Abstract
Platelet adhesion to von Willebrand factor (VWF) activates αIIbβ3, a prerequisite for thrombus formation. However, it is unclear whether the primary VWF receptor, glycoprotein (GP) Ib-IX-V, mediates αIIbβ3 activation directly or through other signaling proteins physically associated with it (eg, FcR γ-chain), possibly with the contribution of other agonist receptors and of VWF signaling through αIIbβ3. To resolve this question, human and GP Ibα transgenic mouse platelets were plated on dimeric VWF A1 domain (dA1VWF), which engages only GP Ib-IX-V, in the presence of inhibitors of other agonist receptors. Platelet adhesion to dA1VWF induced Src kinase-dependent tyrosine phosphorylation of the FcR γ-chain and the adapter molecule, ADAP, and triggered intracellular Ca2+ oscillations and αIIbβ3 activation. Inhibition of Ca2+ oscillations with BAPTA-AM prevented αIIbβ3 activation but not tyrosine phosphorylation. Pharmacologic inhibition of protein kinase C (PKC) or phosphatidylinositol 3-kinase (PI 3-kinase) prevented αIIbβ3 activation but not Ca2+ oscillations. Inhibition of Src with 2 distinct compounds blocked all responses downstream of GP Ib-IX-V under static or flow conditions. However, dA1VWF-induced responses were reduced only slightly in GP Ibα transgenic platelets lacking FcR γ-chain. These data establish that GP Ib-IX-V itself can signal to activate αIIbβ3, through sequential actions of Src kinases, Ca2+ oscillations, and PI 3-kinase/PKC. (Blood. 2004;103:3403-3411)
Introduction
The initial adhesion of platelets to the extracellular matrix of injured blood vessels is mediated at high shear rates by von Willebrand factor (VWF) interaction with glycoprotein (GP) Ib-IX-V.1 Additionally, engagement of GP Ib-IX-V by VWF is thought to contribute to stable platelet adhesion by generating intracellular signals necessary for activation of αIIbβ3. Indeed, αIIbβ3 activation and platelet thrombus formation have been observed under a number of experimental conditions following platelet interaction with VWF.2-6 Furthermore, specific biochemical responses have been documented under the same conditions, including induction of Ca2+ fluxes and activation of tyrosine, serine-threonine, and lipid kinases.7,8 Consequently, GP Ib-IX-V may function as a signaling receptor and an adhesion receptor.
GP Ib-IX-V is a complex of 4 transmembrane polypeptides.9-11 Although the cytoplasmic tail of each subunit lacks a catalytic domain, each may interact directly or indirectly with proteins that can transmit intracellular signals. For example, the cytoplasmic tail of GP Ibα can interact directly with filamin, GP Ibα and Ibβ with 14-3-3-ζ, and GP Ibβ and GP V with calmodulin.12-15 GP Ib-IX-V can be coimmunoprecipitated from platelets with signaling molecules, including Src family kinases,16 phosphatidylinositol 3-kinase (PI 3-kinase)17 and Src homology 2 domain-containing inositol polyphosphate 5-phosphatase-2 (SHIP-2).18 Furthermore, VWF-dependent platelet activation may require localization of GP Ib-IX-V to lipid rafts, membrane structures implicated in cellular signaling.19
Although there is good evidence for a functional link between GP Ib-IX-V and αIIbβ3, 2 critical questions remain: Is GP Ib-IX-V itself capable of transducing signals in platelets, and, if so, to what extent do these signals participate in the activation of αIIbβ3? Several factors have conspired to make it difficult to answer these questions. First, a subpopulation of GP Ib-IX-V in platelets may be associated with immunoreceptor tyrosine activation motif (ITAM)-bearing proteins that can signal in their own right, including the FcγRIIA receptor and the FcR γ-chain.20,21 Second, platelets express numerous receptors for soluble and matrix-associated agonists, and some of the agonists (eg, adenosine diphosphate [ADP], thromboxane A2) are released by adherent platelets.22 Third, VWF not only interacts with GP Ib-IX-V through its A1 domain but also with αIIbβ3 through its C1 domain.1 Thus, outside-in signals stimulated by VWF binding to αIIbβ3 can confound analysis of GP Ib-IX-V signaling.23 Finally, studies of GP Ib-IX-V signaling under static conditions have frequently utilized nonphysiological mediators, such as botrocetin or ristocetin, to promote VWF binding to GP Ib-IX-V, complicating data interpretation further.
Thus, despite the publication of many important studies on the molecular contributors to signaling responses downstream of GP Ib-IX-V, most to date have failed to consistently employ conditions to avoid platelet activation through other costimulatory receptors. The present study has employed these conditions stringently, enabling us to provide an updated working model of GP Ib-IX-V signaling to αIIbβ3. To avoid the experimental complications inherent in using VWF, human or GP Ibα transgenic mouse platelets were exposed to highly purified dimeric von Willebrand factor A1 domain (dA1VWF), which recognizes GP Ib-IX-V but not αIIbβ3.24 In addition, studies were conducted without ristocetin or botrocetin and under conditions in which platelet stimulation through other agonist/receptor pairs was effectively prevented. The results establish that GP Ib-IX-V can generate specific intracellular signals that promote the activation of αIIbβ3, and they identify a hierarchy of the signaling reactions involved in this process.
Materials and methods
Reagents and antibodies
Preparation of dA1VWF (spanning residues 445 to 732),25 human plasma VWF,26 and antibodies or Fab fragments against GP Ibα (AP-1)27 and activated αIIbβ3 (PAC-1, POW-2)28,29 have been described. Monoclonal antibody 1B5 against murine αIIbβ3 was from Dr Barry Coller (Rockefeller University, New York, NY)30 ; polyclonal antibody against the adapter molecule ADAP was from Dr Gary Koretsky (University of Pennsylvania, Philadelphia)31 ; polyclonal antibody against FcR γ-chain and monoclonal antibody 4G10 to phosphotyrosine were from Upstate Biotechnology (Lake Placid, NY); monoclonal antiphosphotyrosine antibody PY20 was from BD Biosciences (San Diego, CA); and monoclonal antibodies to Syk and Fyn were from Santa Cruz Laboratories (Santa Cruz, CA). SU-6656 and PP2 against Src family kinases, PP3, the inactive analog, bisindolylmaleimide I, a general protein kinase C (PKC) inhibitor and its control, bisindolylmaleimide V, were from Calbiochem (San Diego, CA). The nonhydrolyzable adenosine 3′,5′-cyclic monophosphate (cAMP) analog, c-BIMPS (5,6 dichlorobenzimidazole-1-b-d-ribofuranosyl-3′,5′-mono-phosphorothioate, Sp-isomer), was from Biolog Life Sciences (Bremen, Germany).32 MRS-2216 and ARC-69931MX against the P2Y1 and P2Y12 receptors, respectively, were from Dr Kenneth Jacobson (National Institutes of Health [NIH], Bethesda, MD)33 and Astra Zeneca (Wilmington, DE).34 Inhibitors of ligand binding to αIIbβ3, Ro 44-9883 ((1-(N-p-amidinobenzoyl)-l-tyrosyl)-4-piperidinyl(oxy)acetic acid) and Integrilin, were from Dr Beat Steiner (Roche, Basel, Switzerland)35 and Dr David Phillips (Cor Therapeutics, South San Francisco, CA),36 respectively. Oregon Green, Fura Red, BAPTA-AM, and Alexa-488 were from Molecular Probes (Eugene, OR). SuperSignal WestPico reagent was from Pierce Chemicals (Rockford, IL). All other reagents were from Sigma Chemical (St Louis, MO).
Mouse strains
Platelet preparation
Whole blood from healthy, drug-free donors was anticoagulated with acid-citrate-dextrose (ACD), and washed platelets were prepared in Tyrode or Walsh buffer (137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2 · 6H2O, 3.3 mM NaH2PO4 · H2O, 3.8 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 0.1% bovine serum albumin [BSA], 0.1% dextrose).39 Mouse blood was obtained by retro-orbital bleeds into ACD. Whole mouse blood was diluted with an equal volume of modified Tyrode, pH 6.5, containing ACD and 1.5 U/mL apyrase and centrifuged for 2 minutes at 450g. Platelet-rich plasma was removed, centrifuged for 4 minutes at 1200g in the presence of 0.5 U/mL apyrase and 1 μM prostaglandin E1 (PGE1), and platelet pellets were resuspended in Tyrode or Walsh buffer.
Calcium transients
Intracellular free calcium was assessed by a dual ratiometric method using confocal microscopy and the calcium indicator dyes, Oregon Green and Fura Red.40 Platelets were washed once and resuspended to 5 × 107/mL in Tyrode buffer with probenecid. After addition of 2 U/mL apyrase, 10 μM Integrilin, and 10 μM indomethacin, platelets were sedimented at 80g for 4 minutes onto dA1VWF-coated coverslips. Platelets loaded with indicator dyes were viewed using a Bio-Rad (Hercules, CA) confocal microscope equipped with a 60 × Olympus Uplan Apo aqueous immersion lens. Images were acquired simultaneously in both fluorescence channels at 2-second intervals over a period of 40 seconds. When compared in preliminary experiments with data acquired at 1-second intervals, similar results were obtained. Fluorescence intensity was determined using Image-Pro Plus Software (Media Cybernetics, Carlsbad, CA) and converted to intracellular free calcium concentration, (Ca2+)i.40 To determine specificity of calcium responses, cells were allowed to settle onto dA1VWF in the presence or absence of a function-blocking GP Ibα antibody, AP-1.27 When inhibitors and pharmacologic agents were used, platelets were preincubated for 10 to 20 minutes in their presence before plating onto dA1VWF. A minimum of 80 platelets per experimental condition were used to calculate mean (Ca.2+)i, and data from at least 20 platelets (400 experimental points) were used to track calcium oscillations.
Studies of intracellular calcium changes under conditions of flow were performed as described.41 Briefly, platelets were loaded with Fluo-3 AM, suspended with homologous plasma and washed red cells, and perfused for 90 seconds over immobilized multimeric VWF at a shear rate of 3000 s-1. (Ca2+)i of surface-interacting platelets was monitored in real time for the next 30 seconds during translocation. Rapid increases in (Ca2+)i, between 0.4 μM and 2 μM, were designated α peaks; peaks less than 0.4 μM were designated β peaks; and prolonged peaks, more than 2 to 3 μM, were designated γ peaks.41 To assess the role of Src family kinases in Ca2+ responses under flow, platelets were preincubated for 10 minutes with PP2, PP3, or dimethyl sulfoxide (DMSO) vehicle before perfusion.
Analysis of protein tyrosine phosphorylation
One hundred-millimeter bacterial culture plates were precoated at room temperature for 1 hour with 12 μg/mL dA1VWF, 10 mg/mL BSA, or 100 μg/mL fibrinogen.42 After blocking with heat-denatured BSA, 4.5 × 108 platelets in 1.5 mL Walsh buffer containing 2 U/mL apyrase, 10 μM Ro 44-9883, and 10 μM indomethacin were added for the indicated periods at 37°C in a CO2 incubator. For studies examining the role of specific signaling intermediates, platelets were preincubated with additional relevant inhibitors for 10 to 20 minutes prior to plating. Platelets adherent to dA1VWF or fibrinogen were washed gently with phosphate-buffered saline (PBS) and then solubilized on ice in Nonidet P-40 (NP-40) buffer (0.5% NP-40, 50 mM NaCl, 50 mM Tris [tris(hydroxymethyl)aminomethane], pH 7.4, “Complete” protease inhibitor, 1 mM sodium vanadate, 1 mM sodium fluoride, 0.5 mM leupeptin, 100 μg/mL aprotinin, and 0.25 mg/mL Pefabloc). Nonadherent cells from BSA plates were sedimented at 13 000g for 2.5 seconds in a microcentrifuge, and platelet pellets were lysed immediately in NP-40 buffer. Detergent lysates were clarified by centrifugation at 13 000g for 15 minutes at 4°C and subjected to immunoprecipitation, polyacrylamide gel electrophoresis, and Western blotting.43,44
Measurements of αIIbβ3 affinity state
Binding of PAC-1 to human platelets or POW-2 Fab to mouse platelets adherent to dA1VWF was evaluated by confocal microscopy.39 Glass coverslips were incubated with 15 μg/mL dA1VWF or with 1% BSA for 2 hours at room temperature. After washing and blocking with 1% denatured BSA, cells were plated onto the coverslips for 40 minutes at 37°C in a CO2 incubator, routinely in the presence of PAC-1 or POW-2, 2 U/mL apyrase, 10 μM indomethacin, 1 μM ARC-69931MX, and 12 μM MRS-2216. Nonspecific antibody binding was assessed in the presence of 10 μM Ro 44-9883 for human platelets and 5 mM EDTA (ethylenediaminetetraacetic acid) for murine platelets. In some cases, platelets were preincubated with additional selective inhibitors for 10 to 20 minutes prior to plating. Platelets adherent to dA1VWF-coated coverslips were washed gently with PBS, fixed with 3.7% formaldehyde, and stained with fluorescein isothiocyanate (FITC)-conjugated antimurine immunoglobulin M (IgM) (for PAC-1) or Alexa-488-conjugated antimurine H + L chain-specific IgG (for POW-2 Fab). Platelets incubated over BSA were sedimented directly in the presence of formaldehyde. Platelet fluorescence intensity in confocal micrographs was calculated using Image-Pro Plus Software, with 50 to 300 cells included for each experimental condition. The population distribution for antibody binding was represented in a cumulative fluorescence distribution plot, which relates a given fluorescence value with the percentage of platelets expressing that value or lower. Statistical analyses were performed using the Student t test.
Results
Platelet calcium oscillations are triggered directly through GP Ib-IX-V
To evaluate GP Ib-IX-V signaling without a contribution from other receptors, platelets were allowed to adhere to dA1VWF, a dimeric form of the VWF A1 domain. In contrast to VWF, dA1VWF recognizes GP Ib-IX-V but not αIIbβ3. Experiments were routinely conducted in the presence of inhibitors designed to prevent signaling through αIIbβ3 and ADP and thromboxane A2 receptors. Inhibitor efficacy was assessed in preliminary studies and showed that (1) activation of αIIbβ3 by 10 μM ADP or 5 μM U46619, assessed by binding of FITC-fibrinogen or FITC-PAC-1, was prevented; and (2) αIIbβ3 failed to coimmunoprecipitate with Syk, a proximal response to ligand binding to αIIbβ3.45
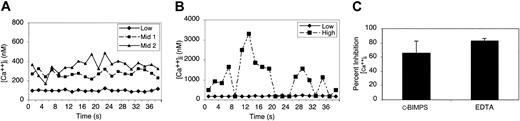
Because an increase in (Ca2+)i is required for αIIbβ3 activation in response to VWF binding GP Ib-IX-V,2,5,40 we first examined whether the adhesion of human platelets to dA1VWF triggers changes in intracellular free Ca2+ concentration. Under static conditions, individual platelets exhibited oscillatory increases in calcium concentration immediately after contact with immobilized dA1VWF, which continued for at least 30 minutes. As routinely measured starting 5 minutes after plating, Ca2+ oscillations in dA1VWF-adherent platelets were dependent on GP Ib-IX-V, because no oscillations were observed in platelets that failed to adhere to a BSA-coated surface or to dA1VWF in the presence of anti-GP Ibα antibody AP-1. Calcium responses to dA1VWF were heterogeneous. Although more than 80% to 95% of platelets showed some increase in (Ca2+)i above baseline (50 to 100 nM), 15% to 20% of platelets showed levels minimally over baseline (low), and another 50% to 60% cycled between 300 and 600 nM (mid) (Figure 1A). Approximately 15% of the platelet population showed major, sudden, and more sustained increases in (Ca2+)i (high) (Figure 1B), reminiscent of the γ peaks that have been reported following stable platelet adhesion to VWF under shear flow.41 These results indicate that adhesion of platelets to dA1VWF is sufficient to trigger calcium oscillations through GP Ib-IX-V.
Intracellular calcium oscillations in dA1VWF-adherent platelets. As described in “Materials and methods,” washed platelets loaded with Oregon Green and Fura Red were plated onto coverslips coated with dA1VWF in the presence of inhibitors of signaling through αIIbβ3 and ADP and thromboxane A2 receptors. After 5 minutes, confocal images were acquired serially and (Ca2+)i was quantified for individual platelets. (A) Typical (Ca2+)i changes occurring in most platelets showed oscillations either just over baseline (low) ( ) or within an intermediate range (mid) (▴, ▪). (B) Approximately 15% of platelets showed more sustained, high-amplitude calcium oscillations reaching several μM (high). Results are representative of 10 separate experiments. (C) dA1VWF-dependent changes in (Ca2+)i are regulated by cyclic AMP and Ca2+ influx. Platelets were allowed to adhere to dA1VWF in the presence of 400 μM c-BIMPS to increase cyclic AMP or 5 mM EDTA to block Ca2+ influx. Results are expressed as a percent of the dA1VWF-mediated response in the absence of these 2 inhibitors. Data represent means ± SEM of 3 experiments.
) or within an intermediate range (mid) (▴, ▪). (B) Approximately 15% of platelets showed more sustained, high-amplitude calcium oscillations reaching several μM (high). Results are representative of 10 separate experiments. (C) dA1VWF-dependent changes in (Ca2+)i are regulated by cyclic AMP and Ca2+ influx. Platelets were allowed to adhere to dA1VWF in the presence of 400 μM c-BIMPS to increase cyclic AMP or 5 mM EDTA to block Ca2+ influx. Results are expressed as a percent of the dA1VWF-mediated response in the absence of these 2 inhibitors. Data represent means ± SEM of 3 experiments.
Intracellular calcium oscillations in dA1VWF-adherent platelets. As described in “Materials and methods,” washed platelets loaded with Oregon Green and Fura Red were plated onto coverslips coated with dA1VWF in the presence of inhibitors of signaling through αIIbβ3 and ADP and thromboxane A2 receptors. After 5 minutes, confocal images were acquired serially and (Ca2+)i was quantified for individual platelets. (A) Typical (Ca2+)i changes occurring in most platelets showed oscillations either just over baseline (low) ( ) or within an intermediate range (mid) (▴, ▪). (B) Approximately 15% of platelets showed more sustained, high-amplitude calcium oscillations reaching several μM (high). Results are representative of 10 separate experiments. (C) dA1VWF-dependent changes in (Ca2+)i are regulated by cyclic AMP and Ca2+ influx. Platelets were allowed to adhere to dA1VWF in the presence of 400 μM c-BIMPS to increase cyclic AMP or 5 mM EDTA to block Ca2+ influx. Results are expressed as a percent of the dA1VWF-mediated response in the absence of these 2 inhibitors. Data represent means ± SEM of 3 experiments.
) or within an intermediate range (mid) (▴, ▪). (B) Approximately 15% of platelets showed more sustained, high-amplitude calcium oscillations reaching several μM (high). Results are representative of 10 separate experiments. (C) dA1VWF-dependent changes in (Ca2+)i are regulated by cyclic AMP and Ca2+ influx. Platelets were allowed to adhere to dA1VWF in the presence of 400 μM c-BIMPS to increase cyclic AMP or 5 mM EDTA to block Ca2+ influx. Results are expressed as a percent of the dA1VWF-mediated response in the absence of these 2 inhibitors. Data represent means ± SEM of 3 experiments.
GP Ib-IX-V-mediated calcium oscillations require Src kinases but not PKC or PI 3-kinase
Cyclic AMP and cyclic AMP-dependent protein kinase negatively regulate platelet responses to soluble agonists,46 and they may also modulate GP Ib-IX-V functions.47,48 To test whether cyclic AMP can affect GP Ib-IX-V-mediated calcium oscillations, cells were plated on dA1VWF in the presence of c-BIMPS, a nonhydrolyzable cAMP analog. This blocked mean increases in (Ca2+)i by 66% (P < .05) (Figure 1C), and it eliminated almost all of the sustained, high Ca2+ peaks. Moreover, chelation of extracellular calcium with EDTA reduced mean (Ca2+)i by 83% (P < .001) and eliminated the high-end calcium peaks. Complete quenching of (Ca2+)i with 40 μM BAPTA-AM eliminated all Ca2+ oscillations and reduced calcium concentrations to less than 50 nM. These results suggest that Ca2+ oscillations triggered through GP Ib-IX-V result from a combination of calcium influx and mobilization and are negatively regulated by cyclic AMP.
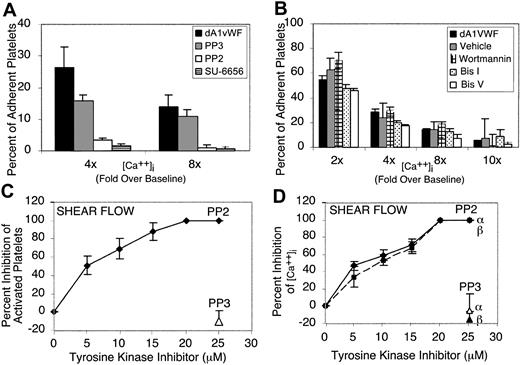
Platelet interaction with VWF leads to activation of one or more Src family kinases and their recruitment to GP Ib-IX-V.16 To determine whether Src kinases are required for dA1VWF-induced Ca2+ oscillations, cells were plated on dA1VWF in the presence of 1 μM SU-6656 or 5 μM PP2, chemically distinct, selective inhibitors of the Src family kinases present in platelets.45,49 When the percentage of adherent platelets exhibiting calcium elevations more than 4-fold and 8-fold over baseline was quantified, SU-6656 and PP2 each blocked increases in (Ca2+)i by more than 80% (P < .001) (Figure 2A). On the other hand, PP3, an inactive analog of PP2, had no effect. Blockade of PI 3-kinase with 100 nM wortmannin or PKC with 12 μM bisindolylmaleimide I had no significant effect on dA1VWF-induced Ca2+ oscillations (Figure 2B).
Roles of Src, PI 3-kinase, and PKC in calcium fluxes induced by platelet adhesion to dA1VWF. Platelets were studied in the presence of inhibitors of signaling through αIIbβ3 and ADP and thromboxane A2 receptors. (A-B) Platelets under static conditions were allowed to adhere to dA1VWF in the presence or absence of (A) Src family kinase inhibitors PP2 (5 μM) or SU-6656 (1 μM), or 5 μM PP3 as a negative control, or (B) 100 nM wortmannin to block PI 3-kinase, wortmannin vehicle control, 12 μM bisindolylmaleimide I (Bis I) to block PKC, or 12 μM bisindolylmaleimide V (Bis V), an inactive congener. Results are expressed arbitrarily as the percent of cells showing intracellular calcium levels more than 2-fold to 10-fold over baseline. (C-D) Shear flow. Platelets in homologous blood were perfused over immobilized multimeric VWF, and (Ca2+)i of surface-interacting platelets was monitored as described in “Materials and methods.” Shown are the inhibitory effects of PP2 on the percent of activated platelets (C) and on α and β Ca2+ peaks in activated platelets (D). PP3 was used as negative control. Values shown are the mean ± SD of 3 to 4 experiments.
Roles of Src, PI 3-kinase, and PKC in calcium fluxes induced by platelet adhesion to dA1VWF. Platelets were studied in the presence of inhibitors of signaling through αIIbβ3 and ADP and thromboxane A2 receptors. (A-B) Platelets under static conditions were allowed to adhere to dA1VWF in the presence or absence of (A) Src family kinase inhibitors PP2 (5 μM) or SU-6656 (1 μM), or 5 μM PP3 as a negative control, or (B) 100 nM wortmannin to block PI 3-kinase, wortmannin vehicle control, 12 μM bisindolylmaleimide I (Bis I) to block PKC, or 12 μM bisindolylmaleimide V (Bis V), an inactive congener. Results are expressed arbitrarily as the percent of cells showing intracellular calcium levels more than 2-fold to 10-fold over baseline. (C-D) Shear flow. Platelets in homologous blood were perfused over immobilized multimeric VWF, and (Ca2+)i of surface-interacting platelets was monitored as described in “Materials and methods.” Shown are the inhibitory effects of PP2 on the percent of activated platelets (C) and on α and β Ca2+ peaks in activated platelets (D). PP3 was used as negative control. Values shown are the mean ± SD of 3 to 4 experiments.
Under shear flow conditions, platelets adherent to VWF exhibit a series of Ca2+ oscillations described temporally as α/β and γ peaks.41 Defining dA1VWF-adherent platelets as activated if they showed at least one Ca2+ oscillation of the α/β type, PP2 was found to inhibit both the percentage of activated platelets (Figure 2C) and the mean (Ca2+)i (Figure 2D) in a dose-dependent fashion. In the presence of the αIIbβ3 antagonist, Ro 44-9883, γ peaks were completely blocked. Taken together, these results indicate that one or more Src family kinases, but not PI 3-kinase or PKC, regulate platelet Ca2+ oscillations triggered by ligand engagement of GP Ib-IX-V under static or flow conditions.
GP Ib-IX-V signals to the molecular adapter, ADAP
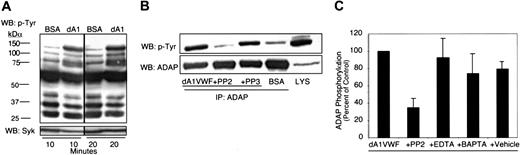
To begin to identify substrates of Src family kinases that might be situated directly downstream of GP Ib-IX-V, tyrosine-phosphorylated proteins from dA1VWF-adherent platelets were examined. Induction of tyrosine phosphorylation in platelet lysates was detectable as soon as 2 minutes after platelet attachment to dA1VWF (not shown). By 10 minutes, several protein bands in the range of 70 to 130 kDa had become tyrosine phosphorylated, but there was no inducible phosphorylation in platelets incubated over a BSA-coated matrix (Figure 3A). Based on previous studies with VWF, Syk (76 kDa) may represent one of the immunoreactive bands.50,51 The most prominent phosphotyrosine band induced by platelet adhesion to dA1VWF migrated at 130 kDa. This was identified by immunoprecipitation as ADAP, an adapter molecule also known as SLAP-130/FYB (Figure 3B), which has not been implicated previously in GP Ib-IX-V signaling. ADAP forms a complex with Syk and SLP-76 during cytoskeletal reorganization in platelets stimulated through αIIbβ3.52 ADAP phosphorylation in dA1VWF-adherent platelets was dependent on Src, because it was inhibited by PP2 but not PP3 (Figure 3B-C). Furthermore, ADAP could be coimmunoprecipitated with the Src kinase, Fyn, both before and after platelet adhesion to dA1VWF (not shown). Tyrosine phosphorylation of ADAP was not dependent on calcium fluxes, because it was not affected by 5 mM EDTA or 1 μM BAPTA-AM (Figure 3C). These data suggest that GP Ib-IX-V ligation activates one or more Src kinases, presumably including Fyn, resulting in phosphorylation of Src substrates, including Syk and ADAP.
Platelet adhesion to dA1VWF induces tyrosine phosphorylation. Human platelets adherent to dA1VWF or incubated over a BSA matrix were lysed and analyzed by Western blotting as described in “Materials and methods.” (A) Platelets adherent to dA1VWF (dA1) or incubated over BSA for 10 or 20 minutes were lysed, and lysates were then probed with antiphosphotyrosine antibodies. Membranes were reprobed for Syk. (B) Platelets were allowed to adhere to dA1VWF with or without 5 μM PP2 or PP3. Then lysates (LYS) were immunoprecipitated with an antibody to ADAP and probed for phosphotyrosine. (C) The effect of PP2, EDTA, and 1 μM BAPTA-AM on tyrosine phosphorylation of ADAP. Data represent ADAP phosphotyrosine band intensities, determined by densitometry, and are expressed relative to results obtained in the absence of inhibitor. Data represent means ± SEM of 3 experiments.
Platelet adhesion to dA1VWF induces tyrosine phosphorylation. Human platelets adherent to dA1VWF or incubated over a BSA matrix were lysed and analyzed by Western blotting as described in “Materials and methods.” (A) Platelets adherent to dA1VWF (dA1) or incubated over BSA for 10 or 20 minutes were lysed, and lysates were then probed with antiphosphotyrosine antibodies. Membranes were reprobed for Syk. (B) Platelets were allowed to adhere to dA1VWF with or without 5 μM PP2 or PP3. Then lysates (LYS) were immunoprecipitated with an antibody to ADAP and probed for phosphotyrosine. (C) The effect of PP2, EDTA, and 1 μM BAPTA-AM on tyrosine phosphorylation of ADAP. Data represent ADAP phosphotyrosine band intensities, determined by densitometry, and are expressed relative to results obtained in the absence of inhibitor. Data represent means ± SEM of 3 experiments.
GP Ib-IX-V signals to αIIbβ3
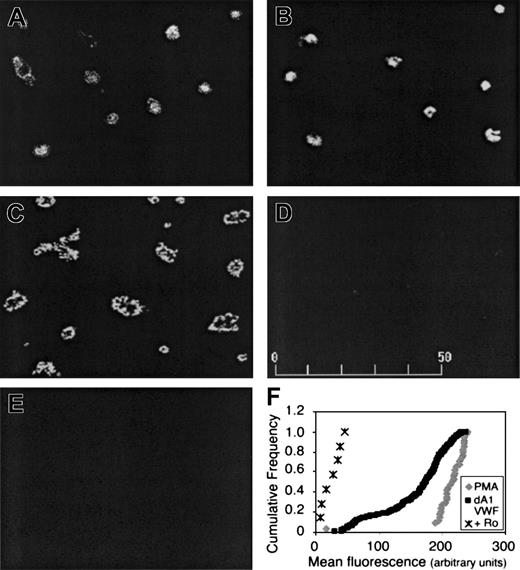
Activation of αIIbβ3 in dA1VWF-adherent platelets was assessed by confocal microscopy using PAC-1, an activation-dependent antibody.28 In this case, because PAC-1 is a ligand mimetic, the αIIbβ3 antagonist, Integrilin, was omitted. Platelets adherent to dA1VWF exhibited PAC-1 binding, even in the presence of inhibitors of signaling through ADP and thromboxane A2 receptors (Figure 4A). In the absence of these inhibitors, PAC-1 binding was even greater (Figure 4B), consistent with the known costimulatory functions of these G protein-linked receptors. When adherent platelets were stimulated with phorbol myristate acetate (PMA) to activate PKC, PAC-1 binding increased further and platelets underwent full spreading (Figure 4C). PAC-1 binding was specific, because it was blocked by Ro 44-9883 (Figure 4D), and platelets suspended over BSA showed no PAC-1 binding (Figure 4E). As observed in the calcium studies, PAC-1 responses were heterogeneous (Figure 4F). Thus, ligation of GP Ib-IX-V leads to a level of αIIbβ3 activation that is readily detectable with PAC-1 but is submaximal when compared with that obtained in the presence of additional agonists.
Platelet adhesion to dA1VWF causes αIIbβ3 activation. Cells were plated over coverslips coated with dA1VWF (A-D) or BSA (E) for 40 minutes in the presence of PAC-1. Platelets were then fixed and stained with an FITC-conjugated antibody against PAC-1 and imaged by confocal microscopy. (A) Platelets adherent to dA1VWF in the presence of inhibitors of signaling through ADP and thromboxane A2 receptors. (B) Same as panel A but no such inhibitors. (C) Platelet adhesion to dA1VWF in the presence of 200 nM PMA to activate PKC. (D) Platelet adhesion to dA1VWF in the presence of αIIbβ3 antagonist, Ro 44-9883 (Ro; 10 μM). (F) Heterogeneity of αIIbβ3 activation in the platelet population under conditions in which signaling through ADP and thromboxane A2 receptors was inhibited. Depicted is the cumulative frequency distribution of fluorescence values within the platelet population.
Platelet adhesion to dA1VWF causes αIIbβ3 activation. Cells were plated over coverslips coated with dA1VWF (A-D) or BSA (E) for 40 minutes in the presence of PAC-1. Platelets were then fixed and stained with an FITC-conjugated antibody against PAC-1 and imaged by confocal microscopy. (A) Platelets adherent to dA1VWF in the presence of inhibitors of signaling through ADP and thromboxane A2 receptors. (B) Same as panel A but no such inhibitors. (C) Platelet adhesion to dA1VWF in the presence of 200 nM PMA to activate PKC. (D) Platelet adhesion to dA1VWF in the presence of αIIbβ3 antagonist, Ro 44-9883 (Ro; 10 μM). (F) Heterogeneity of αIIbβ3 activation in the platelet population under conditions in which signaling through ADP and thromboxane A2 receptors was inhibited. Depicted is the cumulative frequency distribution of fluorescence values within the platelet population.
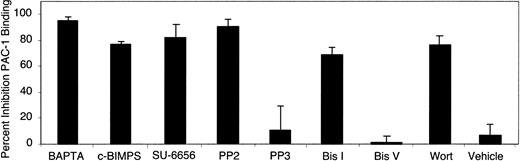
Next, the role of specific signaling molecules in dA1VWF-dependent αIIbβ3 activation was examined (Figure 5). Chelation of intracellular calcium with 1 μM BAPTA-AM blocked dA1VWF-induced PAC-1 binding completely. c-BIMPS blocked PAC-1 binding by 77%. Inhibition of Src kinases by PP2 or SU-6656 inhibited PAC-1 binding by 82% and 75%, respectively. In contrast to the lack of effects of wortmannin and bisindolylmaleimide I on dA1VWF-induced Ca2+ oscillations (Figure 2B), these inhibitors blocked PAC-1 binding by 77% and 73%, respectively (Figure 5C). Altogether, these results indicate that GP Ib-IX-V-mediated activation of αIIbβ3 is dependent sequentially on Src kinases, Ca2+ oscillations, and PI 3-kinase/PKC.
Signaling intermediates involved in GP Ib-IX-V signaling to αIIbβ3. Platelets adherent to dA1VWF were prepared as in Figure 4 in the presence of inhibitors to block signaling through ADP and thromboxane A2 receptors as well as with either 1 μM BAPTA-AM, 400 μM c-BIMPS, 1 μM SU-6656, 5 μM PP2 or PP3, 12 μM bisindolylmaleimide I or V, 100 nM wortmannin, or vehicle control. Data represent means ± SEM of 3 experiments.
Signaling intermediates involved in GP Ib-IX-V signaling to αIIbβ3. Platelets adherent to dA1VWF were prepared as in Figure 4 in the presence of inhibitors to block signaling through ADP and thromboxane A2 receptors as well as with either 1 μM BAPTA-AM, 400 μM c-BIMPS, 1 μM SU-6656, 5 μM PP2 or PP3, 12 μM bisindolylmaleimide I or V, 100 nM wortmannin, or vehicle control. Data represent means ± SEM of 3 experiments.
The FcR γ-chain is not required for GP Ib-IX-V signaling
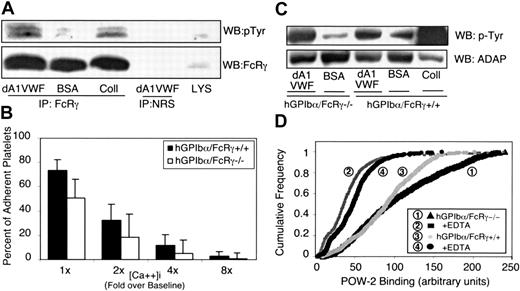
The FcR γ-chain can be coimmunoprecipitated with GP Ib-IX-V 21 and with GP VI.53 It is a substrate for Src kinases54 and an important signaling subunit for GP VI.55 The 14 kDa FcR γ-chain was inducibly tyrosine phosphorylated in dA1VWF-adherent platelets (Figure 6A). Therefore, we asked if FcR γ-chain was required for GP Ib-IX-V responses by studying transgenic mouse platelets expressing human GP Ibα (hGP Ibα) but lacking the FcR γ-chain. Unlike human platelets, mouse platelets lack FcγRIIA. Expression of hGP Ibα enabled the platelets to adhere to dA1VWF. The absence of FcR γ-chain was confirmed by Western blotting (not shown). In hGP Ibα/FcR γ+/+ platelets, intracellular calcium oscillations were somewhat lower than in normal human platelets, and peaks were sustained for shorter periods (eg, 4 to 6 versus 10 to 14 seconds). Strikingly, platelets from hGP Ibα/FcR γ-/- mice underwent calcium oscillations not unlike hGP Ibα/FcR γ+/+ platelets. However, there was a decrease in the percentage of hGP Ibα/FcR γ-/- platelets exhibiting high-end intracellular calcium concentrations (P < .01) (Figure 6B). For example, the percentage of hGP Ibα/FcR γ-/- platelets showing Ca2+ responses more than 4-fold over baseline was reduced by about 50%, and responses more than 8-fold over baseline were reduced by 70%. Thus, FcR γ-chain contributes to maximal intracellular Ca2+ elevations in dA1VWF-adherent platelets, but it is not required for the initiation of Ca2+ responses.
Role of the FcR γ-chain in GP Ib-IX-V signaling. (A) Human platelets were plated on dA1VWF, BSA, or collagen (Coll) for 30 minutes. Platelets were lysed, and FcR γ-chain was immunoprecipitated. Western blots were probed for phosphotyrosine and reprobed for FcR γ-chain. As a control, lysate was immunoprecipitated with normal rabbit serum (NRS). (B) Washed platelets were prepared from transgenic mice expressing human GP Ibα (hGP Ibα/FcR γ+/+) or from the same transgenic mice with a targeted deletion of the FcR γ-chain (hGP Ibα/FcR γ-/-). After loading with calcium indicator dyes, cells were plated on dA1VWF and intracellular calcium concentrations were determined as in Figure 1. Results are representative of 4 experiments. (C) Role of FcR γ-chain in ADAP tyrosine phosphorylation. Human GP Ibα/FcR γ-/- platelets were plated for 20 minutes on dA1VWF, BSA, or collagen. Cells were lysed, immunoprecipitated for ADAP, and Western blots were probed for phosphotyrosine and then reprobed for ADAP. This result is representative of 3 experiments. (D) To determine if there is a role for FcR γ-chain in αIIbβ3 activation, POW-2 Fab binding to mouse platelets was determined in the absence of an αIIbβ3 antagonist. Quantitative representation, as in Figure 4F, of POW-2 Fab binding is based on 4 separate experiments.
Role of the FcR γ-chain in GP Ib-IX-V signaling. (A) Human platelets were plated on dA1VWF, BSA, or collagen (Coll) for 30 minutes. Platelets were lysed, and FcR γ-chain was immunoprecipitated. Western blots were probed for phosphotyrosine and reprobed for FcR γ-chain. As a control, lysate was immunoprecipitated with normal rabbit serum (NRS). (B) Washed platelets were prepared from transgenic mice expressing human GP Ibα (hGP Ibα/FcR γ+/+) or from the same transgenic mice with a targeted deletion of the FcR γ-chain (hGP Ibα/FcR γ-/-). After loading with calcium indicator dyes, cells were plated on dA1VWF and intracellular calcium concentrations were determined as in Figure 1. Results are representative of 4 experiments. (C) Role of FcR γ-chain in ADAP tyrosine phosphorylation. Human GP Ibα/FcR γ-/- platelets were plated for 20 minutes on dA1VWF, BSA, or collagen. Cells were lysed, immunoprecipitated for ADAP, and Western blots were probed for phosphotyrosine and then reprobed for ADAP. This result is representative of 3 experiments. (D) To determine if there is a role for FcR γ-chain in αIIbβ3 activation, POW-2 Fab binding to mouse platelets was determined in the absence of an αIIbβ3 antagonist. Quantitative representation, as in Figure 4F, of POW-2 Fab binding is based on 4 separate experiments.
FcR γ-chain in complex with GP VI promotes collagen-induced tyrosine phosphorylation of Syk, SLP-76, and ADAP.56 Adhesion of hGP Ibα/FcR γ+/+ platelets to dA1VWF stimulated tyrosine phosphorylation of ADAP, as in normal human platelets (Figure 6C). In addition, ADAP phosphorylation was observed in hGP Ibα/FcR γ-/- platelets, although densitometry analysis indicated that the response was reduced slightly when compared with that of hGP Ibα/FcR γ+/+ platelets. To establish whether FcR γ-chain is required for αIIbβ3 activation downstream of GP Ib-IX-V, integrin activation was monitored with POW-2 Fab, an engineered version of PAC-1 specific for high-affinity murine αIIbβ3.29 Human GP Ibα/FcR γ-/- platelets adherent to dA1VWF exhibited POW-2 binding, albeit to a slightly lesser degree than hGP Ibα/FcR γ+/+ platelets (Figure 6D). Thus, while the FcR γ-chain is phosphorylated in dA1VWF-adherent platelets, this protein is not required for GP Ib-IX-V signaling to αIIbβ3.
Discussion
To fulfill their roles in hemostasis, platelets must transform from a resting to an activated state, bind multivalent adhesive ligands, and undergo aggregation. Numerous studies have implicated VWF-bound GP Ib-IX-V in signaling to αIIbβ3 under various experimental conditions.1,7,8 However, the use of VWF is problematic in this context because it also recognizes αIIbβ3, and ligand-occupied αIIbβ3 signals in its own right.23,57 Moreover, platelet activation in vivo can be initiated by many soluble or matrix-associated agonists, and activation can be reinforced by agonists released from activated platelets (ADP) or generated de novo (thromboxane A2). Thus, it can be difficult to determine the extent to which VWF binding to GP Ib-IX-V, per se, initiates platelet signaling.
To address this question without such complications, we exposed platelets to immobilized dA1VWF, a dimerized form of the VWF A1 domain, which recognizes GP Ib-IX-V exclusively and promotes platelet adhesion without the need for modulators. Experiments were routinely conducted in the presence of effective concentrations of inhibitors of signaling through αIIbβ3 and ADP and thromboxane A2 receptors. In addition, selected studies were carried out with GP Ibα transgenic mouse platelets that lack the FcR γ-chain and FcγRIIA, 2 ITAM-bearing moieties that could help to mediate VWF/GP Ib-IX-V signaling because they are associated with GP Ib-IX-V.21,58,59 We have conclusively demonstrated that the interaction of GP Ib-IX-V with dA1VWF is sufficient to trigger a sequence of reactions in platelets that leads to activation of αIIbβ3, even when other costimulatory inputs are blocked. This pathway includes activation of Src kinases, intracellular Ca2+ oscillations, and the actions of PI 3-kinase and PKC.
Two distinct Src inhibitors, PP2 and SU-6656, were used here to establish a necessary role for Src family kinases in initiating signaling from GP Ib-IX-V to αIIbβ3. In dA1VWF-adherent platelets, tyrosine phosphorylation of ADAP, calcium oscillations, and αIIbβ3 activation were all blocked by these inhibitors, suggesting that a Src kinase is interposed between GP Ib-IX-V and these responses. The inhibition of calcium oscillations by PP2 was observed under static conditions with dA1VWF as well as under shear with VWF. Which of the several Src family members present in platelets mediates these responses remains to be determined. In this context, both c-Src and Lyn have been reported to coimmunoprecipitate with GP Ib-IX-V when platelets bind soluble VWF in response to botrocetin. This interaction is dependent on the Src SH3 domain and the p85 regulatory subunit of PI 3-kinase, and p85 may associate constitutively, if indirectly, with GP Ib-IX-V.16,60
Two issues to be resolved are how GP Ib-IX-V regulates Src activity and which Src effectors are involved in stimulating the calcium oscillations needed for activation of αIIbβ3. In quiescent or unstimulated cells, including resting platelets, Src kinases are membrane tethered and in an autoinhibited state such that the SH2 and SH3 domains interact with the catalytic lobes, and the kinase activation loop occludes substrate access to the active site.61 Several events in dA1VWF-adherent platelets might regulate Src by releasing these intermolecular constraints: (1) The binding of Src SH3 to a PI 3-kinase/GP Ib-IX-V complex16 could displace SH3 from the catalytic lobe; (2) a GP Ib-IX-V-associated tyrosine phosphatase could disrupt binding of the SH2 domain to the Src C-terminus62 ; and (3) ligand-induced clustering of GP Ib-IX-V (and associated Src) could stimulate autophosphorylation of the Src activation loop in trans. In fact, conditional clustering of GP Ib-IX-V is sufficient to trigger Src-dependent protein tyrosine phosphorylation, both in platelets and in a Chinese hamster ovary (CHO) cell model system.51,63
Based on information to date, we suggest that ligation of GP Ib-IX-V by dA1VWF triggers the assembly of a receptor-proximal signaling complex that includes activated kinases (Src, Syk, PI 3-kinase) and tyrosine-phosphorylated adapters (SLP-76 and ADAP). Analogous to the function of similar Src- and Syk-based complexes associated with GP VI or αIIbβ3,45,52,64,65 signal output from the GP Ib-IX-V signaling complex would result in tyrosine phosphorylation and activation of phospholipase Cγ (PLCγ), a key regulator of platelet Ca2+ fluxes.66,67 Consistent with this formulation, treatment of platelets with VWF plus ristocetin (or botrocetin) causes tyrosine phosphorylation of PLCγ2 and generation of inositol 1,4,5-triphosphate (IP3).67,68 Recent studies using ristocetin to induce VWF binding to platelets suggest that lipid rafts may serve as platforms for assembly and regulation of GP Ib-IX-V-based signaling complexes.64,69-71 Additional studies will be required to establish with certainty how GP Ib-IX-V signaling is initiated and how other GP Ib-IX-V-associated proteins, such as filamin, calmodulin, and 14-3-3ζ, impact this process.
The tyrosine phosphorylation of ADAP observed in dA1VWF-adherent platelets (Figure 3) identifies a Src substrate not previously recognized as a potential effector in GP Ib-IX-V signaling. ADAP has been implicated in the regulation of β1 and β2 integrin clustering in lymphocytes and mast cells.72,73 Although affinity modulation rather than clustering and avidity modulation appears to be the dominant mode of regulation of αIIbβ3,74 a possible role for ADAP in GP Ib-IX-V signaling to αIIbβ3 warrants further investigation. Were ADAP to be involved in αIIbβ3 activation, its effect would be restricted to GP Ib-IX-V signaling because ADAP-deficient mouse platelets exhibit normal fibrinogen and POW-2 binding in response to ADP or thrombin (A. Obergfell, K. Eto, S. J. Shattil, and G. Koretzky, unpublished observations).
A close functional, if not physical, relationship between GP Ib-IX-V and FcR γ-chain or FcγRIIA has been posited in human platelets.20,21 FcR γ-chain is also a functional subunit of GP VI, and both FcR γ-chain and FcγRIIA are substrates of Src kinases in platelets.20,65,75 We found that the FcR γ-chain was not required for dA1VWF-dependent ADAP phosphorylation, Ca2+ oscillations, or activation of αIIbβ3 in mouse platelets, which constitutively lack FcγRIIA, although some of these responses were slightly impaired in the absence of FcR γ-chain (Figure 6). Although we did not formally evaluate FcγRIIA in human platelets, there is an at least 10-fold excess of GP Ib-IX-V over either FcR γ-chain or FcγRIIA in these cells.65,76 At the very least, the present studies indicate that these 2 ITAM-bearing proteins are dispensable for initiation of GP Ib-IX-V signaling in mouse platelets.
Previous studies have established the importance of calcium fluxes in mediating signals from VWF/GP Ib-IX-V to αIIbβ3, with Ca2+ release from internal stores appearing to be more important than Ca2+ influx.8,40,41 The current work adds to this by demonstrating that induction of calcium oscillations by GP Ib-IX-V can occur independently of other receptors and that those changes in (Ca2+)i are upstream of PI 3-kinase and PKC in a pathway leading to activation of αIIbβ3. The apparent requirement for ADP, PI 3-kinase, and outside-in signals through αIIbβ3 in the induction of sustained, high-amplitude Ca++ oscillations (γ peaks) observed under shear flow conditions40,41 might at first seem contradictory with the sustained high calcium peaks observed here in dA1VWF-adherent platelets under static conditions in which ADP, PI 3-kinase, and αIIbβ3 were blocked (Figure 2). However, a major effect of inhibiting these pathways may be to impede maximal activation of αIIbβ3, which is required for resistance of platelets to shear stress in flow. Because platelets that are not fully activated might be removed prematurely from the adhesive surface under flow, γ peaks would not be detected.
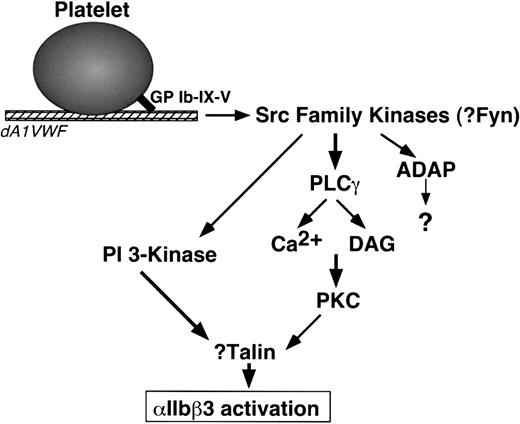
The present studies indicate that PI 3-kinases and PKC are required for GP Ib-IX-V-mediated αIIbβ3 activation but not for GP Ib-IX-V-mediated Ca2+ oscillations. p85 PI 3-kinases are regulated by H-Ras and by phosphorylation and recruitment of the p85 regulatory subunit to membranes.77 Because GP Ib-IX-V can interact with both p85 and 14-3-3ζ, a potential H-Ras regulatory protein,16,60 it is plausible that a relevant pool of PI 3-kinase for αIIbβ3 regulation is activated in proximity to GP Ib-IX-V. What is the connection between GP Ib-IX-V and PKC? Conventional PKC isoforms, such as PKCα and PKCβ, are activated by Ca2+ and diacylglycerol, effectors of phospholipase C.78 Intriguingly, phospholipase Cγ may lie downstream of Src in GP Ib-IX-V signaling.67 PKCα and PKCβ associate with αIIbβ3 in response to agonist-induced platelet activation,79 and PKCα has been implicated in Ca2+-mediated, αIIbβ3-dependent platelet aggregation.80 Beause talin, a PKC substrate, regulates integrin affinity by direct interaction with integrin β tails,81-84 an outline of a signal relay between GP Ib-IX-V and αIIbβ3 can be proposed (Figure 7). Additional studies will be required to firm up these speculations and to determine the precise contribution of GP Ib-IX-V signaling to platelet thrombus formation in vivo.
Model of signaling from GP Ib-IX-V to αIIbβ3 in dA1VWF-adherent platelets. DAG indicates diacylglycerol. See “Discussion.” Not shown are FcR γ-chain and FcγRIIA, signaling proteins associated with GP Ib-IX-V that may participate in GP Ib-IX-V signaling but are not required for it.
Model of signaling from GP Ib-IX-V to αIIbβ3 in dA1VWF-adherent platelets. DAG indicates diacylglycerol. See “Discussion.” Not shown are FcR γ-chain and FcγRIIA, signaling proteins associated with GP Ib-IX-V that may participate in GP Ib-IX-V signaling but are not required for it.
Prepublished online as Blood First Edition Paper, January 15, 2004; DOI 10.1182/blood-2003-10-3664.
Supported by the National Institutes of Health (grants HL-48728, HL-56595, HL-42846, HL-31950, and RR0833 to the General Clinical Research Center of Scripps Clinic and Research Foundation), ESA (AO-LS-99-MAP-MED-007), and the Stein Endowment Fund.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful for the technical assistance of Susan Russell, Bill Kiosses, and Will Cassavant.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal