Abstract
The physiologic significance of MHC-peptide complex presentation by endothelial cells (ECs) to trafficking T lymphocytes remains unresolved. On the basis of our observation that cognate recognition of ECs enhanced transendothelial migration of antigen-specific T lymphocytes in vitro, we have proposed that by displaying antigenic peptides from the underlying tissue, ECs promote the recruitment of antigen-specific T cells. In this study, we have tested this hypothesis by comparing the trafficking of HY-specific T lymphocytes into antigenic and nonantigenic tissue using in vivo models of T-cell recruitment. Up-regulated expression of H2 molecules presenting endogenous antigen in the peritoneal mesothelium and vessels led to the local recruitment of HY-specific T cells in male, but not female, mice. Intravital microscopy experiments analyzing EC–HY-specific T-cell interactions in the cremasteric vascular bed revealed that cognate recognition of the endothelium results in enhanced diapedesis of T cells into the tissue, while not affecting rolling and adhesion. Our results are consistent with the hypothesis that, under inflammatory conditions, antigen presentation by the endothelium contributes to the development and specificity of T-cell–mediated inflammation by favoring the selective migration of antigen-specific T cells. (Blood. 2004;103:3111-3116)
Introduction
Lymphocyte recirculation and the localized recruitment and retention of antigen-specific T cells to sites of inflammation are key events in immune surveillance. While T-lymphocyte recirculation is constitutively regulated and occurs in the absence of inflammation, the development of an immune response depends on the recruitment and retention of specific T cells at antigenic sites,1 thus minimizing collateral damage caused by non–antigen-specific inflammatory cells. T-cell migration into inflamed tissues involves sustained adhesive interactions with the microvascular endothelium that constitutively expresses major histocompatibility complex (MHC) molecules. Given that endothelial cells (ECs) may display tissue-specific as well as foreign peptides,1,2 antigen presentation to migrating T cells is likely to occur during the adhesive interactions required for extravasation. The participation of T-cell receptor (TCR)–derived signals in actively promoting T-cell migration has been suggested by the observations that TCR triggering can induce integrin activation3 and immobilize migrating T cells.4 In addition, chemokine receptor expression by T cells is susceptible to TCR-mediated modulation.5 A role for TCR signaling in the control of T-cell motility has also emerged from our recent studies on the functional consequences of antigen presentation by ECs to CD4+ and CD8+ T cells6,7 in both human and murine systems. In addition, we showed that TCR triggering by the endothelium directly increased T-cell migration by inducing integrin activation.6 A recent study has provided indirect evidence that insulin-specific clonal CD8+ T cells require cognate recognition of pancreatic microvascular endothelium to gain access to pancreatic islets.2 In this study, using in vivo models of T-cell recruitment, we have tested the possibility that cognate recognition of the endothelium by T lymphocytes may directly regulate their recruitment by monitoring the trafficking of HY-specific T cells in antigenic and nonantigenic tissue. Our results are consistent with the hypothesis that, at least in conditions of relatively mild inflammation, cognate recognition of the endothelium contributes to the recruitment of antigen-specific T cells by selectively enhancing their diapedesis.
Materials and methods
Mice
C57BL/6, B10.BR, BALB/c, and CBA/Ca mice were purchased from Olac Harlan (Bicester, United Kingdom) and used at 6 to 8 weeks of age.
MHC class I tetramers and antibodies
Soluble MHC/peptide tetramers were produced as previously described.8 A fluorescein isothiocyanate (FITC)–conjugate rat antimouse CD8 antibody (clone 53-6.7) was purchased from Becton Dickinson Biosciences (BD Biosciences) (Cowley, Oxford, United Kingdom). The following purified monoclonal antibodies (mAbs) were used in functional assays: anti–H2-Kk (HB25; American Type Culture Collection [ATCC], Manassas, VA) and anti–H2-Db (clone 28-14-8; BD Biosciences).
Endothelial cell culture
Murine microvascular ECs were purified and cultured from murine lung tissue as previously described.9 ECs derived from different mouse strains constitutively expressed low levels of CD54 and CD102 and intermediate levels of CD106.9 For functional assays, the ECs were used between passage 4 and 6 and treated with 300 U/mL murine interferon-γ (IFN-γ) (PeproTech, Peterborough, United Kingdom) for 72 hours prior to use in experiments. This led to up-regulation of MHC class I and CD54 expression to similar levels in all the ECs cultures employed in this study (data not shown).
T cells
The CD8+ T-cell clone C6, specific for HY peptide epitope TENSGKDI presented by H2-Kk, was maintained in culture by restimulation every 2 weeks with splenocytes from CBA/Ca male mice and recombinant interleukin-2 (rIL-2) (20 U/mL) (Boehringer Mannheim Biochemicals, Mannheim, Germany) in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) (Globepharm, Guildford, United Kingdom), 2 mM glutamine (Gibco, Paisley, United Kingdom), 50 IU/mL penicillin (Gibco), 50 μg/mL streptomycin (Gibco), and 50 mM 2-ME (Gibco). Memory CD8+ T cells specific for the male-specific minor transplantation antigen HY in the context of H2-Db molecules were obtained by intraperitoneal (IP) immunization of female C57BL/6 mice male splenocytes on 2 occasions 2 weeks apart, as previously described,8 followed by 2 rounds of restimulation in vitro with male splenocytes. Flow cytometric analysis of the T cells by using tetrameric H2b/HY epitopes, WMHHNMLDI (Uty epitope),10 and KCSRNRQYL (Smcy epitope)11 revealed that at this stage 95% of the T cells were HY specific (65% binding the Uty tetramer and 30% binding the Smcy tetramer; data not shown).
PKH26 labeling
T cells were washed and resuspended at a concentration of 107/mL in phosphate-buffered saline (PBS). The PKH26 Cell Linker (Sigma, Poole, United Kingdom) was added at a final concentration of 5 μM, and cells were incubated at 37°C for 30 minutes, then washed 3 times before injection.
Lymphocyte adhesion and transmigration assays
Short-term adhesion assays were carried out according to a previously described protocol.12 Briefly, murine ECs (2 × 104 per well) were seeded onto flat-bottomed 96-well plates (Costar, High Wycombe, United Kingdom) overnight to form a monolayer. The following day, T cells were labeled with 3 μM Calcein am (acetoxymethil) (Molecular Probes, Leiden, The Netherlands), washed, and added to the EC monolayers (5 × 104 per well). Following incubation for 45 minutes at 37°C, nonadherent cells were removed by gentle washing, and T-cell adhesion was measured by a fluorimeter (Cytofluor 2300; Waters, Milford, MA). Wells containing no T cells or unwashed wells constituted the 0% and 100% adhesion, respectively. T-cell adhesion was calculated with the following formula: Fluorescence experimental - Fluorecence 0%/Fluorescence 100% - Fluorescence 0% × 100. T-cell adhesion was then expressed as a percentage of adhesion.
Transmigration experiments were carried out with the use of EC monolayers grown on Costar Transwell tissue-culture well inserts (diameter, 12 mm), which contained polycarbonate membranes with a 3-μm pore size (Costar), as previously described.6,7 We seeded 105 ECs onto fibronectin-coated (50 μg/mL) (Sigma) polycarbonate membranes overnight to form a monolayer. Prior to the assay, the EC monolayers were gently washed with warm medium. Purified C6 CD8+ T cells (7 × 105) in RPMI 1640 supplemented with 10% FCS were added into each insert and left to migrate through the monolayer; the well content was replaced with fresh medium. After 1 hour, the number of migrated T cells was determined by counting the lymphocytes present in the well media. This was done at different time points for the next 6 hours. In these experiments, results are expressed as the percentage of transmigrated cells.
Peritoneal recruitment of circulating T cells
PKH26-labeled T cells (107 per mouse) were injected intravenously into male or female mice that had received an IP injection of IFN-γ (PeproTech, Peterborough, United Kingdom) (600 U) 72 hours earlier. After 24 hours, mice were killed, and infiltration of the peritoneal membrane by labeled T cells was analyzed by means of wide-field fluorescence microscopy (Zeiss Axiovert S100 with Metamorph software [Zeiss, Welwyn Garden City, United Kingdom]). A quantitative assessment of the T-cell infiltration in the peritoneal membrane was done by counting the number of red-labeled T cells in 10 randomly chosen 40 ×-magnified fields. The enrichment of labeled T cells in the peritoneal lavage was assessed by flow cytometry.
Wide-field fluorescence microscopy and flow cytometry
Peritoneal membranes and other tissue samples were laid on Polysine Microscope slides (VWR International, Lutterworth, Leicestershire, United Kingdom), left to dry overnight, and then mounted in Vectorshield (Vector Laboratories, Peterborough, United Kingdom) mounting medium for fluorescence with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Vector Laboratories) to enable tissue visualization. Slides were analyzed with a Coolview-12 cooled charge-coupled device (CCD) camera (Photonic Science, Newbury, United Kingdom) mounted over a Zeiss Axiovert S100 microscope equipped with Metamorph software (Zeiss). We used × 10 and × 40 NA 0.6 objectives and a standard epi-illuminating rhodamine fluorescence filter cube were used, and 12 bit image data sets were generated. Tissue infiltration was quantified by randomly selecting ten 40 ×-magnified fields and assessing the number of fluorescent cells in each field, as previously described.13 The total number of infiltrating T cells obtained in 10 fields in at least 3 animals per experimental group were then averaged and assessed statistically. Infiltration is expressed as the mean of total fluorescent cells counted in each animal (ten 40 × fields per mouse) in a given experimental condition ± standard deviation (SD).
For flow cytometry, 105 cells were incubated with the fluoresceinated mAb indicated at 4°C for 30 minutes. An isotype-matched irrelevant antibody was used as a control. Stained cells were analyzed by means of a FACSCalibur (Becton Dickinson, Mountain View, CA).
Intravital microscopy
These experiments involved direct visualization of T cell–EC interactions by means of intravital microscopy using the cremasteric muscle vasculature of mice pretreated with intrascrotal (IS) IFN-γ (72 hours). Intravital microscopy on the mouse cremaster muscle was performed as previously described.14 Briefly, male C57BL/6 mice were injected intrascrotally with murine IFN-γ (600 U). Controls included a group of congenic B10.BR male mice (genetically similar to C57BL/6 but expressing the H-2k haplotype)14 as well as CBA/Ca (H-2k) and BALB/c (H-2d) male mice. At 72 hours after cytokine injection, mice were anesthetized by IP administration of ketamine (100 mg/kg) (Ketalar; Parke-Davis, Cambridge, United Kingdom) and xylazine (10 mg/kg) (Rompun; Bayer, Newbury, United Kingdom) and placed on a custom-built, heated (37°C) microscope stage. The jugular vein was cannulated to enable T-cell injection. The cremaster muscle was exteriorized through an incision in the scrotum. One testis was gently drawn out to allow the cremaster muscle to be opened and pinned out flat over the optical window within the microscope stage. The tissue was kept warm and moist throughout each experiment by superfusion of warmed Tyrode balanced salt solution. PKH26-labeled HY-specific T cells (6 × 106) were injected into the jugular vein, and after 10 minutes, leukocyte-endothelial cell interactions were observed on a Zeiss Axioskop fixed-stage upright fluorescence microscope fitted with water-immersion objectives. Images were captured by means of a Hamamatsu SIT camera and videocassette recorder (Hamamatsu, Welwyn Garden City, Hertfordshire).
Lymphocyte rolling, firm adhesion, and extravasation in postcapillary venules of 20- to 40-μm diameter were quantified as previously described.14 Rolling cells were defined as fluorescent cells moving slower than the flowing erythrocytes, and rolling flux was quantified as the number of rolling cells moving past a fixed point on the venular wall per minute, averaged over 5 minutes. Firmly adherent leukocytes were defined as fluorescent cells that remained stationary for at least 30 seconds within a 400-μm vessel segment. Transmigrated cells were defined as those cells in the extravascular tissue within 50 μm of the 400-μm vessel segments quantified. Responses were quantified every 10 minutes, and at least 2 vessels were studied in each animal. The mice were killed at the end of the observation period.
Statistical analysis
Results are expressed as mean ± standard error of the mean (SEM) or mean ± SD. To test significance, Student unpaired t tests were performed. A P value of less than .05 was regarded as significant.
Results
Cognate recognition of ECs enhances transendothelial migration of HY-specific CD8+ T cells in vitro
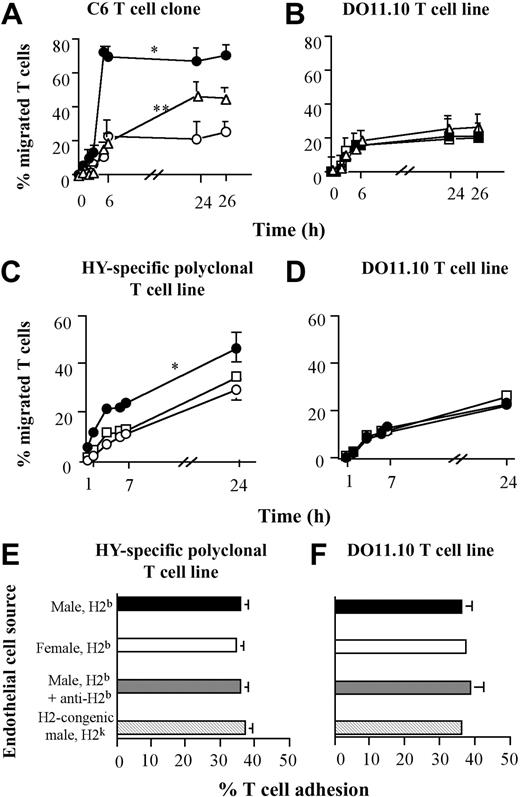
As a prelude to our in vivo experiments, the effect of cognate recognition on migration of HY-specific Kk-restricted CD8+ T-cell clone C6 and HY-specific Db-restricted polyclonal T cells was investigated with the use of a transendothelial migration assay as previously described.7 T cells were used 2 weeks after restimulation, when they failed to kill activated, peptide-pulsed ECs.7 C6 T cells were previously shown in vitro to proliferate in response to IFN-γ–treated male, but not female, ECs in the presence of exogenous IL-2.7 T cells were purified on a Ficoll gradient, and the C6 T-cell clone was seeded onto IFN-γ–treated (300 U/mL) EC monolayers derived from CBA/Ca (H2k) male or female mice grown on a Transwell insert (Figure 1A). In similar experiments (Figure 1B), the polyclonal HY-specific H2-Db–restricted T cells were seeded onto IFN-γ–treated ECs derived from C57BL/6 male or female mice (H2b) and from H2-congenic male B10.BR mice (H2k). The number of transmigrated T cells recovered in the bottom well was monitored hourly.
Effect of antigen presentation by ECs on transendothelial migration of CD8+ T cells. Antigen presentation by ECs selectively enhances transendothelial migration of CD8+ T cells. (A-B) The C6 CD8+ T-cell clone (5 × 105 cells per well) was seeded onto IFN-γ–treated EC monolayers derived from either male (•) or female (○) CBA/Ca mice (H2k) (A). In some experiments, the male CBA/Ca-derived EC monolayer was treated with an anti–H2-Kk mAb (HB25, 1μg/mL, open triangles), for 30 minutes at room temperature, and then washed thoroughly prior to seeding the T lymphocytes. In parallel experiments (B), a CD4+ ovalbumin-specific H2-Ab–restricted DO11.10 TCR-transgenic T-cell line (of irrelevant antigen specificity) was seeded onto duplicate EC monolayers. T-cell migration was monitored for the following 26 hours and is expressed as a percentage of migrated T cells at the specified time points. The average percentage of migrated T cells at the specified time points in 3 experiments with similar design is shown. Standard error bars are shown. *P is significant versus control female CBA/Ca EC monolayer (H2k, P < .003) at the time points between 5 and 26 hours. **P is significant for inhibition compared with male CBA/Ca EC monolayer in the absence of anti–H2-Kk mAb (P < .04) at the time points between 5 and 26 hours. (C) A polyclonal HY-specific, H2-Db–restricted CD8+ T-cell line (5 × 105 cells per well) was seeded onto IFN-γ–treated EC monolayers derived from either female (•) or male (○) C57BL6 mice (H2b) or from H2-congenic male B10.BR mice (H2k, empty □). (D) A DO11.10 TCR-transgenic T-cell line was seeded onto the duplicate EC monolayers. T-cell migration was monitored and expressed as described in “Lymphocyte adhesion and transmigration assays.” The average percentage of migrated T cells at the specified time points in 3 experiments with similar design is shown. Standard error bars are shown. *P is significant versus control female C57BL/6 EC monolayer (H2b, P < .01) and male B10.BR EC monolayer (H2k, P < .03), at all the time points. (E-F) Adhesion of Calcein am–labeled polyclonal HY-specific, H2-Db–restricted CD8+ T-cell line and DO11.10 TCR-transgenic T-cell line (5 × 104 per well), respectively, to IFN-γ–treated EC monolayers (2 × 104 per well) derived from either female or male C57BL/6 mice (H2b) or from congenic male B10.BR mice (H2k). In some experiments, H2b-expressing, male-derived EC monolayers were treated with an anti–H2-Db mAb (clone 28-14-8, 1 μg/mL). Adhesion was measured by fluorimetry, and the average percentage of T-cell adhesion in 3 experiments with similar design is shown. Standard error bars are shown.
Effect of antigen presentation by ECs on transendothelial migration of CD8+ T cells. Antigen presentation by ECs selectively enhances transendothelial migration of CD8+ T cells. (A-B) The C6 CD8+ T-cell clone (5 × 105 cells per well) was seeded onto IFN-γ–treated EC monolayers derived from either male (•) or female (○) CBA/Ca mice (H2k) (A). In some experiments, the male CBA/Ca-derived EC monolayer was treated with an anti–H2-Kk mAb (HB25, 1μg/mL, open triangles), for 30 minutes at room temperature, and then washed thoroughly prior to seeding the T lymphocytes. In parallel experiments (B), a CD4+ ovalbumin-specific H2-Ab–restricted DO11.10 TCR-transgenic T-cell line (of irrelevant antigen specificity) was seeded onto duplicate EC monolayers. T-cell migration was monitored for the following 26 hours and is expressed as a percentage of migrated T cells at the specified time points. The average percentage of migrated T cells at the specified time points in 3 experiments with similar design is shown. Standard error bars are shown. *P is significant versus control female CBA/Ca EC monolayer (H2k, P < .003) at the time points between 5 and 26 hours. **P is significant for inhibition compared with male CBA/Ca EC monolayer in the absence of anti–H2-Kk mAb (P < .04) at the time points between 5 and 26 hours. (C) A polyclonal HY-specific, H2-Db–restricted CD8+ T-cell line (5 × 105 cells per well) was seeded onto IFN-γ–treated EC monolayers derived from either female (•) or male (○) C57BL6 mice (H2b) or from H2-congenic male B10.BR mice (H2k, empty □). (D) A DO11.10 TCR-transgenic T-cell line was seeded onto the duplicate EC monolayers. T-cell migration was monitored and expressed as described in “Lymphocyte adhesion and transmigration assays.” The average percentage of migrated T cells at the specified time points in 3 experiments with similar design is shown. Standard error bars are shown. *P is significant versus control female C57BL/6 EC monolayer (H2b, P < .01) and male B10.BR EC monolayer (H2k, P < .03), at all the time points. (E-F) Adhesion of Calcein am–labeled polyclonal HY-specific, H2-Db–restricted CD8+ T-cell line and DO11.10 TCR-transgenic T-cell line (5 × 104 per well), respectively, to IFN-γ–treated EC monolayers (2 × 104 per well) derived from either female or male C57BL/6 mice (H2b) or from congenic male B10.BR mice (H2k). In some experiments, H2b-expressing, male-derived EC monolayers were treated with an anti–H2-Db mAb (clone 28-14-8, 1 μg/mL). Adhesion was measured by fluorimetry, and the average percentage of T-cell adhesion in 3 experiments with similar design is shown. Standard error bars are shown.
Although the EC monolayers expressed comparable levels of adhesion molecules and MHC molecules,9 the HY-specific C6 T-cell clone and polyclonal HY-specific H2b-restricted T cells displayed a much greater rate of migration through H2k- and H2b-expressing ECs derived from male mice, respectively (Figure 1A,C). The enhanced migration was blocked by pretreating the EC monolayers with an anti-Kk or anti-Db mAb, respectively (Figure 1A and data not shown). As the excess antibody was removed prior to seeding the T cells onto the EC monolayers to avoid effects due to antibody binding to the T cells, the inhibitory effect of MHC blockade on migration became less evident at the late time point of the assay, probably owing to the internalization and recycling of MHC molecules. As expected from the similar pattern of adhesion molecule expression, the EC monolayers used in each group of experiments supported comparable levels of migration of T cells with an irrelevant specificity and MHC restriction (the CD4+ ovalbumin-specific H2Ad-restricted DO11.10-derived T-cell line; Figure 1B and 1D, respectively). This was unaffected by anti-Kk or anti-Db mAbs (Figure 1B and data not shown). These controls also exclude the possibility that differences in the integrity of the EC monolayer used might account for the observed effect.
To investigate whether antigen recognition on the endothelium affected T-cell adhesion or transendothelial migration, short-term adhesion assays were set up in which adhesion of polyclonal HY-specific Db–restricted T cells and the DO11.10 T-cell line to EC monolayers derived from C57BL/6 male and female mice (H2b) and from congenic male B10.BR mice (H2k) was assessed. In some experiments, C57BL/6-derived ECs were pretreated with an anti-Db mAb. As shown in Figure 1E-F, similar levels of T-cell adhesion were observed in all the conditions analyzed.
From these data, it appears that cognate recognition of endogenously derived HY peptide on ECs selectively enhances HY-specific CD8+ T-cell diapedesis in static conditions in vitro.
TCR engagement drives antigen (Ag)–specific T-cell recruitment in the presence of mild inflammation in vivo
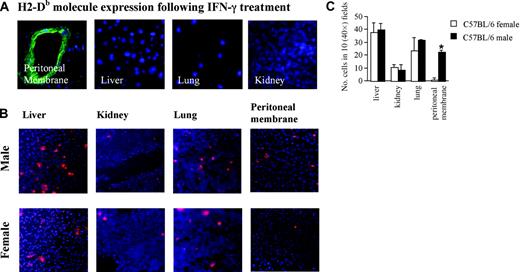
PKH26-labeled polyclonal HY-specific H2-Db–restricted CD8+ T cells (107 per mouse) were injected intravenously into male or female C57BL/6 mice that had received an IP injection of IFN-γ (600 U) 72 hours earlier or PBS. This creates optimal conditions for antigen recognition on peritoneal vessels as it leads to strong MHC class I molecule up-regulation limited to the peritoneal mesothelium and vasculature, while in other sites (such as the lung, liver, and kidney) or in PBS-injected animals (data not shown), MHC class I molecule expression is very low (Figure 2A). Tissue infiltration by labeled T cells and enrichment of HY-specific T cells in the peritoneal lavage were assessed 24 hours later as described in “Materials and methods.” The polyclonal HY-specific H2-Db-restricted CD8+ T cells cells recirculated normally and could be equally well detected in the spleen, lung, liver, and kidney of both male and female mice 6 to 72 hours after injection (Figure 2B-C). The absence of these cells in draining lymph nodes might reflect the lack of expression of CD62L and CCR7 by the polyclonal HY-specific CD8+ T cells (data not shown).15 Most importantly, labeled T cells could be observed only in the peritoneal membrane (Figure 2B-C) and lavage (Figure 3A,E) of IFN-γ-treated male, but not female, mice. In addition, peritoneal recruitment was not observed in male mice that did not receive an IP injection of IFN-γ (Figure 3B).
Peritoneal membrane infiltration by HY-specific T cells in IFN-γ–treated male, but not female, mice. (A) Male and female C57BL/6 mice were injected intraperitoneally with 600 U IFN-γ. This resulted in the selective up-regulation of MHC class I in the peritoneal cavity, as assessed by staining of various tissue samples (obtained 72 hours after IFN-γ injection) with a FITC-conjugated anti–H2-Db mAb (0.5 μg/mL). (B) At 2 days after IFN-γ injection, mice received an intravenous injection of 107 PKH26-labeled Db-restricted HY-specific T cells. The following day, mice were killed, and the presence of fluorescently labeled cells in the liver, kidney, lung, and peritoneal membrane was assessed by wide-field fluorescence microscopy. In both panels A and B, to minimize the effect of arbitrary choice of field, 10 × magnifications are shown. Tissue infiltration was quantified by randomly selecting ten 40 ×-magnified fields and assessing the number of fluorescent cells in each field. (C) The mean and SD of the total number of T cells observed in 10 randomly selected fields in each mouse from at least 3 animals (which gives at least 30 fields analyzed for each experimental group). *P is significant in comparison with female recipients (P = .002).
Peritoneal membrane infiltration by HY-specific T cells in IFN-γ–treated male, but not female, mice. (A) Male and female C57BL/6 mice were injected intraperitoneally with 600 U IFN-γ. This resulted in the selective up-regulation of MHC class I in the peritoneal cavity, as assessed by staining of various tissue samples (obtained 72 hours after IFN-γ injection) with a FITC-conjugated anti–H2-Db mAb (0.5 μg/mL). (B) At 2 days after IFN-γ injection, mice received an intravenous injection of 107 PKH26-labeled Db-restricted HY-specific T cells. The following day, mice were killed, and the presence of fluorescently labeled cells in the liver, kidney, lung, and peritoneal membrane was assessed by wide-field fluorescence microscopy. In both panels A and B, to minimize the effect of arbitrary choice of field, 10 × magnifications are shown. Tissue infiltration was quantified by randomly selecting ten 40 ×-magnified fields and assessing the number of fluorescent cells in each field. (C) The mean and SD of the total number of T cells observed in 10 randomly selected fields in each mouse from at least 3 animals (which gives at least 30 fields analyzed for each experimental group). *P is significant in comparison with female recipients (P = .002).
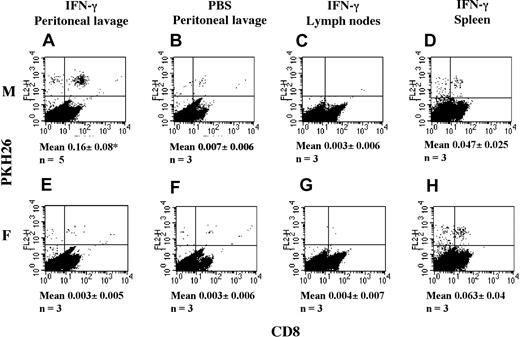
Detection of T cells in the peritoneal lavage. The presence of T cells in the peritoneal lavage is detectable IFN-γ–treated male, but not female, mice. Male and female C57/BL6 mice were injected intraperitoneally with 600 U IFN-γ or PBS. Two days later, mice received an intravenous (IV) injection of 107 PKH26-labeled HY-specific T cells. The following day, mice were killed, and the presence of fluorescently labeled cells in the peritoneal lavage (A-B,E-F) and lymph nodes (from IFN-γ–treated mice; C,G) and spleen (from IFN-γ–treated mice; D,H) was assessed by flow cytometry. To facilitate visualization, cells were double stained with a FITC-conjugated anti-CD8 antibody. The mean and SD of the percentage of labeled cells recovered from the peritoneal lavage of at least 3 animals (n) are shown under the relevant panels. *P is significant versus female mice (P < .02).
Detection of T cells in the peritoneal lavage. The presence of T cells in the peritoneal lavage is detectable IFN-γ–treated male, but not female, mice. Male and female C57/BL6 mice were injected intraperitoneally with 600 U IFN-γ or PBS. Two days later, mice received an intravenous (IV) injection of 107 PKH26-labeled HY-specific T cells. The following day, mice were killed, and the presence of fluorescently labeled cells in the peritoneal lavage (A-B,E-F) and lymph nodes (from IFN-γ–treated mice; C,G) and spleen (from IFN-γ–treated mice; D,H) was assessed by flow cytometry. To facilitate visualization, cells were double stained with a FITC-conjugated anti-CD8 antibody. The mean and SD of the percentage of labeled cells recovered from the peritoneal lavage of at least 3 animals (n) are shown under the relevant panels. *P is significant versus female mice (P < .02).
Cognate recognition of ECs selectively enhances diapedesis in vivo
In a final series of experiments, to directly investigate the role of cognate recognition of the endothelium in lymphocyte transmigration into tissues, intravital microscopy was used to observe T cell–EC interactions in vivo. For this purpose, events within cremasteric venules of C57BL/6 mice, pretreated with intrascrotal administration of IFN-γ (72 hours), were quantified. Control mice included H2-congenic male B10.BR mice that are genetically similar to C57BL/6 except for the MHC region. B10.BR mice carry the H-2k haplotype16,17 and are therefore unable to present cognate peptide to H2-Db–restricted T cells. Another control group included genetically unrelated BALB/c (H-2d) and CBA/Ca (H-2k) male mice. Fluorescently labeled HY-specific H2-Db–restricted polyclonal T cells were injected intravenously, and responses within the microvascular bed viewed for up to 40 minutes after injection of cells. No significant difference in rolling flux (data not shown) or T-cell firm adhesion was observed (Figure 4A) in the different groups. However, as can be seen in Figure 4B, a significant increase in both the magnitude and the rate of T-cell transmigration was selectively observed in C57BL/6 mice. Similar to what we observed in the in vitro studies shown in Figure 1, these results suggest that antigenic endothelium supports T-cell transmigration by specifically regulating the extravasation step of this process. In line with these results, captured in real time, dissected and sectioned cremaster muscles visualized by fluorescent microscopy indicated a large tissue influx of T cells in tissues from C57BL/6 but not control mice (data not shown).
Cognate recognition of ECs enhances antigen-specific T-cell diapedesis in vivo. Cognate recognition of endothelial cells enhances antigen-specific T-cell diapedesis in vivo. Male C57BL/6 (H2b; ▪), congenic male B10.BR (H2k, ▦), or BALB/C and CBA/Ca (H2d, H2k; □) mice were treated with intrascrotal administration of IFN-γ (600 U in 400 μL saline). After 72 hours, fluorescently labeled HY-specific T cells (“Materials and methods”) were injected intravenously after surgical exteriorization of the cremaster muscle and T-cell interactions with venular walls were visualized and quantified by intravital microscopy for up to 40 minutes after injection of the cells (“Materials and methods”). Results are from 3 to 5 mice per group, and significant differences between the H2b in comparison with the H2k and H2d or the H2-congenic H2k strains of mice are shown by asterisk (*P < .05). (A) T-cell firm adhesion. (B) Extravasation.
Cognate recognition of ECs enhances antigen-specific T-cell diapedesis in vivo. Cognate recognition of endothelial cells enhances antigen-specific T-cell diapedesis in vivo. Male C57BL/6 (H2b; ▪), congenic male B10.BR (H2k, ▦), or BALB/C and CBA/Ca (H2d, H2k; □) mice were treated with intrascrotal administration of IFN-γ (600 U in 400 μL saline). After 72 hours, fluorescently labeled HY-specific T cells (“Materials and methods”) were injected intravenously after surgical exteriorization of the cremaster muscle and T-cell interactions with venular walls were visualized and quantified by intravital microscopy for up to 40 minutes after injection of the cells (“Materials and methods”). Results are from 3 to 5 mice per group, and significant differences between the H2b in comparison with the H2k and H2d or the H2-congenic H2k strains of mice are shown by asterisk (*P < .05). (A) T-cell firm adhesion. (B) Extravasation.
Discussion
In this study, we have analyzed the role of antigen recognition in the recruitment and tissue infiltration by T lymphocytes, and in particular, we have assessed the role that cognate recognition of the endothelium plays in the extravasation of antigen-specific T cells. Our results support the hypothesis that TCR triggering by the endothelium significantly amplifies the recruitment of specific T lymphocytes in vivo. These data provide the first direct evidence of antigen-dependent T-cell recruitment and retention into tissues in vivo.
The possibility that antigen expression might regulate specific T-cell localization and tissue retention has been suggested by some studies looking at the migration of self-reactive T cells in autoimmune diseases18 or pathogen-specific T cells in infectious diseases.19 On the basis of our in vitro findings in human and mouse,6,7 we have put forward the hypothesis that the endothelium might increase the recruitment of specific T cells into the tissue by presenting tissue antigen.20 A recent study analyzing pancreas-homing TCR-transgenic insulin-specific CD8+ T cells has provided indirect in vivo evidence in support of this hypothesis, showing that MHC class I expression and insulin production are required for trafficking of these cells into the pancreas, a response accompanied by integrin activation and increased adhesion in vitro.2 This effect was dependent on chemokines and could occur in the absence of inflammation.
Our first experimental approach involved the intravenous administration of HY-specific T cells to male and female mice. The HY-specific polyclonal T cells recirculated normally, but selectively infiltrated and accumulated in the peritoneal cavity of IFN-γ–treated male mice (Figures 2-3). In this model, recruitment of antigen-specific T cells can occur from the bloodstream only through the peritoneal postcapillary venules.
In our study, we have also directly visualized T cell–EC cognate interactions in the vascular bed by intravital microscopy. In line with our in vitro observations, the results suggest that diapedesis rather than adhesion per se is the extravasation step enhanced by TCR triggering. A likely explanation for the selective effect on diapedesis rather than tethering or adhesion is that, owing to their small molecular size, TCR triggering by MHC-peptide complexes can occur only in areas of cell-cell membrane proximity where adhesive intractions have already been established.21 In addition, this effect may be mediated by activation of integrins and/or generation of chemokines specifically involved in the transmigration phase of T-cell migration.22
The mechanism whereby cognate recognition promotes transmigration in vivo has yet to be fully defined, and it is likely to involve TCR-mediated activation of signaling pathways that control adhesion molecule activation and cytoskeletal rearrangements. In our previous in vitro studies, we observed that the enhancement of T-cell migration by cognate recognition of ECs involved a direct effect on T-cell motility and was dependent on integrin activation.6 In this context, recent studies have established a role for adapter proteins such as Vav,23,24 adhesion- and degranulation-promoting adaptor protein (ADAP),25 and Src kinase–associated phosphoprotein of 55 kDa (SKAP-55)26 in the activation of integrins following TCR triggering. The role of these molecules in TCR-driven T-cell extravasation remains to be established. Downstream events in these pathways are likely to involve Ras/Rho family guanosine triphosphatases (GTPases), including Ras, Rho, Rac, and Cdc42.27 Further studies involving the manipulation of such intracellular mediators in vivo will be required to characterize the precise molecular mechanisms of this event.
TCR-mediated control of lymphocyte motility is likely to serve 2 purposes. First, it renders the process of recruitment into, and retention at, antigenic sites of inflammation more efficient by facilitating the entrance of specific T cells, attracted by antigen displayed by the EC surface. Second, during antigen presentation, it allows constitutively mobile T cells to stop and concentrate (by polarization and surface molecule redistribution) on the antigen-presenting cell,28 loaded with optimal antigenic peptides. The observations described in this study have in vivo relevance to the recruitment and retention of either resting memory T cells or recently activated T cells into inflamed tissues. In particular, this mechanism is likely to play a key role in the rejection of vascularized allografts. Cognate recognition of the allogeneic endothelium is likely to enrich for allospecific T cells following their initial activation in lymphoid tissue. Whether the expression of antigenic molecules by high endothelial venules29,30 contributes to the recruitment of naive T cells in secondary lymphoid organs remains an open question that will require further investigations.
Prepublished online as Blood First Edition Paper, December 24, 2003; DOI 10.1182/blood-2003-08-2717.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to S. Puhalla and I. Jackson for their help with the experiments.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal