| 7860 | 1.91 | 2.38 | 42 | 33 |
| 7838 | 4.9 | 5.2 | 38 | 32 |
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal