Abstract
Membrane type 1–matrix metalloproteinase (MT1-MMP) has been suggested to play an important role in angiogenesis, but the mechanisms involved remain incompletely understood. Using an in vitro model of angiogenesis in which cell migration of bovine aortic endothelial cells (BAECs) and their morphogenic differentiation into capillary-like structures on Matrigel are induced by overexpression of MT1-MMP, we show that the platelet-derived bioactive lipid sphingosine 1–phosphate (S1P) is the predominant serum factor essential for MT1-MMP–dependent migration and morphogenic differentiation activities. In the presence of S1P, MT1-MMP–dependent cell migration and morphogenic differentiation were inhibited by pertussis toxin, suggesting the involvement of Gi-protein–coupled receptor-mediated signaling. Accordingly, cotransfection of BAECs with MT1-MMP and a constitutively active Gαi2 (Q205L) mutant increased cell migration and morphogenic differentiation, whereas treatment of BAECs overexpressing MT1-MMP with antisense oligonucleotides directed against S1P1 and S1P3, the predominant S1P receptors, significantly inhibited both processes. These results demonstrate that MT1-MMP–induced migration and morphogenic differentiation involve the cooperation of the enzyme with platelet-derived bioactive lipids through S1P-mediated activation of Gαi-coupled S1P1 and S1P3 receptors. Given the important contribution of platelets to tumor angiogenesis, the stimulation of endothelial MT1-MMP function by S1P may thus constitute an important molecular event linking hemostasis to angiogenesis. (Blood. 2004;103:3020-3028)
Introduction
Angiogenesis, the formation of new vessels from pre-existing endothelium, is an essential component of pathologic conditions such as rheumatoid arthritis, diabetic retinopathies, inflammation, arteriosclerosis and tumor growth, and metastasis.1 Tumor angiogenesis is an extremely complex process in which tumor cells secrete a number of stimulatory cytokines, such as vascular endothelial growth factor (VEGF),2 that induce proliferation, migration, and survival of the endothelial cells (ECs).3 VEGF is also a potent inducer of vascular permeability, resulting in the extravasation of plasma fibrinogen in the extravascular space and in the formation of a cross-linked fibrin provisional matrix that is essential for neovascularization.4 Since new vessels are highly permeable, fibrin is continuously present into the tumor stroma and reflects the activation of the coagulation system,5 suggesting an important regulatory function of the hemostatic system in angiogenesis and tumor growth. Such a close association between human cancer and hemostasis is illustrated by the observation that cancer coagulopathies occur in more than 80% of patients with disseminated cancer and account for a significant percentage of the morbidity and mortality of this disease.6,7 The mechanisms involved in the pathogenesis of hemostatic disorders in cancer remains, however, incompletely understood but likely involve alterations in the function of some components of the coagulation and fibrinolysis systems, such as the vascular endothelium and platelets.8
Fibrin deposition at sites of neovascularization is involved in platelet adhesion and activation. Platelets contain one of the largest stores of angiogenic factors, including VEGF, platelet-derived growth factor (PDGF), transforming growth factor (TGF), interleukin-6 (IL-6),9,10 and therefore, the release of these factors may stimulate ECs and sustain angiogenesis.11 Accordingly, platelets contribute to the stimulation of EC proliferation12 and capillary-like tube formation13 in vitro, and activated platelets are closely associated with microvessels of soft tissue carcinomas in vivo.14 Activation of platelets during coagulation also results in the release of bioactive lipids such as sphingosine 1–phosphate (S1P) and lysophosphatidic acid (LPA).15,16 A role for these platelet-derived lipids in angiogenesis was initially suggested by the identification of endothelial differentiation gene 1 (EDG1)/S1P1, a gene induced during differentiation of human ECs into capillary-like structures,17 as a high-affinity receptor for S1P.18 Five members of the S1P receptor family have since been identified (S1P1-5)19 and the binding of S1P to S1P1 and S1P3 seems to account for most of the effects of this lipid on EC proliferation, migration, and formation of tubular structures in vitro.20-25 These effects of S1P are likely to contribute to neovascularization in vivo, since most EC chemoattractive activities generated during blood clotting are due to platelet-derived S1P.26
Extensive remodeling of the extracellular matrix (ECM) surrounding blood vessels by matrix metalloproteinases (MMPs) also represents an essential process underlying angiogenesis, enabling the migration and formation of new vessels.27 Among members of the MMP family, membrane type 1-MMP (MT1-MMP) is increasingly recognized as an essential enzyme involved in matrix remodeling and in the migration of a variety of cancer28-31 and endothelial32-34 cells. MT1-MMP contains a transmembrane domain that allows its localization at the cell surface35 as well as a short cytoplasmic domain that appears important for the activation of cell migration by the enzyme.28,29,36 MT1-MMP was first identified as a cellular receptor and activator for proMMP-235 but is also itself a potent matrix-degrading enzyme that proteolyses a broad spectrum of ECM proteins,37,38 including fibrin.39 MT1-MMP–dependent invasion of fibrin barriers by ECs may play an important role in angiogenesis,39 as reflected by the induction of EC migration and tubulogenesis in fibrin gels in vitro.40,41 An important role of MT1-MMP in in vivo angiogenesis is also supported by the observation that MT1-MMP–null mice failed to exhibit an angiogenic response to fibroblast growth factor 2 (FGF-2) in the corneal angiogenesis assay.42 However, the means by which MT1-MMP integrates signals from the extracellular milieu to activate these processes and induces cell locomotion and capillary-like structure formation remains poorly understood.
In this work, we investigated the potential relationship between platelet-derived angiogenic factors and MT1-MMP–induced migration and morphogenic differentiation of ECs. Our data show that MT1-MMP predominantly cooperates with S1P to stimulate EC migration and morphogenic differentiation and that these processes involve S1P-mediated activation of G-protein–mediated signaling. The identification of S1P as a major activator of MT1-MMP function in endothelial cells may thus represent a novel pathway linking hemostasis to angiogenesis.
Materials and methods
Materials
Matrigel was from BD Biosciences (Mississauga, ON, Canada). Transwell migration chambers (6.5-mm diameter, 8-μm pore size) were purchased from Costar (Cambridge, MA). S1P, LPA, and pertussis toxin (PTX) were obtained from Sigma (St Louis, MO). Recombinant human insulin-like growth factor 1 (IGF-1), PDGF-BB (PDGF B-subunit homodimer), TGF-β2, and VEGF were from R&D Systems (Minneapolis, MN). Basic FGF (bFGF) was from Upstate Biotechnology (Lake Placid, NY), epidermal growth factor (EGF) was purchased from BD Biosciences Discovery Labware (Bedford, MA), and thrombin was from Amersham Biosciences (Baie d'Urfé, QC, Canada). DMS (N, N-dimethylsphingosine) was from BIOMOL Research Laboratories (Plymouth Meeting, PA), and PTK787/ZK 222584 was provided by Novartis AG (Basel, Switzerland). All products for electrophoresis were purchased from Bio-Rad (Hercules, CA). The anti–MT1-MMP polyclonal antibody AB815 was from Chemicon (Temecula, CA). Horseradish peroxidase–conjugated donkey antirabbit secondary antibody was obtained from Jackson Immunoresearch Laboratories (Mississauga, ON, Canada). FUGENE-6 Transfection Reagent was purchased from Roche (Laval, QC, Canada). Oligofectamine, Trizol reagent, and the Superscript One-step reverse transcriptase–polymerase chain reaction (RT-PCR) kit were obtained from Invitrogen (Burlington, ON, Canada).
Cell culture
Bovine aortic endothelial cells (BAECs) and human endothelial cells from umbilical vein (HUVECs) were purchased from Clonetics (Walkersville, MD) and cultured under an air/CO2 (19:1) atmosphere. BAECs were grown in Dulbecco modified Eagle medium (DMEM) supplemented with 10% (vol/vol) heat-inactivated bovine calf serum (BCSi), 4 mM glutamine, 100 IU/mL penicillin, and 100 mg/mL streptomycin, and the cells were used up to passage 12. HUVECs were grown in endothelial cell growth medium BulletKit (EGM-2) supplemented with 2% (vol/vol) fetal bovine serum (FBS), human EGF (hEGF), hydrocortisone, VEGF, human bFGF (hFGF-B), IGF-1, ascorbic acid, heparin, gentamicin, and amphotericin-B. HUVECs were used up to the sixth passage.
Transfection method
The cDNAs encoding the human MT1-MMP and its cytoplasmic domain-deleted (CΔ20) and catalytically inactive (E240A) mutants have been described.36 The cDNAs encoding wild-type or constitutively active (Q205L) Gαi2 proteins were kindly provided by Dr Bradley Denker. Transient transfections and cotransfections of the plasmids in subconfluent BAECs were performed using the FUGENE-6 transfection reagent. All experiments using these cells were performed 40 hours after transfection.
Treatment with antisense oligonucleotides
Antisense phosphorothioate oligodeoxyribonucleotides (PTOs) against human MT1-MMP,31 S1P1,20 and S1P323 and their scrambled controls were custom synthesized based on published sequences. To reduce MT1-MMP expression, HUVECs grown to approximately 50% confluence were incubated with 200 nM of either the control or MT1-MMP antisenses, according to the manufacturer's protocol. After a 72-hour incubation, cells were used for cell migration and morphogenic differentiation assays, as described in “Morphogenic differentiation assays.”
To reduce S1P1 and S1P3 expression, BAECs were first transfected with MT1-MMP, and 16 hours after transfection the cells were treated with the antisense PTOs specific for these proteins. After a 24-hour incubation, cells were used for cell migration and morphogenic differentiation assays.
Morphogenic differentiation assays
Matrigel was added into flat-bottomed 96-well plates and allowed to gel for 20 minutes at 37°C before cell seeding. Transfected BAECs (2.5 × 104 cells) were added atop the Matrigel in serum-free media with or without the tested serum factors (10% serum [BCSi], 1 μM S1P, 10 μM LPA, 50 ng/mL VEGF, 20 ng/mL bFGF, 20 ng/mL IGF-1, 20 ng/mL TGF-β2, 20 ng/mL EGF, 20 ng/mL PDGF-BB, or 1 U/mL thrombin) and incubated at 37°C for 6 hours. In experiments using HUVECs, the cells (1.5 × 104 cells) were added atop Matrigel in the presence or in the absence of different serum factors (2% serum [FBS], 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF) and incubated at 37°C for 4 to 8 hours. For inhibition studies, cells were pretreated for 2 hours with BB94 (5 μM) or for 30 minutes with PTX (10 ng/mL), PTK787/ZK 222584 (1 μM), or DMS (1 μM).
Capillary-like structures formed by HUVECs and BAECs were examined microscopically and pictures (original magnification × 50) were taken using a Retiga 1300 camera (QImaging, Burnaby, BC, Canada) and a Zeiss Axiovert S100 microscope (Thornwood, NY). The extent to which capillary-like structures formed in the gel was quantified by analysis of digitalized images to determine the thread length of the capillary-like network, using a commercially available image analysis program (Northern Eclipse 6.0; Empix Imaging, Mississauga, ON, Canada). Results are expressed as x-fold induction compared with control and are the means of at least 2 different experiments.
Cell migration assays
BAEC and HUVEC migration were performed on transwells precoated with 0.15% gelatin. The transwells were assembled in 24-well plates and the lower chambers filled with 600 μL of media containing (or lacking) the serum factors described in “Morphogenic differentiation assays.” Transfected BAECs were harvested by trypsinization, centrifuged, resuspended in 100 μL of fresh DMEM media at 2.5 × 105 cells/mL, and inoculated into the upper chamber of each transwell.
HUVECs treated with control or antisense PTOs were harvested by trypsinization, centrifuged, resuspended in fresh DMEM media at 5 × 105 cells/mL, and 100 μL was inoculated into the upper chamber of each transwell. The lower chambers were filled with 600 μL of EGM-2 supplemented (or not) with 2% serum (FBS), 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF. For inhibition studies, cells were pretreated for 2 hours with BB94 (5 μM) or for 30 minutes with PTX (10 ng/mL), PTK787/ZK 222584 (1 μM), or DMS (1 μM). The plates were then placed at 37°C in 5% CO2/95% air for 3 hours. Cells that had migrated to the lower surface of the filters were fixed and stained with 0.1% crystal violet/20% MeOH. The migration was quantified using computer-assisted imaging, and data are expressed as the average density of migrated cells per 4 fields (original magnification × 50).
Western blot procedures and gelatin zymography
Whole-cell lysates and isolated crude membrane fractions were prepared as described previously.43 Equal amounts of protein from treated or transfected cells were resuspended in sample buffer and separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis using 9% acrylamide gels. After electrophoresis, proteins were electrotransferred to a 0.45-μm pore size polyvinylidene difluoride membrane and immunoreactive material was visualized by enhanced chemiluminescence. Activation of proMMP-2 by transfected BAECs was detected by gelatin zymography using a 7.5% polyacrylamide gel containing 1 mg/mL gelatin, as previously described.43,44
Results
S1P, LPA, and VEGF stimulate MT1-MMP–dependent morphogenic differentiation and EC migration
Bovine aortic endothelial cells (BAECs), which express low levels of MT1-MMP and which do not spontaneously form capillary-like structures on Matrigel,45 were used to determine the effect of MT1-MMP overexpression on the migration and morphogenic differentiation of ECs. BAECs were transiently transfected with MT1-MMP and subsequently seeded on Matrigel in media containing 10% serum. Under these experimental conditions, overexpression of MT1-MMP induced the formation of a well-defined capillary network (Figure 1A), indicating that MT1-MMP can confer upon these cells the capacity to form capillary-like structures. Since directional EC migration occurs in the early stages of capillary network formation,46 the effect of MT1-MMP on BAEC migration was also evaluated. As shown in Figure 1B, overexpression of MT1-MMP caused a marked increase in the migratory potential of BAECs in the presence of serum.
MT1-MMP induces morphogenic differentiation and endothelial cell migration in cooperation with serum factors. (A) BAECs were transfected with empty vector (pcDNA3.1) or with an MT1-MMP construct and allowed to recover for 40 hours. (i) Transfected cells were plated on Matrigel in serum-free media and were either left unstimulated or were stimulated with either 10% serum (BCSi), 1 μM S1P, 10 μM LPA, 50 ng/mL VEGF, 20 ng/mL bFGF, 20 ng/mL IGF-1, 20 ng/mL TGF-β2, 20 ng/mL EGF, 20 ng/mL PDGF, or 1 U/mL thrombin. Original magnification, × 50. (ii) Formation of capillary-like structures was recorded after 6 hours and quantified using a computer-based program. Results are expressed as x-fold induction ± SD of nonstimulated control (Ctl) and are the means of 3 different experiments. (B) Transfected BAECs were subjected to migration assay for 3 hours in serum-free media containing either 10% serum (BCSi), 1 μM S1P, 10 μM LPA, 50 ng/mL VEGF, 20 ng/mL bFGF, 20 ng/mL IGF-1, 20 ng/mL TGF-β2, 20 ng/mL EGF, 20 ng/mL PDGF, or 1 U/mL thrombin. Cell migration was quantified using a computer-based program. Data are expressed as x-fold induction ± SD of nonstimulated control (Ctl). Results are the means of 3 independent experiments. (C) HUVECs were pretreated for 2 hours with BB94 (5 μM) or dimethyl sulfoxide (DMSO). Cells were then harvested and plated on Matrigel in serum-free media containing either 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF. A representative of 2 independent experiments is shown. Original magnification, × 50. (D) HUVECs were treated with human MT1-MMP antisense (MT1-MMP AS) or control oligonucleotides (Ctl AS) for 72 hours. Cells were plated on Matrigel in serum-free media containing 1 μM S1P. A representative of 2 independent experiments is shown. Original magnification, × 50. The inhibition of MT1-MMP expression after 72 hours of antisense oligonucleotide treatment was monitored by Western blotting. TD indicates immunodetection. (E) (Left) HUVECs were pretreated for 2 hours with BB94 (5 μM) or DMSO and allowed to attach to filters. The medium in the lower chambers was then replaced with serum-free media containing either 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF and were incubated for 3 hours. (Right) HUVECs were treated with human MT1-MMP antisense (MT1-MMP AS) or control oligonucleotides (Ctl AS) for 72 hours and submitted to migration assay for 3 hours in the presence of 1 μM S1P. Cell migration was quantified as described and data are expressed as x-fold induction ± SD of nonstimulated control (Ctl). Results are the means of 2 independent experiments.
MT1-MMP induces morphogenic differentiation and endothelial cell migration in cooperation with serum factors. (A) BAECs were transfected with empty vector (pcDNA3.1) or with an MT1-MMP construct and allowed to recover for 40 hours. (i) Transfected cells were plated on Matrigel in serum-free media and were either left unstimulated or were stimulated with either 10% serum (BCSi), 1 μM S1P, 10 μM LPA, 50 ng/mL VEGF, 20 ng/mL bFGF, 20 ng/mL IGF-1, 20 ng/mL TGF-β2, 20 ng/mL EGF, 20 ng/mL PDGF, or 1 U/mL thrombin. Original magnification, × 50. (ii) Formation of capillary-like structures was recorded after 6 hours and quantified using a computer-based program. Results are expressed as x-fold induction ± SD of nonstimulated control (Ctl) and are the means of 3 different experiments. (B) Transfected BAECs were subjected to migration assay for 3 hours in serum-free media containing either 10% serum (BCSi), 1 μM S1P, 10 μM LPA, 50 ng/mL VEGF, 20 ng/mL bFGF, 20 ng/mL IGF-1, 20 ng/mL TGF-β2, 20 ng/mL EGF, 20 ng/mL PDGF, or 1 U/mL thrombin. Cell migration was quantified using a computer-based program. Data are expressed as x-fold induction ± SD of nonstimulated control (Ctl). Results are the means of 3 independent experiments. (C) HUVECs were pretreated for 2 hours with BB94 (5 μM) or dimethyl sulfoxide (DMSO). Cells were then harvested and plated on Matrigel in serum-free media containing either 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF. A representative of 2 independent experiments is shown. Original magnification, × 50. (D) HUVECs were treated with human MT1-MMP antisense (MT1-MMP AS) or control oligonucleotides (Ctl AS) for 72 hours. Cells were plated on Matrigel in serum-free media containing 1 μM S1P. A representative of 2 independent experiments is shown. Original magnification, × 50. The inhibition of MT1-MMP expression after 72 hours of antisense oligonucleotide treatment was monitored by Western blotting. TD indicates immunodetection. (E) (Left) HUVECs were pretreated for 2 hours with BB94 (5 μM) or DMSO and allowed to attach to filters. The medium in the lower chambers was then replaced with serum-free media containing either 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF and were incubated for 3 hours. (Right) HUVECs were treated with human MT1-MMP antisense (MT1-MMP AS) or control oligonucleotides (Ctl AS) for 72 hours and submitted to migration assay for 3 hours in the presence of 1 μM S1P. Cell migration was quantified as described and data are expressed as x-fold induction ± SD of nonstimulated control (Ctl). Results are the means of 2 independent experiments.
MT1-MMP–dependent induction of morphogenic differentiation and migration were found to be strictly dependent upon the presence of serum (Figure 1A-B), suggesting the cooperation of MT1-MMP with serum factors. In order to identify these factors, several serum components known to play a role in angiogenesis, such as S1P, LPA, VEGF, bFGF, IGF-1, TGF-β2, EGF, PDGF-BB, and thrombin, were tested for their ability to induce these MT1-MMP–dependent processes. Figure 1A shows that none of these factors were solely capable of increasing morphogenic differentiation with control cells in this system. However, the formation of capillary-like structures by MT1-MMP-transfected BAECs was markedly stimulated by S1P (1 μM) and to a lesser extent by LPA (10 μM) and VEGF (50 ng/mL). As shown in Figure 1B, S1P significantly induced basal migration of control BAECs (12.7 ± 4.1-fold increase) to a level that is similar to that observed with 10% serum (10.3 ± 2.0-fold). This stimulatory effect of S1P on migration and morphogenic differentiation was observed at physiologic concentrations (0.5 μM) and was maximal at 1 μM. LPA (3.7 ± 0.4-fold) and VEGF (1.5 ± 0.3-fold) also stimulated basal migration but their effects were much weaker than that of S1P. Importantly, a strong induction of MT1-MMP–dependent BAEC migration was observed in the presence of S1P (32.9 ± 2.3-fold) and to a lesser extent with LPA (10.4 ± 0.7-fold) and VEGF (2.5 ± 0.4-fold) (Figure 1B). There was no significant stimulation of these MT1-MMP–dependent processes by the 6 other serum factors tested (Figure 1A-B). These results strongly suggest that MT1-MMP–induced morphogenic differentiation and EC migration involve the cooperation of the enzyme with S1P, LPA, and VEGF, the strongest interaction of MT1-MMP being observed with S1P in both processes.
Role of MT1-MMP in S1P-induced morphogenic differentiation and migration of HUVECs
The cooperation of MT1-MMP with S1P, LPA, and VEGF in the induction of morphogenic differentiation and EC migration was further studied using HUVECs. As shown in Figure 1C, the addition of 1 μM S1P stimulated the formation of capillary-like structures by HUVECs, as reported previously.20,21 S1P also strongly stimulated the motility of HUVECs (8.6 ± 1.7-fold; Figure 1E). However, LPA and VEGF were weak inducers of morphogenic differentiation and cell migration in this system (Figure 1C,E). Interestingly, S1P-induced morphogenic differentiation and cell migration of HUVECs involved MMP activity, since batimastat (BB94; 5 μM), a broad-spectrum MMP inhibitor, completely abolished the formation of capillary-like structures by S1P (Figure 1C) and reduced S1P-induced migration by 44% (Figure 1E). The compound, however, had no effect on that stimulated by VEGF (Figure 1E).
The sensitivity of S1P-induced migration and differentiation of HUVECs led us to evaluate the contribution of MT1-MMP in these processes using antisense PTOs directed against this enzyme. Incubation of HUVECs in the presence of MT1-MMP antisense induced a 50% inhibition of S1P-stimulated capillary-like structure formation (Figure 1D) as well as a 52% reduction in S1P-dependent cell migration (Figure 1E). The expression of MT1-MMP was significantly diminished after treatment with the MT1-MMP antisense but not with the control PTO, as monitored by immunoblotting (Figure 1D).
S1P-, LPA-, and VEGF-induced MT1-MMP–dependent morphogenic differentiation and EC migration involve its catalytic activity and cytoplasmic domain
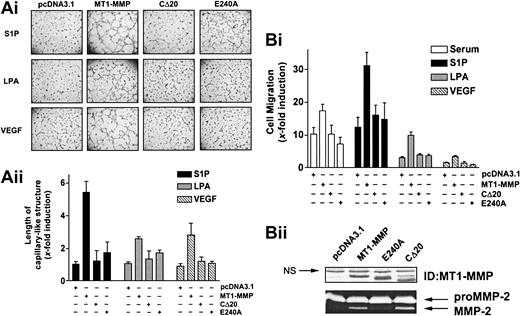
As a first step to determine the mechanisms involved in the cooperation of MT1-MMP with S1P, LPA, or VEGF, we next examined the roles of the catalytic and cytoplasmic domains of the enzyme in the induction of morphogenic differentiation and migration. BAECs were transfected with the wild-type, cytoplasmic domain-deleted (CΔ20), or catalytically inactive (E240A) MT1-MMP constructs, and the extent to which morphogenic differentiation and EC migration were induced by serum, S1P, LPA, or VEGF was monitored. Under all these stimulatory conditions, inactivation of MT1-MMP catalytic activity by site-directed mutagenesis (E240A mutant) abolished morphogenic differentiation (Figure 2A), indicating an essential role of its enzymatic activity for this process. Interestingly, removal of the cytoplasmic domain of the protein (CΔ20) also resulted in a marked reduction of capillary-like structures, suggesting a potential participation of this domain in morphogenic differentiation. Similar to these results, MT1-MMP–dependent migration of BAECs was abolished in cells expressing the CΔ20 or E240A mutants (Figure 2B). These effects were not related to reduced expression of the mutants, as monitored by immunoblotting and gelatin zymography (Figure 2B).
S1P-, LPA-, and VEGF-induced MT1-MMP–dependent morphogenic differentiation and endothelial cell migration involve the catalytic activity and cytoplasmic domain of the protein. (A) BAECs were transfected with empty vector (pcDNA3.1) or with the various MT1-MMP constructs and allowed to recover for 40 hours. (i) Transfected cells were plated on Matrigel in media and stimulated with either 10% serum (BCSi), 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF. Original magnification, × 50. (ii) Formation of capillary-like structure was recorded after 6 hours and quantified using a computer-based program. Results are expressed as x-fold induction ± SD of control (pcDNA3.1) and are the means of 2 independent experiments. (B) (i) Transfected BAECs were allowed to migrate for 3 hours in media containing either 10% serum (BCSi), 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF. Cell migration was quantified as described. Data are expressed as x-fold induction ± SD of control (pcDNA3.1). Results are the means of 2 independent experiments. (ii) Forty hours after transfection, crude membrane fractions were isolated and the expression levels of MT1-MMP constructs were monitored by Western blotting using anti–MT1-MMP polyclonal antibodies. NS indicates not specific. The ability of MT1-MMP constructs to induce proMMP-2 activation was monitored by gelatin zymography.
S1P-, LPA-, and VEGF-induced MT1-MMP–dependent morphogenic differentiation and endothelial cell migration involve the catalytic activity and cytoplasmic domain of the protein. (A) BAECs were transfected with empty vector (pcDNA3.1) or with the various MT1-MMP constructs and allowed to recover for 40 hours. (i) Transfected cells were plated on Matrigel in media and stimulated with either 10% serum (BCSi), 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF. Original magnification, × 50. (ii) Formation of capillary-like structure was recorded after 6 hours and quantified using a computer-based program. Results are expressed as x-fold induction ± SD of control (pcDNA3.1) and are the means of 2 independent experiments. (B) (i) Transfected BAECs were allowed to migrate for 3 hours in media containing either 10% serum (BCSi), 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF. Cell migration was quantified as described. Data are expressed as x-fold induction ± SD of control (pcDNA3.1). Results are the means of 2 independent experiments. (ii) Forty hours after transfection, crude membrane fractions were isolated and the expression levels of MT1-MMP constructs were monitored by Western blotting using anti–MT1-MMP polyclonal antibodies. NS indicates not specific. The ability of MT1-MMP constructs to induce proMMP-2 activation was monitored by gelatin zymography.
Overall, these data show that the induction of EC migration and their morphogenic differentiation involve the cooperation of the enzyme with serum-derived factors, predominantly S1P, and that both the catalytic activity and cytoplasmic domain of the enzyme are required for this process.
S1P- and VEGF-mediated MT1-MMP–dependent morphogenic differentiation and EC migration involve different pathways
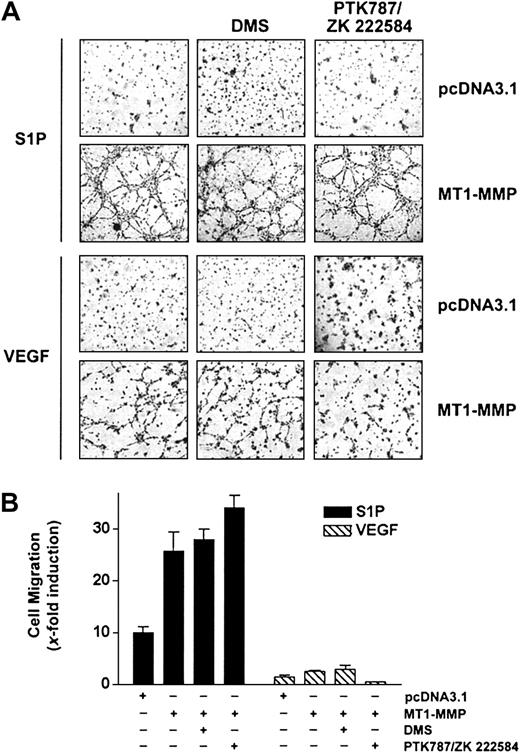
A close cooperation between the S1P- and the VEGF-mediated signaling cascades has been recently identified, based on the observation that VEGF receptor-2 (VEGFR-2) is transactivated following stimulation of ECs by S1P47 and that VEGF induces up-regulation of the S1P1 receptor48 as well as an activation of sphingosine kinase (SK), leading to an increase in the intracellular levels of S1P.49 Since MT1-MMP cooperates with both S1P and VEGF to induce migration and morphogenic differentiation, this suggests that both stimuli may use similar pathways to elicit their MT1-MMP–dependent effects. To investigate this possibility, the effects of inhibitors specific to VEGF receptors or to SK were investigated, using BAECs overexpressing MT1-MMP. As shown in Figure 3A-B, S1P-mediated migration and morphogenic differentiation of these cells were unaffected by treatment with the VEGFR inhibitor PTK787/ZK 222584,50 whereas this compound inhibited these VEGF-dependent effects. Incubation of MT1-MMP-transfected cells in the presence of DMS (N, N-dimethylsphingosine), an SK inhibitor,51 had, however, no effect on either S1P- or VEGF-induced migration and morphogenic differentiation, suggesting that a possible increase in intracellular S1P levels does not contribute to enhanced migration and differentiation induced by these agents. This lack of inhibitory activity of DMS was not related to the concentration used, since, under these conditions, DMS completely inhibited PDGF-induced migration of HUVECs, in agreement with the reported potent activation of SK activity by this cytokine52 (results not shown). Taken together, these results suggest that MT1-MMP cooperates with S1P and VEGF through distinct mechanisms and that S1P-dependent activation of migration and morphogenic differentiation does not involve the VEGF-mediated signaling pathways.
S1P-mediated MT1-MMP–dependent EC migration and morphogenic differentiation does not involve VEGF receptors or sphingosine kinase activity. (A) BAECs were transfected with empty vector (pcDNA3.1) or with MT1-MMP construct and allowed to recover for 40 hours. Transfected cells were allowed to adhere to Matrigel and PTK787/ZK 222 584 (1 μM), DMS (1 μM), or DMSO were added 30 minutes before the addition of 1 μM S1P or 50 ng/mL VEGF. Formation of capillary-like structures was recorded after 6 hours. A representative of 2 independent experiments is shown. Original magnification, × 50. (B) Transfected BAECs were allowed to attach to filters and were then pretreated with PTK787/ZK 222 584 (1 μM), DMS (1 μM), or DMSO (30 minutes). The medium in the lower chambers was replaced with serum-free medium containing either 1 μM S1P or 50 ng/mL VEGF and incubated for 3 hours. Cell migration was quantified and data are expressed as x-fold induction ± SD of nonstimulated control. Results are the means of 2 independent experiments.
S1P-mediated MT1-MMP–dependent EC migration and morphogenic differentiation does not involve VEGF receptors or sphingosine kinase activity. (A) BAECs were transfected with empty vector (pcDNA3.1) or with MT1-MMP construct and allowed to recover for 40 hours. Transfected cells were allowed to adhere to Matrigel and PTK787/ZK 222 584 (1 μM), DMS (1 μM), or DMSO were added 30 minutes before the addition of 1 μM S1P or 50 ng/mL VEGF. Formation of capillary-like structures was recorded after 6 hours. A representative of 2 independent experiments is shown. Original magnification, × 50. (B) Transfected BAECs were allowed to attach to filters and were then pretreated with PTK787/ZK 222 584 (1 μM), DMS (1 μM), or DMSO (30 minutes). The medium in the lower chambers was replaced with serum-free medium containing either 1 μM S1P or 50 ng/mL VEGF and incubated for 3 hours. Cell migration was quantified and data are expressed as x-fold induction ± SD of nonstimulated control. Results are the means of 2 independent experiments.
S1P-stimulated MT1-MMP–dependent morphogenic differentiation and EC migration proceed through heterotrimeric Gi protein and S1P1 and S1P3 receptors
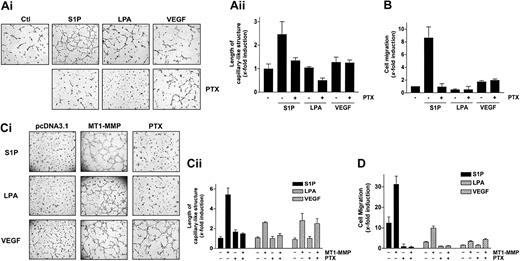
It is now well established that the S1P-mediated mitogenic and motogenic actions on ECs preferentially involve its binding to S1P1 and S1P3, the 2 receptors predominantly expressed by ECs.19,20,21 Since these receptors have been shown to be coupled to pertussis toxin–sensitive Gi proteins, the inhibitory effect of this toxin on the cooperation of S1P with MT1-MMP–dependent processes was investigated in BAECs and HUVECs. First, treatment of HUVECs with PTX almost completely abolished S1P-stimulated capillary-like structure formation (Figure 4A) as well as S1P-induced migration, in agreement with studies showing the involvement of these receptors and of PTX-sensitive Gαi proteins in these processes.20,21 By contrast, VEGF-induced migration, which involves receptor tyrosine kinase signaling,3,23 was preserved (Figure 4B). We next evaluated the importance of Gi proteins in these S1P-stimulated, MT1-MMP–dependent processes using transfected BAECs. As shown in Figure 4C-D, both S1P- and LPA-induced MT1-MMP–dependent morphogenic differentiation and EC migration were abolished by PTX, again suggesting the participation of S1P receptors that are coupled to a PTX-sensitive Gi protein. As expected, VEGF-induced morphogenic differentiation and EC migration were unaffected by PTX (Figure 4C-D), further supporting the involvement of different signaling mechanisms in this effect.
S1P- and LPA-stimulated MT1-MMP–dependent morphogenic differentiation and EC migration involve heterotrimeric Gi protein. (A) (i) After adhesion of HUVECs to Matrigel, PTX (10 ng/mL) was added 30 minutes before the addition of either 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF. Original magnification, × 50. (ii) Formation of capillary-like structures was recorded and quantified using a computer-based program. Data are expressed as x-fold induction ± SD of nonstimulated control. Results are the means of 2 independent experiments. (B) HUVECs were allowed to attach to filters and were pretreated with PTX (10 ng/mL) for 30 minutes. The medium in the lower chambers was then replaced with serum-free media containing either 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF and incubated for 3 hours. Cell migration was quantified using a computer-based program and data are expressed as x-fold induction ± SD of nonstimulated control. Results are the means of 2 independent experiments. (C) BAECs were transfected with empty vector (pcDNA3.1) or with MT1-MMP construct and allowed to recover for 40 hours. (i) Transfected cells were allowed to adhere to Matrigel and PTX (10 ng/mL) was added 30 minutes before the addition of 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF. Original magnification, × 50. (ii) Formation of capillary-like structures was recorded after 6 hours and quantified using a computer-based program. Data are expressed as x-fold induction ± SD of nonstimulated control. Results are the means of 2 independent experiments. (D) Transfected BAECs were allowed to attach to filters and were then pretreated with PTX (10 ng/mL, 30 minutes). The medium in the lower chambers was replaced with serum-free medium containing either 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF and incubated for 3 hours. Cell migration was quantified and data are expressed as x-fold induction ± SD of nonstimulated control. Results are the means of 2 independent experiments.
S1P- and LPA-stimulated MT1-MMP–dependent morphogenic differentiation and EC migration involve heterotrimeric Gi protein. (A) (i) After adhesion of HUVECs to Matrigel, PTX (10 ng/mL) was added 30 minutes before the addition of either 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF. Original magnification, × 50. (ii) Formation of capillary-like structures was recorded and quantified using a computer-based program. Data are expressed as x-fold induction ± SD of nonstimulated control. Results are the means of 2 independent experiments. (B) HUVECs were allowed to attach to filters and were pretreated with PTX (10 ng/mL) for 30 minutes. The medium in the lower chambers was then replaced with serum-free media containing either 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF and incubated for 3 hours. Cell migration was quantified using a computer-based program and data are expressed as x-fold induction ± SD of nonstimulated control. Results are the means of 2 independent experiments. (C) BAECs were transfected with empty vector (pcDNA3.1) or with MT1-MMP construct and allowed to recover for 40 hours. (i) Transfected cells were allowed to adhere to Matrigel and PTX (10 ng/mL) was added 30 minutes before the addition of 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF. Original magnification, × 50. (ii) Formation of capillary-like structures was recorded after 6 hours and quantified using a computer-based program. Data are expressed as x-fold induction ± SD of nonstimulated control. Results are the means of 2 independent experiments. (D) Transfected BAECs were allowed to attach to filters and were then pretreated with PTX (10 ng/mL, 30 minutes). The medium in the lower chambers was replaced with serum-free medium containing either 1 μM S1P, 10 μM LPA, or 50 ng/mL VEGF and incubated for 3 hours. Cell migration was quantified and data are expressed as x-fold induction ± SD of nonstimulated control. Results are the means of 2 independent experiments.
The participation of Gi-mediated signaling was also investigated using overexpressed Gαi2 proteins. A constitutively active Gαi2 mutant (Gi2-Q205L) as well as a wild-type Gαi2 protein were coexpressed with MT1-MMP in BAECs, and the extent of cell migration and morphogenic differentiation was monitored. As shown in Figure 5, cotransfection of the mutant Gαi2(Q205L) protein, but not wild-type Gαi2, along with MT1-MMP, was sufficient to induce morphogenic differentiation (Figure 5A) and cell migration (Figure 5B), even in the absence of S1P or LPA. Taken together, these results confirm that a Gi-linked signaling pathway is involved in S1P- and LPA-induced MT1-MMP–dependent morphogenic differentiation and EC migration and that this stimulatory effect likely involves the αi subunit of the G protein.
Cooperation between MT1-MMP and Gαi for the induction of morphogenic differentiation and EC migration. (A) BAECs were cotransfected with either an empty vector (pcDNA3.1) or an MT1-MMP construct, and with either pcDNA3.1, a constitutively active Gαi2 mutant (Gi2-Q205L), or wild-type Gαi2 and allowed to recover for 40 hours. Cotransfected cells were plated on Matrigel in serum-free medium. Original magnification, × 50. (ii) Formation of capillary-like structures was recorded after 6 hours and quantified using a computer-based program. Data are expressed as x-fold induction ± SD of control (pcDNA3.1). Results are the means of 2 independent experiments. (B) Cotransfected cells were allowed to migrate for 3 hours in serum-free medium. Cell migration was quantified. Data are expressed as x-fold induction ± SD of control (pcDNA3.1). Results are the means of 2 independent experiments.
Cooperation between MT1-MMP and Gαi for the induction of morphogenic differentiation and EC migration. (A) BAECs were cotransfected with either an empty vector (pcDNA3.1) or an MT1-MMP construct, and with either pcDNA3.1, a constitutively active Gαi2 mutant (Gi2-Q205L), or wild-type Gαi2 and allowed to recover for 40 hours. Cotransfected cells were plated on Matrigel in serum-free medium. Original magnification, × 50. (ii) Formation of capillary-like structures was recorded after 6 hours and quantified using a computer-based program. Data are expressed as x-fold induction ± SD of control (pcDNA3.1). Results are the means of 2 independent experiments. (B) Cotransfected cells were allowed to migrate for 3 hours in serum-free medium. Cell migration was quantified. Data are expressed as x-fold induction ± SD of control (pcDNA3.1). Results are the means of 2 independent experiments.
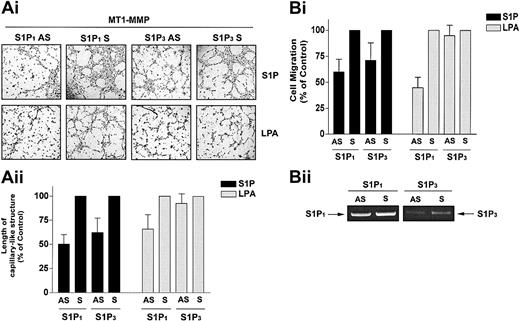
We next tested the hypothesis that the cooperation of MT1-MMP with S1P and LPA occurs through activation of S1P1 and S1P3 using MT1-MMP-transfected BAECs that were incubated with antisense oligonucleotides specific for S1P1 and S1P3. S1P1 antisense PTOs reduced S1P-stimulated MT1-MMP–dependent morphogenic differentiation and migration by 49.9% (Figure 6A) and 40.0% (Figure 6B) compared with scrambled PTOs. S1P3 antisense PTOs reduced S1P-stimulated MT1-MMP–dependent morphogenic differentiation and migration by 37.7% (Figure 6A) and 28.6% (Figure 6B). These results are consistent with prior studies showing that in HUVECs, S1P-induced migration23,24 and morphogenic differentiation20 are impaired by antisense oligonucleotides against these proteins. LPA has been shown to stimulate S1P1 as a low-affinity agonist53 but does not act via the S1P3 receptor. Accordingly, LPA-mediated MT1-MMP–dependent morphogenic differentiation and cell migration were reduced by 34.3% (Figure 6A) and 55.1% (Figure 6B) by S1P1 antisense, whereas the S1P3 antisense had no effect. Predictably, neither S1P1 nor S1P3 antisense pretreatment altered VEGF-stimulated morphogenic differentiation and EC migration (data not shown). These results indicate that specific binding of S1P to G-protein–coupled S1P1 and S1P3 receptors evokes MT1-MMP–mediated effects on EC motility and morphogenic differentiation.
S1P-stimulated MT1-MMP–dependent morphogenic differentiation and EC migration proceed through S1P1 and S1P3 receptors. (A) Sixteen hours after transfection with MT1-MMP, BAECs were incubated with either S1P1 and S1P3 antisense PTOs (S1P1 and S1P3 AS) or their respective scrambled controls (S1P1 and S1P3 S; 200 nM, 24 h). (i) Cells were plated on Matrigel in serum-free media and stimulated with 1 μM S1P or 10 μM LPA. Original magnification, × 50. (ii) Formation of capillary-like structures was recorded after 6 hours and quantified. Results are expressed as a percentage of capillary-like structure formed by cells treated with S1P1 and S1P3 antisense PTOs compared with cells treated with scrambled oligonucleotides controls. Results are the means of 2 independent experiments. (B) Sixteen hours after transfection, BAECs were incubated with the S1P1 and S1P3 antisense PTOs or their respective scrambled controls, as described above. (i) Cells were allowed to migrate for 3 hours in media containing either 1 μM S1P or 10 μM. Results are expressed as a percentage of serum factor–induced cell migration of cells treated with S1P1 and S1P3 antisense PTOs compared with cells treated with scrambled oligonucleotides controls. Results are the means of 3 independent experiments.
S1P-stimulated MT1-MMP–dependent morphogenic differentiation and EC migration proceed through S1P1 and S1P3 receptors. (A) Sixteen hours after transfection with MT1-MMP, BAECs were incubated with either S1P1 and S1P3 antisense PTOs (S1P1 and S1P3 AS) or their respective scrambled controls (S1P1 and S1P3 S; 200 nM, 24 h). (i) Cells were plated on Matrigel in serum-free media and stimulated with 1 μM S1P or 10 μM LPA. Original magnification, × 50. (ii) Formation of capillary-like structures was recorded after 6 hours and quantified. Results are expressed as a percentage of capillary-like structure formed by cells treated with S1P1 and S1P3 antisense PTOs compared with cells treated with scrambled oligonucleotides controls. Results are the means of 2 independent experiments. (B) Sixteen hours after transfection, BAECs were incubated with the S1P1 and S1P3 antisense PTOs or their respective scrambled controls, as described above. (i) Cells were allowed to migrate for 3 hours in media containing either 1 μM S1P or 10 μM. Results are expressed as a percentage of serum factor–induced cell migration of cells treated with S1P1 and S1P3 antisense PTOs compared with cells treated with scrambled oligonucleotides controls. Results are the means of 3 independent experiments.
Discussion
There is considerable evidence that EC-associated MT1-MMP plays an important role in angiogenesis, based on its ability to induce EC locomotion,32-34 invasion of fibrin matrices,39-41,54 and morphogenic differentiation of ECs into capillary-like structures in vitro.32,34 At the present time, the stimulation of these processes by MT1-MMP has been mostly studied in terms of its enzymatic activity toward fibrin substrates, whereas no information is available on the regulation of this activity by soluble angiogenic factors. Since the release of S1P by platelets during coagulation accounts for most chemoattractive activity of serum for ECs26 and S1P is a potent inducer of EC morphogenic differentiation,20-22 we suspected that MT1-MMP–mediated effect on EC migration and morphogenic differentiation may involve a cooperation of the enzyme with S1P.
To study the influence of several well-described angiogenic stimuli on MT1-MMP–dependent migration and morphogenic differentiation, we developed a model in which the enzyme is overexpressed in an EC line (BAEC) that expresses relatively low levels of MT1-MMP and that does not spontaneously form capillary-like structures in vitro. Using this model, we observed that induction of migration and morphogenic differentiation by MT1-MMP absolutely require the presence of specific serum factors, of which S1P was identified to be the most potent activator of these MT1-MMP–dependent processes, with an extent of activation similar to that observed with serum. The validity of this model was further strengthened by the observation that, in HUVECs, preincubation with the MMP inhibitor BB94 or with MT1-MMP antisense oligonucleotides significantly reduced S1P-induced cell migration and morphogenic differentiation. LPA and VEGF were also found to stimulate these MT1-MMP–dependent processes but to a much lower extent, whereas the other tested angiogenic factors were ineffective, further emphasizing the preferential cooperation of MT1-MMP with S1P. Interestingly, we observed that MT1-MMP–dependent migration and morphogenic differentiation involve, as expected, the catalytic activity of the enzyme but that this activity is not the sole determinant involved in these MT1-MMP–dependent processes, since removal of the C-terminal intracellular sequence of the enzyme (CΔ20 mutant) abolished cell migration and morphogenic differentiation to a similar extent. This requirement of the cytoplasmic domain led us to suspect that this domain may be involved in the cooperation of the enzyme with intracellular signaling events triggered by serum-derived angiogenic factors such as S1P.
The effects of S1P on ECs involve its binding to S1P1 and S1P3, 2 key members of the EDG family that are coupled to the heterotrimeric G-protein Gi.19 We provide several lines of evidence indicating that the S1P signals involved in MT1-MMP–dependent EC migration and morphogenic differentiation are transduced via the coupling of these receptors to αi. MT1-MMP–dependent BAEC migration and morphogenic differentiation induced in the presence of S1P and LPA, but not of VEGF, were abolished by preincubation with PTX, demonstrating the involvement of a Gi-coupled receptor. Furthermore, coexpression of a constitutively active mutant of Gαi2 with MT1-MMP was sufficient to induce morphogenic differentiation and migration of BAECs in the absence of stimulation by serum, S1P, or LPA. Activation of Gαi by S1P is likely to involve the binding of the lipid to both S1P1 and S1P3, since down-regulation of both receptors by antisense oligonucleotides markedly reduces MT1-MMP–dependent processes. These results indicate that MT1-MMP is closely linked to the S1P-mediated activation of S1P receptors and may thus play a major role in the angiogenic properties of this lipid. Since S1P1-null mice undergo normal vasculogenesis and angiogenesis but are severely impaired in vessel maturation due to a defect in the recruitment of mural cells to vessel walls,55 it is tempting to speculate that close cooperation between S1P-mediated signals and MT1-MMP–dependent proteolytic remodeling of the ECM could play an important role in the 3-dimensional organization of neovessels. In agreement to this, MT1-MMP was demonstrated in the newly vascular structures of a recanalized mural thrombus, indicating that MT1-MMP participates to neovascularization in vivo.41
Although S1P was found to be the predominant activator of MT1-MMP–dependent migration and morphogenic differentiation, stimulation of these processes was also observed with LPA and VEGF, raising the possibility that these angiogenic stimuli may act on the S1P-mediated signaling pathways. LPA has been shown to act as a low-affinity ligand for the S1P1 receptor53 and it is thus likely that this property is responsible for its effects, since stimulation by this lipid was significantly inhibited by antisense PTOs against S1P1. There is also recent evidence that VEGF interacts with signaling pathways triggered by S1P. For example, S1P induces activation of VEGFR-2,47 and VEGF was shown to stimulate S1P1 transcription and expression48 as well as to stimulate sphingosine kinase (SK) activity,49 leading to increased synthesis of S1P by ECs. However, these properties are very unlikely to contribute to the observed increase in migration and morphogenic differentiation induced by VEGF in cooperation with MT1-MMP, since the SK inhibitor DMS had no effect on these VEGF-mediated processes and that PTX or S1P1 antisense, which would block signaling originating from newly synthesized S1P1 or secreted S1P, did not inhibit the stimulatory effects of VEGF. Moreover, S1P-induced migration and morphogenic differentiation of MT1-MMP-transfected BAECs were not inhibited by a VEGFR inhibitor, further supporting the existence of different mechanisms involved in the coupling of MT1-MMP to these important angiogenic stimuli.
To the best of our knowledge, our results present the first evidence of control of MT1-MMP activity by receptor-mediated G-protein–dependent signaling and highlight the complex role of this enzyme in angiogenesis. The mechanisms involved in the coupling between S1P-mediated signals and MT1-MMP activity remain to be elucidated. MT1-MMP–dependent migration has been shown to involve its matrix-degrading activity,39,56,57 suggesting that the lipid induces a specific activation of the intrinsic activity of MT1-MMP toward ECM proteins or of various non-ECM proteins, including chemokines and growth factor receptors, as well as a number of surface adhesion receptors.58-60 Since cooperation between MT1-MMP and the S1P-mediated signaling events requires the catalytic activity of the enzyme, this suggests that MT1-MMP–dependent cleavage of cell-associated or ECM proteins is important for mediating morphogenic differentiation and EC migration. In addition, since the cytoplasmic domain of MT1-MMP is also required for S1P-induced EC migration and morphogenic differentiation, it is likely that S1P1- and S1P3-dependent G-protein signaling leads to activation of key signaling intermediates that result in the activation of MT1-MMP through this domain. Although this cytoplasmic region lacks motifs known to play a role in the binding and/or recruitment of signaling intermediates, it has been suggested to play an important role in the function of MT1-MMP, such as endocytosis of the protein,61,62 and the activation of the extracellular signal-related protein kinase (ERK) signaling cascade.36 Inhibition of these 2 processes upon deletion of the cytoplasmic domain were correlated with decreased cell migration,29,36,61,62 and it is thus possible that the stimulatory effect of S1P on MT1-MMP–dependent migration involves an increase in the dynamic turnover of the protein at the cell surface, resulting in the relocalization of the protein at the migrating edge of the cells.34 Whether this relocalization of MT1-MMP is a consequence of S1P activation of a number of signaling pathways, including Rho-dependent activation of integrins24 and Src family kinase–dependent tyrosine phosphorylation of key signaling intermediates,25,63 remains to be established. Work aimed at the identification of substrates cleaved by MT1-MMP in response to S1P as well as on the signaling pathways that are activated under these conditions is currently underway and should provide interesting new information on the mechanisms involved.
In summary, our results indicate that MT1-MMP–dependent activation of migration and morphogenic differentiation of ECs involves its cooperation with serum factors, predominantly S1P. During angiogenesis, the leakiness of tumor vessels and the resulting extravasated fibrin provides an ideal substratum for platelet binding, leading to their activation and to the release of bioactive lipids such as S1P and LPA15,16 and of some angiogenic cytokines, including VEGF.14 ECs are then accessible to these platelet-derived ligands at sites of neovessel formation and it is thus possible that these factors cooperate with MT1-MMP to stimulate the angiogenic process. Given the important role of the hemostatic system in the regulation of angiogenesis,7 these results suggest that activation of endothelial cell–associated MT1-MMP activity by platelet-derived angiogenic factors could represent a key molecular event linking hemostasis and angiogenesis.
Prepublished online as Blood First Edition Paper, December 11, 2003; DOI 10.1182/blood-2003-08-2968.
Supported by a grant from the Canadian Institute of Health Research (R.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We are grateful to Dr Brad Denker for his generous gift of plasmids and to Dr Georges-Etienne Rivard for helpful discussions.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal