Abstract
Thrombophilic dysfibrinogen Tokyo V was identified in a 43-year-old man with recurrent thromboembolism. Based on analyses of the patient fibrinogen genes, the amino acid sequence of the aberrant fibrinogen peptide, and deglycosylation experiments, fibrinogen Tokyo V was shown to have an amino acid substitution of γ Ala327Thr and possibly extra glycosylation at γ Asn325 because the mutation confers the N-linked glycosylation consensus sequence Asn-X-Thr. The mutation resulted in impaired function and hypofibrinogenemia (hypodysfibrinogen). Polymerization of fibrin monomers derived from patient fibrinogen was severely impaired with a partial correction in the presence of calcium, resulting in very low clottability. Additionally, a large amount of soluble cross-linked fibrin was formed upon thrombin treatment in the presence of factor XIII and calcium. However, Tokyo V–derived fibrin was resistant to degradation by tissue plasminogen activator (tPA)–catalyzed plasmin digestion. The structure of Tokyo V fibrin appeared severely perturbed, since there are large pores inside the tangled fibrin networks and fiber ends at the boundaries. Taken together, these data suggest that Tokyo V fibrin clots are fragile, so that fibrinolysis-resistant insoluble fibrin and soluble fibrin polymers may be released to the circulation, partly accounting for the recurrent embolic episodes in the patient. (Blood. 2004;103:3045-3050)
Introduction
Fibrinogen is a 340-kDa plasma protein that participates in the final step of blood coagulation and also plays an important role in platelet aggregation.1,2 Fibrinogen is composed of 2 identical molecular halves, each being composed of an Aα, a Bβ, and a γ chain. These polypeptides are encoded by 3 independent genes, clustered on chromosome 4. During synthesis in the liver, these chains are translated, processed, and assembled to the mature molecules.3 Many reports have shown that a variety of genetic mutations result in molecular defects in fibrinogen molecules (dysfibrinogen)4,5 or decreased levels of fibrinogen in blood (afibrinogenemia and hypofibrinogenemia).6
Analyses of molecular and functional abnormalities of dysfibrinogens participate in elucidation of structure-function relationships and fibrin polymerization mechanisms. Patients with dysfibrinogen show bleeding tendencies, thromboembolisms, or no apparent symptoms, depending on the molecular abnormality. Thrombophilic dysfibrinogen is a rare molecular abnormality.5 Here we report a new thrombophilic dysfibrinogen with an amino acid substitution of γ Ala327Thr, inserting a possible extra N-glycosylation site at γ Asn325. The defective fibrinogen results in formation of fragile but fibrinolysis-resistant clots caused by conformation defects in the vicinity of the “a” polymerization pocket, the high-affinity calcium ion binding site, and the tissue plasminogen activator (tPA) binding site of the γ chain.
Patients, materials, and methods
Description of the patient
A 43-year-old man, who suffered from recurrent thromboembolic episodes such as cerebral infarction at the age of 36 years and pulmonary embolism at the age of 42 years, was admitted to a hospital because of severe abdominal pain. Echography and computed tomographic (CT) scan analyses showed that thrombi were present in the thoracic and abdominal aorta and the superior mesenteric artery of the patient. The patient was suspected to have dysfibrinogen based upon coagulation studies conducted on admission. There was a marked discrepancy between fibrinogen levels in plasma determined by the thrombin time method (< 1.47 μM) and the turbidimetric method (4.00 μM) on admission. This was reproduced in another plasma sample in which there also was a discrepancy between fibrinogen levels determined by the thrombin time method (0.61 μM) and the immunologic method (2.16 μM). Thus, the patient fibrinogen was designated as fibrinogen Tokyo V. These data also suggested that the patient had not only dysfibrinogenemia, but also hypofibrinogenemia. Other coagulation studies including plasma levels of protein C, antithrombin III, plasminogen, and protein S were within normal ranges.
Analysis of purified fibrinogen
Fibrinogen was purified from plasma of healthy subjects or patient plasma as described previously.7-10 All samples were obtained with informed consent provided by the patient according to the Declaration of Helsinki, and the study was approved by the Bioethics Committee for Gene Analysis at Jichi Medical School. Clotting of patient-derived fibrinogen was compared with normal fibrinogen by the thrombin time method in the absence or presence of calcium. The aggregation profiles of healthy subject– and patient-derived fibrin monomers were monitored at 350 nm according to the method of Gralnick as described previously.7-10 To study effect of Tokyo V fibrin on normal fibrin polymerization, normal fibrin monomers and Tokyo V fibrin monomers at the same concentration were mixed at ratios of 9:1, 3:1, and 1:1, then processed for measuring fibrin monomer aggregation profiles as described earlier in this paragraph. For the control, normal fibrin monomers were mixed with buffer at ratios of 9:1, 3:1, and 1:1, then processed for measuring aggregation profiles of fibrin monomers. Apparent molecular mass of patient fibrinogen was analyzed by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions and was compared with normal fibrinogen. Identification of the γ chain was carried out by Western blotting using γ chain–specific monoclonal antibody JIF 25 as described.8 Purified fibrinogen (1 mg/mL) was incubated with thrombin (1 U/mL) in the absence or presence of 2 mM calcium to investigate release of fibrinopeptides A and B and the cross-linking profiles of γ chains (γ-dimer formation) and α chains (α-polymer formation) as described previously.7-10 To assess clottability of fibrinogen, purified fibrinogen (1 mg/mL) was incubated in Tris-buffered saline containing 1 U/mL thrombin in the presence of 1 U/mL factor XIII (FXIII) and 2 mM calcium. After incubation at 37 °C for 60 minutes, fibrin clots were squeezed with bamboo sticks and the residual protein amount in supernatants was quantified by measuring absorbance at 280 nm. Relative amounts of β chain of insoluble fibrin clots and of soluble fibrin in the supernatant were quantified using a densitometer to assess the clottability of Tokyo V fibrinogen. Supernatants were analyzed by SDS-PAGE under reducing and nonreducing conditions for semiquantitation of unclotted fibrin and for analysis of soluble fibrin species.
Determination of the nucleotide sequences of fibrinogen genes
Patient genomic DNA was isolated from peripheral leukocytes by standard procedure as described previously.7,9,10 All exons and exon-intron boundaries of fibrinogen genes were amplified by polymerase chain reaction (PCR) using Bucabest DNA polymerase (Takara, Kyoto, Japan) and appropriate primers (Invitrogen Japan, Tokyo, Japan).10 PCR-amplified DNA fragments were isolated after agarose gel electrophoresis using a rapid gel extraction kit (Invitrogen Japan), and their nucleotide sequences were determined directly by the dideoxynucleotide termination reaction method using BigDye cycle sequencing kit and a DNA sequencer model ABI 310 (Applied Biosystems Japan, Tokyo, Japan)10,11 or using Sequenase (US Biochemical, Cleveland, OH) and 35S-dATP (Amersham Biosciences K. K., Tokyo, Japan).7,9
Fibrin degradation by tPA-catalyzed plasmin digestion and tPA-mediated plasmin generation
Purified fibrinogen (1 mg/mL) was incubated with tPA (1.25 U/mL), plasminogen (20 μg/mL), FXIII (1 U/mL), and thrombin (1 U/mL) in the presence of 2 mM calcium. After incubation at 37°C, the reaction was terminated by incubating samples in buffer containing 2% SDS and 10 mM dithiothreitol (DTT) at 98°C for 2 minutes and samples were analyzed by SDS-PAGE under reducing conditions. Fibrinogen (1 mg/mL), increasing concentrations of plasmin (0.3-2.4 casein U/mL), FXIII (1 U/mL), and thrombin (1 U/mL) were incubated at 37°C for 30 minutes in the presence of calcium, and fibrin degradation profiles were analyzed by SDS-PAGE as described in “Analysis of purified fibrinogen” to study Tokyo V fibrin degradation by plasmin without the plasminogen activation process by tPA. tPA-mediated plasmin generation in the presence of fibrin was studied using the synthetic plasmin substrate S-2251 as described.8 Healthy subject or patient fibrinogen was incubated with plasminogen (20 μg/mL), tPA (4 U/mL), FXIII (1 U/mL), thrombin (1 U/mL), and S-2251 (1 mM) in the presence of 2 mM calcium in microtiter plates. Hydrolysis of S-2251 was monitored by measuring absorbance at 405 nm.
Enzymatic removal of N-linked oligosaccharides in fibrinogen
Purified fibrinogen was incubated with N-glycopeptidase F (Roche Diagnostics, Mannheim, Germany) to remove N-linked oligosaccharides in fibrinogen molecules as described previously.9 Removal of oligosaccharides from fibrinogen molecules was confirmed by SDS-PAGE analysis. Identification of the γ chain was conducted by the Western blot analysis as described in “Analysis of purified fibrinogen.”
Scanning electron microscopy of fibrin clots
To investigate the structure of Tokyo V fibrin, clots were formed on carbon-formvar–coated gold grids and processed for scanning electron microscopy using JEOL JSM6300F Field Emission Scanning Electron Microscope (Japan Electron Optics Laboratory, Tokyo, Japan) as described previously.9
Results
Abnormalities of purified fibrinogen Tokyo V
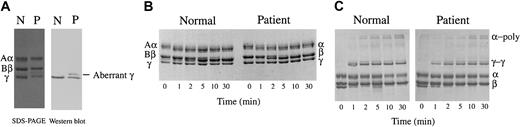
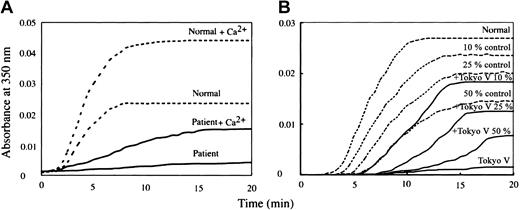
The clotting time of patient-derived fibrinogen (Tokyo V fibrinogen) with thrombin was remarkably prolonged to 42.0 seconds with calcium, and was not measurable in the absence of calcium (Table 1). These data confirmed that fibrinogen Tokyo V was a dysfibrinogen. Apparent mobility of purified fibrinogen Tokyo V subunit polypeptides on SDS-PAGE was indistinguishable from normal fibrinogen polypeptides (Figure 1A). There was a faint polypeptide band that migrated between the γ chain and the Bβ chain on SDS-PAGE in both the healthy subject-derived fibrinogen preparation and the patient-derived fibrinogen preparation. Western blotting analyses of purified fibrinogen preparations showed that the faint polypeptide band migrating between the γ chain and the Bβ chain in the Tokyo V fibrinogen preparation was recognized by γ chain-specific monoclonal antibody JIF25 but the band in the normal fibrinogen preparation was not, indicating the presence of a small amount of aberrant γ chain with slower mobility on SDS-PAGE in Tokyo V fibrinogen (Figure 1A). The amount of the aberrant γ chain band on the Western blot, quantified using a densitometer, was approximately 22% of the total amount of the Tokyo V γ chain. In comparison with normal fibrinogen or fibrin, conversion of Tokyo V fibrinogen Aα and Bβ chains to α and β chains by thrombin was similar to normal fibrinogen, as was cross-linking of the γ chain, indicating that release of fibrinopeptides A and B and FXIIIa-mediated γ chain cross-linking of fibrin were not impaired (Figure 1B-C). In contrast, cross-linking of the Tokyo V fibrin α chain was delayed (Figure 1C). Polymerization profiles of fibrin monomers derived from patient fibrinogen were remarkably impaired (Figure 2A). In particular, polymerization of Tokyo V fibrin did not proceed without calcium, confirming that the patient-derived fibrinogen was dysfunctional. Even with 2 mM calcium, absorbance of the Tokyo V fibrin monomer solution increased much slower than the normal fibrin monomer solution, and the maximum absorbance of the Tokyo V fibrin monomer solution was approximately one-third of that of the normal fibrin monomer solution, suggesting that the fibrin polymerization was severely perturbed (Figure 2A). To further study characteristics of Tokyo V fibrin, polymerization of normal fibrin monomers was studied in the presence of increasing concentrations of Tokyo V fibrin monomers. As shown in Figure 2B, polymerization of normal fibrin monomers was inhibited in a dose-dependent manner by the presence of Tokyo V fibrin monomers.
Clotting times of purified fibrinogen with thrombin
. | Fibrinogen Tokyo V . | Normal fibrinogen . |
|---|---|---|
| Without Ca2+, s | > 300.0 | 11.3 |
| With 2 mM Ca2+, s | 42.0 | 8.7 |
. | Fibrinogen Tokyo V . | Normal fibrinogen . |
|---|---|---|
| Without Ca2+, s | > 300.0 | 11.3 |
| With 2 mM Ca2+, s | 42.0 | 8.7 |
SDS-PAGE analysis of purified fibrinogen. Fibrinogen was purified from citrated plasma obtained from the patient (P) and healthy subjects (N) and was studied for the apparent molecular mass, the release of fibrinopeptides A and B by thrombin, and cross-linking of γ and α chains. (A) SDS-PAGE analyses (SDS-PAGE) of purified fibrinogen and identification of the γ chain by Western blotting using the γ chain–specific monoclonal antibody (Western blot). (B) Conversion of Aα and Bβ chains to α and β chains by thrombin treatment in the absence of calcium. (C) Formation of γ dimer (γ-γ) and α polymer (α-poly) upon thrombin treatment in the presence of FXIII and 2 mM calcium was analyzed by SDS-PAGE.
SDS-PAGE analysis of purified fibrinogen. Fibrinogen was purified from citrated plasma obtained from the patient (P) and healthy subjects (N) and was studied for the apparent molecular mass, the release of fibrinopeptides A and B by thrombin, and cross-linking of γ and α chains. (A) SDS-PAGE analyses (SDS-PAGE) of purified fibrinogen and identification of the γ chain by Western blotting using the γ chain–specific monoclonal antibody (Western blot). (B) Conversion of Aα and Bβ chains to α and β chains by thrombin treatment in the absence of calcium. (C) Formation of γ dimer (γ-γ) and α polymer (α-poly) upon thrombin treatment in the presence of FXIII and 2 mM calcium was analyzed by SDS-PAGE.
Polymerization profiles of fibrin monomers. (A) Aggregation of fibrin monomers derived from normal fibrinogen (dotted line) or from patient fibrinogen (solid line) at neutral pH in the absence or presence (+Ca2+) of 2 mM calcium was monitored at 350 nm. (B) Aggregation profiles of Tokyo V fibrin monomers (solid line; Tokyo V) and those of normal fibrin monomers in the absence (dotted line; normal) or the presence of increasing concentrations (10%, 25%, and 50%) of Tokyo V fibrin monomers (solid line; +Tokyo V) without calcium are shown. +Tokyo V 10%, +Tokyo V 25%, and +Tokyo V 50% indicate that ratios of amounts of normal fibrin monomers and Tokyo V fibrin monomers are 9:1. 3:1, and 1:1, respectively. The concentration of fibrin monomers (the sum of normal fibrin monomers and Tokyo V fibrin monomers) of each preparation was the same. For the control, aggregation profiles of normal fibrin monomers at reduced concentrations without addition of Tokyo V fibrin monomers are shown as dotted lines.
Polymerization profiles of fibrin monomers. (A) Aggregation of fibrin monomers derived from normal fibrinogen (dotted line) or from patient fibrinogen (solid line) at neutral pH in the absence or presence (+Ca2+) of 2 mM calcium was monitored at 350 nm. (B) Aggregation profiles of Tokyo V fibrin monomers (solid line; Tokyo V) and those of normal fibrin monomers in the absence (dotted line; normal) or the presence of increasing concentrations (10%, 25%, and 50%) of Tokyo V fibrin monomers (solid line; +Tokyo V) without calcium are shown. +Tokyo V 10%, +Tokyo V 25%, and +Tokyo V 50% indicate that ratios of amounts of normal fibrin monomers and Tokyo V fibrin monomers are 9:1. 3:1, and 1:1, respectively. The concentration of fibrin monomers (the sum of normal fibrin monomers and Tokyo V fibrin monomers) of each preparation was the same. For the control, aggregation profiles of normal fibrin monomers at reduced concentrations without addition of Tokyo V fibrin monomers are shown as dotted lines.
Analysis of soluble Tokyo V fibrin
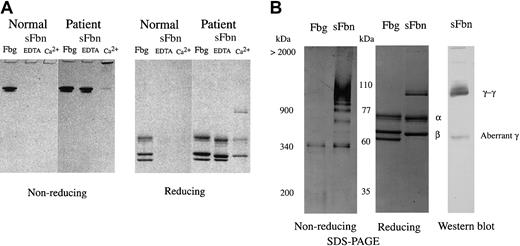
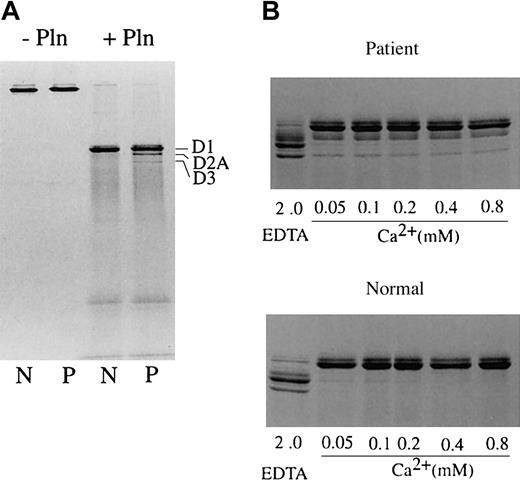
Tokyo V fibrinogen could be converted to fibrin by thrombin treatment, but its polymerization was severely perturbed, suggesting that Tokyo V fibrin might not form solid clots and might circulate as soluble fibrin. To show that soluble fibrin monomers and polymers were formed upon coagulation and incorporated into clots, normal fibrinogen and Tokyo V fibrinogen were treated with thrombin and FXIII in the presence of calcium and the formed clots were squeezed to separate clots and soluble components. Cross-linked fibrin clots and soluble components were analyzed by SDS-PAGE. The supernatant of a normal fibrin preparation did not contain soluble fibrin even in the presence of EDTA (ethylenediamenetetraacetic acid; Figure 3A). In contrast, a large amount (78.2%) of Tokyo V fibrin remained in the soluble fraction in the presence of EDTA (Figure 3A). Cross-linked soluble Tokyo V fibrin was present in the supernatants of clots formed in the presence of calcium. Analysis of the soluble fraction containing cross-linked soluble fibrin by 3% SDS-PAGE showed that cross-linked soluble Tokyo V fibrin consisted of monomers and a variety of fibrin polymers (Figure 3B). The Western blot analysis of the soluble Tokyo V fibrin showed that the majority of the γ chain was present as the cross-linked γ dimer (γ-γ) but a significant amount of the aberrant γ chain remained as the non–cross-linked form, suggesting that the soluble Tokyo V fibrin monomer consisted of abnormal molecules.
SDS-PAGE analysis of soluble Tokyo V fibrin. Tokyo V fibrinogen was incubated with thrombin (1 U/mL) and FXIII (1 U/mL) in the presence of 2 mM calcium (Ca2+) or 2 mM EDTA (EDTA) at 37°C for 1 hour. Formed clots were squeezed using bamboo sticks to separate soluble fibrin (sFbn) from fibrin clots. (A) Untreated fibrinogen, insoluble clots, and soluble fractions were analyzed by SDS-PAGE using 7.5% polyacrylamide gels under nonreducing and reducing conditions. (B) For analysis of cross-linked soluble Tokyo V fibrin, the supernatant of the patient fibrin preparation was separated on 3% polyacrylamide gels under nonreducing conditions. Fibrinogen (Fbg) was the control. The reduced supernatant sample was analyzed by Western blotting for identification of γ chain. γ-dimers (γ-γ) and the aberrant γ chain (aberrant γ) were indicated.
SDS-PAGE analysis of soluble Tokyo V fibrin. Tokyo V fibrinogen was incubated with thrombin (1 U/mL) and FXIII (1 U/mL) in the presence of 2 mM calcium (Ca2+) or 2 mM EDTA (EDTA) at 37°C for 1 hour. Formed clots were squeezed using bamboo sticks to separate soluble fibrin (sFbn) from fibrin clots. (A) Untreated fibrinogen, insoluble clots, and soluble fractions were analyzed by SDS-PAGE using 7.5% polyacrylamide gels under nonreducing and reducing conditions. (B) For analysis of cross-linked soluble Tokyo V fibrin, the supernatant of the patient fibrin preparation was separated on 3% polyacrylamide gels under nonreducing conditions. Fibrinogen (Fbg) was the control. The reduced supernatant sample was analyzed by Western blotting for identification of γ chain. γ-dimers (γ-γ) and the aberrant γ chain (aberrant γ) were indicated.
Genetic abnormality of patient fibrinogen genes
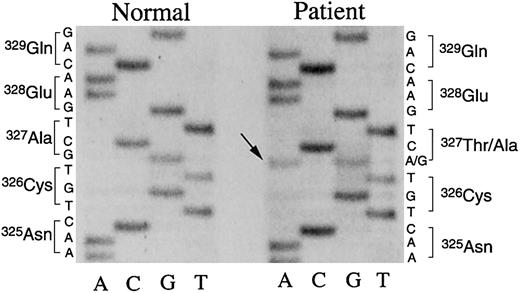
We performed genetic analyses of the 3 patient fibrinogen genes as described.7,9,10 A 5884G>A mutation of the patient fibrinogen γ chain gene was found. The nucleotide mutation was confirmed by 2 independent sequencing methods. As shown in Figure 4, both G and A were found at position 5884 in the directly sequenced PCR-amplified DNA fragments of exon VIII derived from the patient fibrinogen γ chain gene, indicating that the patient had a heterozygous genetic mutation and that this mutation would result in an amino acid substitution of γ Ala327Thr in the affected γ chain. Based on these data, we performed peptide mapping of lysyl-endopeptidase digests of patient fibrinogen γ chain by high-performance liquid chromatography (HPLC) as described7-9 and found a small aberrant peptide peak, suggesting that the abnormal γ chain polypeptide was a small part of the purified fibrinogen molecule. N-terminal amino acid sequencing7-9 of the aberrant peptide found Phe (322), Glu (323), Gly (324), and Thr (327) in the peptide. No Asn was identified in the expected position (325) suggesting that γ Asn325 was glycosylated as a result of the mutation (not shown). The amount of the aberrant peptide was insufficient to confirm the carbohydrate composition linked to the peptide.
Nucleotide sequences of the γ chain gene exon VIII. Single-stranded DNA, amplified by the asymmetrical PCR method,7,8 from the γ chain gene exon VIII derived from a healthy subject or the patient, was subjected to direct nucleotide sequencing using 35S-dATP and Sequenase. The pertinent portion of the autoradiography of urea-polyacrylamide gel electrophoresis is shown. Both G and A (arrow) were detected at position 5884 of the patient γ chain gene, coding for Ala (GCT) and Thr (ACT), whereas only G for Ala (GCT) was identified at the same position of the normal γ chain gene.
Nucleotide sequences of the γ chain gene exon VIII. Single-stranded DNA, amplified by the asymmetrical PCR method,7,8 from the γ chain gene exon VIII derived from a healthy subject or the patient, was subjected to direct nucleotide sequencing using 35S-dATP and Sequenase. The pertinent portion of the autoradiography of urea-polyacrylamide gel electrophoresis is shown. Both G and A (arrow) were detected at position 5884 of the patient γ chain gene, coding for Ala (GCT) and Thr (ACT), whereas only G for Ala (GCT) was identified at the same position of the normal γ chain gene.
Enzymatic removal of N-linked oligosaccharides in fibrinogen
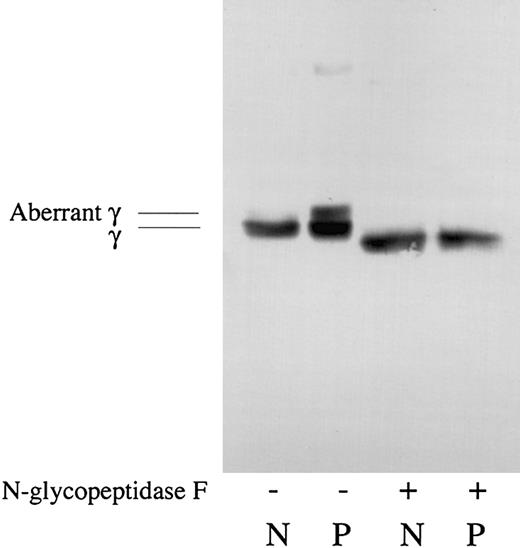
Since there was a higher molecular aberrant γ chain in the Tokyo V fibrinogen preparation, and the presence of an extra oligosaccharide side chain at γ Asn325 of Tokyo V fibrinogen was suggested by the gene analysis and the peptide mapping study, effect of deglycosylation on Tokyo V fibrinogen was studied. As shown in Figure 5, there was the aberrant γ chain in the Tokyo V fibrinogen preparation and this aberrant γ chain band was not detected after N-glycopeptidase F treatment, indicating that the higher molecular aberrant γ chain of Tokyo V fibrinogen migrated to the position similar to the normal γ chain after N-glycopeptidase F treatment. These data strongly suggested the presence of an extra oligosaccharide side chain at γ Asn325 of the abnormal molecule.
Analyses of deglycosylated fibrinogen. Normal fibrinogen (N) and patient fibrinogen (P) were treated with (+) or without (-) N-glycopeptidase F and analyzed by SDS-PAGE and Western blotting using γ chain–specific monoclonal antibody JIF 25. The γ chain (γ) and the aberrant γ chain (aberrant γ) were indicated.
Analyses of deglycosylated fibrinogen. Normal fibrinogen (N) and patient fibrinogen (P) were treated with (+) or without (-) N-glycopeptidase F and analyzed by SDS-PAGE and Western blotting using γ chain–specific monoclonal antibody JIF 25. The γ chain (γ) and the aberrant γ chain (aberrant γ) were indicated.
Analysis of tPA-catalyzed plasmin digestion of fibrin and tPA-mediated plasmin digestion on fibrin surfaces
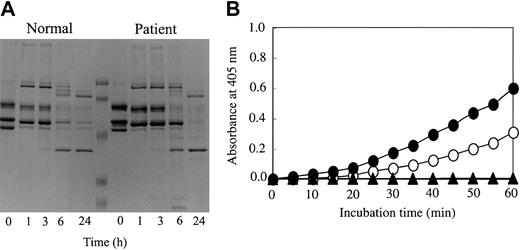
Since the amino acid substitution of Tokyo V fibrinogen resided in the γ chain of the D domain, we speculated that binding of tPA to Tokyo V fibrin may be affected. Thus, tPA-catalyzed plasmin digestion of Tokyo V fibrin and tPA-mediated plasmin generation on Tokyo V fibrin surfaces were studied. As shown in Figure 6A, the disappearance of the Tokyo V fibrin α chain was similar to that of normal fibrin, but degradation of the γ chain and β chain was delayed after a 6 hour incubation. This suggested that tPA-dependent fibrinolysis of Tokyo V fibrin was impaired, whereas digestion of Tokyo V fibrin by plasmin without the plasminogen activation process by tPA was virtually the same as that of normal fibrin (not shown). In accordance with these results, tPA-mediated plasmin generation on Tokyo V fibrin surfaces was prolonged compared with that on normal fibrin surfaces (Figure 6B), suggesting that Tokyo V fibrin would be resistant to physiologic fibrinolysis.
Analysis of tPA-catalyzed plasmin digestion of fibrin and tPA-mediated plasmin generation on fibrin surfaces. (A) Normal or patient fibrinogen was incubated with plasminogen, tPA, FXIII, and thrombin in the presence of calcium. After the indicated time periods, samples were heated at 98°C in the presence of 2% SDS and 10 mM DTT for 5 minutes to terminate the reaction and then analyzed by SDS-PAGE. (B) Normal fibrinogen (•) or patient fibrinogen (○) was incubated with plasminogen, tPA, FXIII, thrombin, and S-2251 in the presence of 2 mM calcium. As a control, normal fibrinogen (▴) was incubated in buffer containing plasminogen, tPA, and S-2251 or in buffer containing plasminogen, tPA, and thrombin. The changes in absorbance at 405 nm were monitored. Background absorbance determined with samples incubated in the absence of S-2251 at 405 nm were subtracted from all values.
Analysis of tPA-catalyzed plasmin digestion of fibrin and tPA-mediated plasmin generation on fibrin surfaces. (A) Normal or patient fibrinogen was incubated with plasminogen, tPA, FXIII, and thrombin in the presence of calcium. After the indicated time periods, samples were heated at 98°C in the presence of 2% SDS and 10 mM DTT for 5 minutes to terminate the reaction and then analyzed by SDS-PAGE. (B) Normal fibrinogen (•) or patient fibrinogen (○) was incubated with plasminogen, tPA, FXIII, thrombin, and S-2251 in the presence of 2 mM calcium. As a control, normal fibrinogen (▴) was incubated in buffer containing plasminogen, tPA, and S-2251 or in buffer containing plasminogen, tPA, and thrombin. The changes in absorbance at 405 nm were monitored. Background absorbance determined with samples incubated in the absence of S-2251 at 405 nm were subtracted from all values.
Digestion of fibrinogen in the presence of Ca2+ ions with plasmin
Since the mutation substituted a Thr at position 327 of the γ chain and possibly added oligosaccharides to Asn325, it may also have affected high-affinity calcium ion binding to the γ chain. Thus, Tokyo V fibrinogen was studied for its resistance to plasmin in the presence of calcium (Figure 7). Normal or Tokyo V fibrinogen was incubated with purified plasmin in the presence of increasing concentrations of calcium and degradation of fibrinogen was analyzed by SDS-PAGE. Normal fibrinogen was converted to the fragment D1 upon incubation with plasmin, but no further degradation of D1 was observed in the presence of 2 mM calcium (Figure 7A). This protection by calcium was observed at low calcium concentration (0.05 mM; Figure 7B). When Tokyo V fibrinogen was incubated with plasmin, fragments D2A and D3 were identified in addition to fragment D1, even at the high calcium concentration (2 mM). The amounts of D2A and D3 were approximately 15% to 20% of the sum of fragments D1, D2A, and D3. These data suggested that the calcium ion–dependent integrity of the Tokyo V fibrinogen conformation was not sufficient to protect the γ chain of fragment D1 from plasmin. The amino acid substitution of Ala to Thr at γ chain 327 and the extra oligosaccharide at Asn325 likely caused structural alternation of the high-affinity calcium binding site in the γ chain. Additionally, approximately 10% to 20% of the purified patient fibrinogen may consist of abnormal molecules because the amount of fragment D1 was not decreased further upon longer incubation.
Digestion of fibrinogen in the presence of calcium with plasmin. Fibrinogen (N: normal fibrinogen, P: patient fibrinogen) was treated with plasmin in Tris-buffered saline containing 2 mM calcium (A) or increasing concentrations of calcium or 2 mM EDTA (B) at 37°C for 1 hour. Untreated fibrinogen and plasmin digests of fibrinogen were analyzed by SDS-PAGE under nonreducing conditions. Fragments D1, D2A, and D3 are indicated. The relative amounts of Tokyo V fibrinogen fragments D2A and D3 were quantified by densitometry, and were approximately 17.5% of the sum of fragments D1, D2A, and D3 (mean of 2 experiments).
Digestion of fibrinogen in the presence of calcium with plasmin. Fibrinogen (N: normal fibrinogen, P: patient fibrinogen) was treated with plasmin in Tris-buffered saline containing 2 mM calcium (A) or increasing concentrations of calcium or 2 mM EDTA (B) at 37°C for 1 hour. Untreated fibrinogen and plasmin digests of fibrinogen were analyzed by SDS-PAGE under nonreducing conditions. Fragments D1, D2A, and D3 are indicated. The relative amounts of Tokyo V fibrinogen fragments D2A and D3 were quantified by densitometry, and were approximately 17.5% of the sum of fragments D1, D2A, and D3 (mean of 2 experiments).
Scanning electron microscopy of fibrin clots
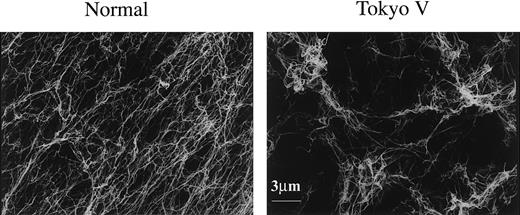
Scanning electron microscopy was carried out to investigate the structure of Tokyo V fibrin clots. In 2 experiments, a major part of Tokyo V fibrin clots appeared normal at a glance but other parts of Tokyo V fibrin clots looked very loose. That part of Tokyo V fibrin clots was composed of tangled fibers in a variety of diameters, and fibers were highly branched (Figure 8B). Additionally, there were many large pores inside the fibrin networks and many fiber ends were observed. In another scanning electron microscopy experiment, whole Tokyo fibrin clots consisted of very thin and highly branched fibers. There also were large holes in the fibrin networks and many fiber ends were observed at the boundaries (not shown). As described earlier in this paragraph, a variety of fibrin structures were observed in Tokyo V fibrin clots. The presence of fiber ends in the networks may represent early termination of protofibril polymerization. Consistent with the structure of Tokyo V fibrin clots, clottability of Tokyo V fibrin was very low. These findings suggested that Tokyo V fibrin would be fragile.
Scanning electron microscopy of fibrin clots. Scanning electron microscopy images of normal fibrin clots (left) and Tokyo V fibrin clots (right) are shown (bar equals 3 μm).
Scanning electron microscopy of fibrin clots. Scanning electron microscopy images of normal fibrin clots (left) and Tokyo V fibrin clots (right) are shown (bar equals 3 μm).
Discussion
Fibrinogen Tokyo V has an amino acid substitution in which the γ chain Ala327 is changed to Thr, thereby inserting an N-linked glycosylation consensus sequence and presumably an extra oligosaccharide side chain at γ Asn-325. This mutation is associated with a thrombophilia because fibrinogen Tokyo V was found in a 43-year-old male patient with recurrent thromboembolic episodes, including cerebral infarction at age 36 years, pulmonary embolism at age 42 years, and thrombi in the aorta and the superior mesenteric artery at age 43 years when the patient was admitted to a hospital. Thrombophilic dysfibrinogen is a rare molecular abnormality and mechanisms for thrombophilia may be assigned to defective binding of thrombin to abnormal fibrin, which leads to increased thrombin levels, or to defective stimulatory function of abnormal fibrin in tPA-mediated fibrinolysis.5
Data from SDS-PAGE analyses of purified fibrinogen followed by Western blotting using γ chain–specific monoclonal antibody JIF 25, peptide mapping analysis of patient fibrinogen γ chain, and plasmin degradation of the fibrinogen D1 fragment in the presence of calcium suggested that the molecular abnormality of fibrinogen Tokyo V was impaired function and hypofibrinogenemia, even though the patient was heterozygous for the mutation. Although the molecular mechanism for decreased plasma levels of the Tokyo V fibrinogen molecule is not studied yet, a decreased assembly, an intracellular transport defect, or hypercatabolism in the circulation of the abnormal molecules may be responsible for hypodysfibrinogenemia. As reported previously, even a single amino acid substitution can develop an intracellular transport defect of a variety of abnormal molecules including dysfibrinogens.12,13 Thus, the transport defect of Tokyo V fibrinogen molecules inside the cells would be most likely. Elucidation of the molecular mechanism of hypodysfibrinogenemia of this molecule would be the future study. The genetic analysis indicated the formation of a new N-linked glycosylation consensus sequence in the affected γ chain. Addition of extra oligosaccharides to γ Asn325 was suggested by the amino acid sequence analysis but was not confirmed because of low recovery of the aberrant peptide. Since N-linked glycosylation is a cotranslational event in the endoplasmic reticulum, preceding molecular folding catalyzed by chaperones, Asn325 of the affected γ chain may be glycosylated. We found an aberrant faint polypeptide band, which migrated between the γ chain and the Bβ chain, and the polypeptide was recognized by γ chain–specific monoclonal antibody JIF 25, indicating the presence of a small amount of aberrant γ chain with a higher molecular mass. Analyses of deglycosylated Tokyo V fibrinogen strongly suggested that the aberrant γ chain had an extra oligosaccharide side chain.
The C-terminal region of the γ chain consists of the D domain and has a variety of functional sites including the “a” polymerization pocket for D:E contact, the lateral association site for D:D contact, the tPA binding site, and the high-affinity calcium binding site. The Tokyo V fibrinogen amino acid mutation and the possible presence of oligosaccharide in that region affected these important fibrinogen/fibrin functions directly or indirectly as shown in this study. Based on the crystal structure of the D domain of fibrinogen,14-17 γ Ala327 resides near the calcium ion binding site and the “a” polymerization pocket. Thus, substitution of γ Ala327Thr would likely interfere with calcium binding to this region and also affect the polymerization pocket. A new carbohydrate linked to γ Asn325 also would quite likely disrupt the adjacent region, resulting in stronger interference with D-to-E contact than the amino acid substitution of γ Ala327Thr. Since γ Asn325 is off to the side of the buried interface, a γ Asn325–linked carbohydrate may not interfere with the D:D association directly, but may have an indirect influence.
Fibrinogen Tokyo V has the paradoxical feature of decreased functional fibrinogen in the presence of recurrent thrombosis. The polymerization defect of Tokyo V fibrinogen (Figure 2) would account for the decreased fibrinogen level as determined by the thrombin time method (Table 1) and very low clottability (Figure 3). A significant amount of Tokyo V fibrin monomers and polymers remains in the soluble fraction and as these soluble molecules are incorporated into fibrin networks, they may interfere with formation of solid fibrin clots. As evidenced by the scanning electron microscopy analysis showing the loose appearance of and the presence of large pores and many fiber ends in Tokyo V fibrin clots, the structure of Tokyo V fibrin clots may be severely perturbed compared with the normal fibrin clots. Thus, Tokyo V fibrin clots would be fragile, and insoluble fibrin clots may be easily liberated from clots to the circulation. But such Tokyo V fibrin clots also undergo impaired fibrinolysis due to decreased tPA-catalyzed plasmin generation, thereby partly accounting for thrombi formation. Inasmuch as insoluble clots are resistant to fibrinolysis, once insoluble fibrin clots are liberated from thrombi, they may form emboli. The presence of soluble fibrin in the circulation would also contribute to development of a prothrombotic state as well.
Upon conversion of fibrinogen to fibrin, tPA binds to fibrin via Aα and γ chains. Polypeptide segments Aα 148-16018 and γ 311-33619 are involved in tPA binding. The amino acid substitution and the presence of possible extra oligosaccharide of Tokyo V fibrinogen/fibrin reside in the proposed tPA binding site in the γ chain, suggesting that tPA binding to the abnormal molecule may be affected directly. A recent report showed that tPA binding segments are buried even in DD fragments, but reversibly expressed on surfaces of DD-E upon association of DD fragments with the E fragments.20 Thus, severely impaired fibrin polymerization will also influence tPA-mediated plasmin digestion of the mutant fibrin, even though the estimated amounts of abnormal fibrinogen molecules are approximately one-fifth those of normal fibrinogen.
Even with the presence of small amounts of abnormal fibrinogen molecules in the purified material, polymerization of patient fibrin was severely perturbed (Figure 2). During fibrin polymerization, the ratio of abnormal molecule incorporation into polymers could be one-tenth to one-twentieth. Since the molecular abnormality was present in the γ chain that consists of the D domain, expression of the “A” polymerization site in the E domain of the Tokyo V fibrin α chain may not be impaired. Thus, Tokyo V fibrin may have normal “A” knobs in the E domain and dysfunctional “a” polymerization pocket(s) in the D domain(s). Given that the D:E contact would be severely perturbed, extension of protofibrils would be terminated once the abnormal fibrin molecule was associated with normal molecules. If extension of protofibrils does not reach over 600 nm due to incorporation of abnormal molecules, protofibrils may not aggregate to form clots.21 Inhibition of normal fibrin monomer polymerization by addition of Tokyo V fibrin monomers, the presence of a variety of soluble fibrin polymer species in the supernatant of the patient fibrin preparation, and the presence of fiber ends in fibrin networks support this notion.
In conclusion, fibrinogen Tokyo V is a thrombophilic hypodysfibrinogen with an amino acid substitution of γ Ala327Thr and a possible extra glycosylation at γ Asn325. The thromboembolism may be accounted for by fibrinolysis-resistant fragile clots and formation of a large amount of soluble fibrin caused by conformation defects in the vicinity of the “a” polymerization pocket, the high-affinity calcium ion binding site, and the tPA-binding site of the γ chain.
Supported by Grants-in-Aid for Scientific Research no. 12670687 to J.M. from the Ministry of Education and Science.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, January 8, 2004; DOI 10.1182/blood-2003-07-2569.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal