Abstract
The Stro-1 antigen potentially defines a mesenchymal stem cell (MSC) progenitor subset. We here report on the role of human ex vivo-expanded selected Stro-1+ or Stro-1- MSC subsets on the engraftment of human CD34+ cord blood cells in the nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse model. The data show that cotransplantation of expanded Stro-1- cells with CD34+ cells resulted in a significant increase of human CD45, CD34, CD19, and CD11b cells detected in blood or in bone marrow (BM) and spleen as compared with the infusion of CD34+ cells alone. Infusion into mice of expanded Stro-1+ and Stro-1- cells (without CD34+ cells) showed that the numbers of Stro-1+-derived (as assessed by DNA analysis of human β-globin with quantitative polymerase chain reaction [PCR]) were higher than Stro-1--derived cells in spleen, muscles, BM, and kidneys, while more Stro-1--derived than Stro-1+-derived cells were found in lungs. The transduction of expanded Stro-1+ cells with an enhanced green fluorescent protein (eGFP) gene did not modify their cytokine release and their homing in NOD/SCID mouse tissues. The difference between the hematopoietic support and the homing capabilities of expanded Stro-1+ and Stro-1- cells may be of importance for clinical therapeutic applications: Stro-1+ cells may rather be used for gene delivery in tissues while Stro-1- cells may rather be used to support hematopoietic engraftment. (Blood. 2004;103:3313-3319)
Introduction
Recent results have shown that cotransplantation of human ex vivo-expanded mesenchymal stem cells (MSCs) together with hematopoietic stem cells hastens hematopoietic recovery following a bone marrow (BM) transplantation in animal models1-4 and in humans.5-7 Human BM contains 2 cell compartments, the hematopoietic cell compartment and the stromal cell compartment, which constitute MSCs.8,9 MSCs are able to give rise to multiple mesodermal tissue types, including bone, cartilage, tendon, muscles, cardiomyocytes, fat, and brain,10-16 and a marrow stromal connective tissue that supports the differentiation of hematopoietic stem cells (HSCs).17,18 However, MSCs are heterogeneous, and little is known on the role of MSC subsets in the hematopoietic engraftment support and in their homing in various tissues.19 Stro-1 antigen is present on fibroblast colony-forming unit (CFU-F) cells in adult human BM and potentially defines a MSC precursor subpopulation.20-22 The aim of the present study was to evaluate the role of ex vivo-expanded Stro-1+ and Stro-1- MSCs on engraftment of human CD34+ cord blood cells in nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mice. Our data showed that the levels of human hematopoietic engraftment (as assessed by the presence of CD45, CD34, CD19, and CD11b cells) in the blood, spleen, and mouse BM were higher when Stro-1--derived cells were coinfused with CD34+ cells than when Stro-1+-derived cells were used.
In a second step, we investigated the homing of expanded Stro-1+ and Stro-1- cells (infused without CD34+ cells) in BM, spleen, liver, brain, heart, lungs, kidneys, and muscles of NOD/SCID mice. Eight-week-old NOD/SCID mice received 3.5 Gy irradiation, and 24 hours later the cells were infused. We analyzed the homing of cells by polymerase chain reaction (PCR) quantitation of DNA of human β-globin. Results showed that the DNA amount from expanded Stro-1+ cells was higher than that of expanded Stro-1- cells in spleen (× 8), muscles (× 6), BM (× 2), liver (× 1.5), and kidneys (× 1.5). No significant difference was observed in brain, while more Stro-1- than Stro-1+ cell DNA was found in lungs (× 3.5).
In conclusion, expanded Stro-1+ cells better migrated than expanded Stro-1- cells in most mouse tissues. This indicated that Stro-1+ cells would be potentially a good vector to bring specific therapeutic genes into tissues. To test this hypothesis, we infused into NOD/SCID mice expanded Stro-1+ cells transfected with an enhanced green fluorescent protein (eGFP) gene. The specific eGFP DNA was found in every investigated tissue—namely, BM, liver, brain, heart, spleen, kidneys, muscles, and lungs.
The difference between the hematopoietic support and the homing capacities of Stro-1+ and Stro-1- cells may be of importance for clinical application of MSCs12 : Stro-1+ cells would be rather used for gene delivery in tissues and Stro-1- cells (or unseparated MSCs because they contain around 90% Stro-1- cells) for hematopoietic engraftment support.
Patients, materials, and methods
Collection and isolation of CD34+ cells from human umbilical cord blood (hUCB)
Human umbilical cord blood (hUCB) samples were obtained from full-term deliveries after informed consent of the mother and were used in accordance with the procedures approved by the human experimentation and ethics committees of Hopital St Antoine in Paris. The hUCB was collected in bags (Macopharma, Tourcoing, France) containing heparin and processed within 24 hours. Samples were diluted 1:2 in phosphate-buffered saline (PBS) without Mg2+/Ca2+ (B. Braun Medical, Boulogne, France). Low-density mononuclear cells (MNCs) were collected after centrifugation on Ficoll-Paque density gradient (1.077 g/L; Biochrom, Berlin, Germany) and washed in PBS. CD34 cells were isolated using the MACS cell isolation kit and MidiMACS columns (Miltenyi Biotec, Bergisch Gladbach, Germany) according to the manufacturer's instruction. A purity of more than 95% was currently obtained. Selected CD34 cells are referred to as “CD34+ cells” in the following. They were frozen in fetal calf serum supplemented with 10% dimethylsulfoxide (B. Braun Medical) until use.
Isolation and growth of human bone marrow (BM) MSCs
BM cells were obtained from iliac crest aspirates from healthy donors giving their BM for allogeneic transplantation purposes, after informed consent, and were used in accordance with the procedures approved by the human experimentation and ethics committees of Hopital St Antoine. For the study of Stro-1+ cell proportions in normal BM, aliquots of 2 mL were taken from 10 different BM collections. For cotransplantation and homing studies, 50 mL BM was taken from 2 different donors. BM samples were diluted 1:3 in PBS without Mg2+/Ca2+. MNCs were separated on Ficoll-Paque density gradient (1.077 g/mL) and washed in PBS. They were plated at a concentration of 1 × 106 to 10 × 106 MNCs per milliliter in T-75 cm2 tissue culture flasks in Dexter medium (McCoy 5A medium supplemented with 12.5% heat-inactivated fetal calf serum, 12.5% heat-inactivated horse serum, 1% sodium bicarbonate, 1% sodium pyruvate, 0.4% minimum essential medium [MEM] nonessential amino acids, 0.8% MEM essential amino acids, 1% MEM vitamin solution, 1% l-glutamine [200 mM], 1% penicillin-streptomycin solution [all from Invitrogen, Groningen, The Netherlands], 106 M hydrocortisone [Stem Cell Technologies, Vancouver, BC], 2 ng/mL human basic recombinant fibroblast growth factor [FGFb; R&D Systems, Abington, United Kingdom]) and incubated at 37°C and 5% CO2 in a humidified atmosphere. After 1 week, nonadherent cells were removed, and the complete medium (except hydrocortisone) was used. When 50% confluence was obtained, cells were harvested after a 2-minute incubation with 0.25% trypsin and 1 mM EDTA (ethylenediaminetetraacetic acid) (Stem Cell Technologies) at 37°C. The collected adherent cells were incubated with anti-Stro-1 antibody, and the Stro-1+ cells were separated using immunomagnetic beads (as described in “Immunomagnetic selection with an anti-Stro-1 antibody”). Stro-1+ and Stro-1- cells were harvested and cultured in complete medium without hydrocortisone. When 90% confluency was observed, cells were detached by trypsin incubation and replated at 1:3 dilution in T-75 cm2 flasks. These expanded cells will be referred to as “expanded Stro-1+” or “expanded Stro-1-” MSCs.
Immunomagnetic selection with an anti-Stro-1 antibody
Stro-1+ cells were isolated by magnetic immunobeads (Dynabead M450, Dynal Asa, Oslo, Norway) linked to Stro-1 antibody (produced by one of us [P.C.] and purified by Biocytex, Marseille, France) as previously described.22,23 Briefly, anti-Stro-1--coated beads were added to the stromal cell suspension, and positive cells were recovered with a magnetic particle concentrator (MPC-1) and washed 3 times before use.
FACS analysis
Phycoerythrin (PE)-labeled monoclonal antibodies used in this study were anti-CD34, anti-CD45, anti-CD19, anti-CD11b, anti-CD105 (SH2), anti-CD73 (SH3) (BD Pharmingen, San Diego, CA). Cells were incubated with antibodies in PBS supplemented with 0.5% bovine serum albumin (BSA; Sigma Chemicals, St Louis, MO) for 20 minutes at 4°C. Cells were washed, resuspended in 200 μL PBS, 0.5% BSA, and analyzed with a FACSCalibur (BD Pharmingen, San Diego, CA) with the acquisition of at least 10 000 events per test.
NOD/SCID mouse model
All experiments and procedures were performed in compliance with the French Ministry of Agriculture regulations for animal experimentation (Act no. 87-847, October 19, 1987; modified May 2001). NOD-LtSz-scid/scid (NOD-SCID) mice, from breeding pairs originally purchased from Jackson Laboratories (Bar Harbor, ME), were bred in our pathogen-free unit and maintained in sterile microisolator cages. Eight-week-old mice were sublethally irradiated with 3.5 Gy from a 137Cs source (2.115 Gy/min).
Cotransplantation of CD34+ cells plus expanded Stro-1+ or Stro-1- MSCs. Twelve to 24 hours after irradiation, 1 × 106 expanded Stro-1+ or Stro-1- MSCs resuspended in PBS were injected into the retro-orbital plexus of mice together with or without 1 × 105 CD34+ cells. Control groups included mice receiving only selected CD34+ cells or receiving no cells. Therefore, 4 groups of mice (8 mice per group) were defined: “CD34+ + Stro-1+,” “CD34+ + Stro-1-,” “CD34+ alone,” and “no cells.” At 3, 6, 9, and 12 weeks after transplantation, 50 μL peripheral blood was collected from the retro-orbital plexus, and the percentage of human hematopoietic CD45 cells in murine blood cells was counted by fluorescence-activated cell sorter (FACS) analysis. Differences in the human CD45 cell engraftment were made by calculating the areas under the curves (AUCs) at different time points (3, 6, 9, and 12 weeks).
Twelve weeks after injection, mice were killed, and blood, spleen, and BM were collected. Cells were isolated, resuspended in PBS, and the percentages of human CD34, CD45, CD19, and CD11b measured by FACS.
Infusion of expanded Stro-1+ or Stro-1- MSCs for homing studies. Twelve to 24 hours after irradiation, 1 × 106 expanded Stro-1+ or Stro-1- MSCs resuspended in PBS were injected into the retro-orbital plexus of mice. Twelve weeks later, mice were killed, and blood, spleen, BM, lungs, heart, brain, liver, kidneys, and leg muscles were collected for DNA extraction and PCR analysis to detect the presence of human MSC DNA in these tissues.
DNA extraction and PCR analysis
Genomic DNA for PCR analysis was prepared from tissues using phenol chloroform extraction after overnight incubation at 65°C in lysis buffer as previously described.24 The DNA concentration and purity was estimated by optical density (OD) measurement. DNA analyses were performed by real-time quantitative PCRs (TaqMan technology and ABI PRISM 7700; Altera, Norwalk, CT). Amplification was performed using manufacturer-provided reagents following the standard recommended amplification conditions (Applied Biosystems, Foster City, CA) as previously described.25,26 One hundred nanograms of purified DNA from various tissues were amplified using TaqMan universal PCR master mix 4304437 (Applied Biosystems). The primers and probes were designed with Primer Express software (Applied Biosystems). The primers and probe for β-globin were forward primer 5′-GTGCACCTGACTCCTGAGGAGA3-′ and reverse primer 5′-CCTTGATACCAACCTGCCCAG3-′; the probe labeled with fluorescent reporter and quencher was 5′-FAM-AAGGTGAACGTGGATGAAGTTGGTGG-TAMRA-3′. FAM (6-carboxy-fluorescein) was used as a reporter fluorochrome, and TAMRA (6-carboxy-tetramethyl-rhodamine) was used as quencher. As internal control, endogenous mouse RAPSYN gene (receptor-associated protein at the synapse) was also amplified. The primers and probe for RAPSYN gene were forward primer 5′-ACCCACCCATCCTGCAAAT-3′ and reverse primer 5′-ACCTGTCCGTGCTGCAGAA-3′.3 Probe was chosen in order to hybridize to an internal sequence of the PCR target sequence. At each PCR cycle, the fluorescence intensity of additional reporter dye molecules was monitored. Threshold cycles (Ct) were selected in the line in which all samples were in logarithmic phase. The quantity of PCR product was calculated by Ct value. To determine the efficiency of amplification and the assay precision, calibration curves for β-globin and RAPSYN were constructed with an 0.99 correlation (r2) and an efficiency superior to 98%. The reagent control consisted of all constituents of the PCR reaction mixture except template DNA. The negative control DNA was isolated from the same tissues from NOD/SCID mice that did not undergo transplantation. The positive control DNA was isolated from human MSCs. Evaluation of human specificity of β-globin amplification was demonstrated using 10-fold dilution for 100 ng to 0 ng human MSC DNA per assay in PCR-grade water and in 0 to 100 ng murine DNA per assay. No cross-reactivity between human and murine genomic DNA was observed when amplification of human β-globin or murine RAPSYN was performed with our primer-probe set. All samples were also amplified to detect the RAPSYN as an internal control for the presence of amplifiable DNA in the real-time PCR chimerism assay. To quantify the number of human cells in mouse tissue, the number of copies of β-globin and RAPSYN were normalized. Amplifiable DNA input in each sample was assayed by means of an active reference system, and a pair of primers and TaqMan probe specific for constant gene were used as the active reference in the real-time PCR chimerism assay. Ratio between human and murine DNA copy numbers was expressed in percentage of human β-globin DNA copies in RAPSYN DNA copies. Human cells contained 2 copies of β-globin, and murine cells contained 2 copies of RAPSYN. Therefore, we can express the ratio of human-murine DNA copy numbers in numbers of human cells per murine cells.
Retroviral gene transduction
Expanded Stro-1+ MSCs were transduced with an eGFP gene to check if a gene transduction would modify the ex vivo cytokine release of the transduced cells and their homing after their injection into NOD/SCID mice, as compared with nontransduced cells. The eGFP gene was introduced into expanded Stro-1+ MSCs after the first culture passage using the pG13 packaging cell line producing recombinant pSF vector carrying the eGFP gene. The pSF-eGFP vector is based on the Friend mink cell focus-forming/murine embryonic stem cell virus (FMEV). It contains the eGFP gene under the transcriptional control of the spleen focus-forming virus long-term repeat (LTR), which has been combined with a permissive leader sequence of the murine embryonic stem cell virus (MESV) to overcome transcriptional repression of U3-mediated gene expression. Viral supernatants were passed through a 0.45 μm filter to remove cells and cellular debris before use. MSCs at 20% of confluence were exposed to viral supernatant in the presence of 1 μg/mL polybrene (Sigma) for 5 hours, and this was repeated until stromal cells reached confluence. Transduction efficiency, evaluated 2 to 14 days after the last infection, was determined by FACS and microscopic analysis. Enhanced GFP detection in tissues was assessed by PCR; positive controls consisted of human eGFP-expanded Stro-1+ cells, and negative controls consisted of murine genomic DNA extracted concurrently with each set of test samples and a reagent control. The primers for eGFP were forward primer 5′-ctcgtgaccaccctgacctac-3′ and reverse primer 5′-aagaagatggtgcgctccg-3′.
Immunohistology
The presence of GFP protein on eGFP-tranduced cells in mouse tissue sections was assessed by a rabbit anti-GFP antibody. The human origin of these cells on mouse tissues was assessed by an antibody directed against human β2-microglobulin. Tissues were fixed in 4% neutral buffered formaldehyde for 16 hours at room temperature and were then embedded in paraffin. Sections (5 μm) were deparaffinized and rehydrated and then permeabilized for 5 minutes in 0.1% Triton/PBS at room temperature. For eGFP detection, the immunocytochemistry was performed on a NexES IHC automat (Ventana, Illkirch, France) using p-Dimethylaminobenzene (DAB) detection kit (no. 22495, Ventana) with a 2% trypsin digestion step for 30 minutes. Slides were incubated for 30 minutes with rabbit anti-GFP polyclonal antibody (product no. AB3080, Chemicon International, Temecula, CA) diluted at 1:1000 (product no. E3432, Novocastra, Newcastle upon Tyne, United Kingdom). A biotin-labeled goat antirabbit immunoglobulin G (IgG) antibody was used. Immunoreactivities were visualized by avidin-horseradish peroxidase (avidin-hrpo) DAB detection. Negative control consisted of rabbit IgG diluted at 1:1000 in antibody diluent (no. 107784, Ventana). The slides were counterstained with hematoxylin and dehydrated. For β2-microglobulin, the immunocytochemistry was also performed on a NexES IHC automat (Ventana). The monoclonal anti-β2-microglobulin antibody (product no. E3432, Novocastra) was applied at 1:50 in antibody diluent. A secondary antibody composed of biotynyled antirabbit IgG was used. Immunoreactivities were visualized by Ventana kit to make alkaline phosphatase reaction with Fast Red (no. 760031, Ventana) substrate followed by counterstaining with hematoxylin.
Statistical analysis
Statistical analyses were performed using table curve software (SPSS, Paris, France). The kinetics of human CD45 cell engraftment was evaluated by calculating areas under the curves (AUCs) at different time points (3, 6, 9, and 12 weeks). Differences in CD45+ cell AUCs between mouse groups were assessed using the Mann-Whitney rank sum test. Other statistics tests were performed using Sigmastats software (SPSS). When indicated, values are reported as mean ± standard deviation (SD). Statistical significance was set for P < .05.
Results
Separation and flow cytometric analysis of expanded Stro-1+ and Stro-1- cells
Ten BM samples were studied. After a 1-week culture period, nonadherent cells containing hematopoietic cells were discarded. The adherent cells, detached with trypsin, contained a median of 6% Stro-1+ cells (mean, 7% ± 6%; range, 1%-25%). Cells were then separated with anti-Stro-1-coated beads, and the Stro-1+ and Stro-1- cells were further seeded for expansion. Confluence was usually reached after 3 days. Cells were reseeded at least twice. The total median time of culture was 15 days. Cells were then collected by trypsinization and analyzed by flow cytometry. Results showed that expanded Stro-1+ cells were 30% to 56% SH2+ (CD105) and 87% to 97% SH3+ (CD73). Expanded Stro-1- cells were 12% to 42% SH2+ and 71% to 83% SH3+. None of the fractions contained CD45 (hematopoietic) or CD34 cells.
In 2 independent experiments, the Stro-1+ and Stro-1- cells were expanded by the same technique and further used for infusion to NOD/SCID mice (see below).
Cotransplantation of CD34+ cells plus expanded Stro-1+ or Stro-1- MSCs
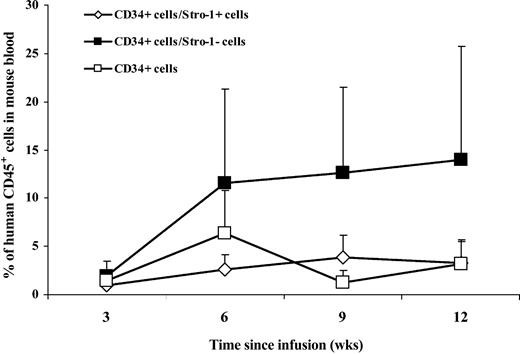
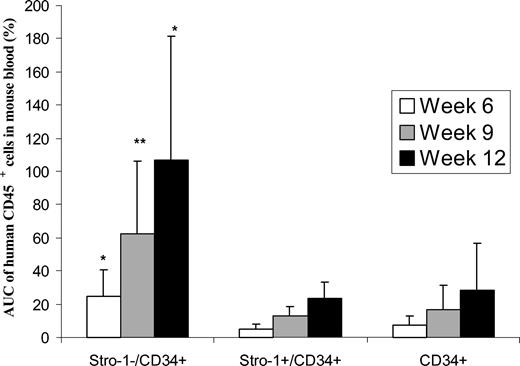
Human CD45 cell engraftment in blood. Eight-week-old mice were sublethally irradiated with 3.5 Gy. Twelve to 24 hours after irradiation, CD34+ with expanded Stro-1+ or Stro-1- cells were injected into the retro-orbital plexus of mice. Controls consisted of mice receiving only CD34+ cells or no cells. The purity of hUCB CD34+ cells used in these 2 series of experiments was 93% and 98%. At weeks 3, 6, 9, and 12 after transplantation, blood was collected from the retro-orbital plexus. The percentages of CD45 cells were determined by FACS analysis, and the data from the 2 experiments were aggregated and plotted according to time (Figure 1) (no CD45 cells were found in the control group of mice receiving no human cells). The data showed that the percentages of human CD45 cells in peripheral blood of NOD/SCID mice were higher at 6, 9, and 12 weeks for the group of mice receiving the combination of “CD34+ + Stro-1-” cells, as compared with mice receiving only CD34+ cells. The differences are highly significant when comparing the areas under the curve (AUCs) for each group of mice (Figure 2) (P = .02, P = .009, and P = .01 at weeks 6, 9, and 12, respectively). The differences were also significant when the data of the “CD34+ + Stro-1-” group was compared with the “CD34+ + Stro-1+” group (P = .005, P = .004, and P = .02 at weeks 6, 9, and 12, respectively). No difference was observed between the groups “CD34+ + Stro-1+” versus “CD34+ alone.” These data of human CD45 cell engraftment in peripheral blood indicated that expanded Stro-1- cells sustained human hematopoietic engraftment in NOD/SCID mice and that no advantage was brought by coinfusion of expanded Stro-1+ cells over infusion of CD34+ cells alone.
Percentages of human CD45 cells in the blood of NOD/SCID mice at 3, 6, 9, and 12 weeks after infusion of human cord blood CD34+ cells with or without ex vivo-expanded Stro-1+ or Stro-1-cells. Each point corresponds to the mean ± SD of CD45 cell percentages in 8 mice.
Percentages of human CD45 cells in the blood of NOD/SCID mice at 3, 6, 9, and 12 weeks after infusion of human cord blood CD34+ cells with or without ex vivo-expanded Stro-1+ or Stro-1-cells. Each point corresponds to the mean ± SD of CD45 cell percentages in 8 mice.
Percentages of human CD45 cells detected in peripheral blood of NOD/SCID mice. Columns represent the mean value (from 8 animals) with standard deviations of the areas under the curve (AUC) of CD45 cell percentages in the blood of NOS/SCID mice. *A statistically significant difference with P < .05 as compared with infusion of “CD34+alone”; **a difference with P < .01.
Percentages of human CD45 cells detected in peripheral blood of NOD/SCID mice. Columns represent the mean value (from 8 animals) with standard deviations of the areas under the curve (AUC) of CD45 cell percentages in the blood of NOS/SCID mice. *A statistically significant difference with P < .05 as compared with infusion of “CD34+alone”; **a difference with P < .01.
Engraftment of human CD34, CD45, CD19, and CD11b cells in BM, spleen, and blood 12 weeks after infusion. Animals of the above groups were killed at 12 weeks, and cells recovered from the BM, spleen, and peripheral blood were analyzed for the presence of human CD34, CD45, CD19, and CD11b cells.
Cotransplantation of “CD34+ + Stro-1-” cells resulted in BM in an increase (as compared with the group “CD34+ alone”) in the percentage of human CD34 (8.7% vs 4.3%, P = .04), CD45 (48% vs 27%, P = .04), and CD19 (37% vs 20%, P = .03) (Table 1). No difference was found for CD11b.
Percentages of human CD45, CD19, CD11b, and CD34 in BM, spleen, and blood of animals 12 weeks after cotransplantation of 1 × 105 hUCB CD34+ cells and 1 × 106 expanded Stro-1+ or Stro-1− cells in NOD/SCID mice
Tissue . | CD34+ Stro-1− . | CD34+ Stro-1+ . | CD34+ . |
|---|---|---|---|
| BM | |||
| CD45 | 48 ± 22 | 33 ± 16 | 27 ± 25 |
| CD19 | 38 ± 13 | 21 ± 13 | 20 ± 17 |
| CD11b | 3 ± 1 | 4 ± 1 | 3 ± 3 |
| CD34 | 9 ± 3 | 5 ± 2 | 4 ± 4 |
| Spleen | |||
| CD45 | 35 ± 25 | 14 ± 8 | 15 ± 16 |
| CD19 | 19 ± 23 | 7 ± 6 | 11 ± 12 |
| CD11b | 8 ± 10 | 0.8 ± 0.7 | 3 ± 4 |
| Blood | |||
| CD45 | 14 ± 12 | 3 ± 2 | 4 ± 4 |
| CD19 | 9 ± 7 | 1.6 ± 1.3 | 3 ± 3 |
| CD11b | 4 ± 6 | 0.25 ± 0.25 | 1 ± 1 |
Tissue . | CD34+ Stro-1− . | CD34+ Stro-1+ . | CD34+ . |
|---|---|---|---|
| BM | |||
| CD45 | 48 ± 22 | 33 ± 16 | 27 ± 25 |
| CD19 | 38 ± 13 | 21 ± 13 | 20 ± 17 |
| CD11b | 3 ± 1 | 4 ± 1 | 3 ± 3 |
| CD34 | 9 ± 3 | 5 ± 2 | 4 ± 4 |
| Spleen | |||
| CD45 | 35 ± 25 | 14 ± 8 | 15 ± 16 |
| CD19 | 19 ± 23 | 7 ± 6 | 11 ± 12 |
| CD11b | 8 ± 10 | 0.8 ± 0.7 | 3 ± 4 |
| Blood | |||
| CD45 | 14 ± 12 | 3 ± 2 | 4 ± 4 |
| CD19 | 9 ± 7 | 1.6 ± 1.3 | 3 ± 3 |
| CD11b | 4 ± 6 | 0.25 ± 0.25 | 1 ± 1 |
In the spleen, there was a trend for higher percentages of CD45, CD19, and CD11b in the group “CD34+ + Stro-1-” as compared with “CD34+ alone,” but differences were not significant (P = .08, P = .4, and P = .2 for CD45, CD19, and CD11b, respectively). In blood, in addition to higher levels of CD45 as reported in Figure 1, there was a trend for an increase of CD19 and CD11b cell percentages in the group receiving “CD34+ + Stro-1-” cells as compared with the group “CD34+ alone” (P = .2 for both CD19 and CD11b). In contrast, no significant differences in the percentages of human hematopoietic cells were observed between “CD34+ + Stro-1+” and “CD34+ alone” groups.
Homing of Stro-1+ and Stro-1- MSCs in mouse tissues
In a second series of experiments, NOD/SCID mice receiving expanded Stro-1+ or Stro-1- MSCs (without CD34+ cells) or no cells were killed at 12 weeks, and DNAs extracted from the BM, spleen, peripheral blood, brain, heart, liver, lungs, kidneys, and leg muscles were analyzed for the presence of specific human DNA by detection of human β-globin gene. The RAPSYN murine gene probe was used as an internal control to normalize the amount of human DNA as compared with murine DNA in quantitative PCR. The ratio of human-murine DNA copy numbers was expressed in numbers of human cells per 10 000 murine cells. Human Stro-1+ and Stro-1- cell DNAs were found in variable amounts in BM, spleen, brain, heart, liver, lungs, kidneys, and muscles but not in blood (Table 2). Results showed that the amount of Stro-1+ cell DNA was higher than that of Stro-1- DNA in spleen (× 8), muscles (× 6), BM (× 2), liver (× 1.5), and kidneys (× 1.5). No significant difference was observed in brain, while more Stro-1- than Stro-1+ DNA was found in lungs (× 3.5). These data indicate that Stro-1+-derived cells migrated better than Stro-1--derived cells in most mouse tissues.
Homing of expanded Stro-1+ or Stro-1− cells in mouse tissues
Tissues . | Stro-1+ cells . | Stro-1− cells . |
|---|---|---|
| Spleen | 8 ± 5 | 1.5 ± 1 |
| BM | 13 ± 7 | 6 ± 3 |
| Brain | 4 ± 5 | 4 ± 0.6 |
| Heart | 1.6 ± 1.1 | 2 ± 2 |
| Liver | 0.6 ± 0.4 | 0.4 ± 0.6 |
| Lungs | 2 ± 3 | 7 ± 11 |
| Kidneys | 2.9 ± 1.9 | 1.9 ± 1.7 |
| Muscles | 2 ± 2 | 0.2 ± 0.3 |
Tissues . | Stro-1+ cells . | Stro-1− cells . |
|---|---|---|
| Spleen | 8 ± 5 | 1.5 ± 1 |
| BM | 13 ± 7 | 6 ± 3 |
| Brain | 4 ± 5 | 4 ± 0.6 |
| Heart | 1.6 ± 1.1 | 2 ± 2 |
| Liver | 0.6 ± 0.4 | 0.4 ± 0.6 |
| Lungs | 2 ± 3 | 7 ± 11 |
| Kidneys | 2.9 ± 1.9 | 1.9 ± 1.7 |
| Muscles | 2 ± 2 | 0.2 ± 0.3 |
The results are expressed as numbers of equivalent human cells per 10 000 murine cells (as evaluated by number of copies of human β-globin DNA in mouse tissue DNA) in each analyzed tissue.
Injection into NOD/SCID mice of expanded Stro-1+ cells transduced with eGFP
To check whether gene transduction might modify the cytokine release and homing functions of expanded Stro-1+ cells, the cells were transduced with the gene encoding for the green fluorescent protein (eGFP). In 2 separate experiments, eGFP gene marking efficiency was 78% and 73%. The eGFP expression was unchanged after 4 additional weeks of culture, and the eGFP-transduced Stro-1+ cells showed in vitro by enzyme-linked immunosorbent assay (ELISA) similar cytokine (macrophage colony-stimulating factor [M-CSF], granulocyte-macrophage [GM]-CSF, G-CSF, thrombopoietin [TPO], interleukin-6 [IL-6], leukemia inhibitory factor [LIF]) release than nontransduced Stro-1+ cells (results not shown). eGFP-transduced cells were infused into the irradiated NOD/SCID mouse. At 12 weeks after injection, animals were killed, and blood, spleen, liver, BM, brain, heart, lungs, muscles, and kidneys were harvested and DNA extracted. In BM, eGFP cells were assessed by fluorescence in FACS analysis, and no eGFP-positive cell could be detected. In contrast, using a more sensitive technique, PCR, eGFP-positive DNA was found in BM from 5 of 5 tested mice. Similarly, most of the tissues tested in PCR were found eGFP-positive (lungs, 4 of 5 mice; liver, 3 of 5; muscles, 5 of 5; kidneys, 4 of 4; spleen, 5 of 5; brain, 3 of 5; and heart, 5 of 5), indicating that the gene transfection did not alter the capacity of expanded Stro-1+ cells to migrate.
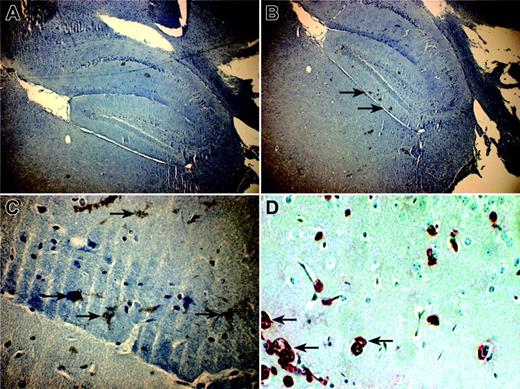
In situ localization of eGFP-transduced cells was studied in tissues by microscope examination to detect GFP green fluorescence. No fluorescence was detected in any examined tissue. To improve the sensitivity of detection, the presence of GFP protein on eGFP-tranduced cells in mouse tissue sections was then assessed by a rabbit anti-GFP antibody (revealed with DAB). In parallel, the human origin of these cells on mouse tissues was assessed by an antibody directed against human β2-microglobulin. Nevertheless, eGFP cells remained undetectable in most tissue cross sections, except in brain, where eGFP-marked cells were observed into the dentate gyrus area (Figure 3). The human origin of these cells was independently confirmed with an antihuman β2-microglobulin antibody revealed with alkaline phosphatase (Figure 3).
Distribution of human eGFP MSC-derived cells in brain of NOD/SCID mice. Pictures were taken in confocal microscopy from 5 μm-thin sagittal sections of brain of NOD/SCID mice. (A) A × 40 magnification of a cross section incubated with a nonrelevant antibody (negative control). (B) Cross sections, magnified × 40 and (C) × 400, of brain incubated with anti-GFP antibody counterstained with hematoxylin. (D) A × 400 magnification of brain cross section incubated with anti-β2-microglobulin antibody counterstained with hematoxylin. Black arrows indicate positive GFP cells (B-C) and human β2-microglobulin-positive cells (D) in the dentate gyrus area.
Distribution of human eGFP MSC-derived cells in brain of NOD/SCID mice. Pictures were taken in confocal microscopy from 5 μm-thin sagittal sections of brain of NOD/SCID mice. (A) A × 40 magnification of a cross section incubated with a nonrelevant antibody (negative control). (B) Cross sections, magnified × 40 and (C) × 400, of brain incubated with anti-GFP antibody counterstained with hematoxylin. (D) A × 400 magnification of brain cross section incubated with anti-β2-microglobulin antibody counterstained with hematoxylin. Black arrows indicate positive GFP cells (B-C) and human β2-microglobulin-positive cells (D) in the dentate gyrus area.
Discussion
Engraftment and initiation of hematopoiesis by tranplanted HSCs depend on complex processes. Transplanted cells must home into the BM microenvironment and lodge in the appropriate niches before they proliferate and differentiate.18,27,28 Stromal cells present in this microenvironment, now commonly called MSCs, are thought to play an important role in hematopoietic stem cell engraftment. Marrow stromal cells are a heterogeneous population of cells, including reticular endothelial cells, fibroblasts, adipocytes, and osteogenic precursor cells, which provide growth factors, cell-to-cell interactions, and matrix proteins.
Previous studies have demonstrated the capacity of MSCs to improve hematopoietic engraftment when they are coinfused with hematopoietic stem cells,1,3 the first demonstration being made by Anklesaria et al29 in a mouse model. In the humanized sheep model of HSC transplantation, cotransplantation of BM-derived stromal cells resulted in enhancement of BM engraftment and increased levels of human cells circulating early after transplantation.30 Recently, MSCs derived from human fetal lungs have been shown to promote engraftment of human umbilical cord blood CD34+ cells in a mouse model.4 However, MSCs are heterogeneous, and little is known on the respective role of the various subpopulations of MSCs in hematopoietic homing and engraftment. Simmons et al20,21 and then Dennis et al22 showed that the Stro-1 antigen is expressed in a progenitor MSC subset that is enriched in CFU-F progenitors. Given their multipotentiality, Stro-1+ cells appear as good candidates for cell and gene therapy. The aim of the present study was to know the functions of these cells, as compared with those lacking the Stro-1 antigen. Phenotypically, the 2 cell subsets did not show any significant difference in the expression of several membrane antigens (in addition to SH2 and SH3 reported here, we tested integrin β1, integrin α1, integrin α3, integrin α5, integrin αvβ3, integrin αvβ5, CD44, endoglin, SH4, S-endo, Thy-1; P.C., unpublished results, March 2003). In contrast, we found that the levels of human hematopoietic engraftment (as assessed by the presence of human CD34, CD45, CD19, and CD11b cells) achieved in the blood, spleen, and BM of NOD/SCID mice were higher when expanded Stro-1- cells were cotransplanted with cord blood CD34+ cells as compared with Stro-1+ cells.
For decades, studies on MSCs were hampered by the lack of sufficient numbers of human cells to infuse in humans. The possibility to expand these cells ex vivo has actually renewed the clinical interest for MSCs. Indeed, the spatial organization of stem cells in the marrow, mediated by the hematopoietic microenvironment and extracellular matrix, may be crucial for hematopoietic regeneration after HSC transplantation.18,27,28 Based on animal data, including the results presented here, it is postulated that stromal cells infused into humans can engraft in the marrow microenvironment that has been damaged by chemotherapy or irradiation and therefore would improve the hematopoietic recovery after HSC transplantation. A series of studies has brought evidence that these cells do play a role.5,6 However, randomized studies in humans (that are in progress) need to be done to clearly prove this point.
In a second part of our study, we analyzed the homing of human Stro-1- selected MSCs in tissues of mice. By quantitative PCR analysis, we found that expanded Stro-1+ cells migrated better than expanded Stro-1- cells in BM, spleen, muscles, liver, and kidneys. No difference was observed in brain, while more expanded Stro-1- than Stro-1+ cells were found in lungs. This latter result in lungs fitted with the data reported by Noort et al4 although their data were obtained with fetal lung MSCs. The homing of MSCs in various tissues has been earlier reported,31,32 but this is the first observation of a difference of migration of MSC subsets based on Stro-1 antigen expression.
We recently demonstrated that allogeneic MSCs were able to engraft in a patient with aplastic anemia, to improve the stromal microenvironment, and to contribute to donor stromal cell regeneration.33 The fact that, in this observation, allogeneic MSCs were only detectable by real-time PCR in the BM recipient did not mean that the cells did not repopulate host tissues. Indeed, Horwitz et al34 found less than 2% infused MSCs in children suffering from osteogenesis imperfecta, while the biologic effect of this infusion was impressive on the correction of the disease, particularly in the new dense bone formation. Similarly, Koç et al,35 despite finding less than 2% donor cells in the BM-derived MSCs of patients with Hurler syndrome and metachromatic leukodystrophy infused with expanded MSCs, noted a reversal of disease pathophysiology in some tissues.
The high plasticity of MSCs has opened new areas of clinical applications.12,19 Transplantation of MSCs would attenuate or possibly correct genetic disorders of bone, cartilage, and muscle, as previously published.34,35 Here, to ensure that the gene transduction would not modify Stro1+ MSC functions, the cells were transduced with the eGFP gene and analyzed for ex vivo cytokine release and homing in NOD/SCID mice: The eGFP-transduced Stro-1+ cells had the same cytokine release and migrated to the same tissues (as assessed by PCR) in NOD/SCID mice as the nontransduced cells.
Noteworthy, we did not report on the presence of eGFP cells detected by microscope examination in tissue sections because this technique had proven to have, in preliminary testing, a very low sensitivity. Visualization of eGFP-positive cells in tissue cross sections was difficult and was only revealed by immunostaining with a rabbit anti-GFP antibody in the dentate gyrus brain section.
The presence of eGFP-positive cells was thus assessed by a technique more sensitive, PCR: by this technique, most of the tissues were found positive. In particular, the BM that was found eGFP-negative by direct microscope examination and by FACS analysis was actually eGFP-positive by PCR in 5 of 5 tested mice. In a baboon model, Devine et al32 also found positive homing of eGFP cells using a sensitive quantitative PCR technique. These results were also obtained by our team36 in irradiated macaques.
Very rare pluripotent stem cells persist into the stromal cell compartment of adult BM.37,38 In given circumstances (lesion, new environment) they may proliferate and differentiate. Interestingly, in our nonhuman primate model,36 infused MSCs homed preferentially to altered tissues, which is promising for specific tissue repair. However, it's remained unknown if these MSCs were engrafted or just resident in the tissues where they migrated. We are presently investigating if the expanded MSCs that home in altered tissues of NOD/SCID mice actually differentiate into true local MSCs and acquire the specific tissue antigen or just remain in the tissue without changing their phenotype.
Our data may be interesting for gene therapy: Stro-1+ cells that were not modified by gene transduction would probably be used in humans for targeting tissues. Indeed, the potential of MSCs as vehicles for gene delivery or protein production has been indicated by many authors.12-19,23 Nolta et al1 have shown that MSCs transfected with the IL-3 gene can improve engraftment of human HSCs in immunodeficient beige-nude-xid (bnx) mice by producing the IL-3 protein in vivo. The same result was obtained by Brouard et al3 in the NOD/SCID mouse model with a murine stromal cell line.
In conclusion, the present data demonstrate that the functions of MSCs depend on their Stro-1 phenotype: expanded Stro-1+ home in higher numbers than expanded Stro-1- cells in most mouse tissues but support less engraftment of hematopoietic progenitors. In contrast to hematopoietic support, for which selection of Stro-1+ cells seems unnecessary (around 90% of unselected MSCs are Stro-1-), a preselection before ex vivo gene tranfection for in vivo gene delivery appears useful to improve MSC homing, at least in NOD/SCID mice.
Prepublished online as Blood First Edition Paper, January 8, 2004; DOI 10.1182/blood-2003-04-1121.
Supported by grants from the Association pour la Recherche sur les Myopathies/Institut National de la Santé et de la Recherche Médicale (AFM/INSERM no. 4CS02F), Etablissement Français des Greffes, and the Association Combattre la Leucémie (M.B.).
M.L. and D.T. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank C. Joubert and C. Beaudelin for animal care.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal