Inflammatory cytokines can stimulate the release of ultralarge VWF multimers from endothelial cells, as well as affect ADAMTS-13 cleavage of VWF, thus demonstrating another link between inflammation and thrombosis.
Systemic inflammation shifts the hemostatic balance in the direction of thrombosis. This has long been recognized by physicians who treat patients with cancer, infections, autoimmune diseases, and pregnancy, as these systemic conditions (as well as many others) are associated with a hypercoagulable state. Thus the link between thrombosis and inflammation continues to be the focus of considerable attention, both in the laboratory and in the clinic.
Interactions between platelets, leukocytes, and the endothelium lie at the interface between thrombosis and inflammation, and these interactions are profoundly influenced by the actions of inflammatory cytokines. Examples of cytokines with procoagulant influence include tumor necrosis factor α (TNF-α), interleukin-6 (IL-6), and IL-8, which, among many effects, are known to stimulate expression of tissue factor by monocytes and macrophages (TNF-α),1 increase platelet reactivity and induce fibrinogen and plasminogen activator inhibitor-1 (PAI-1) expression by the liver (IL-6),1,2 and activate endothelial cells (TNF-α and IL-8).2,3 FIG1
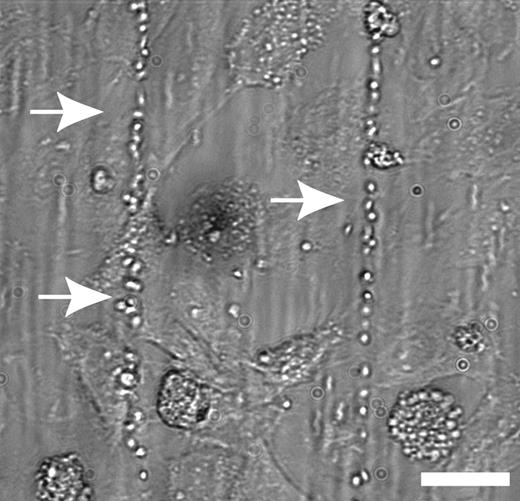
IL-8 and TNF-α, but not IL-6, stimulated the release of ULVWF multimers. See the complete figure in the article beginning on page 100.
IL-8 and TNF-α, but not IL-6, stimulated the release of ULVWF multimers. See the complete figure in the article beginning on page 100.
Building on this theme, Bernardo and colleagues in this issue of Blood (page 100) increase our understanding of the mechanisms by which TNF-α, IL-6, and IL-8 exert their procoagulant influence, which turns out to involve the blood clotting protein von Willebrand factor (VWF) and ADAMTS-13, an enzyme involved in the proteolytic processing of VWF and deficient in the disease thrombotic thrombocytopenic purpura (TTP).
VWF synthesized by endothelial cells either is constitutively secreted into the circulation in the form of low-molecular-weight multimers or is stored in Weibel-Palade bodies, and in response to endothelial cell agonists is released in the form of ultralarge multimers (ULVWF). ULVWF is the most adhesive and reactive form of VWF and may lead to spontaneous platelet aggregation if not further processed by the ADAMTS-13 metalloprotease. Lack of ULVWF cleavage by ADAMTS-13 is thought to be the primary defect underlying TTP.
In this study, Bernardo and colleagues investigated whether ULVWF release from endothelial cells and its subsequent cleavage by ADAMTS-13 are affected by the inflammatory cytokines TNF-α, IL-6, and IL-8, as these processes could represent potential links between inflammation and thrombosis. First, using an elegant system in which cultured endothelial cells are subjected to defined flow stress in vitro, the authors demonstrate that TNF-α and IL-8 (but not IL-6) stimulate the release of ULVWF strings from human umbilical vein endothelial cells (HUVECs) in a dose-dependent manner. As HUVECs lack an IL-6 receptor, the absence of response with IL-6 was not surprising; when the experiment was repeated with IL-6 precomplexed with soluble IL-6 receptor, ULVWF release was seen (although not to the level of the other cytokines).
The authors then turn their attention to whether these cytokines can alter the ability of ADAMTS-13 to cleave ULVWF. They show that IL-6 (but not TNF-α or IL-8) greatly abrogates the ability of ADAMTS-13 to cleave ULVWF strings under flowing, but not static, assay conditions. As this interesting effect is still observed when partially purified ADAMTS-13 is pretreated with IL-6, the authors speculate that IL-6 may physically impair binding of ADAMTS-13 to ULVWF under the shear stress of flowing conditions. As the authors point out, this observation is especially interesting in light of previous studies demonstrating elevated IL-6 levels in patients with TTP,4 and in coronary thrombi and atherosclerotic plaques.5
This important study provides insight into potential new mechanisms by which the inflammatory process is able to shift the hemostatic balance in favor of thrombosis. Cytokines present in a variety of pathologic conditions may influence both the release of ULVWF from endothelial cells and its subsequent processing by ADAMTS-13, thus allowing ULVWF to persist long enough to induce platelet adhesion and aggregation, and ultimately lead to thrombosis. New information regarding regulation of the interface between inflammation and thrombosis is always welcome as it eventually may lead to new points of therapeutic intervention for patients with inflammation-associated coagulopathies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal