Abstract
T-cell clones generated from both CD4+CD25+ and CD8+CD25+ human thymocytes were assessed for their ability to suppress the proliferative response to allogeneic stimulation of type 1 T-helper (Th1) or type 2 T-helper (Th2) clones derived from autologous CD4+CD25- thymocytes. Both CD4+ and CD8+ T-regulatory (Treg) cells completely suppressed the proliferation of Th1 clones but exhibited significantly lower suppressive activity on the proliferation of Th2 clones. The partial suppressive effect on Th2 cells was further reduced by the addition in culture of interleukin-4 (IL-4), whereas it was increased in the presence of an anti–IL-4 monoclonal antibody (mAb). The suppressive activity on Th2 clones was also completely inhibited by the addition of IL-7, IL-9, and IL-15 but not of IL-2, whereas the suppressive effect on Th1 clones was only reverted by the addition of IL-15. Of note, Th2 clones expressed significantly higher amounts of mRNA for IL-4 receptor (IL-4R) and IL-9R α chains than Th1 clones, whereas the expression of mRNA for IL-2R, IL-7R, and IL-15R α chains was comparable. Taken together, these findings demonstrate that Th2 cells have a lower susceptibility than Th1 cells to the suppressive activity of human CD25+ regulatory thymocytes, because they are able to produce, and to respond to, growth factors distinct from IL-2, such as IL-4 and IL-9. (Blood. 2004; 103:3117-3121)
Introduction
CD4+CD25+ T-regulatory (Treg) cells have been isolated from the periphery and thymus of mice and humans.1-4 Recently, a small subset of CD8+CD25+ cells sharing similar characteristics and functions with CD4+CD25+ thymocytes has been detected in human postnatal thymuses.5 Both CD4+CD25+ and CD8+CD25+ human thymocytes constitutively expressed Foxp3 and glucocorticoid-induced tumor necrosis factor (TNF) receptor (GITR) mRNA, as well as surface CCR8, surface TNF receptor 2 (TNFR2), and cytoplasmic CTLA-4 (cytotoxic T-lymphocyte–associated antigen-4) proteins, which are usually a feature of Treg cells.5-8 Following activation, they did not produce cytokines, but many of them expressed both CTLA-4 and TGF-β1 (transforming growth factor-β1) on their surface.4,5 These cells did not proliferate but suppressed the proliferation of autologous CD4+CD25- thymocytes to allogeneic stimulation by a contact-dependent mechanism. Their suppressive activity appeared to be related to the combined action of surface CTLA-4 and TGF-β1 that inhibited the expression of the interleukin-2 receptor (IL-2R) α chain on target T cells.4,5 Treg cells that develop in, and emerge from, the thymus are certainly responsible for the maintenance of self-tolerance and prevention of autoimmune disorders, which often result from type 1 T-helper (Th1)–mediated immune responses against autoantigens.9 Thus, the suppressive effect of CD4+CD25+ on Th1 cells is predictable.
To establish whether CD4+CD25+ T cells exert the same regulatory activity even on Th2 cells, T-cell clones from both CD4+CD25+ and CD8+CD25+ human thymocytes were generated and assessed for their suppressive activity on the proliferative response to allogeneic stimulation of T-cell clones showing Th1 or Th2 profile of cytokine production, which were contemporaneously derived from autologous CD4+CD25- thymocytes. The results showed that the proliferation of Th1 clones was completely suppressed by both CD4+ and CD8+ autologous Treg cells, whereas the same Treg cells exhibited much lower suppressive activity on the proliferation of Th2 clones. This difference was due to the ability of Th2 cells to produce cytokines that allow them to proliferate even in the absence of responsiveness to IL-2. Indeed, the suppressive activity on Th2 clones was enhanced by neutralization in culture of IL-4, whereas it was completely blocked by the addition of IL-4, IL-7, IL-9, and IL-15 but not of IL-2. By contrast, the proliferation of Th1 cells was only restored by the addition of IL-15. Of note, Th2 clones expressed significantly higher levels of IL-4R and IL-9R mRNA than Th1 clones, whereas the expression on Th1 and Th2 cells of IL-2R, IL-7R, and IL-15R was comparable.
Materials and methods
Reagents and Abs
The medium used was RPMI 1640 (Seromed, Berlin, Germany), supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 1% pyruvate, 2 × 10-5 M 2-mercaptoethanol (2-ME) (all from Gibco Laboratories, Grand Island, NY), and 10% fetal calf serum (FCS; HyClone, Logan, Utah). Unconjugated and fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, allophycocyanin (APC)–, or peridin chlorophyll protein (PerCP)–conjugated anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD16, anti-CD19, anti-CD25, anti-CD34, anti-CD56, anti-TCRγδ (T-cell receptor γδ), anti–IL-4, and anti–IFN-γ (interferon-γ) monoclonal antibodies (mAbs) were purchased from BD Biosciencies (Mountain View, CA). The anti–glicophorin A, B mAb was from Sigma Chemical (St Louis, MO). The anti-TCR Vβ mAbs were from Serotec (Oxford, United Kingdom). Conjugated and unconjugated isotype-matched control Abs were purchased from Southern Biotechnology Associated (Birmingham, AL). Human rIL-2 was a kind gift from Eurocetus (Milan, Italy). Human rIL-4, rIL-7, rIL-9, and rIL-15 were purchased by R&D System (Minneapolis, MN). The goat antimouse immunoglobulin G (IgG) and anti-CD25 Abs conjugated with magnetic beads were obtained from Milteny Biotec (Bisley, Germany).
Human thymuses
Healthy postnatal thymus specimens were obtained from children, aged between 5 days and 3 years, who underwent corrective cardiac surgery at the Apuanic Pediatric Hospital of Massa Carrara, Italy. The procedures followed in the study were in accordance with the ethical standards depicted in the Declaration of Helsinki and accepted by the Regional Committee on Human Experimentation.
Isolation of CD4+CD25-, CD4+CD25+, CD8+CD25-, and CD8+CD25+ thymocytes
Negative selection of CD4+ and/or CD8+ single-positive (SP) thymocytes was first performed by high-gradient magnetic cell sorting, as described elsewhere.4,5 Briefly, thymic mononuclear cell (MNC) suspensions were incubated for 20 minutes with anti-CD8 (or anti-CD4), anti-CD14, anti-CD16, anti-CD19, anti-CD34, anti-CD56, anti-TCRγδ, anti–glycophorin A, B mAbs, extensively washed, and then incubated for an additional 20 minutes with goat antimouse polyclonal Ab conjugated to colloidal super-paramagnetic microbeads, according to the magnetic cell sorter (MACS) system (Milteny Biotec). After washing, cells were separated on a CS+ column. The remaining SP CD4+ or CD8+ T cells were then separated into CD25+ or CD25- by positive selection by the use of anti-CD25 MACS microbeads. The positive selections were performed on LS+ columns. Aliquots of CD4+CD25--purified thymocytes were stored in frozen state to be used as target cells for the evaluation of suppressive activity of T-cell clones generated from CD4+CD25+ or CD8+CD25+ autologous thymocytes.
Cloning of thymocytes
For the cloning procedure, CD4+CD25+, CD4+CD25-, CD8+CD25+, and CD8+CD25- thymocytes from one postnatal human thymus were seeded under limiting-dilution conditions (0.5 cell/well) in round-bottom microwell plates (Nunc, Rochester, NY), containing 105 irradiated (60 Gy [6000 rad]) allogeneic peripheral blood mononuclear cells (PBMCs) as feeder cells, 1% phytohemagglutinin (PHA; vol/vol), and rIL-2 (20 U/mL), as reported.5
Flow cytometry analysis
Assessment of suppressive activity
The assessment of suppressive activity by thymocyte suspensions or T-cell clones was performed by using a proliferation assay, as detailed in previous papers.4,5 Briefly, 5 × 104 CD4+CD25- thymocytes were cultured with 105 irradiated T-cell–depleted allogeneic PBMCs and soluble anti-CD3 (1 μg/mL) in the presence of different numbers of CD4+CD25+ autologous thymocytes (target–regulatory cell ratio 1:1, 2:1, 4:1). In some experiments, mixed lymphocyte cultures (MLCs) were performed in presence or absence of IL-2 (10 IU/mL), IL-4 (2 ng/mL), IL-7 (1 ng/mL), IL-9 (2 ng/mL), or IL-15 (7 ng/mL). On day 5, after 8 hours of pulsing with 0.5 μCi (0.0185 MBq) 3H-TdR/well (Amersham), cultures were harvested, and radionuclide uptake was measured by scintillation counting. The average levels of proliferation, measured as cpm (± SD), were 41.375 ± 13.499 for the 8 Th1 clones, 36.553 ± 14.133 for the 9 Th2 clones, and 570 ± 370 for the 3 Treg clones. The suppressive activity was expressed as percentage of inhibition of proliferation.
Real-time quantitative RT-PCR (TaqMan)
TaqMan reverse transcription–polymerase chain reaction (RT-PCR) was performed as described elsewhere.5 Quantitative analysis of Foxp3, GITR, and IL-2R, IL-4R, IL-7R, IL-9R, and IL-15R α chains was performed by using Assay on Demand (Applied Biosystems, Warrington, United Kingdom). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) quantitative analysis was performed by using predeveloped TaqMan assay reagents target kits (Applied Biosystems). GAPDH was used for normalization.
Results
Generation and characterization of human T-cell clones
CD4+CD25+, CD4+CD25-, CD8+CD25+, and CD8+CD25- thymocyte populations were first purified (purity > 98%) and then cloned by limiting dilution. Twelve T-cell clones (clonal efficiency, 3.2%) were generated from CD4+CD25+, 11 (clonal efficiency, 2.9%) from CD8+CD25+, 53 (clonal efficiency, 18.2%) from CD4+CD25-, and 38 (clonal efficiency, 10%) from CD8+CD25- thymocytes. All clones from each thymocyte population were isolated and expanded for analysis of their function. The CD4+CD25+ and the CD8+CD25+ T-cell clones were heterogeneous in their ability to inhibit the proliferative response of autologous CD4+CD25- thymocytes in response to allogeneic stimulation (Figure 1A), whereas none of the T-cell clones derived from CD4+CD25- or CD8+CD25- thymocytes showed any suppressive effect (data not shown). However, none of the clones derived from CD4+CD25+ or CD8+CD25+ thymocytes produced IL-4 or IFN-γ following stimulation with phorbol 12-myristate 13-acetate (PMA) plus ionomycin, as evaluated by analysis of intracellular synthesis at the single cell level (Figure 1B), whereas all clones generated from CD4+CD25- thymocytes produced detectable amounts of IL-4 and/or IFN-γ in response to stimulation with PMA plus ionomycin (Figure 1B). On the basis of their cytokine production profile, 8 CD4+ T-cell clones were classified as Th1 (production of IFN-γ but no IL-4), 9 as Th2 (production of IL-4 but no IFN-γ), and 36 as Th0 (production of both IL-4 and IFN-γ).
Characterization of T-cell clones generated from CD4+CD25+, CD8+CD25+, CD4+CD25- thymocyte suspensions. (A) T-cell clones generated from purified CD4+CD25+ or CD8+CD25+ thymocytes were assessed for their ability to inhibit the proliferative response of CD4+CD25- autologous thymocytes to allogeneic stimulation, as described in “Materials and methods.” Results are expressed as mean percentage of inhibition of proliferation obtained in triplicate cultures of each CD4+ or CD8+ T-cell clone. (B) T-cell clones generated from CD4+CD25+, CD8+CD25+, CD4+CD25- thymocytes were assessed for their ability to produce IL-4 and/or IFN-γ by flow cytometry analysis at single cell level. Production of cytokines by each clone was considered as noteworthy when the proportion of producer T-cell blasts was higher than 10%.
Characterization of T-cell clones generated from CD4+CD25+, CD8+CD25+, CD4+CD25- thymocyte suspensions. (A) T-cell clones generated from purified CD4+CD25+ or CD8+CD25+ thymocytes were assessed for their ability to inhibit the proliferative response of CD4+CD25- autologous thymocytes to allogeneic stimulation, as described in “Materials and methods.” Results are expressed as mean percentage of inhibition of proliferation obtained in triplicate cultures of each CD4+ or CD8+ T-cell clone. (B) T-cell clones generated from CD4+CD25+, CD8+CD25+, CD4+CD25- thymocytes were assessed for their ability to produce IL-4 and/or IFN-γ by flow cytometry analysis at single cell level. Production of cytokines by each clone was considered as noteworthy when the proportion of producer T-cell blasts was higher than 10%.
Suppressive activity of CD4+CD25+ and CD8+CD25+ T-cell clones on the proliferation of autologous Th1 or Th2 clones
One CD8+ (no. 9) and 2 CD4+ (no. 2 and no. 8) clones showing strong suppressive activity on autologous CD4+CD25- thymocytes, as well as the expression of Foxp3 and GITR mRNA (data not shown), were selected. As target cells, 2 Th1 and 2 Th2 clones were chosen (Figure 2A). The TCR usage of all clones was determined by flow cytometry and revealed that all T-cell blasts of each clone expressed a single Vβ chain, thus confirming their common clonal origin (data not shown). The 2 Th1 and the 2 Th2 clones were then stimulated with irradiated allogeneic PBMCs for 5 days in absence or presence of different concentrations of T-cell blasts from the 3 suppressive clones. The results of this experiment are depicted in Figure 2B. At 1:1 target–regulatory cell ratio, all 3 clones strongly suppressed the proliferation of Th1 clones (between 80% and 98%), but they exerted much lower suppressive effect on the proliferation of Th2 clones (between 35% and 63%) (Figure 2B). As shown in Figure 2C, the mean suppressive activity of the CD4+CD25+ clone no. 8 was significantly higher on the 8 Th1 clones than on the 9 Th2 clones not only at 1:1 but also at 2:1 target–regulatory cell ratio. Both the CD4+CD25+ no. 2 and the CD8+CD25+ no. 9 clones also exerted similar effects (data not shown). The suppressive activity of the CD4+CD25+ clone no. 8 was also assessed on the proliferation of 10 randomly selected Th0 clones. The mean of inhibition (± SD) of proliferation was equal to 66% ± 9%.
Lower susceptibility of Th2 than Th1 clones to the suppressive activity of autologous Treg clones. (A) Cytokine profile of 2 Th1 (i-ii) and 2 Th2 (iii-iv) T-cell clones used as target of Treg clones, as assessed by flow cytometry at single cell level. (B) Suppressive effect of T-cell blasts from 3 Treg clones on the proliferative response to allogeneic stimulation of T-cell blasts from the 2 Th1 and the 2 Th2 clones. Results are expressed as percentage of inhibition of proliferation obtained in triplicate cultures. (C) Suppressive effect of the Treg clone CD4+CD25+ no. 8 on the proliferation of 8 Th1 or 9 Th2 clones at different target–regulatory cell ratios. Results are expressed as mean values (+ SD) of percentage of inhibition of proliferation obtained in triplicate cultures from the 8 Th1 and the 9 Th2 clones. **P < .0005; *P < .05.
Lower susceptibility of Th2 than Th1 clones to the suppressive activity of autologous Treg clones. (A) Cytokine profile of 2 Th1 (i-ii) and 2 Th2 (iii-iv) T-cell clones used as target of Treg clones, as assessed by flow cytometry at single cell level. (B) Suppressive effect of T-cell blasts from 3 Treg clones on the proliferative response to allogeneic stimulation of T-cell blasts from the 2 Th1 and the 2 Th2 clones. Results are expressed as percentage of inhibition of proliferation obtained in triplicate cultures. (C) Suppressive effect of the Treg clone CD4+CD25+ no. 8 on the proliferation of 8 Th1 or 9 Th2 clones at different target–regulatory cell ratios. Results are expressed as mean values (+ SD) of percentage of inhibition of proliferation obtained in triplicate cultures from the 8 Th1 and the 9 Th2 clones. **P < .0005; *P < .05.
Lower susceptibility of Th2 clones to the suppressive activity of Treg clones is at least in part due to their ability to produce IL-4
We asked, therefore, whether the lower susceptibility of Th2 cells to the suppressive activity of either CD4+CD25+ or CD8+CD25+ thymocytes could be related to their ability to produce a T-cell growth factor distinct from IL-2, such as IL-4. To test this hypothesis, we assessed the suppressive activity of the suppressive CD4+CD25+ no. 8 clone on the proliferative response to allogeneic stimulation of the 8 Th1 and the 9 Th2 clones, in the absence or the presence of IL-4 or an anti–IL-4 mAb. Neither the addition of IL-4 nor the addition of anti–IL-4 mAb had any effect on the suppressive activity exerted by this clone on the proliferation of Th1 cells, whereas the addition of IL-4 strongly reduced its suppressive activity on the proliferation of Th2 cells. Moreover, the addition of anti–IL-4 mAb consistently enhanced the suppressive activity exerted by the same clone on Th2 cells (Figure 3A). Because IL-4 has been reported to revert the anergic state of CD4+CD25+ Treg cells,3 the possibility that the lower suppressive effect exerted on Th2 clones was due to IL-4–induced Treg cell proliferation was investigated. To this end, all the 3 Treg clones were assessed for their ability to proliferate in response to exogenous IL-4. As shown in Figure 3B, IL-4 had virtually no effect on the proliferative response of Treg clones.
Effect of the addition of IL-4 or anti–IL-4 mAb on the suppressive activity exerted by a Treg clone on the proliferation of Th1 and Th2 cells. (A) T-cell blasts from 8 Th1 and 9 Th2 clones were stimulated for 5 days with 105 allogeneic irradiated peripheral blood mononuclear cells (PBMNCs) with or without 3 different concentrations of T-cell blasts (target–regulatory cell ratio 1:1, 2:1, and 4:1) from the autologous Treg clone CD4+CD25+ no. 8, in the absence (medium; ▪) or the presence of rIL-4 (▦), anti-IL-4 mAb (□) or isotype control mAb (▨). Results are expressed as mean values (+ SD) of percentage of inhibition of proliferation obtained in triplicate cultures from the 8 Th1 and the 9 Th2 clones. *P < .01. (B) T-cell blasts from both Treg and Th2 clones were cultured for 5 days alone or with 105 allogeneic-irradiated T-cell–depleted PBMNCs in the absence (medium; ▪) or the presence of rIL-4 (▦; 2 ng/mL). One representative experiment is shown.
Effect of the addition of IL-4 or anti–IL-4 mAb on the suppressive activity exerted by a Treg clone on the proliferation of Th1 and Th2 cells. (A) T-cell blasts from 8 Th1 and 9 Th2 clones were stimulated for 5 days with 105 allogeneic irradiated peripheral blood mononuclear cells (PBMNCs) with or without 3 different concentrations of T-cell blasts (target–regulatory cell ratio 1:1, 2:1, and 4:1) from the autologous Treg clone CD4+CD25+ no. 8, in the absence (medium; ▪) or the presence of rIL-4 (▦), anti-IL-4 mAb (□) or isotype control mAb (▨). Results are expressed as mean values (+ SD) of percentage of inhibition of proliferation obtained in triplicate cultures from the 8 Th1 and the 9 Th2 clones. *P < .01. (B) T-cell blasts from both Treg and Th2 clones were cultured for 5 days alone or with 105 allogeneic-irradiated T-cell–depleted PBMNCs in the absence (medium; ▪) or the presence of rIL-4 (▦; 2 ng/mL). One representative experiment is shown.
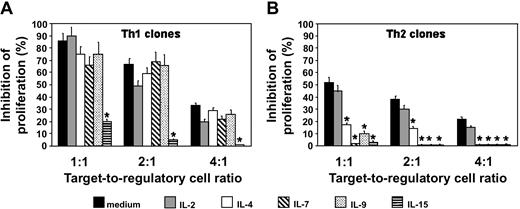
Not only IL-4, but also IL-7, IL-9, and IL-15, but not IL-2, bypass the suppressive activity of Treg cells on the proliferation of Th2 cells, whereas only IL-15 restores the proliferation of Th1 cells
The effect in the same system of other cytokines, such as IL-7, IL-9, and IL-15, which also share the γ but not the α chain with the IL-2R, was then investigated. Not only IL-4 but also IL-7, IL-9, and IL-15, but not IL-2, completely restored the proliferative response of Th2 cells, whereas only IL-15 exhibited the same restoring effect on the proliferation of Th1 cells (Figure 4). Of note, neither IL-7 nor IL-9 were able, even in the presence of IL-4, to induce the proliferation of CD4+ or CD8+ Treg clones (data not shown).
Effect of different cytokines on the suppressive activity of the Treg clone CD4+CD25+ no. 8 on the proliferation of Th1 or Th2 cells. T-cell blasts from 8 Th1 and 9 Th2 clones were stimulated for 5 days with 105 allogeneic-irradiated T-cell–depleted PBMNCs with or without 3 different concentrations of T-cell blasts (target–regulatory cell ratio 1:1, 2:1, and 4:1) from the autologous Treg clone CD4+CD25+ no.8, in the absence (medium) or the presence of rIL-2 (10 U/mL), rIL-4 (2 ng/mL), rIL-7 (1 ng/mL), rIL-9 (2 ng/mL), or rIL-15 (7 ng/mL). Results are expressed as mean values (+ SD) of percentage of inhibition of proliferation obtained in triplicate cultures of the 8 Th1 and the 9 Th2 clones. *P < .01.
Effect of different cytokines on the suppressive activity of the Treg clone CD4+CD25+ no. 8 on the proliferation of Th1 or Th2 cells. T-cell blasts from 8 Th1 and 9 Th2 clones were stimulated for 5 days with 105 allogeneic-irradiated T-cell–depleted PBMNCs with or without 3 different concentrations of T-cell blasts (target–regulatory cell ratio 1:1, 2:1, and 4:1) from the autologous Treg clone CD4+CD25+ no.8, in the absence (medium) or the presence of rIL-2 (10 U/mL), rIL-4 (2 ng/mL), rIL-7 (1 ng/mL), rIL-9 (2 ng/mL), or rIL-15 (7 ng/mL). Results are expressed as mean values (+ SD) of percentage of inhibition of proliferation obtained in triplicate cultures of the 8 Th1 and the 9 Th2 clones. *P < .01.
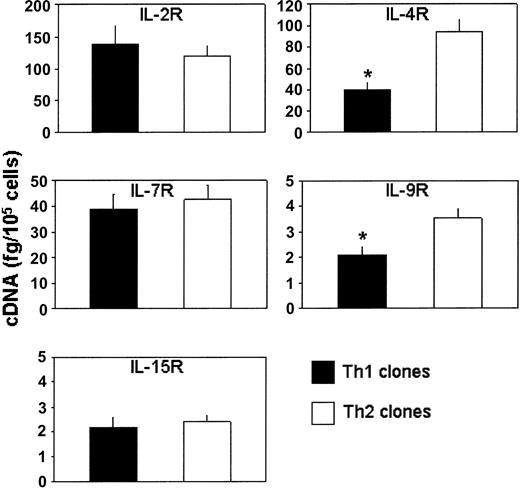
The expression of private α chains of receptors for IL-2, IL-4, IL-7, IL-9, and IL-15 was also evaluated by measuring the amount of their mRNAs by real-time quantitative RT-PCR on T-cell blasts from the 8 Th1 and the 9 Th2 clones before their testing in the suppressive assays. Th1 and Th2 clones showed the expression of comparable amounts of mRNA for IL-2R, IL-7R, and IL-15R α chains, whereas the expression of mRNA for IL-4R and IL-9R α chains was significantly higher in Th2 than in Th1 cells (Figure 5).
Measurement of mRNA for the α chain of IL-2, IL-4, IL-7, IL-9, or IL-15 receptors in Th1 and Th2 clones. Columns represent mean values (+ SD) of mRNA levels detected by real-time quantitative RT-PCR on 8 Th1 and 9 Th2 clones, which were assessed before their testing in the suppressive assays. *P < .05.
Measurement of mRNA for the α chain of IL-2, IL-4, IL-7, IL-9, or IL-15 receptors in Th1 and Th2 clones. Columns represent mean values (+ SD) of mRNA levels detected by real-time quantitative RT-PCR on 8 Th1 and 9 Th2 clones, which were assessed before their testing in the suppressive assays. *P < .05.
Discussion
In this study, we have compared the suppressive effect of CD4+CD25+ or CD8+CD25+ T-regulatory thymocytes on the proliferative response of Th1 and Th2 lymphocytes. Three selected Treg clones (2 CD4+ and 1 CD8+), generated from purified CD25+ human thymocyte suspensions, showed the ability to virtually abolish the proliferative response of autologous Th1 clones, whereas the same Treg clones exhibited only partial inhibitory effect on the proliferation of Th2 clones. An intermediate suppressive effect on the proliferation of Th0 clones was observed.
In previous studies,4,5 we demonstrated that the combined activity of CTLA-4 and TGF-β1 expressed by activated CD4+CD25+ thymocytes was able to inhibit the expression of the α chain of the IL-2R on target T cells.10 This effect made these cells unresponsive to the growth activity of IL-2 but not of IL-15, a T-cell growth factor that shares with IL-2 the γ chain of its receptor, but possesses its own private α chain.10 We, therefore, hypothesized that the lower susceptibility of Th2 cells to the suppressive activity exerted by Treg thymocytes was related to the peculiar ability of these cells in comparison with Th1 cells to produce, and to respond to, growth factors distinct from IL-2, such as IL-4 and IL-9, whose receptors share the γ chain with IL-2R but possess a distinct α chain.11,12
The results of this study provide convincing evidence on the validity of this hypothesis. First, the suppressive activity of Treg clones on the proliferation of Th2 cells was strongly increased by the addition in culture of an anti–IL-4 Ab but was further reduced by the addition of IL-4. More important, not only IL-4 and IL-15 but also IL-7 and IL-9 inhibited the suppressive activity of Treg clones on the proliferation of Th2 cells, whereas only IL-15 showed this effect on Th1 cells. Finally, at least before their testing in the suppressive assays, Th2 clones exhibited higher amounts of mRNA for IL-4R and IL-9R α chains than Th1 clones, whereas the amounts of IL-2R, IL-7R, and IL-15R α chain mRNAs were comparable. A possible explanation for the inhibitory effect exerted by IL-7 only on the suppressive activity by Treg cells on Th2 cells, despite the similar expression of the IL-7R in both Th1 and Th2 clones, may be that IL-7 is an early inducer of IL-4 production.13 Thus, because IL-15 is a T-cell growth factor produced by non-T cells, such as monocytes, dendritic cells, and other cell types,14,15 it is possible to hypothesize that the suppressive activity exerted by CD4+CD25+ or CD8+CD25+ that emerges from thymus can be bypassed when these cells operate under in vivo conditions characterized by the presence of high IL-15 production. By contrast, it is likely that the suppressive activity of Treg cells on Th2 lymphocytes can be inhibited not only under in vivo conditions characterized by the production by non-T cells of either IL-7 or IL-15 in their microenvironment but also by the ability of Th2 cells to produce, and to respond to, both IL-4 and IL-9. The results of this study are apparently at variance with previous reports, showing that both IL-2 and IL-4 partially inhibit the suppressive activity of CD4+CD25+ human Treg cells by inducing their proliferation.3,16 This discrepancy probably rests on the different experimental models used (ie, circulating CD4+CD25+ T cells versus thymus-derived CD25+ Treg clones). Circulating CD4+CD25+ T cells, unlike thymus-derived CD25+ Treg clones, consist not only of Treg cells but also of in vivo–activated T-cell effectors, which are highly susceptible to the activity of growth factors, such as IL-2 and IL-4.
The observation that both CD4+CD25+ and CD8+CD25+ Treg cells that emerge from the thymus and then colonize secondary lymphoid organs can exert strong suppressive activity on Th1, but much lower on Th2, cells is of great potential interest. The resident Treg cells that develop in the thymus17 and are exported to peripheral tissues are certainly responsible for the maintenance of self-tolerance and prevention of autoimmune diseases,7,18-21 which are thought to result from Th1-mediated immune responses.9 By contrast, Th2 cells are more rarely detectable in tissues affected by autoimmune disorders, and their cytokines can even play a protective role by either counteracting the development and function of Th1 cells or dampening the activity of Th1-activated macrophages.22-25 Thus, it is reasonable that Th2 cells are less susceptible than Th1 cells to the suppressive activity of “natural” Treg cells.
Another important implication emerging from the results of this study deals with the current debate on whether missing immune deviation or reduced immune suppression accounts for the increased prevalence of allergy (a Th2-mediated disorder), which has been attributed to the reduced microbial burden in childhood (“hygiene hypothesis”).25,26 Recently, on the basis of epidemiologic observations showing a contemporaneous increase in the prevalence of type 1 diabetes mellitus, multiple sclerosis, and Crohn disease (which are considered as Th1-mediated disorders), the role of reduced immune suppression has been emphasized.27,28 However, the results of this study provide clear evidence that at least CD4+CD25+ (and also CD8+CD25+) Treg cells cannot be considered as equally responsible for the suppression of Th1- or Th2-mediated immune responses, because Th2 cells are poorly susceptible to, and can easily escape from, their activity. This result is consistent with the observation that depletion in mice of CD4+CD25+ T cells prevents antigen-induced Th2 differentiation by increasing the differentiation of Th1 cells.29 Obviously, the possibility that Th2 responses are suppressed by other types of Treg cells, which are generated from mature T cells under certain conditions of antigenic stimulation and exert their suppressive activity by way of the production of cytokines, such as IL-10 and TGF-β,21 cannot be excluded. However, the results of this study indicate that different regulatory mechanisms are involved in dampening Th1 and Th2 responses; therefore, it is probably incorrect to evoke the same explanation for the increased prevalence of both autoimmune and allergic disorders.
Prepublished online as Blood First Edition Paper, December 24, 2003; DOI 10.1182/blood-2003-09-3302.
Supported by funds from the European Community (project no. QLK3-CT02-02026), the Italian Association for Cancer Research (AIRC), and the Ministry of Health of Tuscan Region.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal