Abstract
As mouse models have become commonplace for studying hemostasis and thrombosis, we considered whether the mouse system had utility for assessing genetic alterations in platelet receptors. Platelets from 5 mouse strains (C57BL/6 [C57], FVB/N [FVB], BALB/c, C3H/He, and 129Sv) showed only minor differences in the expression of integrin αIIb, integrin β3, glycoprotein (GP) Ibα, or GPVI across strains. However, FVB platelets expressed approximately 50% the level of integrin α2 as platelets from other strains (P < .0001). We bred FVB mice with C57 and assessed α2 expression in FVB/C57xFVB/C57 (F2) offspring. Linkage analysis demonstrated the gene responsible for α2 levels is tightly linked to the D13mit260 marker (log odds [lod] score 6.7) near the α2 gene. FVB platelets showed reduced aggregation and a longer lag phase to collagen. FVB and C57 platelets aggregated similarly to collagen-related peptide, but FVB platelets showed a reduction in rhodocytin-induced Syk and PLCγ2 tyrosine phosphorylation. Thus, FVB platelets express half the level of α2 as other mouse strains, a trait linked to the α2 gene and seemingly responsible for reduced platelet aggregation to collagen. These strain differences serve as a useful model for the 2-fold difference in human platelet α2β1 expression and demonstrate that α2β1 participates in signaling during platelet activation. (Blood. 2004;103:3396-3402)
Introduction
Formation of a thrombus at the site of an injured vessel requires the coordinated action of critical platelet plasma membrane adhesion molecules.1 The most important initial contact of platelets with the exposed endothelial collagen and von Willebrand factor (VWF) involves the binding of glycoprotein (GP) Ibα to immobilized VWF. The VWF-GPIbα interaction is “fast-on” and relatively “fast-off,” and results in a rolling of platelets along the exposed subendothelium.2,3 This slowing of the platelets allows binding of the activating collagen-receptor, GPVI, to its ligand, resulting in activation of platelet integrins and subsequent firm adhesion, where the reactions between receptor and ligand are relatively “slow-on” but irreversible.4 The binding of integrin α2β1 to collagen and αIIbβ3 to VWF and fibrinogen are the principal interactions underlying firm adhesion.3 Intracellular signaling between and through these adhesive receptors plays a crucial role in platelet adhesion and aggregation.5 The importance of the GPIb-IX-V and αIIbβ3 in normal hemostasis is underscored by the bleeding diatheses that have been reported in patients with quantitative or qualitative deficiencies of the genes that encode them.6
Mouse models are now commonplace for studying hemostasis and thrombosis, and important insights pertaining to the major platelet adhesive receptors have been gleaned from mouse studies involving targeted disruptions of the genes for GPIbα,7 GPVI,8 and integrin chains α2,9,10 β1,4 αIIb11 and β3.12 A variety of different mouse strains have been used to assess hemostasis. For example, the FVB strain is typically used for transgenic experiments, the 129/Sv strain is used to derive embryonic stem (ES) cells, and the C57 strain is used for uniform background breeding studies. Different strains may exhibit different levels of gene expression, a feature that has been used to elucidate crucial gene regions regulating transcription.13
We and others have previously studied how genetic changes exert quantitative and qualitative alterations in human platelet adhesive receptors.14 Polymorphisms of both integrin α2 and GPIbα have been associated with quantitative differences in receptor levels in healthy individuals.15,16 The variation of integrin α2 in the normal population is 5-fold, and some portion of this variability has been associated with a C/T polymorphism at nucleotide 807. Individuals homozygous for the 807C or 807T alleles have an average 2-fold difference in platelet α2β1 levels,17 and this difference has been linked to increased adhesion to collagen and clinical thrombotic events.18 Comparable alterations in platelet adhesion receptor expression have not been assessed in different mouse strains. Assessing the functional consequences of subtle genetic variations in humans is challenged by numerous gene-gene and gene-environment interactions, and studies in mice can greatly minimize these confounding variables. In addition, comparative sequence analyses between species and between nonhuman primates have proved useful for identifying sequences that affect function and expression.19 Thus, in the case of platelet adhesion receptors, knowing mouse strain differences in expression levels might be valuable for defining the responsible quantitative trait loci as well as affecting strain choice for particular functional experiments.
Materials and methods
Chemicals and antibodies
Fibrillar type I equine tendon collagen was purchased from Helena Laboratories (Beaumont, TX). Soluble type I calf skin collagen was purchased from Bio/Data (Horsham, PA). Rhodocytin was kindly provided by Dr T. Morital (Tokyo, Japan). Fluorescein isothiocyanate (FITC) antimouse integrin α2 (Hal/29), phycoerythrin (PE) antirat integrin α2 (HMα2) that cross-reacts with mouse α2, PE antimouse CD61 (2C9.G2), FITC antimouse CD41 (MWReg30), and polyclonal rabbit antihuman P-selectin were from BD Biosciences (San Diego, CA). We have previously described FITC-labeled antimouse GPVI (JAQ1).20 Polyclonal rabbit antimouse integrin α2 antisera was kindly provided by U. Mayer (Manchester, United Kingdom). PE antimouse GPIbα (P0p4) was prepared as previously described.21 Rabbit polyclonal antisera against Syk (N-19) and against PLCγ2 (Q-20) were from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse antihuman phosphotyrosine (4G10) was from Upstate Biotechnology (Charlottesville, VA). Mouse antihuman β-actin, horseradish peroxidase-conjugated goat antirabbit immunoglobulin G (IgG), horseradish peroxidase-conjugated goat antimouse IgG, and horseradish peroxidase-conjugated goat antirabbit IgG were from Sigma (St Louis, MO).
Animals and blood sampling
We obtained 5 strains of normal inbred laboratory mice (C57BL/6, FVB/N, BALB/c, C3H/He, and 129Sv) from The Jackson Laboratory (Bar Harbor, ME). For brevity—especially in the figures—these strains will be abbreviated C57, FVB, BALB, C3H, and 129, respectively. In some experiments FVB and C57 mice were cross-bred, and the resulting FVB/C57 (F1) offspring were also cross-bred to yield the FVB/C57xFVB/C57 (F2). At 8 to 12 weeks of age mice were subjected to isoflurane anesthesia and whole blood (∼700 μL) was collected from the inferior vena cava into acid-citric acid-dextrose (ACD; 85 mM trisodium citrate, 71 mM citric acid, and 111 mM dextrose) at a ratio of 1:6 (vol/vol) for flow cytometry studies and 3.8% sodium citrate solution at 1:9 (vol/vol) for all other studies. Mean platelet volume (MPV) was measured with a Coulter Z1 Particle Counter (Beckman Coulter, Miami, FL).
Tail bleeding times
Mice were anesthetized by an intraperitoneal injection of approximately 0.125 mL Rodent III (100 mg/mL ketamine, 20 mg/mL xylametazalone, 10 mg/mL acepromazine in H2O; Northwest Pharmacy, Houston, TX). Several minutes later the mice were immobilized and 5 mm of the tip of the tail was cut off with a sharp scalpel. The tail was immediately dipped into a 15-mL tube containing 0.9% NaCl at 37°C and the time to cessation of bleeding was recorded.
Flow cytometry
Flow cytometry was performed essentially as previously described.9 Briefly, whole blood was diluted 1:50 with modified Tyrode buffer (12 mM NaHCO3, 138 mM NaCl, 5.5 mM glucose, 2.9 mM KCl, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4) and incubated with saturating concentrations of fluorescently labeled antiplatelet antibodies, and directly analyzed on a Coulter Epics XL flow cytometer (Beckman Coulter). The background mean fluorescence intensity (MFI) of all control antibodies was less than 4% of the test antibody, and therefore was not considered in the analyses.
Western blotting and immunoprecipitation
Whole blood was diluted with an equal volume of modified Tyrode buffer and centrifuged at 160g for 15 minutes at room temperature to obtain platelet-rich plasma (PRP). Platelets in PRP were counted and resuspended in Tyrode buffer with 1 mM Mg2+ at a concentration of 2.5 × 108/mL, and allowed to recover for 1 hour at room temperature before the experiment. Western immunoblotting with polyclonal rabbit antihuman P-selectin and polyclonal rabbit antimouse integrin α2 antisera was performed as previously described.9 Films were scanned using Photoshop version 5.5 software (Adobe), and densitometric quantification of the signals was performed using National Institutes of Health (NIH) Image software (Scion Image beta version 4.0.2; Scion, Frederick, MD). For rhodocytin-induced platelet activation, rhodocytin was added to PRP in an aggregometer, stirred for the indicated times, and the reaction terminated with an equal volume of ice-cold 2% Igepa (Sigma), 2 mM sodium orthovanadate, 20 mM sodium fluoride, 10 mM sodium pyrophosphate, and 10% protease inhibitor cocktail (Sigma). One portion of the total lysate was used for immunoblotting total tyrosine phosphorylation or for immunoprecipitation. For the latter, a total of 5 × 107 platelets were lysed, and the lysate was precleared with protein A-sepharose beads for 30 minutes at 4°C, incubated with antibodies to either Syk or PLCγ2 for 1 hour at 4°C, and precipitated with protein A-sepharose beads. The immunoprecipitated proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes and immunoblotted with 4G10 to detect tyrosine phosphorylation and also with antisera to Syk and PLCγ2 to assess total protein.
mRNA quantification
Total RNA was extracted from mouse platelets using TRIzol reagent (Invitrogen, Carlsbad, CA), and reverse transcribed into cDNA using oligo (dT)12-18 and Moloney murine leukemia virus (MMLV) reverse transcriptase (RT) (Invitrogen).
Primers and probes
The fluorogenic probes contained a covalently linked 5′ reporter dye (FAM, 6-carboxy-fluorescein) and a covalently linked 3′ quencher dye (TAMRA, 6-carboxy-tetramethyl-rhodamine). Integrin αIIb polymerase chain reaction (PCR) primers were 5′-GCTCGCGTTCCAATTGAGAA-3′ (sense) and 5′-TGGAAACATGCCACAAAAGG-3′ (antisense); the integrin αIIb probe was 5′(6FAM)-CATCTCCAGCTACCGCCCGGGTA-(TAMRA)-3′. Integrin α2 PCR primers were 5′-TGTCACGATTCCCCTCATGA-3 (sense), 5′-TGCAGTCATAGCCAACAGCAA-3′ (antisense); probe was 5′-(TET)-AA-TGAAACCCACGGAGAAAGCAGAAGTACC-(TAMRA)-3′.
PCR amplification and cDNA quantification
PCR reactions contained a final concentration of 400 nM sense and antisense primers, 200 nM TaqMan probe, TaqManPCR Master Mix, and 4 μL cDNA in a total volume of 25 μL. Each PCR amplification was performed in quadruplicate wells in an ABI Prism 7700 Sequence Detector (Perkin Elmer, Foster City, CA): 50°C for 2 minutes and 95°C for 10 minutes, followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute. Reagents were obtained from ABI (Foster City, CA). For real-time mRNA quantification, an ABI Prism 7700 Sequence Detection System (Perkin Elmer) was used. Data were analyzed according to the manufacturer's recommendations. Levels of integrin α2 mRNA were compared among platelets of different mouse strains by the relative quantification (2-ΔΔCT) approach of Livak and Schmittgen.22
Genotyping for FVB and C57
Several public databases were queried for polymorphic microsatellite markers in or near the mouse integrin α2(ITGA2) gene. Preliminary studies showed that microsatellite marker “D13Mit260” developed at the White-head Institute/Massachusetts Institute of Technology Center for Genome Research and obtained from Research Genetics (Huntsville, AL) was able to amplify genomic DNA from both FVB and C57 mice and yield different length PCR products. The PCR primer sequences flanking this microsatellite were 5′-TAAATTTGGATGCAGACAATGG-3′ (sense) and 5′-TTAAAAAATAGAAATGGCTCTGTGTG-3′ (antisense). Mouse genomic DNA (100 ng) was amplified in 20 μL containing 10 mM Tris-HCl (pH 8.0), 50 mM KCl, 1 μM of each primer, 2 mM MgCl2, 250 μM of each dNTP (deoxynucleoside triphosphate), and amplified in a thermal cycler as follows: 1 cycle of 95°C for 5 minutes, 55°C for 2 minutes, and 72°C for 3 minutes, followed by 30 cycles of 95°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute, followed by a final elongation step at 72°C for 10 minutes. DNA products were run in a 2% high-resolution agarose gel.
Linkage analysis
Quantitative and qualitative linkage analysis was carried out using ILINK of the FASTLINK computer package (National Center for Biotechnology Information [NCBI], http://www.ncbi.nlm.nih.gov/CBBresearch/Schaffer/fastlink.html).23 For the quantitative linkage analysis, the NOCOM program (Ott, ftp://linkage.rockefeller.edu/software/utilities)24,25 was used to estimate the number of components, the means for each of these components, and the standard deviation (equal for each mean). The estimates of the means for each of the 3 components (genotypes) and the variance were used as starting values in the ILINK program (NCBI). Integrin α2 expression levels were not available on the parental mice, therefore the mean values of the heterozygous mice were used as the parental quantitative trait values. Since the matings were heterozygous crosses, a disease allele frequency of 0.5 was used in the analysis. The log natural (ln) likelihood was estimated with θ= 0.5, allowing for the estimation of 3 genotypic means (μ1, μ2, and μ3) and the variance (σ). The analysis was carried out again estimating the ln likelihood at the maximum likelihood estimates (MLEs) for θ, μ1, μ2, μ3, and σ. In addition, a qualitative trait analysis was carried out. Each mouse was assigned to 1 of 3 liability classes based on their integrin α2 expression levels. The ranges for integrin α2 expression levels for liability class one ranged from 60.4 to 76.4, for liability class 2 from 87.8 to 102, and for liability class 3 from 126 to 132.2. All of the mice had integrin α2 expression levels that fell into one of these 3 classes. It was assumed that for liability class one and 3 the mice were homozygous and for liability class 2 heterozygous. The parental mice were made unknown for the analysis since their integrin α2 expression levels had not been measured.
Platelet aggregation
PRP was adjusted to a platelet concentration of 2 × 105/μL with Tyrode buffer plus 1 mM MgCl2 and platelet lumiaggregometry was performed in a 4-channel aggregometer (Bio/Data). Maximum aggregation was recorded at 10 minutes and expressed as arbitrary units relative to 100% transmission (adjusted with platelet poor plasma). Lag time was recorded as the time in minutes from the time agonist was added to the time aggregation begins.
Statistical analysis
The phenotypic characteristics of the different mouse strains were compared by analysis of variance (ANOVA), with individual means compared by the Bonnferroni/Dunn procedure. All data are expressed as the mean plus or minus standard deviation (SD). A probability value of less than .05 was considered statistically significant. All analyses were conducted using StatView 5 software (SAS Institute, Cary, NC).
Results
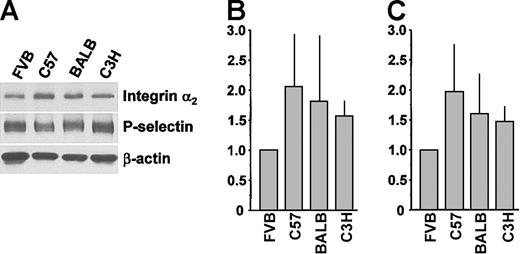
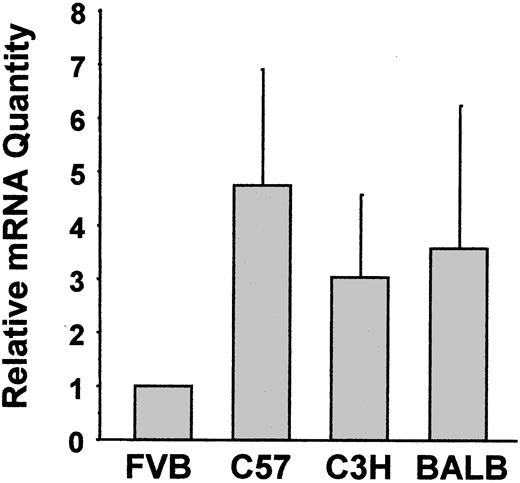
Platelets from 5 mouse strains were screened by flow cytometry for expression levels of GPIbα and integrins αIIb, β3, and α2 (Figure 1). There were small differences in the expression of integrin αIIb, integrin β3, GPIbα, and GPVI across all 5 strains (Figure 1A-C,F). Platelets from C57, C3HJ, BALB, and 129 mice expressed equivalent levels of integrin α2. However, FVB mice expressed only about 50% the level of integrin α2 as the other 4 strains (P < .0001; Figure 1D). A second anti-α2 monoclonal antibody indicated a similar reduced level of expression of integrin α2 in the FVB strain (P < .0001; Figure 1E). There was no significant gender difference in the expression of any receptor in any of the 5 strains (not shown). Subsequent studies did not include the 129 strain because of the very inefficient breeding that is common with this strain. Western immunoblotting of total platelet lysates was consistent with the surface labeling studies (Figure 2). Densitometry normalized to both P-selectin (Figure 2B) and β-actin (Figure 2C) showed FVB integrin α2 levels to be approximately half of the levels in other strains. We also quantified integrin α2 mRNA levels using real-time RT-PCR and observed reduced FVB platelet α2 mRNA compared with platelets from other strains (Figure 3).
Surface expression of platelet adhesion receptors from different mouse strains. Mouse platelets were analyzed by flow cytometry using fluorescently labeled monoclonal antibodies anti-CD41 (A), anti-CD61 (B), Pop4 (C), Hal/29-an antimouse integrin α2 (D), HMα2-an antirat integrin α2 (E), and JAQ1 (F). Analyses in panels A-E were performed 7 times. In panel F, analyses were performed 5 times for FVB and C57, and 2, 3, and 4 times for C3H, BALB, and 129, respectively. MFI indicates mean fluorescence intensity. Data are means ± SD.
Surface expression of platelet adhesion receptors from different mouse strains. Mouse platelets were analyzed by flow cytometry using fluorescently labeled monoclonal antibodies anti-CD41 (A), anti-CD61 (B), Pop4 (C), Hal/29-an antimouse integrin α2 (D), HMα2-an antirat integrin α2 (E), and JAQ1 (F). Analyses in panels A-E were performed 7 times. In panel F, analyses were performed 5 times for FVB and C57, and 2, 3, and 4 times for C3H, BALB, and 129, respectively. MFI indicates mean fluorescence intensity. Data are means ± SD.
Total lysate levels of platelet integrin α2 from different mouse strains. (A) Western blot analysis of platelets from different mouse strains. Platelet lysate (2 μg) was separated by SDS-PAGE, transferred to membranes, and probed with antibodies to integrin α2, P-selectin, and β-actin. (B,C) Densitometric analysis of integrin α2 expression normalized to P-selectin and β-actin expression, respectively (n = 3). Data are mean ± SD.
Total lysate levels of platelet integrin α2 from different mouse strains. (A) Western blot analysis of platelets from different mouse strains. Platelet lysate (2 μg) was separated by SDS-PAGE, transferred to membranes, and probed with antibodies to integrin α2, P-selectin, and β-actin. (B,C) Densitometric analysis of integrin α2 expression normalized to P-selectin and β-actin expression, respectively (n = 3). Data are mean ± SD.
Quantification of integrin α2 mRNA. Platelet total RNA was extracted, reverse transcribed into cDNA, and amplified in an ABI Prism 7700 Sequence Detector. Platelet integrin αIIb was the internal control used to normalize results between samples. Results are expressed as the fold-difference in α2 mRNA expression in platelets from FVB mice. Data are the means ± SD of 4 separate experiments starting at the RNA extraction.
Quantification of integrin α2 mRNA. Platelet total RNA was extracted, reverse transcribed into cDNA, and amplified in an ABI Prism 7700 Sequence Detector. Platelet integrin αIIb was the internal control used to normalize results between samples. Results are expressed as the fold-difference in α2 mRNA expression in platelets from FVB mice. Data are the means ± SD of 4 separate experiments starting at the RNA extraction.
There were no significant differences in platelet counts or tail bleeding times across strains (Table 1). Mean platelet volume of both FVB and C57 was significantly lower than BALB and C3H (P < .0001; Table 1). The mean platelet volume of FVB was no different than that of C57, so subsequent studies compared FVB with C57 to minimize potential influence of platelet size.
Platelet parameters in different mouse strains
. | Strain . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | FVB . | C57 . | BAL-C . | C3H . | |||
| Platelet count, × 103/μL* | 6.22 ± 1.24 | 4.79 ± 1.47 | 5.67 ± 1.70 | 5.96 ± 2.34 | |||
| MPV, μM3† | 4.81 ± 0.48 | 4.96 ± 0.51 | 6.67 ± 0.30 | 6.59 ± 0.82 | |||
| Bleeding time, min* | 7.3 ± 2.7 | 7.3 ± 3.5 | 6.5 ± 3.8 | 7.1 ± 3.6 | |||
. | Strain . | . | . | . | |||
|---|---|---|---|---|---|---|---|
. | FVB . | C57 . | BAL-C . | C3H . | |||
| Platelet count, × 103/μL* | 6.22 ± 1.24 | 4.79 ± 1.47 | 5.67 ± 1.70 | 5.96 ± 2.34 | |||
| MPV, μM3† | 4.81 ± 0.48 | 4.96 ± 0.51 | 6.67 ± 0.30 | 6.59 ± 0.82 | |||
| Bleeding time, min* | 7.3 ± 2.7 | 7.3 ± 3.5 | 6.5 ± 3.8 | 7.1 ± 3.6 | |||
All data are expressed as mean ± SD.
P not significant (NS) for any comparison.
P = NS for FVB versus C57 and for BALB/c versus C3H; P < .0001 for FVB versus BALB and for FVB versus C3H; P < .0001 for C57 versus BALB and for C57 versus C3H.
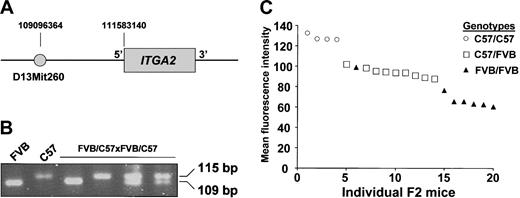
Genetic variation has strong effects on expression,26 but it cannot be assumed that altered levels of gene expression result from genetic variations at a single locus or at a locus in close proximity to the gene of interest.27 We sought to determine if the phenotypic difference in integrin α2 expression between the platelets of the FVB and C57 strains resulted from a single gene or multiple genes, and in particular, if this phenotype was associated with variations near the integrin α2 gene (ITGA2). FVB mice were bred with C57 mice, yielding an F1 generation of FVB/C57 mice. F1 males were then bred with F1 females. Platelets from the F2 offspring of the FVB/C57 x FVB/C57 cross were examined for α2 expression as in Figure 1. Initially, we screened several microsatellite markers within the ITGA2 gene, but none distinguished the 2 mouse strains. We therefore tested the known markers closest to ITGA2, and discovered that D13mit260 (Figure 4A) could distinguish C57 mice from several other mouse strains, although FVB had not been previously characterized with this marker. As shown in Figure 4B, D13mit260 could distinguish FVB from C57 and utilized it to genotype the F2 offspring of the FVB/C57 × FVB/C57 cross. Figure 4C shows the distribution of α2 expression levels according to genotype. For both the quantitative and qualitative linkage analysis a maximum log odds (lod) score of 6.7 was obtained at θ = 0.025. For the quantitative analysis the estimates of the 3 genotypic means were 65.5, 93.8, and 127.8, respectively.
Correlation of mouse strain genotype with integrin α2 expression phenotype. Successive generations of breeding of FVB and C57 mice led to 20 offspring designated as FVB/C57 × FVB/C57 (F2). These mice were phenotyped for platelet integrin α2 expression and genotyped with a marker flanking the integrin α2 gene (ITGA2). (A) Map of the ITGA2 gene showing the position of the primer set (D13mit260) used to distinguish the 2 mouse strains. (B) Ethidium-stained agarose gel of PCR products. The first 2 lanes have products from the F0 parental FVB and C57 strains, the last 4 lanes are 4 of the F2 cross (FVB/C57 × FVB/C57) samples showing the 3 possible genotype patterns. (C) Flow cytometric analysis of platelet surface integrin α2 expression of the 20 F2 offspring. The result of each F2 mouse is shown in descending order of α2 expression.
Correlation of mouse strain genotype with integrin α2 expression phenotype. Successive generations of breeding of FVB and C57 mice led to 20 offspring designated as FVB/C57 × FVB/C57 (F2). These mice were phenotyped for platelet integrin α2 expression and genotyped with a marker flanking the integrin α2 gene (ITGA2). (A) Map of the ITGA2 gene showing the position of the primer set (D13mit260) used to distinguish the 2 mouse strains. (B) Ethidium-stained agarose gel of PCR products. The first 2 lanes have products from the F0 parental FVB and C57 strains, the last 4 lanes are 4 of the F2 cross (FVB/C57 × FVB/C57) samples showing the 3 possible genotype patterns. (C) Flow cytometric analysis of platelet surface integrin α2 expression of the 20 F2 offspring. The result of each F2 mouse is shown in descending order of α2 expression.
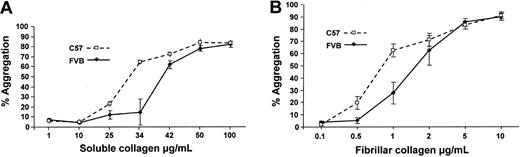
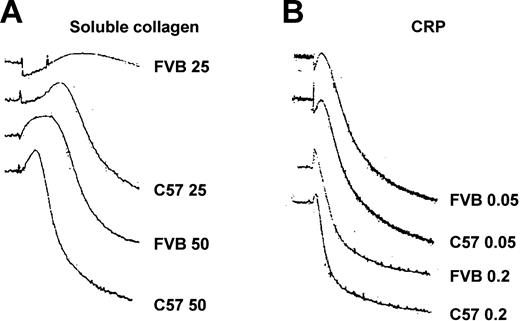
It was of interest to address whether the approximate 2-fold difference in platelet integrin α2 expression could affect platelet function. Compared with platelets from C57 mice, platelets from FVB mice showed a reduced aggregation response to submaximal concentrations of soluble and fibrillar collagens (Figure 5). As shown in Table 2, there was a significant difference in aggregation between platelets from FVB and C57 mice at 34 μg/mL soluble collagen and at 1 μg/mL fibrillar collagen. Figure 6 shows that compared with platelets from C57 mice, platelets from FVB mice had a reduced aggregation response to low-dose soluble collagen, but had a prolonged lag phase to soluble collagen concentrations that produced submaximal and maximal aggregation responses. The platelet GPVI/Fcγ complex is the major collagen signaling receptor, and cross-linked collagen-related peptide (CRP) activates platelets through GPVI and not α2β1.28,29 Figure 6 and Table 2 show that platelets from FVB and C57 mice had equivalent aggregation responses to CRP.
Lumiaggregometry of platelets from FVB and C57 mice. Platelet-rich plasma from each strain was prepared and assessed for maximum percent aggregation in response to a range of concentrations of soluble type I calf skin collagen and fibrillar type I equine collagen. Data are means ± standard error (SE).
Lumiaggregometry of platelets from FVB and C57 mice. Platelet-rich plasma from each strain was prepared and assessed for maximum percent aggregation in response to a range of concentrations of soluble type I calf skin collagen and fibrillar type I equine collagen. Data are means ± standard error (SE).
Aggregation responses to submaximal stimulation in FVB and C57 strain
Assay . | FVB . | C57 . | P . |
|---|---|---|---|
| Soluble collagen, 34 μg/mL, n = 5 | |||
| Maximum aggregation, % | 14.6 ± 26.4 | 64.2 ± 26.3 | < .02 |
| Lag time, min | 8.16 ± 1.8 | 3.98 ± 1.12 | .002 |
| Fibrillar collagen, 1 μg/mL | |||
| Maximum aggregation, % | 21.1 ± 20.6 | 65.2 ± 14.9 | .0003 |
| Lag time, min | 4.36 ± 2.1 | 2.19 ± 0.99 | .02 |
| CRP, 0.5 μg/mL, n = 6 | |||
| Maximum aggregation, % | 74 ± 12.7 | 74.2 ± 11.5 | NS |
| Lag time, min | 0.51 ± 0.07 | 0.5 ± 0.1 | NS |
| Rhodocytin, 20 nM, n = 3 | |||
| Maximum aggregation, % | 73.7 ± 11.6 | 76 ± 9.5 | NS |
| Lag time, min | 1.38 ± 0.22 | 0.97 ± 0.06 | < .04 |
Assay . | FVB . | C57 . | P . |
|---|---|---|---|
| Soluble collagen, 34 μg/mL, n = 5 | |||
| Maximum aggregation, % | 14.6 ± 26.4 | 64.2 ± 26.3 | < .02 |
| Lag time, min | 8.16 ± 1.8 | 3.98 ± 1.12 | .002 |
| Fibrillar collagen, 1 μg/mL | |||
| Maximum aggregation, % | 21.1 ± 20.6 | 65.2 ± 14.9 | .0003 |
| Lag time, min | 4.36 ± 2.1 | 2.19 ± 0.99 | .02 |
| CRP, 0.5 μg/mL, n = 6 | |||
| Maximum aggregation, % | 74 ± 12.7 | 74.2 ± 11.5 | NS |
| Lag time, min | 0.51 ± 0.07 | 0.5 ± 0.1 | NS |
| Rhodocytin, 20 nM, n = 3 | |||
| Maximum aggregation, % | 73.7 ± 11.6 | 76 ± 9.5 | NS |
| Lag time, min | 1.38 ± 0.22 | 0.97 ± 0.06 | < .04 |
All data are expressed as means ± SD.
NS indicates not significant.
Aggregation tracings from FVB and C57 mice. (A) Platelet-rich plasma from FVB and C57 strains of mice was stimulated with 25 μg/mL and 50 μg/mL soluble type I calf skin collagen. (B) Platelet-rich plasma from FVB and C57 strains of mice was stimulated with 0.05 μg/mL and 0.2 μg/mL cross-linked CRP.
Aggregation tracings from FVB and C57 mice. (A) Platelet-rich plasma from FVB and C57 strains of mice was stimulated with 25 μg/mL and 50 μg/mL soluble type I calf skin collagen. (B) Platelet-rich plasma from FVB and C57 strains of mice was stimulated with 0.05 μg/mL and 0.2 μg/mL cross-linked CRP.
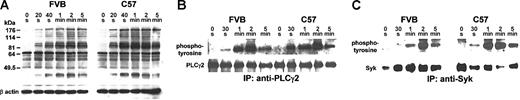
Recent evidence suggests that there is also some platelet signaling through α2β1, although the extent is substantially less than that through GPVI.30 The snake venom toxin rhodocytin has been reported to elicit signals through α2β1,31,32 although this is not essential for the aggregation response induced by the agonist. We found that rhodocytin induced comparable levels of platelet aggregation between the FVB and C57 mice, but the FVB platelets showed a prolonged lag phase (P < .04; Table 2). FVB platelets showed a reduction in rhodocytin-induced protein tyrosine phosphorylation (Figure 7A; note ∼90 kDa-95 kDa, 40 seconds), Syk, and PLCγ2 tyrosine phosphorylation (Figure 7B-C; note 30 seconds and 1 minute).
Platelet protein tyrosine phosphorylation induced by rhodocytin. Platelet-rich plasma from FVB and C57 mice was stimulated with 20 nM rhodocytin for the indicated time points and then solubilized. (A) Total platelet lysates were analyzed by imunoblotting with 4G10 for protein tyrosine phosphorylation (upper panels) and for β-actin (lower panels). (B) Platelet lysates were immunoprecipitated with an antiphospholipase Cγ2 antibody, separated by SDS-PAGE, and imunoblotted with 4G10 for protein tyrosine phosphorylation (upper panels) and for total phospholipase Cγ2 (lower panels). (C) Platelet lysates were immunoprecipitated with an anti-Syk antibody, separated by SDS-PAGE, and imunoblotted with 4G10 for protein tyrosine phosphorylation (upper panels) and for total Syk (lower panels).
Platelet protein tyrosine phosphorylation induced by rhodocytin. Platelet-rich plasma from FVB and C57 mice was stimulated with 20 nM rhodocytin for the indicated time points and then solubilized. (A) Total platelet lysates were analyzed by imunoblotting with 4G10 for protein tyrosine phosphorylation (upper panels) and for β-actin (lower panels). (B) Platelet lysates were immunoprecipitated with an antiphospholipase Cγ2 antibody, separated by SDS-PAGE, and imunoblotted with 4G10 for protein tyrosine phosphorylation (upper panels) and for total phospholipase Cγ2 (lower panels). (C) Platelet lysates were immunoprecipitated with an anti-Syk antibody, separated by SDS-PAGE, and imunoblotted with 4G10 for protein tyrosine phosphorylation (upper panels) and for total Syk (lower panels).
Discussion
We have demonstrated a 2-fold reduction in α2β1 expression in the FVB mouse strain due to a genetic locus in or near the integrin α2 gene, which results in significant differences in collagen-mediated platelet aggregation and rhodocytin-mediated platelet signal transduction. We found no major differences in expression across strains for most of the major platelet adhesion receptors, such that there is no reason to prefer one strain to another for experiments involving GPIbα, GPVI, and integrins αIIb and β3. Our finding of reduced levels of integrin α2 in FVB platelets was not likely due to an altered epitope on integrin α2 affecting antibody binding, since similar results were obtained with a second anti-α2 monoclonal antibody (Figure 1). The strain difference in integrin α2 levels was further supported by additional protein and mRNA assays (Figures 2 and 3). When assessing total integrin α2 levels in platelet lysates we compared the expression to a “housekeeping gene” (β-actin) and to another platelet surface protein (P-selectin) in the event that there might have been a strain difference in the reference protein that could have produced a “false-positive” normalization on densitometry (Figure 2). For quantification of relative levels of integrin α2 mRNA, the reference target was integrin αIIb since the surface expression was so similar. On the basis of quantitative differences in mRNA levels, we conclude that the lower expression of integrin α2 in FVB platelets is most likely due to either reduced transcription or/and RNA stability.
A single locus regulates the integrin α2 expression difference
Multiple loci can affect clinical phenotypes in such human diseases as cancer, hypertension, or arthritis.33-35 In addition, multiple loci can also cause a quantitative difference in expression of human gene products.36 Genetic linkage analysis of different strains of Saccharomyces cervisiae has shown strain difference in expression in approximately 43% of the genome, that these differences are highly heritable, and that multiple loci contribute to the different expression levels in most of the differently expressed genes.27 In the case of the 2-fold difference in α2β1 expression between the FVB and C57 strains, we first identified a polymorphic marker at position 109 096 364 of mouse chromosome 13 that had not previously been shown to distinguish the FVB and C57 genomes. There were statistically significant differences in platelet integrin α2 expression among the D13mit260 genotypes of the F2 offspring. For this data set there was strong evidence that there were 3 genotypic components:
The transcriptional regulation of the human integrin α2 gene has been characterized by several groups,37-39 and work from Jacquelin et al40 suggests that alterations in the 5′ region of the human integrin α2 gene are responsible for the interindividual differences in expression. The current publicly available mouse genome sequence does not include the sequence from the FVB strain. Once available, such a sequence would allow experiments to test putative regulatory regions that might account for the difference in ITGA2 expression between FVB and C57 mouse platelets.
FVB and C57 platelet functional differences
Our findings provided an opportunity to ask whether a 2-fold difference in integrin α2 would have functional consequences. An important limitation of our studies is the inability to exclude strain differences other than α2 levels. However, there are several reasons to study expression differences among different mouse strains: (1) environmental effects are extremely difficult to control in human comparison studies, and (2) mouse strains are not subject to concerns about developmental compensation in knockout mouse systems. Furthermore, the FVB and C57 strains exhibited neither quantitative (Figure 1) nor functional (Figure 6 and Table 2) differences in GPVI, the other major collagen receptor. To assess the functional significance of the difference in integrin α2 expression, we chose the C57 strain because platelets from this strain were otherwise most similar to FVB for the expression of the 3 other surface adhesion receptors and for mean platelet volume. Our studies suggest that the level of α2β1 expression affects platelet function, even at the relatively modest 2-fold difference. This difference causes a prolonged lag phase and reduced level of maximum aggregation to submaximal concentrations of soluble and fibrillar collagen. These results are generally in agreement with Holtkotter et al9 who observed reduced aggregation to low dose soluble collagen in α2 +/-mice and Jarvis et al41 who used α2β1 blocking antibodies. We performed our aggregation studies with several additional concentrations of fibrillar collagen in the low range, which may explain why no difference in aggregation with this collagen according to α2 levels was detected in that previous study. (The original source of the 2 collagens was the same, making differences in this reagent less likely.)
There are a few reports comparing platelet aggregation and bleeding times using different mouse strains,42-44 but we are not aware of any that have specifically examined platelet adhesion receptor expression. We observed no effect of α2β1 levels on the mouse tail bleeding time, a finding consistent with Holtkotter et al.9 However, mouse tail bleeding times appear to be insensitive to defects in collagen receptors, since (1) levels of α2β1 affect both human platelet adhesion to immobilized collagen17 and mouse platelet adhesion to soluble collagen,9,10 and (2) mouse GPVI is not required for a normal tail bleeding time.8
The snake venom toxin, rhodocytin, induces strong platelet activation and aggregation45,46 which has been shown to coincide with,31,32 but is not dependent on,21 α2β1-mediated intracellular signaling events. We found reduced tyrosine phosphorylation of different signaling molecules in response to rhodocytin in platelets with low α2β1 levels. Thus, although further studies are needed to address how rhodocytin activates platelets, our data suggest that rhodocytin-induced aggregation lag time and signaling are partially affected by the level of α2β1 expression. This is also consistent with the idea that some amount of platelet signaling does occur through α2β1.
Many other quantitative platelet phenotype differences are likely to exist in inbred mouse strains, and systematic surveys of the mouse genome may prove a useful tool to identify variants that could be tested for their contribution to human disease. The FVB-C57 strain difference serves as a useful model for the known 2-fold difference in human platelet α2β1 expression and supports a role for α2β1 in signaling events during platelet activation. One could also speculate that the reduced level of integrin α2 could contribute to the relative resistance to atherosclerosis and restenosis after angioplasty observed in FVB mice.47 Although many genes are regulated by multiple loci with varying relative effects on expression, the difference in integrin α2 expression that we found appears to be largely regulated by a single locus with a major effect on expression. This difference in α2 expression levels impacts on submaximal platelet activation, which could be enough of an added thrombosis risk to produce pathologic thrombi upon exposure of subendothelial collagen after an in vivo injury.
Prepublished online as Blood First Edition Paper, January 22, 2004; DOI 10.1182/blood-2003-10-3721.
Supported by the National Institutes of Health (HL65229) (P.F.B.), the National Heart Lung and Blood Institute Programs for Genomic Application (HL66728) (E.M.R.), and the Fondren Foundation.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal