Evidence is growing that many types of ion channels and other molecules once thought to be restricted to the nervous system are expressed in hematopoietic cells, where they may function at various levels of differentiation.
In 1978, Miller et al1 inserted electrodes into a Guinea pig megakaryocyte and showed that when electric current was injected, the cell fired a biphasic action potential exactly as a neuron would do under the same conditions. Voltage-dependent calcium and potassium channels identical to those found in some nerve and muscle cells were later shown to underlie its action potential.2 Long distracted by the dazzling electric currents of neurons and myocytes, electrophysiologists had traditionally dismissed most other tissues—including blood—as “nonexcitable” and therefore unworthy of their attention. But the action potential of the Guinea pig megakaryocyte, which blurred this convenient distinction, was hard to ignore. Why did blood cells need ion channels? What business did they have generating electricity?FIG1
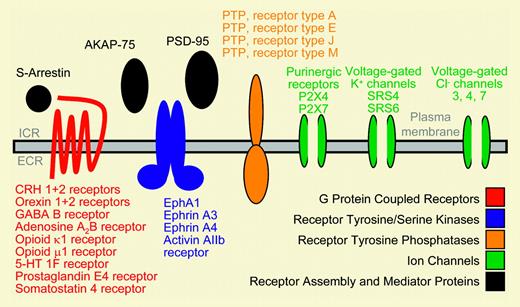
Schematic display of neurobiologic genes expressed in primary human CD34+ hematopoietic stem and progenitor cells. See the complete figure in the article beginning on page 81.
Schematic display of neurobiologic genes expressed in primary human CD34+ hematopoietic stem and progenitor cells. See the complete figure in the article beginning on page 81.
Inspired by the electronics of this curious bone marrow cell, and enabled by refinements in the patch-clamp technique that permitted characterization of single ion channels, electrophysiologists started around 1990 to tinker with other blood cells. They found to their great surprise that many of the same depolarizing (sodium and calcium) and hyperpolarizing (potassium and chloride) channels that had originally been considered the exclusive domain of neurons or muscle cells actually operated in hematopoietic cells as well. We now recognize that blood cells, like neurons, generate and store electric potential energy. Through the cooperative behavior of a panoply of ion channels, they harness this energy to carry out a diverse range of hefty biophysical work, including volume regulation, cell movement, and degranulation. It is likely that whenever a blood cell like a platelet or a neutrophil does any “heavy lifting,” electric energy is spent through the operation of many of the same ion channels that exist in brain and muscle but that perform totally different tasks in those tissues.
In the current issue of Blood, Steidl and colleagues (page 81) take this concept a step further by reporting that human CD34+ cells express not only genes encoding many of the ion channels found in the brain, but also a variety of other proteins whose roles have been primarily defined in the nervous system. These include numerous neuromediators, receptors, kinases, phosphatases, and other proteins involved in the regulation of ion channels, neurotransmitter release, or other aspects of neuronal behavior. Of particular interest is their finding that many components of the exocytic machinery involved in neurotransmitter release at axon termini are ex pressed by CD34+ cells. This observation supports the view that agonist-mediated exocytosis in hematopoietic cells may share more similarities than differences at the molecular level with the highly specialized exocytosis that takes place in neurons. In their paper, Steidl et al go to great lengths to show that many neurobiologic genes are not only expressed at both the mRNA and protein level in CD34+ cells, some of them exhibit their predicted functions in these cells.
Since many of the ion channels that coexist in the brain and the blood carry out disparate tasks in these tissues, it is not surprising that many other “neurobiologic” proteins may have been adapted by nature for different roles in hematopoietic cells. Defining their functions in proliferative and mature blood cells is now the challenge. The blood-brain barrier is weakening.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal