Abstract
A fraction of total cellular tissue factor procoagulant activity remains masked or “encrypted” in intact cells. Decryption of this activity partly involves the extracellular exposure of anionic phospholipids such as phosphatidylserine. Because of the potential association of tissue factor and phospholipid scramblase activity with lipid rafts, we have explored the role of lipid rafts in regulating factor VIIa/tissue factor activity. In HEK293 cells, tissue factor antigen was not stably associated with lipid rafts, yet disruption of rafts with methyl-β-cyclodextrin resulted in a 3-fold stimulation of tissue factor procoagulant activity. Treatment with methyl-β-cyclodextrin was not associated with cytotoxicity and did not result in the exposure of additional tissue factor antigen. Factor VIIa/tissue factor activity decrypted with methyl-β-cyclodextrin was quantitatively similar to that obtained by using lytic concentrations of octyl glucoside but more sensitive to inhibition by cell surface tissue factor pathway inhibitor and the phospholipid binding protein, annexin V. Partial decryption of tissue factor was achieved with methyl-β-cyclodextrin prior to complete disruption of lipid rafts, suggesting the role of an enzyme localized to lipid rafts in the transbilayer transport of phosphatidylserine. We conclude that lipid rafts are required for the maintenance of cellular tissue factor in an encrypted state. (Blood. 2004;103:3038-3044)
Introduction
Tissue factor (TF) is a 45-kDa glycoprotein anchored to the plasma membrane by way of a single transmembrane spanning α-helix.1,2 The extrinsic coagulation cascade is initiated when TF binds and activates circulating factor VIIa (fVIIa) to form a protease complex (fVIIa/TF) that cleaves and activates the zymogens, factor IX and factor X (fX), generating active proteases, factor IXa and factor Xa (fXa), respectively. TF is expressed primarily on subendothelial tissues, but TF expression may be induced on endothelial cells by inflammatory mediators such as tumor necrosis factor α (TNF-α).3,4 TF may also be found circulating on monocytes,5 on microparticles derived from various cellular sources,6-8 and in a soluble form arising from alternate processing of the TF gene.9 Although subendothelial TF may be responsible for initiating fibrin formation at sites of vascular injury, bloodborne TF may be an important contributor to propagation of the developing thrombus.8,10 TF procoagulant activity is regulated by tissue factor pathway inhibitor (TFPI), a Kunitz-type protease inhibitor that binds both fVIIa and Xa11,12 and by availability of anionic phospholipid. Phosphatidylserine (PS), for example, increases the kcat/km of fVIIa/TF by 3 orders of magnitude.13-15
Cell surface TF is fully accessible to fVIIa and anti-TF antibodies, but a fraction of fVIIa/TF activity is suppressed or encrypted.15,16 Decryption of TF has been achieved by treating TF-expressing cells with hydrogen peroxide,17 calcium ionophore,15,18 or lytic concentrations of detergents such as Triton X-100 or octyl glucoside.19,20 One common consequence of these agents is the loss of membrane phospholipid asymmetry with the subsequent exposure of PS. However, blocking exposed PS with saturating concentrations of annexin V fails to fully inhibit decrypted fVIIa/TF activity.18,21 This PS-independent component of TF decryption has not been defined.
Factor VIIa/TF may also be regulated by localization of TF to plasmalemmal caveolae. Mulder et al22,23 demonstrated localization of TF to caveolae in smooth muscle cells by immunogold localization. Posttranslational modification of TF by thioester-linked palmitate24 provides a requisite targeting signal for localization to lipid rafts and caveolae. Sevinsky et al25 were the first to provide evidence that the membrane environment of lipid rafts and caveolae might play an important role in the regulation of TF procoagulant activity. Those researchers showed that down-regulation of TF procoagulant activity was coincident with translocation of the TF/fVIIa/fXa/TFPI complex to lipid rafts and suggested that this membrane environment may augment TFPI inhibition of fVIIa/TF. Our laboratory, however, has recently demonstrated that lipid raft localization does not influence the function of TFPI.26 Chimeric versions of TFPI that targeted exclusively to lipid rafts or to the bulk plasma membrane exhibited identical inhibitory activities. Further, endogenous TFPI activity was unaffected by disruption of lipid rafts with methyl-β-cyclodextrin (MBCD). Although our studies did not indicate a role for the membrane environment in TFPI function, they did suggest lipid rafts may regulate fVIIa/TF activity independent of TFPI activity. Specifically, we showed that MBCD treatment decrypted fVIIa/TF activity that was subsequently less sensitive to TFPI inhibition. The purpose of the current study, therefore, was to further evaluate the role of lipid rafts in the encryption of TF. In HEK293 cells that do not express TFPI, TF antigen did not localize to lipid rafts, but its activity was extremely sensitive to MBCD, suggesting a critical role for lipid rafts in the tonic suppression of TF procoagulant activity.
Materials and methods
Reagents and cells
Monoclonal antibodies to human tissue factor pathway inhibitor and decay-accelerating factor were gifts of Drs George Broze and Douglas Lublin, respectively (Washington University, St Louis, MO). Monoclonal antibody to human tissue factor (HTF1) was a generous gift of Dr Steven Carson (University of Nebraska, Lincoln, NE). Horseradish peroxidase–conjugated secondary antibodies were from Jackson Laboratories (West Grove, PA). Purified human factor VIIa and factor X were from Enzyme Research (South Bend, IN). Factor Xa amidolytic substrate was from American Diagnostica (Greenwich, CT). Recombinant human tissue factor (rTF, Innovin) was from Dade-Behring (Deerfield, IL). Recombinant human TFPI produced in Escherichia coli (rTFPI) was obtained from Dr George Broze. MBCD, α-cyclodextrin, n-octyl-β-d-glucopyranoside (octyl glucoside), and annexin V were from Sigma (St Louis, MO). Annexin V conjugated to phycoerythrin (PE) was from Becton Dickinson (Franklin Lakes, NJ). HEK293 cells and HEK293 cells expressing glycosylphosphatidylinositol (GPI)–linked TFPI26 were maintained in Dulbecco modified Eagle medium supplemented with 10% fetal bovine serum in a 5% CO2/95% humidified air atmosphere at 37°C. Chinese hamster ovary (CHO) cells and adult human dermal fibroblasts (Cambrex, East Rutherford, NJ) were maintained in Ham F12 medium and FGM-2, respectively, under the same culture conditions.
Western blotting
Proteins were separated by nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Membranes were blocked with 3% nonfat milk and incubated with appropriate mouse monoclonal antibody diluted in blocking buffer. Bands were visualized with antimouse immunoglobulin G (IgG)–horseradish peroxidase conjugate and SuperSignal chemiluminescent substrate (Pierce, Chicago, IL).
Flow cytometry
Analysis was performed on a Becton Dickinson FacsCalibur using CELLQUEST software. For analysis of cell surface TF, 106 HEK293 cells were treated with buffer or MBCD and resuspended in 500 μL phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA) and 10 μg mouse monoclonal antibody to human TF, then allowed to incubate for 1 hour at 4°C. Cells were then washed twice in PBS and resuspended in 200 μL PBS containing 1% BSA and 10 μg antimouse IgG-PE and then allowed to incubate an additional 30 minutes at 4°C. For analysis of PS, 105 cells were resuspended in 100 μL binding buffer (10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 140 mM NaCl, 2.4 mM CaCl2, pH 7.4) and incubated for 1 hour at 4°C with 5 μL annexin V–PE prior to analysis.
Isolation of lipid rafts
Rafts were isolated by treatment with Triton X-100 and discontinuous sucrose-density gradient centrifugation by a modified detergent extraction procedure. Cells were washed twice in cold PBS and lysed for 30 minutes at 4°C in MES-buffered saline (50 mM MES [2-(N-morpholino)ethanesulfonic acid], pH 6.5) containing 1% Triton X-100, 10 mM EDTA (ethylenediaminetetraacetic acid), 1 mM phenylmethyl sulfonyl fluoride, 5 μg/mL soybean trypsin inhibitor, 17.5 μg/mL benzamidine, and 5 μg/mL leupeptin. The lysate was adjusted to 40% sucrose by the addition of an equal volume 80% sucrose and loaded beneath a discontinuous sucrose gradient of 30% sucrose and 5% sucrose. Gradients were centrifuged at 100 000g for 3 hours, and fractions were collected from the top.
Preparation of β-cyclodextrin/cholesterol complex
Preparation was performed essentially according to Christian et al.27 Briefly, a conical centrifuge tube was coated with cholesterol by addition of 100 μmol cholesterol in 5 mL ethanol that was then evaporated under nitrogen. MBCD (100 μmol) was dissolved in 5 mL cell buffer (21 mM HEPES, pH 7.4, 137 mM NaCl, 5 mM KCl, 0.75 mM Na2HPO4, 5.5 mM glucose, and 2 mM CaCl2) and then added to the cholesterol-coated tube. The tube was incubated in a bath sonicator for 5 minutes followed by overnight incubation at 37°C under constant rotation. Insoluble material was removed by filtration through a 0.2-μm filter, and the cholesterol content of the filtrate was determined enzymatically following extraction with an equal volume of ether. The MBCD-to-cholesterol ratio in the filtrate was 3.9 ± 0.4 (mean ± SD, n = 3).
TF decryption and fVIIa/TF activity measurement
Decryption of TF and subsequent fVIIa/TF activity measurement were performed in 2 steps. In the first step, 2 million cells were incubated in 0.2 mL cell buffer containing indicated concentrations of cyclodextrin or octyl glucoside for the period of time indicated. In the second step, 5 μL treated cell suspension was transferred to a 96-well plate containing 45 μL cell buffer. Following incubation with 10 nM fVIIa for 30 minutes at 4°C, 1 μM fX and 500 μM Spectrozyme Xa were added simultaneously to a final volume of 70 μL, and A405 (absorbance at 405 nm) was monitored continuously for 1 hour. Factor VIIa/TF activity was determined from the first derivative of the nonlinear increase in fXa during the first 10 to 20 minutes of the reaction when less than 10% of substrate fX had been consumed.28 Absolute rates of fVIIa/TF activity expressed as picomoles per minute squared were generated by using a path length of 0.2 cm and an extinction coefficient for p-nitroaniline of 9.92 L mmol-1 cm-1 at 405 nm.29
This protocol was designed to minimize the direct effect of decrypting agents on fVIIa/TF activity. Following incubation of cells to decrypt TF, the concentration of octyl glucoside or MBCD was diluted by a factor of 14 into the fVIIa/TF activity assay. We evaluated the direct effect of diluted decrypting agents on fVIIa/TF activity by using CHO cells, which neither display nor inhibit human fVIIa/TF activity. CHO cells were treated with decrypting agents and diluted into the fVIIa/TF assay system above in the presence of added human rTF. An amount of rTF was added to wells to reflect that contained in similar incubations with HEK293 cells.
LDH activity
Lactate dehydrogenase (LDH) activity released from cells following treatment with decrypting agents was assessed after removal of cells by centrifugation. Activity was measured by using 50 mM lactate as substrate in the presence of 100 mM Tris (tris(hydroxymethyl)aminomethane) pH 9.3, 150 mM KCl. The reaction was initiated by the addition of 6.5 mM nicotinamide adenine dinucleotide (NAD) and followed for 5 minutes at 340 nm.30 An extinction coefficient of 6.22 L mmol-1 cm-1 was used to calculate activity.
Statistics
Statistical significance was assessed by the use of an unpaired t test (Microsoft Excel 2002). Relevant 2-tailed P values are cited in figure legends.
Results
TF does not localize to lipid rafts in untreated HEK293 cells
Previous reports have provided conflicting evidence regarding TF association with lipid rafts and caveolae. One report suggested a stable association of TF with caveolae,23 whereas another described an inducible translocation of TF to caveolae in complex with fVIIa, fXa, and TFPI.25 We investigated the membrane localization of TF in untreated HEK293 cells by using insolubility in Triton X-100 as a measure of inclusion in lipid rafts. Figure 1A shows that the GPI-anchored protein, decay-accelerating factor, exclusively localized to the buoyant fractions of a sucrose gradient, whereas TF entirely localized to the high density gradient fractions containing solubilized protein. On the basis of its detergent solubility, therefore, we conclude that little uncomplexed TF is associated with lipid rafts in HEK293 cells.
Properties of lipid rafts in HEK293 cells. (A) Tissue factor does not stably associate with lipid rafts. Wild-type HEK293 cells (10 million) were lysed in 0.1 mL MES-buffered saline (pH 6.5) containing 1% Triton X-100, adjusted to 40% sucrose, and placed beneath a step gradient consisting of 1.0 mL 30% sucrose and 0.4 mL 5% sucrose. Following centrifugation, gradient fractions were subjected to SDS-PAGE and Western blot using anti-TF (top blot) and anti-DAF (bottom blot) antibodies. Buoyant, Triton-insoluble material is indicated by DAF distribution. (B) Complete disruption of lipid rafts requires treatment with 10 mM MBCD for 60 minutes. Sixty million HEK293 cells expressing GPI-anchored and hemagglutinin (HA)-tagged TFPI were treated with cell buffer (CB) for 60 minutes (top blot), 10 mM MBCD for 30 minutes (middle blot), or 10 mM MBCD for 60 minutes (bottom blot) before addition of 1 mL MES-buffered saline containing 1% Triton X-100. Lysates were adjusted to 40% sucrose and placed beneath a step gradient consisting of 5 mL 30% sucrose and 3 mL 5% sucrose. Following centrifugation, gradient fractions were subjected to SDS-PAGE and Western blot using anti-HA antibody to detect epitope-tagged TFPI.
Properties of lipid rafts in HEK293 cells. (A) Tissue factor does not stably associate with lipid rafts. Wild-type HEK293 cells (10 million) were lysed in 0.1 mL MES-buffered saline (pH 6.5) containing 1% Triton X-100, adjusted to 40% sucrose, and placed beneath a step gradient consisting of 1.0 mL 30% sucrose and 0.4 mL 5% sucrose. Following centrifugation, gradient fractions were subjected to SDS-PAGE and Western blot using anti-TF (top blot) and anti-DAF (bottom blot) antibodies. Buoyant, Triton-insoluble material is indicated by DAF distribution. (B) Complete disruption of lipid rafts requires treatment with 10 mM MBCD for 60 minutes. Sixty million HEK293 cells expressing GPI-anchored and hemagglutinin (HA)-tagged TFPI were treated with cell buffer (CB) for 60 minutes (top blot), 10 mM MBCD for 30 minutes (middle blot), or 10 mM MBCD for 60 minutes (bottom blot) before addition of 1 mL MES-buffered saline containing 1% Triton X-100. Lysates were adjusted to 40% sucrose and placed beneath a step gradient consisting of 5 mL 30% sucrose and 3 mL 5% sucrose. Following centrifugation, gradient fractions were subjected to SDS-PAGE and Western blot using anti-HA antibody to detect epitope-tagged TFPI.
Complete raft disruption in HEK293 cells requires treatment with 10 mM methyl-β-cyclodextrin for 60 minutes
In our hands, various cell types are differentially sensitive to the effects of treatment with MBCD. We, therefore, investigated the concentration and time dependence of raft disruption in HEK293 cells by using an epitope-tagged chimera of TFPI and decay-accelerating factor (DAF) that is directly GPI-anchored to the plasma membrane. Figure 1B shows that TFPI exclusively localized to the buoyant fractions of the sucrose gradient following a 60-minute treatment with cell buffer. Thirty minutes of treatment with 10 mM MBCD resulted in only a small decrease in the percentage of raft-associated TFPI, whereas 60 minutes of treatment completely abrogated association of TFPI with lipid rafts. Thus, subsequent studies used concentrations of MBCD encompassing 10 mM.
Direct effect of decrypting agents on soluble fVIIa/TF
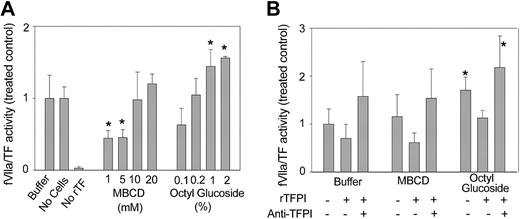
Decrypting agents may directly affect fXa generation by fVIIa/TF and the activity of fXa toward chromogenic substrate, masking their influence on the plasma membrane environment. Therefore, we performed control experiments to define the effects of residual octyl glucoside and MBCD on soluble TF in a suspension of CHO cells. Figure 2A shows that CHO cells displayed no TF procoagulant activity (bar 3) and did not express inhibitory activity toward human fVIIa/TF (bars 1-2). Residual MBCD did not alter fVIIa/TF activity aside from a moderate suppression at 1 and 5 mM. The lowest concentrations of octyl glucoside also had no effect on the activity of soluble fVIIa/TF, but detergent carried over from the treatment of CHO cells with 1% to 2% octyl glucoside resulted in a statistically significant 1.5-fold increase in fVIIa/TF activity. Thus, residual quantities of decrypting agents are generally inert with the exception of a modest stimulation of basal TF procoagulant activity induced by octyl glucoside.
Effect of residual decrypting agents on fVIIa/TF activity and sensitivity to TFPI. The effect of residual decrypting agents on fVIIa/TF activity was assessed by using 5 μL rTF diluted 1:1000 as the source of TF and 5 × 104 CHO cells. Activity measurements were performed as described in the presence of 10 nM fVIIa. (A) Rates of fVIIa/TF activity were determined in the presence (Buffer) or absence (No Cells) of CHO cells and also in the absence of added rTF (No rTF). Bars labeled MBCD and Octyl Glucoside indicate rates of fVIIa/TF activity in the presence of CHO cells incubated in the presence of indicated concentrations of MBCD or octyl glucoside. (B) CHO cells were incubated (107/mL) in cell buffer, cell buffer containing 10 mM MBCD, or cell buffer containing 1% octyl glucoside, and aliquots containing 5 × 104 cells were added to reactions containing rTF. Rates of fVIIa/TF were determined in the presence (+) or absence (-) of 5 nM TFPI or 5 μg anti-TFPI monoclonal antibody. In both panels, rates of fVIIa/TF activity are presented as a ratio to those obtained with buffer-treated CHO cells. Data presented are means ± SDs for at least 4 determinations. *P < .01 versus buffer-treated CHO cells.
Effect of residual decrypting agents on fVIIa/TF activity and sensitivity to TFPI. The effect of residual decrypting agents on fVIIa/TF activity was assessed by using 5 μL rTF diluted 1:1000 as the source of TF and 5 × 104 CHO cells. Activity measurements were performed as described in the presence of 10 nM fVIIa. (A) Rates of fVIIa/TF activity were determined in the presence (Buffer) or absence (No Cells) of CHO cells and also in the absence of added rTF (No rTF). Bars labeled MBCD and Octyl Glucoside indicate rates of fVIIa/TF activity in the presence of CHO cells incubated in the presence of indicated concentrations of MBCD or octyl glucoside. (B) CHO cells were incubated (107/mL) in cell buffer, cell buffer containing 10 mM MBCD, or cell buffer containing 1% octyl glucoside, and aliquots containing 5 × 104 cells were added to reactions containing rTF. Rates of fVIIa/TF were determined in the presence (+) or absence (-) of 5 nM TFPI or 5 μg anti-TFPI monoclonal antibody. In both panels, rates of fVIIa/TF activity are presented as a ratio to those obtained with buffer-treated CHO cells. Data presented are means ± SDs for at least 4 determinations. *P < .01 versus buffer-treated CHO cells.
Figure 2B details the effects of residual decrypting agents on the inhibition of fVIIa/TF by TFPI and the reversal of TFPI activity by monoclonal antibody. CHO cells were treated with MBCD and octyl glucoside at their optimal decrypting concentrations, 10 mM and 1%, respectively. TFPI suppressed equal amounts of fVIIa/TF activity in the presence of residual MBCD or octyl glucoside. Because of the basal stimulation of fVIIa/TF, however, TFPI inhibited a lower percentage of fVIIa/TF activity in the presence of residual octyl glucoside than in the presence of MBCD. Neither octyl glucoside nor MBCD prevented the complete recovery of fVIIa/TF activity in the presence of inhibitory anti-TFPI antibody. The data contained in Figure 2A-B collectively define the direct effects of residual amounts of decrypting agents on fVIIa/TF activity and its inhibition by TFPI. With the exception of higher concentrations of octyl glucoside used, these effects are minimal.
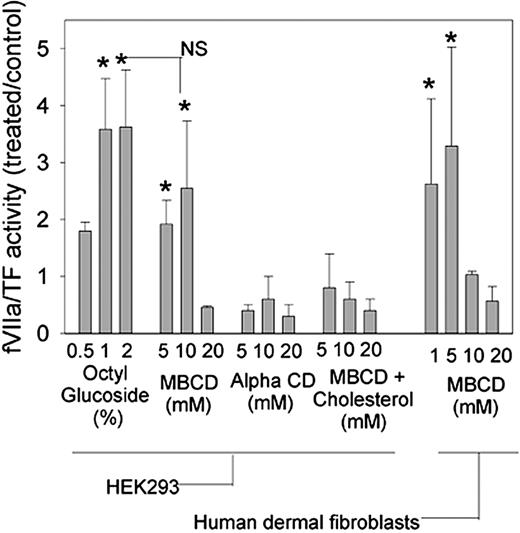
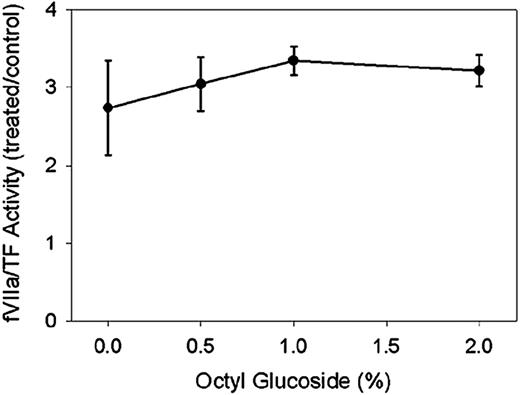
Decryption of cellular TF by MBCD
Wild-type HEK293 cells were primarily used in this study because they endogenously express TF but no detectable TFPI antigen or activity. Figure 3 shows the results of fVIIa/TF activity in cells treated with decrypting agents expressed as a ratio to those cells treated with buffer alone. At lytic concentrations of 1% to 2%, octyl glucoside increased TF procoagulant activity by a factor of 4 over buffer-treated cells. Maximal decryption of TF with MBCD occurred at 10 mM and was not statistically different than that induced by 2% octyl glucoside. Forms of cyclodextrin with a lower cholesterol affinity, α-cyclodextrin, and cholesterol-loaded MBCD did not activate fVIIa/TF activity, confirming that decryption with MBCD is dependent on membrane cholesterol extraction and not the result of an alternative cyclodextrin-mediated mechanism. In contrast to decryption with octyl glucoside, decryption of TF with MBCD did not require cell disruption because (1) more cytotoxic concentrations of MBCD did not further activate fVIIa/TF activity and (2) similar levels of LDH were released from cells treated for 1 hour with MBCD (3.56 ± 0.86 nmol/min, mean ± SD, n = 4) or with buffer (2.25 ± 0.64 nmol/min, mean ± SD, n = 4). Decryption of TF was also observed with primary human dermal fibroblasts. Untreated human dermal fibroblasts displayed similar fVIIa/TF activity to the HEK293 cells (11.6 ± 4.3 pmols/min2 versus 17.6 ± 5.8 pmols/min2, mean ± SD, n ≥ 5). In the fibroblasts, a 3-fold increase in fVIIa/TF activity occurred at 5 mM rather than 10 mM, but the degree of fVIIa/TF activation was similar to that in HEK293 cells.
MBCD decrypts TF in HEK293 cells and primary human dermal fibroblasts. HEK293 cells or human dermal fibroblasts were incubated at 107/mL in cell buffer alone or cell buffer containing indicated concentrations of octyl glucoside, MBCD, α-cyclodextrin, or cholesterol-enriched MBCD. Rates of fVIIa/TF were determined by using a 5-μL aliquot of treated cells and expressed as a ratio to the rate determined by using cells treated with buffer alone. Data presented are means ± SDs for at least 5 determinations. *P < .01 versus buffer-treated cells. Maximal decryption with octyl glucoside was not statistically different from maximal decryption with MBCD indicated by NS.
MBCD decrypts TF in HEK293 cells and primary human dermal fibroblasts. HEK293 cells or human dermal fibroblasts were incubated at 107/mL in cell buffer alone or cell buffer containing indicated concentrations of octyl glucoside, MBCD, α-cyclodextrin, or cholesterol-enriched MBCD. Rates of fVIIa/TF were determined by using a 5-μL aliquot of treated cells and expressed as a ratio to the rate determined by using cells treated with buffer alone. Data presented are means ± SDs for at least 5 determinations. *P < .01 versus buffer-treated cells. Maximal decryption with octyl glucoside was not statistically different from maximal decryption with MBCD indicated by NS.
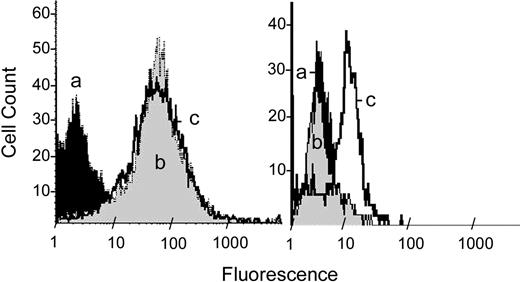
Figure 4 shows the consequences of MBCD treatment on the exposure of TF antigen and PS in HEK293 cells following MBCD treatment. HEK293 cells were treated with buffer or MBCD for 1 hour before staining with anti-TF and antimouse IgG-PE (left panel) or annexin V–PE to detect PS (right panel). HEK293 cells incubated with antimouse IgG-PE served as a negative control for both treatments to assess nonspecific fluorescence. MBCD did not alter the amount of TF that is accessible to anti-TF antibody but did increase exposure of PS that is accessible to binding with annexin V.
MBCD does not affect cell surface TF content but enhances exposure of phosphatidylserine. Wild-type HEK293 cells were treated (107/mL) with cell buffer alone or with cell buffer containing 10 mM MBCD for 1 hour. In both the left and right panels, histogram a (black) represents buffer-treated cells stained with antimouse IgG-PE. Cells treated with MBCD and stained with antimouse IgG-PE displayed a distribution identical to histogram a. (Left) Histograms b (gray) and c (boldface) show cells stained consecutively with monoclonal anti-TF antibody and antimouse IgG-PE following treatment with buffer or 10 mM MBCD, respectively. (Right) Histogram b (gray) and c (boldface) show cells stained with annexin V–PE following treatment with cell buffer or MBCD, respectively.
MBCD does not affect cell surface TF content but enhances exposure of phosphatidylserine. Wild-type HEK293 cells were treated (107/mL) with cell buffer alone or with cell buffer containing 10 mM MBCD for 1 hour. In both the left and right panels, histogram a (black) represents buffer-treated cells stained with antimouse IgG-PE. Cells treated with MBCD and stained with antimouse IgG-PE displayed a distribution identical to histogram a. (Left) Histograms b (gray) and c (boldface) show cells stained consecutively with monoclonal anti-TF antibody and antimouse IgG-PE following treatment with buffer or 10 mM MBCD, respectively. (Right) Histogram b (gray) and c (boldface) show cells stained with annexin V–PE following treatment with cell buffer or MBCD, respectively.
Inhibition of decrypted fVIIa/TF by TFPI and annexin V
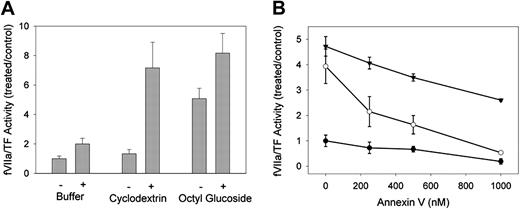
To assess the sensitivity of decrypted fVIIa/TF activity to TFPI, we repeated decryption studies by using a previously characterized HEK293 cell line stably transfected with a GPI-anchored version of TFPI.26 Basal fVIIa/TF activity in these transfected cells was lower than with their untransfected counterparts, so normalized rates of fVIIa/TF activity were considerably higher than those observed with wild-type HEK293 cells. Figure 5A details the sensitivity of basal and decrypted fVIIa/TF activity to TFPI. Treatment with 1% octyl glucoside resulted in a 5-fold increase of TF procoagulant activity, whereas treatment with 10 mM MBCD had little effect. When TFPI was inhibited by the inclusion of 1μg/mL anti-TFPI antibody, MBCD- and octyl glucoside-treated cells displayed similar fVIIa/TF activity. Thus, much of the activity (> 70%) decrypted by octyl glucoside was not inhibited by TFPI, whereas virtually all of the activity decrypted with MBCD was sensitive to inhibition by membrane-bound TFPI.
Sensitivity of decrypted TF to TFPI and annexin V. (A) HEK293 cells stably expressing a GPI-anchored version of TFPI (TFPI-PI) were treated with cell buffer alone or cell buffer containing 10 mM MBCD or 1% octyl glucoside. Aliquots of the treated cells were analyzed for fVIIa/TF activity in the presence (+) or absence (-) of 1 μg/mL inhibitory monoclonal antibody against the first Kunitz domain of TFPI. Rates of fVIIa/TF activity are expressed as ratios to buffer-treated cells in the absence of inhibitory antibody. Data presented are means ± 1 SDs for 6 determinations. (B) Wild-type HEK293 cells were treated with cell buffer alone (•) or cell buffer in the presence of 10 mM MBCD (○) or 1% octyl glucoside (▾). Aliquots of the treated cell suspensions were analyzed for fVIIa/TF activity in the presence of the indicated concentrations of annexin V. Rates of TF activity are expressed as ratios to buffer-treated cells in the absence of annexin V. Data presented are means ± SEM for at least 4 determinations.
Sensitivity of decrypted TF to TFPI and annexin V. (A) HEK293 cells stably expressing a GPI-anchored version of TFPI (TFPI-PI) were treated with cell buffer alone or cell buffer containing 10 mM MBCD or 1% octyl glucoside. Aliquots of the treated cells were analyzed for fVIIa/TF activity in the presence (+) or absence (-) of 1 μg/mL inhibitory monoclonal antibody against the first Kunitz domain of TFPI. Rates of fVIIa/TF activity are expressed as ratios to buffer-treated cells in the absence of inhibitory antibody. Data presented are means ± 1 SDs for 6 determinations. (B) Wild-type HEK293 cells were treated with cell buffer alone (•) or cell buffer in the presence of 10 mM MBCD (○) or 1% octyl glucoside (▾). Aliquots of the treated cell suspensions were analyzed for fVIIa/TF activity in the presence of the indicated concentrations of annexin V. Rates of TF activity are expressed as ratios to buffer-treated cells in the absence of annexin V. Data presented are means ± SEM for at least 4 determinations.
TF procoagulant activity that is decrypted with detergents displays only partial dependence on the exposure of PS. We examined the PS dependence of decrypted fVIIa/TF activity in wild-type HEK293 cells. Cells treated with 10 mM MBCD or 1% octyl glucoside were exposed to 0 to 1 μM annexin V prior to activity measurement. Annexin V binds negatively charged phospholipids in a calcium-dependent manner that alters the affinity of TF for fVIIa, thereby inhibiting TF procoagulant activity.31-33 The results of these studies are shown in Figure 5B. Basal fVIIa/TF activity was almost completely suppressed by 1 μM annexin V. In octyl glucoside-treated cells, fVIIa/TF activity decreased linearly as a function of annexin concentration to 50% of maximal activity at 1 μM. This relationship did not reach an asymptote, suggesting that further inhibition might be achieved by higher annexin concentrations. In contrast to octyl glucoside treatment, fVIIa/TF decrypted with MBCD was completely sensitive to annexin. Inhibition was nearly complete at 1 μM annexin, indicating an exclusive dependence of fVIIa/TF activity decrypted with MBCD on PS exposure.
MBCD and octyl glucoside decrypt the same pool of TF
TF decrypted with MBCD or octyl glucoside is differentially sensitive to inhibition by TFPI and annexin V, suggesting that these 2 agents decrypt different pools of TF. We hypothesized that successive treatment with these agents would result in additive decryption of TF. Thus, we treated wild-type HEK293 cells with 10 mM cyclodextrin for 1 hour followed by a 15-minute exposure to 0% to 2% octyl glucoside. The converse experiment in which cells were treated with octyl glucoside followed by MBCD was not performed because MBCD treatment of detergent micelles is not relevant to our observations in intact cells. Figure 6 displays the effect of octyl glucoside on MBCD-treated HEK293 cells. Cells treated only with 10 mM MBCD showed a characteristic 3-fold increase in fVIIa/TF activity. Subsequent treatment with various concentrations of octyl glucoside did not result in any further increase in TF procoagulant activity. These data suggest that TF is maximally decrypted by either 1% octyl glucoside or 10 mM MBCD.
Octyl glucoside and MBCD decrypt the same pool of TF. Wild-type HEK293 cells were treated with 10 mM MBCD for 1 hour followed by indicated concentrations of octyl glucoside for 15 additional minutes. Activity of fVIIa/TF was then measured in aliquots of treated cell suspensions and expressed as a ratio to activity in cells treated with cell buffer alone for 1 hour. Data presented are means ± SD for 4 determinations.
Octyl glucoside and MBCD decrypt the same pool of TF. Wild-type HEK293 cells were treated with 10 mM MBCD for 1 hour followed by indicated concentrations of octyl glucoside for 15 additional minutes. Activity of fVIIa/TF was then measured in aliquots of treated cell suspensions and expressed as a ratio to activity in cells treated with cell buffer alone for 1 hour. Data presented are means ± SD for 4 determinations.
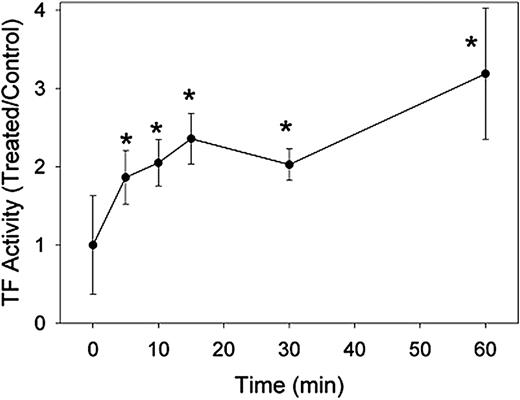
MBCD partially decrypts TF before complete raft disruption
Enhancement of fVIIa/TF activity was maximal when cells were exposed to 10 mM MBCD for 60 minutes. These same conditions are required to completely ablate the association of GPI-linked proteins with lipid rafts in our hands. We further explored the time dependence of MBCD decryption of TF to determine whether complete raft disruption was necessary to activate fVIIa/TF. Wild-type HEK293 cells were incubated with 10 mM cyclodextrin for 5, 10, 15, 30, and 60 minutes prior to measurement of fVIIa/TF. These data are displayed in Figure 7. After 60 minutes, we again observed a characteristic 3- to 4-fold increase in fVIIa/TF activity, but shorter incubations also enhanced fVIIa/TF activity. In as little as 5 minutes, a doubling of fVIIa/TF activity was observed, and maximal activation was approached by 15 minutes. These experiments suggest that, although rafts may be involved in suppressing cell surface fVIIa/TF activity, full raft disruption is not required to decrypt TF.
MBCD decrypts TF activity prior to lipid raft disruption. HEK293 cells were incubated in cell buffer containing 10 mM MBCD for indicated periods of time up to 1 hour. Factor VIIa/TF activity was then analyzed in aliquots of the treated cell suspensions and is expressed as a ratio to that in cells treated with buffer alone. Data presented are means ± 1 SD for 6 determinations. *P < .01 versus buffer-treated cells.
MBCD decrypts TF activity prior to lipid raft disruption. HEK293 cells were incubated in cell buffer containing 10 mM MBCD for indicated periods of time up to 1 hour. Factor VIIa/TF activity was then analyzed in aliquots of the treated cell suspensions and is expressed as a ratio to that in cells treated with buffer alone. Data presented are means ± 1 SD for 6 determinations. *P < .01 versus buffer-treated cells.
Discussion
This study details an important advance in our understanding of the regulation of TF by the plasma membrane environment. Heretofore, maximal decryption of TF has been achieved primarily by complete cell disruption (eg, with lytic concentrations of detergents).19,20 Here, we show that TF decryption can occur in intact cells to an extent similar to that achieved following cell lysis. Importantly, we show that TF is regulated by lipid rafts by a potential enzymatic mechanism. Many previous studies have shown that disruption of lipid rafts influences the function of resident raft proteins.34-39 In the present study, the integrity of lipid rafts appears to be important to maintaining TF in an encrypted state even though TF does not localize to lipid rafts. Specifically, our data suggest that lipid rafts are important in restricting anionic phospholipids to the internal leaflet of the plasma membrane, thus providing a mechanism for the acute regulation of cell surface fVIIa/TF activity in addition to the recognized contribution of TFPI.
The extent of TF decryption observed with MBCD and octyl glucoside must be considered in the context of the direct effect of residual decrypting agents on soluble fVIIa/TF activity. By diluting treated cells by a factor of 14 into the fVIIa/TF activity assay, we minimized these effects in our system. The residual effects of trace MBCD had little influence on fVIIa/TF activity. The residual effects of octyl glucoside on fVIIa/TF were not apparent at the lowest concentrations but resulted in a 1.5- to 2-fold stimulation at higher concentrations. Thus, when HEK293 cells were decrypted with 1% and 2% octyl glucoside, the resulting estimate of decrypted fVIIa/TF activity is exaggerated. Other studies using detergents to decrypt TF activity may require re-interpretation in light of the presence of substantial residual detergent. Our study emphasizes the cellular effect of various decrypting agents because cell suspensions are significantly diluted before measurement of fVIIa/TF activity.
Octyl glucoside and MBCD decrypt TF to the same extent, but the resulting fVIIa/TF activity is differentially sensitive to inhibition by TFPI. Activity decrypted by MBCD is sensitive to TFPI, whereas that decrypted by octyl glucoside is only partially sensitive. The residual presence of octyl glucoside in fVIIa/TF activity assays partially explains this discrepancy. Figure 2B shows that, although TFPI inhibits the same amount of absolute fVIIa/TF activity, basal stimulation of fVIIa/TF by trace amounts of octyl glucoside is not sensitive to TFPI inhibition. The remainder of the fVIIa/TF activity decrypted with octyl glucoside that is insensitive to TFPI is likely the result of membrane dissolution. Consequently, the effective concentration of TFPI relative to TF in octyl glucoside micelles is lower than in MBCD-treated cells in which TFPI concentrations are enhanced because of compartmentalization with TF in the intact plasma membrane.
The activity of fVIIa/TF decrypted by MBCD or octyl glucoside is also differentially sensitive to inhibition by annexin V. Following MBCD or buffer treatment, 1 μM annexin V completely inhibited factor VIIa/TF activity, indicating that decryption occurred exclusively by way of exposure of PS. At the same concentration, annexin V inhibited fVIIa/TF activity by only 50% following octyl glucoside treatment. This result might suggest that 50% of octyl glucoside decryption occurs by way of a PS-independent mechanism. For 2 reasons, we believe that PS-independent mechanisms contribute little to TF decryption by octyl glucoside. First, some PS-independent activity is likely due to the direct effect of octyl glucoside alone on fVIIa/TF activity (Figure 2A). Second, the dose-dependent response of fVIIa/TF activity to annexin shown in Figure 5B never reaches an asymptote, suggesting that annexin is merely unable to saturate available PS-binding sites. The lowered affinity of annexin for PS in mixed octyl glucoside micelles (diameter < 5 nm) is consistent with other results that have shown a dependence of annexin binding and clustering on the curvature of the phospholipid-binding surface.31,40 Andree et al,31 for example, displayed that annexin V inhibited prothrombinase activity by more than 80% in large unilamellar vesicles (diameter 100-200 nm) consisting of equimolar amounts of phosphatidylserine and phosphatidylcholine but only by 20% in small unilamellar vesicles (diameter, 10-50 nm) of identical composition. The membrane curvature of an intact cell is certainly more conducive to annexin binding and clustering than that of a detergent micelle. Thus, our data do not support the existence of a PS-independent mechanism involved in the decryption of TF in HEK293 cells.
Detergent-mediated decryption of TF is a process with little physiologic relevance. The involvement of lipid rafts, however, represents a more realistic mechanism for acute physiologic control of fVIIa/TF activity. Our data suggest an indirect mechanism for TF activation in which the asymmetric distribution of PS in the plasma membrane is controlled by the lipid raft environment. In this model, intact rafts actively maintain a pool of PS in the internal leaflet and may become a conduit for transbilayer transport of PS. In an intact membrane the half time for transbilayer phospholipid flip-flop ranges from hours to days,41-43 yet in our studies MBCD decrypts TF in minutes, implying that PS transport is enzyme catalyzed. Despite early suggestions that lipid rafts were poor sources of PS,44 data suggest that lipid rafts are enriched in PS45,46 that may serve as substrate for candidate enzymes mediating transbilayer phospholipid transport, at least one of which localizes to lipid rafts.47 On the contrary, Kunzelmann-Marche et al48 recently reported that lipid raft disruption inhibits transbilayer movement of PS. With the use of HEL cells, this group showed that calcium ionophore failed to induce PS exposure and activate prothrombinase activity when added to MBCD-treated cells as compared with untreated cells. Differences between our study and that of Kunzelmann-Marche et al48 include (1) the use of a different cell type, (2) stimulation of PS exposure using calcium ionophore, and (3) the assessment of prothrombinase rather than fVIIa/TF activity as a measure of functional exposure of PS. Further study to resolve the discrepant conclusions of these studies is indicated.
Finally, the results of our studies have broad implications for the interpretation of studies using MBCD to disrupt caveolae and lipid rafts. Our study demonstrates that MBCD treatment alters the transbilayer distribution of PS in the bulk membrane environment in addition to disrupting lipid rafts. Alteration of the bulk membrane environment has the potential to alter nonraft-mediated signaling pathways, proteolytic cascades, and endocytic processes. In light of our findings, the importance of caveolae and lipid rafts in biologic processes must be cautiously estimated when based in large part on sensitivity to membrane cholesterol depletion.
Supported by a Scientist Development Grant from the American Heart Association (D.J.D.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, December 30, 2003; DOI 10.1182/blood-2003-07-2399.
We thank Drs Steven D. Carson and George J. Broze Jr for helpful discussions during the course of this project.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal