Abstract
Factor VIII (FVIII) functions as a cofactor within the intrinsic pathway of blood coagulation. Quantitative or qualitative deficiencies of FVIII result in the inherited bleeding disorder hemophilia A. Expression of FVIII (domain structure A1-A2-B-A3-C1-C2) in heterologous mammalian systems is 2 to 3 orders of magnitude less efficient compared with other proteins of similar size compromising recombinant FVIII production and gene therapy strategies. FVIII expression is limited by unstable mRNA, interaction with endoplasmic reticulum (ER) chaperones, and a requirement for facilitated ER to Golgi transport through interaction with the mannose-binding lectin LMAN1. Bioengineering strategies can overcome each of these limitations. B-domain-deleted (BDD)-FVIII yields higher mRNA levels, and targeted point mutations within the A1 domain reduce interaction with the ER chaperone immunoglobulin-binding protein. In order to increase ER to Golgi transport we engineered several asparagine-linked oligosaccharides within a short B-domain spacer within BDD-FVIII. A bioengineered FVIII incorporating all of these elements was secreted 15- to 25-fold more efficiently than full-length FVIII both in vitro and in vivo. FVIII bioengineered for improved secretion will significantly increase potential for success in gene therapy strategies for hemophilia A as well as improve recombinant FVIII production in cell culture manufacturing or transgenic animals. (Blood. 2004;103: 3412-3419)
Introduction
Factor VIII (FVIII) is a large plasma glycoprotein that functions as an essential cofactor for the proteolytic activation of factor X by activated factor IX within the intrinsic pathway of blood coagulation.1 The inherited bleeding disorder, hemophilia A, results from quantitative or qualitative deficiency of coagulation FVIII and affects 1 in 5000 males. There are approximately 17 000 patients with hemophilia in the United States, 80% of whom have hemophilia A.2 This lifelong hemorrhagic diathesis is treated successfully with FVIII replacement either from plasma-derived sources or, for the last decade, primarily with recombinantly derived protein.3 Plasma levels maintained above 2% can effectively prevent most severe hemorrhages,4 so hemophiliaAhas been an attractive target for gene therapy applications.
Recombinant FVIII (rFVIII) therapy has proved to be costly due to the expense of production, purification, and formulation. The manufacturing technology used has required specialized centralized production and distribution, limiting access to developing and third-world countries.5 rFVIII still requires intravenous access for delivery due to limited bioavailability from other delivery routes. Regular prophylactic infusions of rFVIII can effectively prevent joint hemorrhages and prevent the development of hemophilic arthropathy. However, the cost and limited availability of rFVIII has prevented universal implementation of this treatment strategy. In addition, gene therapy applications for hemophilia A have been hampered by inadequate expression in vivo.6,7
Several biochemical characteristics of rFVIII have contributed to the high costs associated with this therapy. Expression of rFVIII within heterologous systems (eg, commercial rFVIII production or gene therapy applications) is 2 to 3 orders of magnitude lower than that of other comparably sized proteins.8 The mRNA is inefficiently expressed,8-10 a significant portion of the primary translation product is misfolded and ultimately degraded,11,12 and FVIII is retained within the endoplasmic reticulum (ER) through interaction with various ER chaperones including immunoglobulin-binding protein (BiP).13-16 Properly folded FVIII requires a facilitated transport mechanism for efficient transport from the ER to the Golgi via interaction with the mannose-binding lectin LMAN1/endoplasmic reticulum Golgi intermediate compartment protein of 53 kDa (ERGIC-53).17-19 Upon secretion from the cell, FVIII requires stabilization by von Willebrand factor (VWF),9,20 which can be provided by supplementation of cell media, by coexpression in the same cells, or by the plasma of the recipient or gene therapy host. Once cleaved and activated by thrombin or factor Xa, the activated form of FVIII (FVIIIa) is unstable, due to spontaneous and proteolytic inactivation.21-23
Insights into the secretion pathway as well as FVIII structure and function have led to opportunities to bioengineer forms of rFVIII with improved efficiency of secretion. FVIII shares an identical domain structure (A1-A2-B-A3-C1-C2) with factor V (FV), another coagulation cofactor.24-26 FVIII and FV share approximately 40% amino acid identity within their A and C domains. The B domains of both cofactors are encoded by unusually large single exons and share no amino acid homology, although they have conserved a high content of asparagine (N)-linked oligosaccharides.27-30 FVIII and FV have 19 and 25 consensus sites for N-linked glycosylation, respectively. Early biochemical studies demonstrated that the FVIII B-domain was dispensable for FVIII cofactor activity.31 B-domain-deleted (BDD)-FVIII also resulted in a 17-fold increase in mRNA levels over full-length wild-type FVIII and a concomitant increase in the amount of synthesized primary translation product.11,32 However, only a 30% increase in secreted protein was observed, suggesting intracellular interactions were limiting efficient secretion. Inefficient secretion of FVIII correlated with interaction with the protein chaperone BiP.13 Consistent with BiP having a peptide-dependent ATPase activity, FVIII release from BiP and transport out of the ER required high levels of intracellular adenosine triphosphate (ATP),15 whereas FV did not detectably associate with BiP and did not require high levels of ATP for secretion.33 An 11-residue hydrophobic beta-sheet within the FVIII A1-domain was predicted to interact with BiP. Significantly, mutation of a single residue, Phe309Ser, within this hydrophobic pocket increased FVIII secretion 3-fold and also reduced the ATP requirement for secretion.34
Further insight into FVIII secretion was gained through the molecular understanding of combined FV and FVIII deficiency. Patients with this rare bleeding disorder have FV and FVIII levels between 5% and 30%; in two thirds of the patients, the disorder is a result of null expression of LMAN1 (previously identified as ERGIC-53).17,18 LMAN1 is a homohexameric type 1 transmembrane protein. It was characterized as a mannose-binding lectin proposed to target specific glycoproteins to coat protein complex II (COP-II)-coated vesicles budding from the ER for transport to the Golgi.35-39 Previous work demonstrated that LMAN1 is required for efficient secretion of FVIII and FV and that this is mediated by oligosaccharide structures within their B domains.40
In this report we show that bioengineered forms of FVIII can be expressed that (1) produce increased mRNA and primary translation product levels by deletion of the majority of the B domain, (2) have improved secretion efficiency by using a targeted A1-domain mutation (Phe309Ser), and (3) exploit the LMAN1-facilitated ER-Golgi transport by retaining several N-linked oligosaccharides within a short B-domain spacer. A bioengineered FVIII with all of these features is expressed 15- to 25-fold more efficiently than full-length wild-type FVIII both in vitro in traditional heterologous expression systems as well as in vivo in a mouse model of hemophilia A.
Materials and methods
Materials
Anti-heavy chain FVIII monoclonal antibody (F-8) conjugated to CL-4B sepharose was a gift from Debra Pittman (Genetics Institute, Cambridge, MA). FVIII-deficient and normal pooled human plasma were obtained from George King Biomedical (Overland Park, KS). Activated partial thromboplastin (automated aPTT reagent) and CaCl2 were purchased from General Diagnostics Organon Teknika (Durham, NC). Dulbecco modified Eagle medium (DMEM), alpha-modified essential medium (alpha-MEM), and fetal bovine serum were purchased from Gibco BRL (Gaithersburg, MD). COAMATIC was purchased from DiaPharma (West Chester, OH). Asserachrom VIIIC:Ag was purchased from Diagnostica Stago (Parsippany, NJ). FVIII:C-EIA was purchased from Enzyme Research Laboratories (South Bend, IN). Plasmid Purification Maxi Kit was purchased from Qiagen (Valencia, CA). FuGENE-6 transfection reagent was purchased from Roche Applied Science (Indianapolis, IN). QuikChange XL Site-Directed Mutagenesis Kit was purchased from Stratagene (La Jolla, CA).
Plasmid mutagenesis
The B-domain variant constructs were prepared by polymerase chain reaction (PCR) using a 5′ oligonucleotide proximal to a native KpnI restriction site within the A2 domain sequence of FVIII and various 3′ oligonucleotides spaced at designated intervals along the FVIII B-domain. The 3′ oligonucleotides included an engineered MluI restriction site that encodes for threonine/arginine and were synthesized such that the Thr/Arg was positioned at the following FVIII residues within the B domain for each construct: residues 769/770 for plasmid 29aa/N1, residues 794/795 for 54aa/N2, residues 857/858 for 117aa/N3, residues 903/904 for 163aa/N4, residues 946/947 for 206aa/N5, residues 966/967 for 226aa/N6, and residues 1009/1010 for 269aa/N8. FVIII wild-type (FVIII WT) was used as the DNA template for each reaction and the PCR products were digested with KpnI and MluI. The restriction digested DNA fragments were ligated into the vector pMT2/1648MluI that encodes for full-length FVIII with an MluI site engineered at residues 1647/1648. Although this creates a nonnative B-domain/A3-domain junction of residues Thr/Arg from Gln/Arg, the expressed protein has similar synthesis, secretion, and activity to FVIII WT.41 The construct F309S/226aa/N6 was generated by subcloning the SpeI-KpnI fragment of plasmid Phe309Ser (generated previously) into the 226aa/N6 plasmid. The construct 226aa/N1 was generated in stages using a combination of overlapping PCR strategy and the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene) such that N-linked glycosylation consensus sites were eliminated by substituting Gln for Asn at residues 757, 784, 828, 900, and 963. All B-domain variants were characterized by restriction enzyme digestion and DNA sequencing.
Plasmid transfection and analysis
Plasmid DNA was transfected into COS-1 cells by the diethylamino ethanol-dextran method as previously described42 and into Chinese hamster ovary (CHO) cells using FuGENE-6 transfection reagent per the manufacturer's recommendations. Conditioned medium was harvested at 60 hours after transfection in the presence of 10% fetal bovine serum.
Factor VIII activity and antigen analysis
FVIII activity was measured by (1) a 1-stage aPTT clotting assay on an MLA Electra 750 fibrinometer (Medical Laboratory Automation, Pleasantville, NY) by reconstitution of human FVIII-deficient plasma or (2) a 2-stage assay using the COAMATIC chromogenic assay according to the manufacturer's instructions. The FVIII plasma standard was FACT plasma (normal pooled plasma) from George King Biomedical for the one-stage aPTT clotting assay. The calibration standard included with the COAMATIC chromogenic assay is assayed according to the Fourth International WHO standard. FVIII antigen was quantified by an anti-FVIII light chain sandwich enzyme-linked immunosorbent assay (ELISA) method using both the Asserachrom VIII:C Ag and FVIII:C-EIA commercial kits according to the manufacturers' instructions.
Metabolic labeling
Protein synthesis and secretion were analyzed by metabolically labeling cells at 60 hours after transfection for 30 minutes with [35S]-methionine/[35S]cysteine (300 μCi/mL [11.1 MBq/mL] in methionine-free medium), followed by a chase for 4 hours in medium containing 100-fold excess unlabeled methionine and 0.02% aprotinin. Cell extracts and conditioned media were harvested and immunoprecipitations were performed and analyzed as described previously.42 Autoradiograph band intensities were analyzed by National Institutes of Health (NIH, Rockville, MD) Image software (public domain).
Generation of CHO cell lines stably expressing FVIII B-domain variants
CHO cell lines stably expressing B-domain variants 226aa/N6 and F309S/226aa/N6 were generated as previously described.42 Briefly, the 226aa/N6 and F309S/226aa B-domain variants within the expression plasmid pED6 were transfected into dihydrofolate reductase-deficient CHO cells using the FuGENE-6 transfection kit according to the manufacturer's instructions. The cells were cultured in alpha-MEM containing 10% heat-inactivated fetal bovine serum, 25 U/mL penicillin, 25 μg/mL streptomycin, and 2 mM/mL l-glutamine. At 48 hours from transfection, the cells were split at 1:10, and maintained for 10 to 14 days, until individual colonies were formed. Approximately 100 colonies were screened for FVIII activity, and 24 colonies that expressed the highest FVIII activities were amplified into 6-well culture plates and then finally were amplified to 100-mm culture dishes. The 3 colonies expressing the highest FVIII activity were chosen for methotrexate selection. Initially, 0.05 μM methotrexate was added to the 100-mm cultured plates. When colonies formed, approximately 100 colonies were chosen for screening again. The dose of methotrexate was gradually increased until the FVIII activity from the amplified colonies was no longer observed to be increasing with the increased concentration of methotrexate. Maximal FVIII expression was observed at 0.65 μM and 0.35 μM methotrexate for the 226aa/N6 and F309S/226aa/N6 cell lines, respectively. All cell lines were split and seeded at 1.5 × 106 into 100-mm culture dishes for preparation for analysis.
Plasmid expression in vivo by hydrodynamic tail-vein injection into a FVIII-/- mouse model
A FVIII knock-out (-/-) mouse model of hemophilia A was used to analyze the in vivo expression of the high-efficiency secretion FVIII B-domain variant constructs. Plasmid DNA (100 μg) was diluted in 2.5 mL lactated Ringer and infused over 10 seconds into the tail vein.43,44 Retro-orbital blood collection was performed at 24 and 48 hours and FVIII secretion analyzed by a human FVIII-specific ELISA (FVIII:C-EIA). Statistics were performed using a 2-tailed homoscedastic (2-sample equal variance) Student t test.
Results
Factor VIII B-domain variants are secreted more efficiently from COS-1 and CHO cells
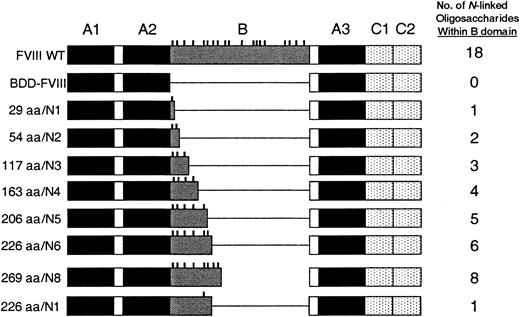
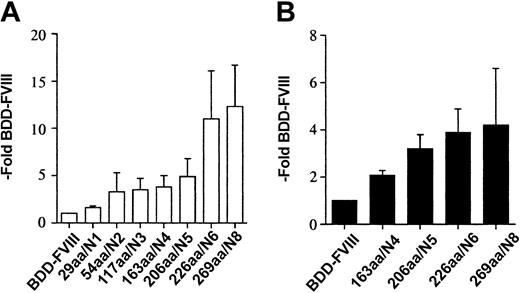
Using oligonucleotide site-directed mutagenesis, we constructed FVIII mutants with variably sized B domains. The constructs were prepared such that with each additional segment of B domain, 1 or 2 additional consensus sites for N-linked glycosylation were included (Figure 1). Amino-terminal B-domain sequence was used and the size ranged from 29 amino acids (aa) to 269 aa beginning with aa 741. The number of consensus sites for N-linked glycosylation ranged from 1 to 8. The BDD-FVIII and B-domain variant plasmids were transfected into COS-1 cells and cell media were harvested from 24 to 60 hours after transfection. The relative efficiency of FVIII secretion was measured by a one-stage clotting assay and a FVIII-specific ELISA (Asserachrom) and compared with expression of BDD-FVIII (Figure 2A-B). We observed a stepwise incremental increase in the amount of FVIII measured within the cell media that correlated with each B-domain size increase and the addition of each additional consensus site for N-linked glycosylation. Average BDD-FVIII expression was 140 mU/mL and 32 ng/mL. The 226aa/N6 variant was expressed with an average 11-fold increase by one-stage clotting activity assay compared with BDD-FVIII. Further increase in the size and oligosaccharide content of the B domain (269aa/N8) did not yield significantly higher expression. Cotransfection of the B-domain variants with a plasmid expressing von Willebrand factor did not yield higher expression, suggesting that VWF was not limiting within the COS-1-conditioned media (data not shown). Relative expression as determined by antigen analysis was not as high (226aa/N6 4-fold that of BDD-FVIII) as determined by one-stage activity analysis. This suggests that these mutants have a slightly increased specific activity or a slight reduction in antigenicity using the particular ELISA reagents.
FVIII constructs containing B-domain variants. Constructs were prepared within B-domain-deleted-FVIII. The context for the B-domain sequence is shown schematically compared with FVIII wild-type (WT). The mutant constructs are named according to the size of the B domain as measured in amino acids (aa) and the number of consensus sites for N-linked glycosylation (vertical dashes).
FVIII constructs containing B-domain variants. Constructs were prepared within B-domain-deleted-FVIII. The context for the B-domain sequence is shown schematically compared with FVIII wild-type (WT). The mutant constructs are named according to the size of the B domain as measured in amino acids (aa) and the number of consensus sites for N-linked glycosylation (vertical dashes).
FVIII B-domain variants are expressed more efficiently in COS-1 cells. One-stage activity (A) and FVIII antigen (B) for FVIII B-domain variant cDNA constructs transiently expressed in COS-1 monkey kidney cells. One-stage activity was determined by aPTT-based assay. FVIII antigen was determined by a commercial ELISA (Asserachrom FVIII:C Ag). The data were obtained from assaying the conditioned medium at 60 hours following transfection and compared with BDD-FVIII control. Data presented are the mean of several independent experiments (n ≥ 3), and the error bars represent the standard deviation.
FVIII B-domain variants are expressed more efficiently in COS-1 cells. One-stage activity (A) and FVIII antigen (B) for FVIII B-domain variant cDNA constructs transiently expressed in COS-1 monkey kidney cells. One-stage activity was determined by aPTT-based assay. FVIII antigen was determined by a commercial ELISA (Asserachrom FVIII:C Ag). The data were obtained from assaying the conditioned medium at 60 hours following transfection and compared with BDD-FVIII control. Data presented are the mean of several independent experiments (n ≥ 3), and the error bars represent the standard deviation.
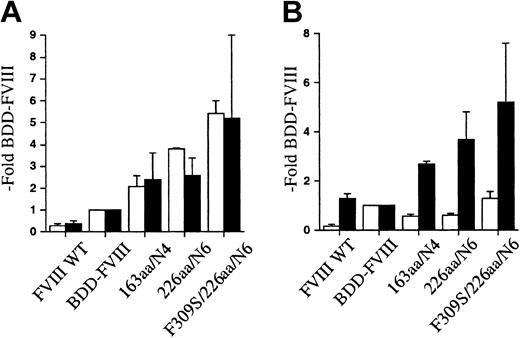
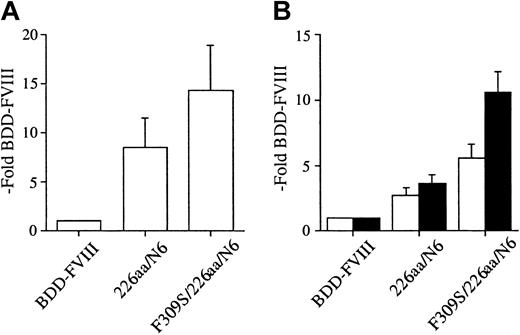
To determine if the observations were cell-line specific, FVIII WT, BDD-FVIII, and the 163aa/N4 and 226aa/N6 B-domain variants were transiently transfected into CHO cells. The conditioned media were collected from 24 to 60 hours following transfection and analyzed for FVIII expression. FVIII WT showed a reduced relative expression compared with BDD-FVIII, consistent with previous reports. This can be primarily attributed to reduced mRNA levels.41 The 163aa/N4 and 226aa/N6 variants were expressed approximately 2-fold and 4-fold higher, respectively, than BDD-FVIII as measured by activity and antigen assays (Figure 3A-B). Similar FVIII activity was obtained by both 1-stage and 2-stage activity analysis. The quantity of FVIII secreted as measured by antigen determination in the CHO cells was ELISA specific. The antigen levels obtained by Asserachrom FVIII:C Ag were much lower than that obtained with the Enzyme Research Laboratories FVIII:C-EIA, again suggesting that there are differences in antigenicity for these B-domain variants with different ELISA reagents. Although there were cell-line-specific differences in the magnitude of the improvement in secretion efficiency for the B-domain variants, the CHO cells still secreted the B-domain variants more efficiently than BDD-FVIII.
FVIII B-domain variants are expressed more efficiently in CHO cells. Both 1- and 2-stage activity (A) and FVIII antigen (B) for FVIII B-domain variant cDNA constructs transiently expressed in CHO cells. One-stage activity (□) was determined by aPTT-based assay; 2-stage activity (▪) was determined by a commercial chromogenic assay (COAMATIC). FVIII antigen was determined by 2 different commercial ELISAs, Asserachrom FVIII:C Ag (□) and FVIII:C-EIA (▪). The data were obtained from assaying the conditioned medium at 60 hours following transfection and compared with BDD-FVIII control. Data presented are the mean of several independent experiments (n ≥ 3), and the error bars represent the standard deviation.
FVIII B-domain variants are expressed more efficiently in CHO cells. Both 1- and 2-stage activity (A) and FVIII antigen (B) for FVIII B-domain variant cDNA constructs transiently expressed in CHO cells. One-stage activity (□) was determined by aPTT-based assay; 2-stage activity (▪) was determined by a commercial chromogenic assay (COAMATIC). FVIII antigen was determined by 2 different commercial ELISAs, Asserachrom FVIII:C Ag (□) and FVIII:C-EIA (▪). The data were obtained from assaying the conditioned medium at 60 hours following transfection and compared with BDD-FVIII control. Data presented are the mean of several independent experiments (n ≥ 3), and the error bars represent the standard deviation.
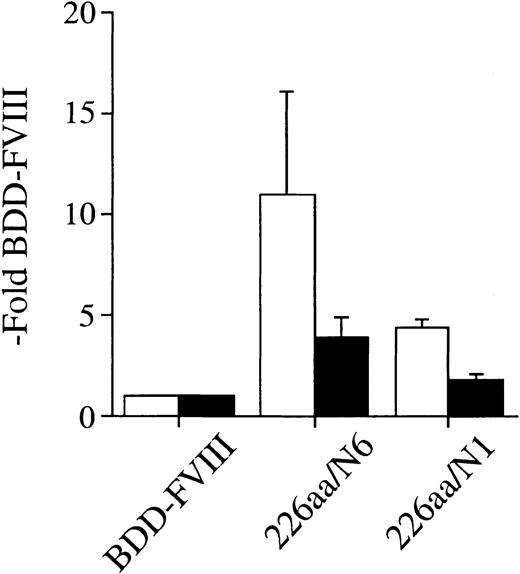
We also prepared an alternate FVIII B-domain variant that encoded for the same 226 aa of B-domain sequence as the 226aa/N6 construct but in which we created Asn → Gln point mutations at the consensus sites for N-linked glycosylation at residues 757, 784, 828, 900, and 963. Although the remaining Asn at residue 943 is a potential consensus site for N-linked glycosylation, previous detailed study of B-domain glycosylation indicates that this site is not used.45 Thus this construct should be fully devoid of N-linked oligosaccharides within the B-domain fragment. This alternative B-domain variant was transfected into COS-1 cells and analyzed for secretion efficiency compared with BDD-FVIII and the 226aa/N6 construct (Figure 4). The 226aa/N1 construct was secreted 4.4-fold more efficiently than BDD-FVIII as determined by one-stage clotting activity assay but was significantly lower than the 226aa/N6 construct (11-fold more efficient than BDD-FVIII), which retained all consensus sites for N-linked glycosylation. Antigen determination by ELISA (Asserachrom) was also significantly lower than the 226aa/N6 construct. Thus the benefit of the B-domain variants to the secretion efficiency of these FVIII constructs is attributable to the N-linked oligosaccharide content. However, there still remains an observed increased secretion efficiency for the 226aa/N1 construct over BDD-FVIII, suggesting a residual benefit of the B-domain primary amino acid sequence as well.
Efficiency of expression of FVIII B-domain variants in COS-1 cells is dependent on oligosaccharide content. One-stage activity (□) and FVIII antigen (▪) for FVIII B-domain variant cDNA constructs transiently expressed in COS-1 monkey kidney cells by transfection. One-stage activity was determined by aPTT-based assay. FVIII antigen was determined by a commercial ELISA (Asserachrom FVIII:C Ag). The data were obtained from assaying the conditioned medium at 60 hours following transfection and compared with BDD-FVIII control. Data presented are the mean of several independent experiments (n ≥ 3), and the error bars represent the standard deviation.
Efficiency of expression of FVIII B-domain variants in COS-1 cells is dependent on oligosaccharide content. One-stage activity (□) and FVIII antigen (▪) for FVIII B-domain variant cDNA constructs transiently expressed in COS-1 monkey kidney cells by transfection. One-stage activity was determined by aPTT-based assay. FVIII antigen was determined by a commercial ELISA (Asserachrom FVIII:C Ag). The data were obtained from assaying the conditioned medium at 60 hours following transfection and compared with BDD-FVIII control. Data presented are the mean of several independent experiments (n ≥ 3), and the error bars represent the standard deviation.
Factor VIII A1-domain mutant/B-domain variant hybrid expression in COS-1 cells
Transport from the ER to the Golgi requires release from ER resident chaperones. The previously described FVIII A1-domain point mutation, Phe309Ser, resulted in 3-fold higher expression of full-length FVIII. We hypothesized that this point mutation would further enhance the secretion of the B-domain variants. We prepared a hybrid construct, F309S/226aa/N6, and performed transient transfection into COS-1 cells. Cell media were collected from 24 to 60 hours after transfection and analyzed for expression by activity and antigen analysis (Figure 5A-B). Results were expressed relative to BDD-FVIII transfected in parallel. The 226aa/N6 construct was expressed an average of 8.5-fold that of BDD-FVIII expression as measured by one-stage activity. There was a further increase in secretion observed for the F309S/226aa/N6 mutant to an average of 14.3-fold BDD-FVIII expression. Similar enhancement in secretion was observed as measured by 2 different FVIII-specific antigen assays. Thus, there was an additive effect of the A1-domain mutation to the secretion advantage of the B-domain variant. This observation for F309S/226aa/N6 within COS-1 cells was also confirmed in similar transfection experiments within CHO cells (Figure 3A-B).
A FVIII A1-domain mutation further enhances expression of FVIII B-domain variants in COS-1 cells. One-stage activity (A) and FVIII antigen (B) for FVIII A1-domain/B-domain variant hybrid transiently expressed in COS-1 monkey kidney cells by transfection. One-stage activity was determined by aPTT-based assay. FVIII antigen was determined by 2 different commercial ELISAs, Asserachrom FVIII:C Ag (□) and FVIII:C-EIA (▪). The data were obtained from assaying the conditioned medium at 60 hours following transfection and compared with BDD-FVIII control. Data presented are the mean of several independent experiments (n ≥ 3), and the error bars represent the standard deviation.
A FVIII A1-domain mutation further enhances expression of FVIII B-domain variants in COS-1 cells. One-stage activity (A) and FVIII antigen (B) for FVIII A1-domain/B-domain variant hybrid transiently expressed in COS-1 monkey kidney cells by transfection. One-stage activity was determined by aPTT-based assay. FVIII antigen was determined by 2 different commercial ELISAs, Asserachrom FVIII:C Ag (□) and FVIII:C-EIA (▪). The data were obtained from assaying the conditioned medium at 60 hours following transfection and compared with BDD-FVIII control. Data presented are the mean of several independent experiments (n ≥ 3), and the error bars represent the standard deviation.
Metabolic labeling of factor VIII B-domain variants
We performed metabolic radiolabeling pulse-chase analysis to confirm the improved secretion observed by activity and antigen analysis. COS-1 cells transiently expressing FVIII WT, Phe309Ser, BDD-FVIII, 226aa/N6, and F309S/226aa/N6 were metabolically labeled with [35S]-methionine/[35S]-cysteine at 60 hours after transfection. Following pulse labeling, the cells were harvested while duplicate plates expressing each FVIII protein were chased for 4 hours in media containing excess unlabeled methionine. Pulse and chase cell extracts and chase cell media were harvested, and immunoprecipitated with a FVIII-specific antibody, and the immunoprecipitates were analyzed by 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Figure 6). FVIII WT was detected as a single-chain polypeptide of approximately 280 kDa within the cell and after 4 hours was observed in the conditioned media as a single chain, heavy chain, and light chain. The Phe309Ser mutation within full-length FVIII was detected within the pulse cell extract of similar size and intensity, demonstrating a similar rate of translation, but was of higher intensity within the cell media, supporting more efficient secretion. Additional lower intensity and faster mobility bands are evident within the cell extracts of both FVIII WT and full-length FVIII. These bands are not identified but are FVIII specific due to their absence from the mock-transfected cell extracts and likely represent FVIII polypeptides generated by proteolysis within their respective B domains. BDD-FVIII was observed as a single-chain polypeptide within the pulse cell extract migrating at the predicted size of 170 kDa. It was of higher intensity compared with FVIII WT consistent with increased synthesis of the primary translation product due to increased mRNA level. BDD-FVIII was observed within the chase cell media as a single chain of approximately 170 kDa. The intracellular forms of both B-domain variants, 226aa/N6 and the hybrid F309S/226aa/N6, were also observed as single-chain polypeptides of approximately 190 kDa and were synthesized at a rate similar to BDD-FVIII. The 226aa/N6 variant was observed within the chase cell media migrating as single chain of predicted size, however, at a much higher intensity (6-fold) compared with BDD-FVIII, consistent with a marked improvement in secretion efficiency. The F309S/226aa/N6 hybrid was observed within the chase cell media at an even higher intensity (10-fold), consistent with a further improvement in secretion efficiency. That the B-domain variants reported here were secreted as single-chain polypeptides was expected, since these constructs do not retain the paired basic amino acid clearing enzyme (PACE)/furin recognition sequence46 present in full-length FVIII at residues 1313 and 1648. The absence of PACE/furin processing has previously been demonstrated to have no effect on the synthesis, secretion, or activation of FVIII variants.33
Metabolic labeling reveals efficient expression of FVIII B-domain variants in COS-1 cells. COS-1 cells either mock transfected (m) or transiently transfected with FVIII WT, F309S, BDD-FVIII, 226aa/N6, and F309S/226aa/N6 plasmids were pulse labeled for 30 minutes with [35S]-methionine and duplicate plates chased for 4 hours with excess unlabeled methionine. Cell extracts were harvested after pulse labeling (P) and following the 4-hour chase, cell extracts (C) and cell media were harvested. [35S]-methionine-labeled proteins were separated by SDS-PAGE. Molecular-weight markers are indicated on the left. Single chain (SC), heavy chain (HC), and light chain (LC) of FVIII WT within the cell media are indicated on the right for comparison with the FVIII B-domain variants.
Metabolic labeling reveals efficient expression of FVIII B-domain variants in COS-1 cells. COS-1 cells either mock transfected (m) or transiently transfected with FVIII WT, F309S, BDD-FVIII, 226aa/N6, and F309S/226aa/N6 plasmids were pulse labeled for 30 minutes with [35S]-methionine and duplicate plates chased for 4 hours with excess unlabeled methionine. Cell extracts were harvested after pulse labeling (P) and following the 4-hour chase, cell extracts (C) and cell media were harvested. [35S]-methionine-labeled proteins were separated by SDS-PAGE. Molecular-weight markers are indicated on the left. Single chain (SC), heavy chain (HC), and light chain (LC) of FVIII WT within the cell media are indicated on the right for comparison with the FVIII B-domain variants.
Efficient, stable expression of factor VIII B-domain variant and A1-domain mutant/B-domain variant hybrid within CHO cells
CHO cell lines stably expressing either 226aa/N6 or F309S/226aa/N6 were prepared as previously described. High-expressing clones were easily identified prior to the addition of methotrexate. Individual clones were further selected through the addition of methotrexate. The highest expressing clones were expanded for analysis. Conditioned media (10 mL) were collected over 48 hours from a 100-mm plate (seeded at 1.5 × 106 cells per plate) containing the cell line stably expressing 226aa/N6 in 0.6 μM methotrexate. This yields FVIII activity by one-stage assay of 11 U/mL and FVIII protein by antigen analysis (Enzyme Research Laboratories) of 1.6 μg/mL. In a similar media collection, the cell line stably expressing F309S/226aa/N6 in 0.3 μM methotrexate yields FVIII activity by one-stage assay of 12 U/mL and 2.6 μg/mL FVIII protein by antigen analysis (Enzyme Research Laboratories). In comparison, previous reports for stable cell lines expressing BDD-FVIII forms generated yields of only approximately 1 U/mL with similar or higher levels of methotrexate selection.47-49 Thus the efficiency of secretion of these mutants leads to the generation of high-expression stable cell lines with relative ease and minimal methotrexate selection.
Factor VIII A1-domain mutant/B-domain variant hybrid expression in vivo
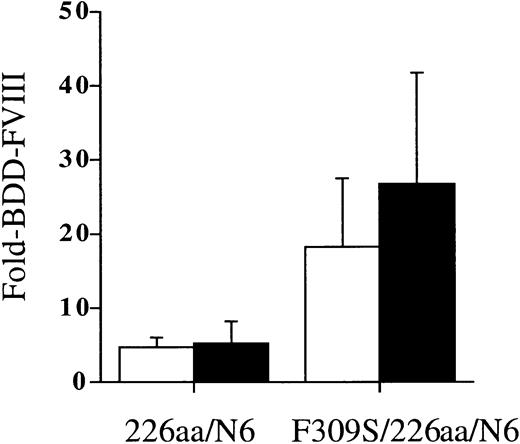
We next tested whether the secretion improvements for these FVIII variants observed in the COS-1 and CHO cells would also be observed in an in vivo heterologous expression system. We induced transient expression of the FVIII variants in the liver of hemophilia A (FVIII-/-) mice using a hydrodynamic tail-vein injection of plasmid DNA. Mice were injected with 100 μg plasmid DNA containing the 226aa/N6 and F309S/226aa/N6 constructs. Expression of the FVIII variants was analyzed from mouse plasma harvested by retro-orbital blood sampling at 24 hours and 48 hours after injection (Figure 7). Results were compared with littermate controls injected with BDD-FVIII. Activity was determined by 2-stage chromogenic activity assay. Average expression of BDD-FVIII was 0.62 U/mL at 24 hours and 48 hours (range, 0.26-2.4 U/mL). The 226aa/N6 variant was expressed 5-fold higher than BDD-FVIII at 24 and 48 hours after injection, whereas the F309S/226aa/N6 was expressed 18-fold and 27-fold higher than BDD-FVIII measured at 24 and 48 hours after injection, respectively. The improved expression for the F309S/226aa/N6 was statistically significantly different from the 226aa/N6 variant at both 24 and 48 hours after injection (P < .05). This suggests that the inherent limitations to FVIII expression in tissue culture systems are also relevant to in vivo expression and can be overcome with similar strategies.
A FVIII B-domain variant and a FVIII A1-domain/B-domain variant hybrid are expressed more efficiently in vivo in FVIII-/- mice. A FVIII B-domain variant and a FVIII A1-domain/B-domain variant hybrid were transiently expressed in vivo following a hydrodynamic tail-vein injection method into FVIII-/- mice. Plasmid DNA (100 μg) containing the FVIII variants diluted in 2.5 mL lactated Ringer solution was injected as a bolus over 10 seconds into the tail vein. Expression of the FVIII variants was analyzed from mouse plasma harvested by retro-orbital blood sampling at 24 hours (□) and 48 hours (▪) after injection. FVIII activity was determined by a 2-stage chromogenic assay and compared with mice injected similarly with BDD-FVIII as a control. Average expression of BDD-FVIII was 0.62 U/mL at 24 hours and 0.62 U/mL at 48 hours. Data presented are the mean of several independent experiments (n ≥ 3), and the error bars represent the standard deviation.
A FVIII B-domain variant and a FVIII A1-domain/B-domain variant hybrid are expressed more efficiently in vivo in FVIII-/- mice. A FVIII B-domain variant and a FVIII A1-domain/B-domain variant hybrid were transiently expressed in vivo following a hydrodynamic tail-vein injection method into FVIII-/- mice. Plasmid DNA (100 μg) containing the FVIII variants diluted in 2.5 mL lactated Ringer solution was injected as a bolus over 10 seconds into the tail vein. Expression of the FVIII variants was analyzed from mouse plasma harvested by retro-orbital blood sampling at 24 hours (□) and 48 hours (▪) after injection. FVIII activity was determined by a 2-stage chromogenic assay and compared with mice injected similarly with BDD-FVIII as a control. Average expression of BDD-FVIII was 0.62 U/mL at 24 hours and 0.62 U/mL at 48 hours. Data presented are the mean of several independent experiments (n ≥ 3), and the error bars represent the standard deviation.
Discussion
The recombinant DNA technology era has enabled manufacturers to increase the production of FVIII concentrates to try to meet the needs of patients with hemophilia A. Although most patients receive FVIII concentrates in response to hemorrhagic complications, prophylaxis is increasingly used as a therapeutic strategy. Prophylaxis is defined as the regular infusion of factor concentrates with the aim of preventing bleeding.4 The published strategies that have shown a significantly reduced rate of joint deterioration on physical and x-ray examination have used 25 to 40 IU FVIII/kg infused on alternate days (minimum 3 days/week).50-54 The increased use of prophylactic strategies to prevent the progression of joint disease in hemophilia A created a demand for FVIII concentrates that could not be met by donor plasma pools and plasma purification techniques. In addition, patients with hemophilia were the most affected by the transmission of infectious agents through plasma-derived factor concentrates in the 1980s. Dramatic improvements in donor plasma pool screening and purification techniques have enabled the production of safe plasma-derived FVIII concentrates. However, over the past 10 years, rFVIII has become the preferred form of factor replacement for patients with hemophilia A.3 Current developments in rFVIII technology have enabled the production of rFVIII devoid of any human protein exposure during the cell culture fermenting process and in final formulation.55 All of these technological advancements increased costs to manufacturers, and FVIII replacement remains a very expensive therapeutic with the average patient using up to $100 000 of FVIII concentrate per year.56 There has been continued interest in the bioengineering of rFVIII with improved function to overcome some of the limitations in current treatment and the high costs of therapy.57,58 It is possible that some of the costs associated with rFVIII production could be reduced with improved efficiency of FVIII expression within the mammalian expression systems currently used (CHO and BHK cells).
The first and only bioengineered FVIII molecule to come to commercial production so far was ReFacto (moroctocog alfa, Wyeth, Cambridge, MA). This was a B-domain deletion of residues 744 to 1637 such that Ser743 was fused to Gln1638 creating a 14-residue B-domain linker between the A2 and A3 domains. This portion of B domain contained no consensus sites for N-linked glycosylation. With deletion of the B domain, ReFacto was observed to be less prone to proteolytic degradation, therefore no addition of plasma-derived albumin was needed for stabilization of the final product.59 This bioengineered BDD-FVIII has undergone extensive biochemical comparison with full-length FVIII.60 It was shown to have a high specific activity of 14 000 IU/mg as measured by a 2-stage chromogenic FVIII assay and had comparable interactions with thrombin and activated protein C. It showed comparable ability to participate as a cofactor in factor Xa generation in a mixture of factors IXa and X, phospholipid, and calcium and good binding capacity for phospholipid vesicles and VWF. Clinical studies demonstrated that ReFacto is a safe, well-tolerated, and effective treatment for hemophilia whether given as on-demand therapy for hemorrhagic complications, administered in routine or intermittent prophylaxis, or used for surgical management.61 Most significantly, rates of inhibitor formation in previously untreated patients with hemophilia A were similar to those observed with full-length rFVIII concentrates.62
Although these studies support that the B domain is dispensable for FVIII procoagulant function and that BDD-FVIII can have unique advantages, some biologic differences remain. Li and Gabriel63 studied the affinity of FVIII and BDD-FVIII for activated platelets and found that unactivated BDD-FVIII bound with tighter affinity than native FVIII (dissociation constant [Kd] = 10.4 nM versus 5.1 nM). Further activation of the BDD-FVIII yielded even higher affinity binding (Kd = 2.1 nM) similar to that observed for thrombin-activated FVIII (Kd = 1.7 nM). These results demonstrated that the binding of FVIII to platelets increases with each activation step largely through release of the B domain and was consistent with the multistate binding of FVIII.64 In addition, FVIII assay discrepancies have been reported in which one-stage clotting assays of ReFacto activity using commercial aPTT reagents are consistently lower, approximately 50%, than that measured by one-stage chromogenic assays.65,66 This assay discrepancy has been reported in vitro as well as ex vivo after plasma analysis from treated patients. Mikaelsson et al65 demonstrated that at low levels of phospholipid, the one-stage activity of ReFacto exceeded the chromogenic result. Further, when mixtures of phosphatidylserine (PS) and phosphatidylcholine were used as the source of phospholipid, the one-stage activity results were in agreement with the chromogenic results as long as the content of PS was maintained below 10%. This was an unexpected observation following deletion of the B domain and the mechanism is as of yet unexplained. In our studies in this report, all the activity measurements were expressed relative to BDD-FVIII so no assay discrepancy would be apparent between the various B-domain variants.
Other BDD-FVIII variants have undergone biochemical characterization and have yielded some important insights. LA-VIII is a deletion of B-domain residues 760 through 1639 and retains one consensus site for N-linked glycosylation at residue 757 at the junction of the deletion.31,32 Following transient transfection into COS-1 cells, the LA-VIII construct yielded a 17-fold increase in mRNA compared with FVIII WT and a 15-fold increase in primary translation product. However, the marked increase in mRNA and primary translation product led to only a 1.3-fold increase in the amount of secreted protein, consistent with a defect in efficient transfer of the primary translation product from the ER to the Golgi.
The goal of the current study was to build on the advantages of BDD-FVIII (increased mRNA and protein synthesis, decreased proteolytic degradation upon final formulation) by increasing the secretion efficiency. We observed that the addition of a short B-domain sequence (optimally 226 aa) with several consensus sites for N-linked glycosylation (optimally 6) resulted in up to a 10-fold increase in secretion compared with BDD-FVIII. Additional incorporation of a previously described point mutation (Phe309Ser) within a putative BiP binding region of the FVIII A1-domain further increased secretion. The advantages of these B-domain variants was observed in both COS-1 and CHO cell lines as well as in vivo using a transient liver expression strategy in the hemophilia A mouse model. This is most significant as it suggests that the processes limiting FVIII expression in transfected cells in vitro are also relevant in vivo. We hypothesize that at least a minimal portion of B-domain sequence containing some N-linked oligosaccharides is necessary to facilitate proper folding through chaperone interactions and to take advantage of facilitated transport from the ER to Golgi through interaction with LMAN1.
Thus, FVIII with minimal B-domain content could provide more efficient expression in vitro to improve the efficiency of commercial production of BDD-FVIII. The ability to produce recombinant FVIII variants such as these with high efficiency would lead to higher yields with current manufacturing strategies, reducing factor costs and increasing product availability. This will of course require further rigorous biochemical characterization to determine bioequivalence of these bioengineered FVIII variants compared with currently approved BDD-FVIII and full-length FVIII recombinant products.
In addition, most gene therapy applications for hemophilia A have relied on BDD-FVIII due to its advantages of reduced overall size of the cDNA for packaging into viral vectors and increased mRNA levels. However, in vivo results have remained poor, with plasma levels typically undetectable (< 1%) or in the 1% to 4% range in a few patients transiently.7,67 Thus a FVIII variant with minimal B domain could provide a significant advantage over BDD-FVIII using currently available gene transfer strategies. Incorporation of these modifications into the previous vectors used in the clinic may increase expression 10-fold, into the therapeutic range.
There remains concern, however, that bioengineered molecules with juxtaposition of novel amino acid sequences could lead to neoantigenicity. This was a concern with the first commercial BDD-FVIII, ReFacto, but was potentially minimized by engineering the B-domain junction sequence to create a fusion of Ser743 to Gln1638. This “SQ” link promotes efficient intracellular cleavage of the primary single-chain translation product of 170 kDa into a dimer of 90 and 80 kDa chains.60 The B-domain variants studied here are secreted as single-chain polypeptides and have novel engineered B-domain junctions. Thus, their potential neoantigenicity would have to be tested within established models to further address this concern.
This study also provides some insights into the previously unknown biologic function of the B domain. Although the B domains of FV and FVIII share no amino acid homology they have a similar concentration of N-linked oligosaccharides. The presence of the B domain and a similar predominance of N-linked oligosaccharides have been conserved among a number of species' FVIII analyzed thus far.31,68,69 FVIII is cotranslationally translocated into the lumen of the ER where it folds and assembles into its tertiary structures. These reactions are facilitated by enzymes and molecular chaperones that interact with folding intermediates of FVIII.70 This mechanism ensures that only completely folded FVIII is transported through the secretion pathway and serves as “quality control.” Properly folded and assembled FVIII molecules are released to transit to the Golgi apparatus. Improperly folded FVIII proteins are recognized by chaperones and are not released, but are rather transferred into degradative pathways.12,71 Our previous studies have highlighted a role for the ER lectin chaperones, calnexin and calreticulin, in this process, both of which display substrate specificity for glycoproteins containing partially glucosylated N-linked core oligosaccharides.71 Recent work has also demonstrated that LMAN1 directly interacts with FVIII and that high mannose-containing oligosaccharides, mostly clustered within the B domain, provide a significant contribution to this interaction.40,72 Thus the N-linked oligosaccharides can participate in the folding interactions within the ER as well as potentially help facilitate ER-Golgi transport. Interestingly, the 226aa/N1 construct, in which 5 of the consensus sites for N-linked glycosylation were mutated, still displayed improved secretion over BDD-FVIII. This suggests that N-linked oligosaccharides outside of the B domain or perhaps protein-protein interactions with LMAN1 are also important. This was also suggested from the observations on LMAN1-FVIII interaction72 and from a recent crystal structure of the carbohydrate recognition domain of p58, a rat homologue of LMAN1.73 That work identified a surface patch of conserved residues on the opposite side of the mannose-binding site that may contribute to ligand-binding specificity through protein-protein interactions.
Other bioengineering strategies have been directed at improving other aspects of FVIII functional properties including increasing the potency, stability, and resistance to inactivation.58 In addition, porcine-human hybrid BDD-FVIII variants have been generated that exhibit reduced antigenicity to inhibitor plasmas providing promise for alternatives for hemophilia patients with established inhibitors or perhaps to reduce the overall immunogenicity of FVIII thus reducing the incidence of new inhibitors.74 These improvements to FVIII functional properties could also be combined with genetic modifications, such as those shown in this study, thus enabling high efficiency secretion along with other bioengineered improvements.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-10-3591.
Supported by the National Heart Lung and Blood Institute (HL52173 and HL57346) to R.J.K., a Research Fellowship from the Heart and Stroke Foundation of Canada to M.A.C., and a National Hemophilia Foundation Career Development Award in Hemostasis to S.W.P.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank Agustin Calatroni for assistance with statistical analysis.

![Figure 6. Metabolic labeling reveals efficient expression of FVIII B-domain variants in COS-1 cells. COS-1 cells either mock transfected (m) or transiently transfected with FVIII WT, F309S, BDD-FVIII, 226aa/N6, and F309S/226aa/N6 plasmids were pulse labeled for 30 minutes with [35S]-methionine and duplicate plates chased for 4 hours with excess unlabeled methionine. Cell extracts were harvested after pulse labeling (P) and following the 4-hour chase, cell extracts (C) and cell media were harvested. [35S]-methionine-labeled proteins were separated by SDS-PAGE. Molecular-weight markers are indicated on the left. Single chain (SC), heavy chain (HC), and light chain (LC) of FVIII WT within the cell media are indicated on the right for comparison with the FVIII B-domain variants.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/9/10.1182_blood-2003-10-3591/6/m_zh80090460720006.jpeg?Expires=1767711227&Signature=GBO6304vt1tP2GMhvcxSbk8w4g-zLvAkhrJ9-WaY~Op1tXB1inToS~6at7sd6-38tbCDtXLtWkv4kbc3ReGFL4XXP0WexTfklpMkCqcp9r0968Wo37-Xt-Q53qbN9LkC~xFXpbXi8aQXlSlVq4YG-F5BvhEGMillOgTITNlW9QjzWdRVlkDQYQlRNTNx6w7nnKahWxDAMmBUcnebMsR6H8mzK7qeO6zVQXiduuucFqgH2SC2-SIncNUzxRrnNyKOlO5ByLPaslBOwRgky6WDle5P1e0twQFjsDLnaOeyD5ZZEYoMYa3CU-80JAUBOAk93n~UMGJiO2rIT6am6jEtCg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal