Abstract
The most prominent cell-surface integrin α4β1 partner, a 70-kDa protein, was isolated from MOLT-4 T leukemia cells, using anti–α4β1 integrin antibody-coated beads. By mass spectrometry, this protein was identified as EWI-2, a previously described cell-surface partner for tetraspanin proteins CD9 and CD81. Wild-type EWI-2 overexpression had no effect on MOLT-4 cell tethering and adhesion strengthening on the α4β1 ligand, vascular cell adhesion molecule-1 (VCAM-1), in shear flow assays. However, EWI-2 markedly impaired spreading and ruffling on VCAM-1. In contrast, a mutant EWI-2 molecule, with a different cytoplasmic tail, neither impaired cell spreading nor associated with α4β1 and CD81. The endogenous wild-type EWI-2–CD81–α4β1 complex was fully soluble, and highly specific as seen by the absence of other MOLT-4 cell-surface proteins. Also, it was relatively small in size (0.5 × 106 Da to 4 × 106 Da), as estimated by size exclusion chromatography. Overexpression of EWI-2 in MOLT-4 cells caused reorganization of cell-surface CD81, increased the extent of CD81-CD81, CD81-α4β1, and α4β1-α4β1 associations, and increased the apparent size of CD81-α4β1 complexes. We suggest that EWI-2–dependent reorganization of α4β1-CD81 complexes on the cell surface is responsible for EWI-2 effects on integrin-dependent morphology and motility functions. (Blood. 2004;103: 3013-3019)
Introduction
The integrin α4β1 (VLA4; CD49d/CD29) is present on mononuclear leukocytes, as well as eosinophils, basophils, and some nonhematopoietic tumor cells. It mediates adhesion to the alternatively spliced CS1 region of fibronectin and to vascular cell adhesion molecule-1 (VCAM-1) on activated endothelium.1,2 Hence, α4β1 plays a key role during immune surveillance, inflammation, hematopoiesis, and melanoma metastasis, while also contributing to cell activation and survival.3,4 A null mutation in the α4 gene results in embryonic lethality in mice, demonstrating a critical role for α4β1 also during development.5 Many in vivo studies show anti-α4 antagonists having positive therapeutic effects in a variety of inflammatory disease models.3,6 For example, blocking of α4β1 function had notably positive clinical effects on Crohn disease and multiple sclerosis in humans.7,8
Studies of α4β1 integrin ligand and divalent cation binding sites,9,10 and cytoplasmic tail interactions,11 have yielded key mechanistic insights. We hypothesized that lateral associations of α4β1 with other proteins may also make critical mechanistic contributions. Here we utilize anti–α4 antibody–coated beads, and mass spectrometry to isolate and identify EWI-2 as the most prominent cell-surface partner for α4β1. EWI-2 is a cell-surface transmembrane protein with 4 immunoglobulin (Ig) domains that was previously discovered as a partner for tetraspanin proteins CD9 and CD81.12,13 Expression of EWI-2 in MOLT-4 cells caused diminished cell spreading and ruffling. In parallel, we observed formation of enlarged and extended EWI-2–CD81–α4β1 cell-surface complexes. We suggest that expanded EWI-2–CD81–α4β1 complexes limit the availability of α4β1 for participation in cell spreading/ruffling functions.
Materials and methods
Reagents and antibodies
Anti-integrin antibodies used in this study were anti-β1 TS2/16 and anti-α4 A4-PUJ1.14 Antitetraspanin mAbs were anti-CD9, ALB6 (Beckman Coulter, Hialeah, FL), anti-CD81, M38,15 and JS64.16 Also used were anti-CD98 antibody, 4F2, and anti–major histocompatibility complex (MHC) class I, W6/32. The M2 anti-FLAG epitope monoclonal antibody (mAb), horseradish peroxidase–conjugated goat antimouse polyclonal antibody and nonimmune mouse IgG were from Sigma (St Louis, MO). Monoclonal antibodies were conjugated to protein A (4F2 and W6/32) or to protein G sepharose (A4-PUJ1 and 5C11) with dimethylpilimidate (Sigma). Recombinant soluble VCAM-Ig was a gift from Dr Roy Lobb (Biogen, Cambridge, MA).
Cell culture
MOLT-4 T leukemic cells (American Type Culture Collection [ATCC], Manassas, VA) were maintained in RPMI medium with 10% fetal bovine serum (Invitrogen), 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, 10 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), and 1 mM sodium pyruvate. K562 cells were maintained in Iscove modified Dulbecco medium (IMDM) with 10% fetal bovine serum. ΦNX-ampho packaging cells (ATCC) were maintained in Dulbecco modified Eagle medium (DMEM) with 10% fetal bovine serum. Transient transfection of ΦNX-ampho cells and retroviral infection of MOLT-4 and K562 cells was performed as described.17 Stable MOLT-4 and K562 infectants were selected in medium containing 200 μg/mL zeocin (Invitrogen) and maintained as a polyclonal cell population.
Purification and identification of α4β1-associated proteins
MOLT-4 cells (4.3 × 1010) were lysed in 100 mL buffer (50 mM HEPES, pH 7.4, 150 mM NaCl, 2 mM MnCl2, 1% Brij 58, and protease inhibitors). Lysis and subsequent steps were performed at room temperature. After centrifugation (30 minutes, 14 000g), the supernatant was extensively precleared by sequential incubations with Sepharose 4B, Staphylococcus aureus (fixed cells), protein G sepharose, protein A sepharose, followed by 3 rounds of immunoprecipitation with unrelated monoclonal antibodies directly coupled to protein A or protein G sepharose. Each 1-hour incubation was followed by centrifugation for 15 minutes at 14 000g. Finally, the lysate was incubated with A4-PUJ1–coupled beads, transferred to a disposable BioRad (Hercules, CA) column, and washed extensively with lysis buffer. Bound proteins were eluted in 1% Triton X-100, 0.2% sodium dodecyl sulfate (SDS) in 50 mM HEPES, pH 7.4, 150 mM NaCl, followed by 100 mM glycine, pH 3.0. Elution fractions and remaining beads were boiled for 5 minutes in Laemmli sample buffer, proteins were resolved by 10% SDS–polyacrylamide gel electrophoresis (PAGE), and gels were stained with Coomassie Blue R. Proteins at approximately 70 kDa and all other areas of the gel containing stained proteins were excised, rinsed with 50% high-performance liquid chromatography (HPLC)–grade acetonitrile (Sigma), and stored at -70°C. Proteins were then digested and identified by mass spectrometry as described.12
Construction and expression of chimeras and fusion proteins
C-terminal FLAG-tagged EWI-2 was previously described.12 Human CD2 cDNA (provided by Dr Ellis Reinherz) was FLAG-tagged using polymerase chain reaction (PCR) primers: sense, 5′-TAAGTCGACCCTAAGATGAGCTTTCCATG-3′, and antisense 5′-GGAATTCCTACTTGTCATCGTCGTCCTTGTAATCATTAGAGGAAGGGGAC-3′. To replace EWI-2 cytoplasmic tail with CD2 tail (EWI-2–CD2 chimera) PCR primers were: EWI-2, sense, 5′-GATATCGTCGACCCACGCG-3′, and antisense, 5′-TCCTTTTGGTGATGGTACCAAGGACAG-3′; CD2, sense, 5′-CTTGGTACCATCACCAAAAGGAAAAAACAG-3′, and antisense, same as for CD2 amplification. Recombinant PCR products were digested with SalI and EcoRI and cloned into SalI/EcoRI sites of the pLXIZ retroviral vector (Clontech, Palo Alto, CA).
Generation of anti–EWI-2 antiserum
EWI-2–Fc fusion protein containing all 4 Ig domains of EWI-2 followed by an Fc fragment from mouse IgG2a was cloned into pLXIZ retroviral vector. Recombinant secreted EWI-2–Fc protein was produced in stably transduced Chinese hamster ovary (CHO)/P cells and purified on a protein A sepharose column. Rabbit polyclonal anti–EWI-2 antibody was prepared by 4 injections of 250 μg fusion protein per animal (Proteintech, Chicago, IL). A mouse IgG2a column was used to remove anti-Fc antibodies, and then anti–EWI-2 antibodies were affinity purified using an EWI-2–Fc column.
Immunoprecipitation and immunoblotting
MOLT-4 cells were washed twice with phosphate-buffered saline (PBS) and lysed in lysis buffer (20 mM HEPES, pH 7.4, 150 mM NaCl, 2 mM MgCl2, 2 mM CaCl2, 1% Brij 58 [Sigma], and protease inhibitors). In some experiments cells were biotinylated with 0.2 mg/mL sulfo-NHS-LC biotin (Pierce, Rockford, IL) in PBS for 1 hour at room temperature; biotinylation reaction was quenched by adding NH4Cl (50 mM), and cells were rinsed 3 times with PBS prior to lysis. After a 1-hour extraction at room temperature, insoluble material was removed by centrifugation, and lysates were precleared for 1 hour with protein G sepharose (Pharmacia, Uppsala, Sweden). M2 anti-FLAG agarose or specific antibodies and protein G sepharose were added, and immune complexes were collected at room temperature for 2 hours. After washing 3 times with lysis buffer, immune complexes were boiled in sample buffer, resolved by SDS-PAGE, and transferred to nitrocellulose. FLAG epitope immunoblots were performed according to the manufacturer's instructions (Sigma).
Gel chromatography
MOLT-4 lysate (0.7 mL from 5 × 106 cells) was passed over a Sepharose CL6B column (Amersham Pharmacia Biotech, Piscataway, NJ; 1 × 25 cm) equilibrated with lysis buffer at room temperature, and 0.7-mL to 0.9-mL fractions were collected. The column was calibrated with blue dextran and phenol red (to define the void volume and small molecule elution volume) and with β-galactosidase (∼ 5.4 × 105 Da).
Shear flow adhesion assay
Glass coverslips were coated with soluble recombinant human VCAM-1 (R&D Systems, Minneapolis, MN) at different concentrations (1-20 μg/mL) and blocked with DPBS, 1% human albumin. Flow assay was performed essentially as described.12,13 Briefly, cells were resuspended at 106 cells/mL in flow buffer (Dulbecco phosphate-buffered saline [DPBS], 0.1% human serum albumin). Cells were perfused at 0.5, 0.75, 1.0, and 2.0 dyne/cm2. For each experimental condition, 5 separate, random areas were analyzed (recording commenced 5 minutes after first appearance of cells; each area recorded for 15 seconds). Following recording of initial cell tethering, shear was increased sequentially to 2, 4, 8, 12, 16, and 20 dyne/cm2 and 3 coverslips per condition were analyzed to determine resistance to detachment (adhesion strengthening).
Time-lapse videomicroscopy
Acid-washed glass coverslips (22 mm2) spanning a 12-mm hole drilled in a 60-mm dish were coated overnight at 4°C with VCAM-Ig in PBS. Coverslips were then blocked for 1 hour at room temperature with serum-free medium (SFM): RPMI supplemented with 5 mg/mL cell-culture–grade bovine serum albumin (ICN Pharmaceuticals, Santa Ana, CA), 50 mM HEPES, pH 7.2, 2 mM MgCl2, 2 mM CaCl2. MOLT-4 cells transduced with vector or expressing wild-type EWI-2 or EWI-2–CD2 chimeric protein were washed twice with SFM, plated at 105 cells/dish in SFM, and maintained in a custom-built stage incubator (humidified 10% CO2, 37°C). IP lab software running on a Macintosh G4 computer drove image acquisition (1 frame/30 seconds) and controlled a VS25 electronic shutter via a Uniblitz D122 controller (Vincent Associates, Rochester, NY) during time-lapse experiments. By visual inspection, cells were judged to be spread when they were no longer phase bright and were increased in area and/or showed obvious asymmetry. The accuracy of this method was validated by quantitation of cell area using Scion Image software (Scion Corp, Frederick, MD), which confirmed that we could readily distinguish cells in which area had increased by more than 1.25-fold.
Results
Identification of EWI-2 as a major α4β1-associated protein
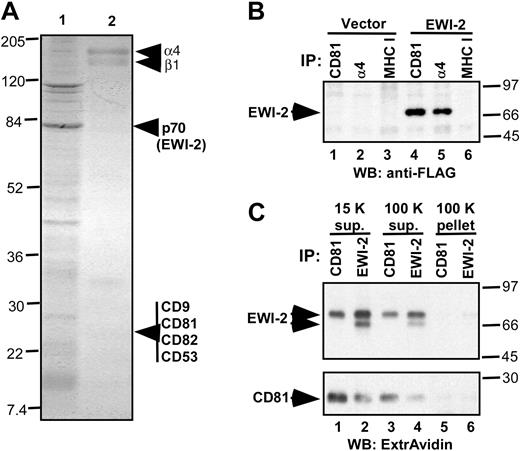
Anti–α4 antibody–coated beads were used to isolate putative α4β1-associated proteins from MOLT-4 cell lysate in relatively mild detergent (1% Brij 58) conditions. A major, approximately 70-kD protein, seen by Coomassie blue staining in Figure 1A (lane 1), was identified by mass spectrometry (Table 1). Ten distinct peptide sequences exactly matched amino acid sequences within EWI-2, a recently discovered Ig super family (SF) protein.12 EWI–2-α4β1 association was confirmed by specific coimmunoprecipitation of FLAG-tagged EWI-2 with α4β1 (Figure 1B, lane 5), but not MHC class I (lane 6). Immunoprecipitation of tetraspanin CD81 also yielded FLAG-tagged EWI-2 (Figure 1B, lane 4), consistent with recent identification of EWI-2 as a major partner for tetraspanins CD9 and CD81.12,13 Because by flow cytometry CD9 was not detected on the surface of MOLT-4 cells, CD81 may provide the major link between EWI-2 and the integrin. Tetraspanins CD53 and CD82 were not considered further because they are not major partners for EWI-2. To exclude the possibility that EWI-2–CD81 complexes occur in the context of large detergent-insoluble raftlike complexes, lysate was ultracentrifuged at 100 000g prior to CD81 and EWI-2 immunoprecipitation. The CD81–EWI-2 complex almost entirely remained in the supernatant (Figure 1C, lanes 3 and 4) and was barely detectable in the 100 000g pellet (lanes 5 and 6).
Identification of EWI-2 as a major α4β1-associated protein. (A) Coomassie blue staining of proteins eluted from anti-α4 antibody beads with Triton X-100 SDS (lane 1) and remaining on the beads (lane 2). The sharp band of approximately 110 kDa corresponds to nonspecifically associated nucleolin. (B) EWI-2 coimmunoprecipitates with α4β1 and CD81. CD81 (mAb M38, lanes 1 and 4), α4β1 (A4PUJ, lanes 2 and 5), and MHC class I (W6/32, lanes 3 and 6) were immunoprecipitated from 1% Brij 58 lysate of MOLT-4 cells stably expressing either EWI-2–FLAG or vector control. EWI-2 protein was visualized using biotinylated anti-FLAG mAb M2. (C) MOLT-4 cells stably transduced with EWI-2–flag construct were cell-surface biotinylated and lysed in 1% Brij 58. Lysate from 107 cells was centrifuged at 15 000g for 15 minutes. The resulting supernatant was used for immunoprecipitation (lanes 1 and 2), or further centrifuged for 45 minutes at 100 000g to yield 100 K supernatant (lanes 3 and 4) and 100 K pellet that was resuspended in 1% Triton X-100, 0.1% SDS (lanes 5 and 6). Immunoprecipitations with anti-CD81 (mAb M38) or anti–EWI-2 pAb were as indicated. Note that anti–EWI-2 pAb recognizes 2 forms of EWI-2 on the cell surface, whereas only one (mature) form of EWI-2 associates with CD81. Numbers to the left (panel A) and right (panels B-C) of the blots indicate the sizes of the molecular weight markers (in kDa).
Identification of EWI-2 as a major α4β1-associated protein. (A) Coomassie blue staining of proteins eluted from anti-α4 antibody beads with Triton X-100 SDS (lane 1) and remaining on the beads (lane 2). The sharp band of approximately 110 kDa corresponds to nonspecifically associated nucleolin. (B) EWI-2 coimmunoprecipitates with α4β1 and CD81. CD81 (mAb M38, lanes 1 and 4), α4β1 (A4PUJ, lanes 2 and 5), and MHC class I (W6/32, lanes 3 and 6) were immunoprecipitated from 1% Brij 58 lysate of MOLT-4 cells stably expressing either EWI-2–FLAG or vector control. EWI-2 protein was visualized using biotinylated anti-FLAG mAb M2. (C) MOLT-4 cells stably transduced with EWI-2–flag construct were cell-surface biotinylated and lysed in 1% Brij 58. Lysate from 107 cells was centrifuged at 15 000g for 15 minutes. The resulting supernatant was used for immunoprecipitation (lanes 1 and 2), or further centrifuged for 45 minutes at 100 000g to yield 100 K supernatant (lanes 3 and 4) and 100 K pellet that was resuspended in 1% Triton X-100, 0.1% SDS (lanes 5 and 6). Immunoprecipitations with anti-CD81 (mAb M38) or anti–EWI-2 pAb were as indicated. Note that anti–EWI-2 pAb recognizes 2 forms of EWI-2 on the cell surface, whereas only one (mature) form of EWI-2 associates with CD81. Numbers to the left (panel A) and right (panels B-C) of the blots indicate the sizes of the molecular weight markers (in kDa).
Peptides recovered from VLA4-associated p70 protein
Peptide . | Corresponding EWI-2 amino acids . |
|---|---|
| EVLVPEGPLYR | 29-39 |
| DTQFSYAVFK | 83-92 |
| VVAGEVQVQR | 95-104 |
| LQGDAVVLK | 105-113 |
| LQAQDAGIYECHTPSTDTR | 117-135 |
| HTHLAVSFGR | 197-206 |
| VLPDVLQVSAAPPGPR | 148-163 |
| STLQEVVGIR | 148-163 |
| LVAQLDTEGVGSLGPGYEGR | 148-163 |
| EEGVVLEAVAWLAGGTVYR | 439-457 |
Peptide . | Corresponding EWI-2 amino acids . |
|---|---|
| EVLVPEGPLYR | 29-39 |
| DTQFSYAVFK | 83-92 |
| VVAGEVQVQR | 95-104 |
| LQGDAVVLK | 105-113 |
| LQAQDAGIYECHTPSTDTR | 117-135 |
| HTHLAVSFGR | 197-206 |
| VLPDVLQVSAAPPGPR | 148-163 |
| STLQEVVGIR | 148-163 |
| LVAQLDTEGVGSLGPGYEGR | 148-163 |
| EEGVVLEAVAWLAGGTVYR | 439-457 |
EWI-2 peptides (accession no. AAL01052) recovered from the major p70 band.
EWI-2 modulates α4β1 functions in leukemic cells
The EWI-2 protein was overexpressed on MOLT-4 cells at a level approximately 4- to 5-fold higher than endogenous EWI-2, but only about 2- to 2.5-fold higher than that seen on other leukemic cell lines such as HPB-MLT and Ramos (not shown). Overexpressed EWI-2 did not alter adhesion of MOLT-4 or α4-transfected K562 cells to α4β1 substrate VCAM-1 under static or shear flow conditions. Indeed, EWI-2 overexpression did not affect either the number of cells attached, or adhesion strengthening (resistance to detachment), as measured using a variety of shear flow forces and substrate concentrations (not shown). However, in MOLT-4 cells overexpressing EWI-2 protein, it was obvious after 5 minutes that cell spreading was greatly impaired (Figure 2A) and this deficiency was maintained over at least 85 minutes (Figure 2B). Ruffling of EWI-2-transfected MOLT-4 cells on VCAM-1 was also largely abolished (supplemental video; see the Supplemental Video link at the top of the online article on the Blood website). Whereas EWI-2 had a pronounced effect on MOLT-4 spreading at low VCAM coating concentrations (eg, 0.5-2.5 μg/mL), this effect was much less obvious at a higher VCAM coating concentration (eg, 10 μg/mL; Figure 2C-D).
EWI-2 effects on α4β1-mediated cell spreading. (A) MOLT-4 cells stably transduced with either EWI-2–flag construct or vector control were plated on VCAM-Ig–coated coverslips (2.5 μg/mL). Original magnification, × 20. (B) Quantitation of results from panel A. For the supplemental time-lapse video, micrographs are taken at 30-second intervals for 65 to 85 minutes. (C) MOLT-4 spreading at different VCAM-Ig coating densities. Original magnification, × 20. (D) Quantitation of results from panel C. Percent spreading for EWI-2-transfected cells (black bars) and control cells (gray bars) is shown at different VCAM-1 coating densities. All results were reproduced in multiple independent experiments. Surface expression levels (in mean fluorescent intensity [MFI] units [control cells, EWI-2 transfectants]) were determined by flow cytometry for α4 integrin (180, 183); β1 integrin (206, 208); and CD81 (215, 210). mAbs were A4-PUJ1, TS2/16, and JS64, respectively.
EWI-2 effects on α4β1-mediated cell spreading. (A) MOLT-4 cells stably transduced with either EWI-2–flag construct or vector control were plated on VCAM-Ig–coated coverslips (2.5 μg/mL). Original magnification, × 20. (B) Quantitation of results from panel A. For the supplemental time-lapse video, micrographs are taken at 30-second intervals for 65 to 85 minutes. (C) MOLT-4 spreading at different VCAM-Ig coating densities. Original magnification, × 20. (D) Quantitation of results from panel C. Percent spreading for EWI-2-transfected cells (black bars) and control cells (gray bars) is shown at different VCAM-1 coating densities. All results were reproduced in multiple independent experiments. Surface expression levels (in mean fluorescent intensity [MFI] units [control cells, EWI-2 transfectants]) were determined by flow cytometry for α4 integrin (180, 183); β1 integrin (206, 208); and CD81 (215, 210). mAbs were A4-PUJ1, TS2/16, and JS64, respectively.
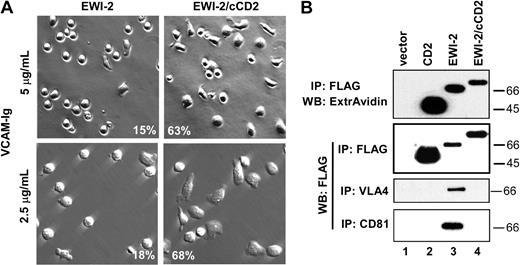
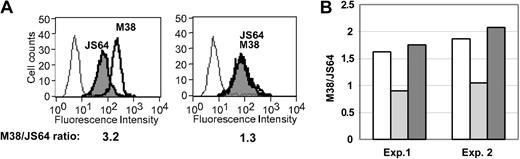
Whereas wild-type EWI-2 reduced cell spreading down to 13% to 15%, a chimeric form of EWI-2 (with CD2 tail replacing EWI-2 tail) allowed cell spreading to remain at 67% to 80% in 2 separate experiments at different VCAM densities (Figure 3A). The lack of spreading inhibition by EWI-2/cCD2 closely correlates with loss of α4β1 and CD81 association. Although the FLAG-tagged EWI-2/cCD2 chimera was well expressed at the cell surface (Figure 3B, top panel) and in the total lysate (Figure 3B, second panel), it did not coimmunoprecipitate with either α4β1 or CD81 (Figure 3B, bottom panels, lane 4). Further evidence for lack of CD81–EWI-2/cCD2 association was obtained using intact cells. In K562 cells stably transfected with α4 integrin (KA4 cells), subsequent expression of EWI-2 caused a selective decrease in exposure of the CD81 mAb M38 epitope, relative to the mAb JS64 epitope (Figure 4A). In parallel, EWI-2 became physically associated with CD81 in the cell lysate (not shown). Thus, a diminished M38/JS64 ratio for CD81 is indicative of EWI-2 association. Indeed, a decreased M38/JS64 ratio was also observed for MOLT-4 cells transfected with wild-type EWI-2 (Figure 4B). However, the ratio did not decrease upon expression of the mutant EWI-2/cCD2 in multiple experiments (Figure 4B), consistent with a lack of CD81 association. In fact, the ratio was slightly increased for MOLT-4 cells expressing EWI-2/cCD2, consistent with possible disruption of endogenous EWI-2–CD81 association.
Mutant EWI-2 with altered spreading and association properties. (A) MOLT-4 cells stably transduced with either EWI-2–FLAG or EWI-2/cCD2 were plated on VCAM-Ig–coated coverslips (5 μg/mL or 2.5 μg/mL), and photographed after 65 minutes using phase contrast (top row) or Hoffman objectives (bottom row). Percent of the spread cells (quantified as described in “Materials and methods”) is indicated in the lower part of the micrographs (original magnification, × 20). (B) MOLT-4 cells stably transduced with vector control (lane 1), CD2 (lane 2), EWI-2 (lane 3), or EWI-2/cCD2 were lysed in 1% Brij 58. Proteins were immunoprecipitated with M2 (anti-FLAG), A4PUJ (α4), or M38 (CD81) mAbs. In the first panel, cells were surface biotinylated prior to lysis. Sizes (kDa) of molecular weight markers are shown on the right.
Mutant EWI-2 with altered spreading and association properties. (A) MOLT-4 cells stably transduced with either EWI-2–FLAG or EWI-2/cCD2 were plated on VCAM-Ig–coated coverslips (5 μg/mL or 2.5 μg/mL), and photographed after 65 minutes using phase contrast (top row) or Hoffman objectives (bottom row). Percent of the spread cells (quantified as described in “Materials and methods”) is indicated in the lower part of the micrographs (original magnification, × 20). (B) MOLT-4 cells stably transduced with vector control (lane 1), CD2 (lane 2), EWI-2 (lane 3), or EWI-2/cCD2 were lysed in 1% Brij 58. Proteins were immunoprecipitated with M2 (anti-FLAG), A4PUJ (α4), or M38 (CD81) mAbs. In the first panel, cells were surface biotinylated prior to lysis. Sizes (kDa) of molecular weight markers are shown on the right.
Mutant EWI-2 fails to alter exposure of M38 epitope. (A) K562 cells transfected with α4 integrin (KA4; left) and KA4–EWI-2 (right) cells were analyzed by flow cytometry with anti-CD81 mAbs JS64 (gray histogram) and M38 (bold histogram). The open histogram shows staining with a negative control mAb. Cell-surface expression of α4β1 integrin was identical in both transfected cell lines (not shown). (B) MOLT-4 cells were analyzed as for panel A. The M38/JS64 ratio from 2 independent experiments is shown for control cells (white bars), EWI-2 (light gray bars), and EWI-2/cCD2 (dark gray bars) transfectants.
Mutant EWI-2 fails to alter exposure of M38 epitope. (A) K562 cells transfected with α4 integrin (KA4; left) and KA4–EWI-2 (right) cells were analyzed by flow cytometry with anti-CD81 mAbs JS64 (gray histogram) and M38 (bold histogram). The open histogram shows staining with a negative control mAb. Cell-surface expression of α4β1 integrin was identical in both transfected cell lines (not shown). (B) MOLT-4 cells were analyzed as for panel A. The M38/JS64 ratio from 2 independent experiments is shown for control cells (white bars), EWI-2 (light gray bars), and EWI-2/cCD2 (dark gray bars) transfectants.
EWI-2 promotes extended CD81-α4β1 complex formation
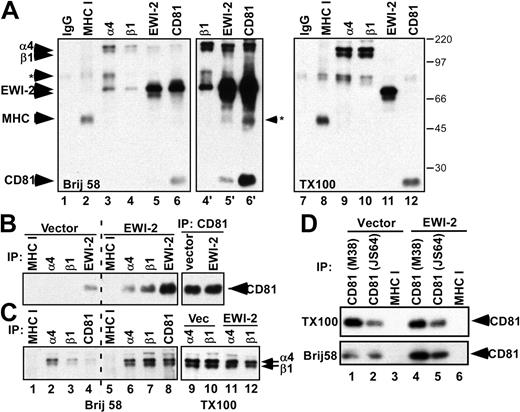
Immunoprecipitation of α4 and β1 from 1% Brij 58 detergent lysate of surface biotin-labeled vector-transfected MOLT-4 cells yielded the expected target integrin subunits, plus associated endogenous EWI-2 (Figure 5A, lanes 3, 4, 4′), while immunoprecipitation of CD81 yielded both EWI-2 and α4β1 (lane 6′). Conversely, immunoprecipitation of EWI-2 yielded associated α4β1 and CD81 (lane 5′). Addition of 1% Triton X-100 to the Brij 58 lysis buffer completely disrupted associations among α4β1, CD81, and EWI-2 (Figure 5A, compare lanes 9-12 with lanes 3-6). These results, together with Figure 1D, establish that 1% Brij 58 conditions are suitable for measuring EWI-2–CD81–α4β1 associations in the soluble phase, and without interference from other cell-surface biotin-labeled proteins. Biotinylated negative control MHC class I was well expressed (Figure 5, lanes 2 and 8) but did not associate with EWI-2, CD81, or α4β1 (lanes 2-6). Higher resolution SDS-PAGE analysis revealed that the faint band seen in lane 6′ (marked with *) does not comigrate with MHC class I (not shown). Results in Figure 5A show that α4β1, CD81, and EWI-2 are essentially the only major surface-labeled proteins that coimmunoprecipitate with each other. Hence, although Brij 58 may be less stringent than Triton X-100, the complexes observed are nonetheless highly specific. EWI-2, CD81, and α4β1 associations were observed not only using anti–EWI-2 polyclonal antibody (pAb), anti-CD81 mAb M38, anti-α4 mAb PUJ-4, and anti-β1 mAb TS2/16 (Figure 5A), but also with anti-CD81 mAb JS64 and anti-FLAG mAb to detect FLAG-EWI-2. Hence, observation of EWI-2–CD81–α4β1 complexes is highly reproducible.
EWI-2 promotes increased CD81-α4β1 complex formation. (A) MOLT-4 cells were biotinylated and lysed in 1% Brij 58 (lanes 1-6) or in 1% Brij 58 followed by preclearing and addition of 1% Triton X-100 (lanes 7-12). Lanes 4′ to 6′ are longer esposures of lanes 4 to 6. Proteins were immunoprecipitated as indicated and visualized with ExtrAvidin-HRP (Sigma). Note that CD81 is not detected in Figure 5A, lanes 3 and 4, because associated proteins shield it from biotin labeling. The background band of approximately 50 kDa precipitated with EWI-2 (lane 5′) is not MHC class I because it does not comigrate in higher resolution SDS-PAGE (not shown). Numbers to the right of panel A indicate the sizes of molecular weight markers (in kDa). (B-C) MOLT-4 cells were surface biotinylated and lysed in 1% Brij 58 (lanes 1-8) or Brij 58 followed by Triton X-100 (lanes 9-12). The indicated proteins were immunoprecipitated using the following antibodies: W6/32 (MHC I), A4PUJ (α4), TS2/16 (β1), M38 (CD81), and polyclonal anti–EWI-2 antiserum. CD81 (panel B) and α4β1 (panel C) were visualized by ExtrAvidin-HRP. (D) MOLT-4 cells were biotinylated and lysed in 1% Brij 58 or in 1% Brij 58 followed by preclearing and addition of 1% Triton X-100 (TX100). Proteins were immunoprecipitated with either anti-CD81 (M38, JS64) or anti–HLA I (W6/32) mAbs.
EWI-2 promotes increased CD81-α4β1 complex formation. (A) MOLT-4 cells were biotinylated and lysed in 1% Brij 58 (lanes 1-6) or in 1% Brij 58 followed by preclearing and addition of 1% Triton X-100 (lanes 7-12). Lanes 4′ to 6′ are longer esposures of lanes 4 to 6. Proteins were immunoprecipitated as indicated and visualized with ExtrAvidin-HRP (Sigma). Note that CD81 is not detected in Figure 5A, lanes 3 and 4, because associated proteins shield it from biotin labeling. The background band of approximately 50 kDa precipitated with EWI-2 (lane 5′) is not MHC class I because it does not comigrate in higher resolution SDS-PAGE (not shown). Numbers to the right of panel A indicate the sizes of molecular weight markers (in kDa). (B-C) MOLT-4 cells were surface biotinylated and lysed in 1% Brij 58 (lanes 1-8) or Brij 58 followed by Triton X-100 (lanes 9-12). The indicated proteins were immunoprecipitated using the following antibodies: W6/32 (MHC I), A4PUJ (α4), TS2/16 (β1), M38 (CD81), and polyclonal anti–EWI-2 antiserum. CD81 (panel B) and α4β1 (panel C) were visualized by ExtrAvidin-HRP. (D) MOLT-4 cells were biotinylated and lysed in 1% Brij 58 or in 1% Brij 58 followed by preclearing and addition of 1% Triton X-100 (TX100). Proteins were immunoprecipitated with either anti-CD81 (M38, JS64) or anti–HLA I (W6/32) mAbs.
EWI-2 not only associated with CD81 and α4β1, but also caused the formation of extended CD81-α4β1 complexes. Evidence for extended complexes (α4β1-CD81, Figure 5B; CD81-α4β1, Figure 5C; α4β1-α4β1, Figure 5C; and CD81-CD81, Figure 5D) could be seen using Brij 58 conditions. For example, EWI-2-transfected cells showed markedly increased recovery of CD81 upon precipitation of α4β1 integrin subunits (Figure 5B, compare lanes 6 and 7 with lanes 2 and 3). Likewise, EWI-2-transfected cells showed enhanced recovery of α4β1 subunits upon precipitation of CD81 (Figure 5C, compare lane 8 with lane 4). In another example, EWI-2-transfected cells showed substantially increased recovery of α4β1 subunits upon precipitation of α4 or β1 (Figure 5C, compare lanes 6 and 7 with lanes 2 and 3), and finally, EWI-2-transfected cells showed markedly increased recovery of CD81 upon precipitation of CD81 using 2 different antibodies of differing immunoprecipitation efficiency (Figure 5D, lower panel, compare lane 5 with lane 2, and lane 4 with lane 1).
The amplified α4β1-CD81, CD81-α4β1, α4β1-α4β1, and CD81-CD81 associations seen in Figure 5B-D are not simply due to more of these components being present in the initial lysate from EWI-2-transfected cells compared with vector control MOLT-4 cells. Indeed, the subsequent addition of 1% Triton X-100 to the Brij 58 lysate allowed verification that actual levels of total recoverable α4β1 and CD81 were not increased. For example, in Figure 5B, compare lanes 9 and 10; in Figure 5C, compare lanes 9 through 12; and in Figure 5B (upper panel), compare lanes 4 and 5 with lanes 1 and 2. Furthermore, by flow cytometry, comparable levels of α4 integrin and CD81 were present on both vector control MOLT-4 cells and EWI-2 transfectants (see Figure 2 legend). In additional control experiments, precipitation of endogenous EWI-2 yielded a modest amount of associated CD81 (Figure 5B, lane 4), whereas EWI-2 from stable EWI-2–expressing MOLT-4 cells yielded much more CD81 (Figure 5B, lane 8), as expected. Also, precipitation of MHC class I yielded no detectable CD81 or α4β1 (Figure 5B-C, lanes 1 and 5; Figure 5D, lanes 3 and 6).
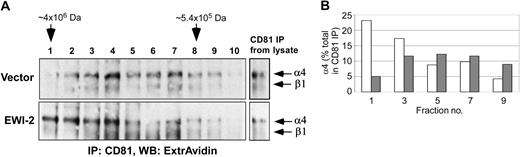
The results in Figure 5 suggest that EWI-2 strongly enhances formation of an extended network of CD81 and α4β1 interactions. To address this further, we performed gel permeation chromatography of Brij 58 lysates of MOLT-4 using Sepharose CL6B columns, followed by immunoprecipitation of CD81 complexes (Figure 6). As indicated from visualization of cell-surface biotin-labeled α4β1, CD81-α4β1 complexes from MOLT-4 vector control cells were readily retained within the included volume of the column (Figure 6A, top panel). In contrast, CD81-α4β1 complexes from EWI-2-transfected cells were considerably increased in size (Figure 6A, bottom panel), although still mostly retained within the included volume of the column. Densitometric quantitation confirms that CD81-α4β1 complexes are increased in size upon EWI-2 overexpression (Figure 6B).
Gel filtration of CD81-α4β1 complexes. (A) MOLT-4 cells (5 × 106 cells) stably transduced with vector control or EWI-2–flag were surface biotinylated and lysed in 1% Brij 58. The extracts were fractionated by size exclusion chromatography on Sepharose CL6B. CD81 was immunoprecipitated from each fraction with M38 mAb, and the associated α4β1 integrin was visualized with ExtrAvidin-HRP. (B) Quantitation of results from panel A. ▦ indicates vector; □, EWI-2.
Gel filtration of CD81-α4β1 complexes. (A) MOLT-4 cells (5 × 106 cells) stably transduced with vector control or EWI-2–flag were surface biotinylated and lysed in 1% Brij 58. The extracts were fractionated by size exclusion chromatography on Sepharose CL6B. CD81 was immunoprecipitated from each fraction with M38 mAb, and the associated α4β1 integrin was visualized with ExtrAvidin-HRP. (B) Quantitation of results from panel A. ▦ indicates vector; □, EWI-2.
At the transcriptional level, EWI-2 is differentially expressed in various lymphoid cells and tissues. Quantitation of multiple tissue expression array results revealed relative EWI-2 levels (in arbitrary units) of 4.8 (lymph node), 2.7 (thymus), 1.7 (spleen), 0.6 (bone marrow), and 0.4 (peripheral blood lymphocytes). Among leukemic cell lines, levels were 1.7 (MOLT-4), 1.8 (Daudi), 1.1 (HL60), 0.9 (K562), and 0.8 (Raji). The α4β1–CD81–EWI-2 complex was also detected in other leukemic cell lines tested, including Jurkat, Daudi, and HPB-MLT (not shown).
Discussion
Characterization of EWI-2–CD81–α4β1 complexes
Our results indicate that EWI-2 is perhaps the most prominent cell-surface protein partner for integrin α4β1 on hematopoietic cells. Indeed, EWI-2 was the major α4β1-associated cell-surface protein stained by Coomassie blue. Furthermore, all Coomassie-stained associated proteins were analyzed by mass spectrometry, but none yielded nearly as many distinct peptides as obtained from EWI-2. Cell-surface tetraspanin CD81 is also a major partner for α4β1 integrin (this study and Serru et al18 ), but it is small (∼ 22 kDa) and difficult to detect by surface labeling, especially when associated with other proteins (T.V.K. et al, our unpublished results, 1996-2004).
Several results point to tetraspanin CD81 providing a link between EWI-2 and α4β1. First, anti-CD81 antibody coimmunoprecipitated both EWI-2 and α4β1 far in excess of any other surface-labeled proteins from Brij 58 detergent lysate. Second, a mutation of EWI-2 that abolished CD81 association also abolished α4β1 association. Third, direct association of EWI-2 with CD81 has been observed in the absence of integrins,12 and specific association of α4β1 with CD81 was seen in K562 cells in the absence of EWI proteins,19 and we have not seen an EWI-2–α4β1 complex in the absence of CD81 (in Figure 5A, lanes 3 and 4, CD81 was present but not biotin labeled). Hence, the evidence strongly supports an EWI-2–CD81–α4β1 complex, with CD81 playing a central linking role.
Considering that CD81 has been reported to associate with so many different proteins,20 the EWI-2–CD81–α4β1 complex was remarkably specific. In Brij 58 detergent lysate of MOLT-4 cells, EWI-2, α4β1, and CD81 were essentially the only 3 cell-surface biotin-labeled proteins found to associate with each other. Evidence for the EWI-2–CD81–α4β1 complex was obtained using 6 different antibodies (2 each against each component). Besides observing the complex in EWI-2-transfected MOLT-4 cells and α4-transfected K562 cells, we also observed totally endogenous EWI-2–CD81–α4β1 complex in the MOLT-4, Daudi, Jurkat, and HPB-MLT lymphoid cell lines. Besides being very specific and highly reproducible, the EWI-2–CD81–α4β1 complex remained almost completely in solution upon ultracentrifugation at 100 000g, and the endogenous complex was relatively small in size (0.5 × 106 to 4 × 106) as seen by gel filtration. In contrast, another tetraspanin-integrin complex isolated in CHAPS (3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonic acid) detergent was found to be in excess of 107 Da.21 We conclude that the EWI-2–CD81–α4β1 complex is not an artifact due to incomplete Brij 58 detergent lysis, and its appearance in lysate does not depend on lipid raft/membrane vesicles being present.
Functional effects of EWI-2
Expression of EWI-2 at a level only approximately 4- to 5-fold above endogenous in MOLT-4 cells had dramatic functional consequences on cell spreading and ruffling morphology. This is not an aberrantly high level of EWI-2 expression since other leukemic cells (and perhaps also lymphoid tissues) expressed EWI-2 endogenously at nearly these levels. Despite the dramatic morphologic effects, a number of parameters did not change, indicating that EWI-2 effects were decidedly selective. Cell-surface expression levels of α4β1 and CD81 were not changed, and initial α4β1 integrin-mediated cell tethering and adhesion strengthening on immobilized VCAM-1 were unaltered by EWI-2 in either MOLT-4 or K562 cell transfectants in shear flow assays. The lack of an EWI-2 effect on α4β1 in shear flow contrasts markedly with the effects of CD81 on α4β1. CD81 was recently shown to promote rapid α4β1-mediated adhesion strengthening to VCAM-1 under shear flow.22 EWI-2 can also form specific complexes with α3β1 integrin in multiple cell lines, with CD81 and/or CD9 serving a linker function.23 Consistent with results shown here for the ruffling of MOLT-4 cells, the presence of EWI-2 inhibited α3β1-dependent motility functions of epidermoid carcinoma A431 cells.23
Mechanistic insights into EWI-2 function
The functional consequences of EWI-2 overexpression are closely linked to EWI-2–CD81–α4β1 complex formation, since a well-expressed EWI-2 mutant that does not associate with CD81-α4β1 proteins had no functional effects. Our results provide a few clues as to how EWI-2 is affecting the CD81-α4β1 complex, thereby affecting function. First, EWI-2 expression markedly enhanced the formation of extended EWI-2–CD81–α4β1 complexes, in which CD81-CD81, CD81-α4β1, and α4β1-α4β1 associations were specifically increased, and the apparent size of the complex was also increased as seen by gel filtration chromatography. We suggest that EWI-2 stabilizes and expands a network of interactions, such that immunoprecipitation of any 1 of the 3 components would increase the recovery of all 3 components. Our recent demonstration that CD81 exists as a constitutive homodimer24 adds to the potential of this molecule to play a central role in such a network. Second, EWI-2 not only altered CD81-α4β1 complexes in the cell lysate, but also reorganized CD81 on the surface of intact cells. EWI-2 caused the M38 epitope on CD81 to be selectively obscured, relative to the JS64 epitope. Importantly, mutant EWI-2 that did not associate with CD81-α4β1 in the lysate, in parallel did not decrease the M38/JS64 epitope ratio. Hence, our conclusions from biochemical and immunochemical results converge.
A consequence of altered CD81-α4β1 complex organization on the cell surface might be that downstream signaling is altered, leading to impaired cell spreading. For example, EWI-2–dependent reorganization of CD81 should be accompanied by reorganization of CD81-associated signaling proteins, such as protein kinase C (PKC)25 and phosphatidylinositol 4-kinase.26,27 Alternatively, the availability of α4β1 could become limited as it is sequestered away within extended EWI-2–CD81–α4β1 complexes. This would be particularly important at low ligand densities, when spreading is more dependent on the availability of α4β1. Indeed, the suppressive effects of EWI-2 on cell spreading were most acute at low VCAM densities. In results elsewhere, the effects of CD81 on rapid α4β1 adhesion strengthening were more obvious at higher VCAM densities.22 This provides additional evidence that effects of EWI-2 and CD81 on α4β1 function are quite distinct. We have found elsewhere that EWI-2 modifies α3β1-dependent cell motility in epidermoid carcinoma cells, while causing CD81 to be redirected to cell filopodia, into greater physical proximity with α3β1 integrin.23 These results are consistent with our conclusion here that EWI-2 is reorganizing CD81-α4β1 complexes, thereby altering downstream α4β1-dependent functions.
Implications and conclusions
There is precedent for cell-surface IgSF proteins modulating integrin-dependent functions. For example, CD47 associates with and modulates the function of integrin αVβ3,28 and also can modulate the function of α4β1.29 However, in the latter case there is no evidence for physical CD47 association with α4β1, and in neither case is there evidence for an involvement of tetraspanins. In an example of a tetraspanin modulating integrin-dependent functions, CD151 affects α3β1 and α6β1 integrins, not by altering initial ligand interactions, but by modulating subsequent adhesion strengthening.30 Now we extend those examples to include a structural and functional link between EWI-2 and α4β1. This has likely relevance to the immune system since we and others31 have found variable expression of EWI-2 mRNA and protein on various lymphoid cells and tissues, thus suggesting a proportional regulation of α4β1 integrin function in those locations.
Although EWI-2 is an IgSF protein and might possibly have a counter-receptor, our results indicate that modulation of α4β1 function is likely independent of counter-receptor activity (since our EWI-2/cCD2 mutant would presumably retain counter-receptor activity). It remains to be determined whether EWI-F, another member of the EWI protein family that can associate with CD81,28,32 will also be recruited into functionally important complexes with integrins. In conclusion, our newly described EWI-2–CD81–α4β1 complex extends our understanding of α4β1 in the lateral dimension, and thus complements other recent discoveries of functionally important proteins such as paxillin, that associate directly with the α4 cytoplasmic tail.33
Prepublished online as Blood First Edition Paper, December 18, 2003; DOI 10.1182/blood-2003-07-2201.
Supported by grants from the National Institutes of Health (GM38903) to M.E.H. and fellowships from the Leukemia Lymphoma Society (T.V.K.) and the Medical Foundation Charles A. King Trust (C.S.S.).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 2. EWI-2 effects on α4β1-mediated cell spreading. (A) MOLT-4 cells stably transduced with either EWI-2–flag construct or vector control were plated on VCAM-Ig–coated coverslips (2.5 μg/mL). Original magnification, × 20. (B) Quantitation of results from panel A. For the supplemental time-lapse video, micrographs are taken at 30-second intervals for 65 to 85 minutes. (C) MOLT-4 spreading at different VCAM-Ig coating densities. Original magnification, × 20. (D) Quantitation of results from panel C. Percent spreading for EWI-2-transfected cells (black bars) and control cells (gray bars) is shown at different VCAM-1 coating densities. All results were reproduced in multiple independent experiments. Surface expression levels (in mean fluorescent intensity [MFI] units [control cells, EWI-2 transfectants]) were determined by flow cytometry for α4 integrin (180, 183); β1 integrin (206, 208); and CD81 (215, 210). mAbs were A4-PUJ1, TS2/16, and JS64, respectively.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/8/10.1182_blood-2003-07-2201/6/m_zh80080459750002.jpeg?Expires=1765898147&Signature=K4f6IorVcTw6bGfSfAa7Pyh~RvD~hVOtF9A1EihnyaZm6tf1M9EKKJ0VzK3o3az0JWyWW4JmIKXDNE9ImOdz7M3tJco2QELJ9ZnnzCQqeJBCtBeQWE3R8oMhQuzdil5bPRZMPdXVAEtGd4WsLOHhaV8cKAII4JUGiF1ymssnlYffm5j6jLZQ5CdP2yY5cwj0yWO~gSSSDVnW94IBhONZO2fG1VsWOQ6fP8TbQEXJLFIkQJYTtc8sF0qEOg3Fx3twMMA~amnlDa9TDUfvcOdOa33oOhs~-zpal3SqGmofCnIPTNnnaKApeZEw5mQc0ijZSRHRlfzFmG5qslq~fq~Y1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal