Abstract
Signal transducer and activator of transcription-5 (STAT5) plays an important role in repopulating activity of hematopoietic stem cells (HSCs). However, the relationship of STAT5 activation with early acting cytokine receptors is not well established. We have directly compared bone marrow (BM) from mice mutant for STAT5a and STAT5b (STAT5ab-/-) with that from mice lacking c-Mpl (c-Mpl-/-), the thrombopoietin receptor. Both STAT5 and c-Mpl deficiency only mildly affected committed myeloid progenitors assayed in vitro, but STAT5ab-/- BM showed lower Gr-1+ (4.4-fold), B220+ (23-fold), CD4+ (20-fold), and Ter119+ (17-fold) peripheral blood repopulating activity than c-Mpl-/- BM against wild-type competitor in long-term repopulating assays in vivo. Direct head-to-head competitions of STAT5ab-/- BM and c-Mpl-/- BM showed up to a 25-fold reduction in STAT5ab-/- contribution. Differences affecting reconstitution of primitive c-Kit+Lin-Sca-1+ multipotent progenitor (MPP)/HSC (1.8-fold) and c-Kit+Lin-Sca-1- oligopotent progenitor BM fractions (3.3-fold) were more modest. In serial transplantation experiments, STAT5ab-/- and c-Mpl-/- BM both failed to provide consistent engraftment in tertiary hosts and could not radioprotect lethally irradiated quaternary recipients. These results indicate substantial overlap in c-Mpl-STAT5 signaling defects at the MPP/HSC level but indicate that STAT5 is activated independent of c-Mpl to promote multilineage hematopoietic differentiation. (Blood. 2004;103:2965-2972)
Introduction
Cytokine signaling pathways are important mediators of signals promoting hematopoietic stem cell (HSC) and progenitor cell survival, proliferation, and differentiation. Although deficiency of some cytokines has revealed striking redundancy, other early acting hematopoietic cytokines have more essential roles in HSCs and multipotent progenitors. Stem cell factor (SCF),1 thrombopoietin (TPO),2,3 and Flt3 ligand4 are produced by the stem cell microenvironment. Although SCF has generally been recognized as a stem cell active cytokine for many years, the role of TPO as a potent early acting cytokine has been described more recently. TPO is capable of synergistically promoting survival and proliferation of primitive hematopoietic cells in vitro.5-10 SCF and TPO play important roles in early differentiation from the HSC, and mutant mice with defects in SCF (c-Kit)11 or TPO (c-Mpl)12 receptor function show significant defects in lymphomyeloid repopulating activity. Although c-Kit and c-Mpl are not exclusive to murine HSCs, the receptors are most highly expressed in populations enriched for HSCs.13-15 In contrast, Flt3 is not expressed on murine HSCs but rather is a marker of differentiation toward the common lymphoid progenitor and associated loss of self-renewal capacity.16-18
Signal transducer and activator of transcription-5 (STAT5) can be activated by a wide range of cytokines, and STAT5a/STAT5b double mutant mice (STAT5ab-/-) have significant hematopoietic repopulating defects.19-21 The upstream factors that might use STAT5 activation to promote early hematopoietic development are thus important to define. Because TPO is produced by bone marrow (BM) stromal cells22 and is important for HSC expansion in vivo,23 we compared STAT5ab-/- BM24 with BM from mutant mice lacking expression of c-Mpl12,25,26 (c-Mpl-/-). The activation of STAT5 by c-Mpl has been documented in purified murine megakaryocytes27 and human platelets,28 as well as human erythroleukemia and mouse megakaryoblastic cell lines.29-31 Although Janus kinase-2 (JAK2)32,33 mediated STAT5 activation is dominant in c-Mpl signaling, mutation of tyrosine residues in the cytoplasmic domain of c-Mpl does not completely abrogate STAT5 activation, indicating that STAT5 can be activated by other pathways downstream of c-Mpl.34 Activation of the Shc adapter protein33 and the mitogen-activated protein kinase (MAPK) pathways35 by c-Mpl suggests the potential for redundancy in downstream signaling. Mutant c-Mpl receptors can also use the phosphatidylinositol-3 kinase (PI3-K) pathway as an alternative to STAT5 for activation of anti-apoptosis signaling.36 Despite what is known about signaling in differentiated cell types, in primitive hematopoietic precursors c-Mpl/STAT5 signaling has not been well characterized. We reasoned that STAT5-deficient phenotypes might be due primarily to defects in c-Mpl signal transduction at stages of hematopoiesis in which c-Mpl expression was highest.
The temporal pattern of c-Mpl expression throughout hematopoiesis has recently been more clearly defined. c-Mpl signaling is important for multipotent progenitor (MPP) self-renewal and expansion,37 and JAK2-mediated self-renewal of MPP fractions is dependent on STAT5.38 c-Mpl mutant mice have been shown to have defects in HSCs and committed progenitors, and it is, therefore, likely that STAT5ab-/- and c-Mpl-/- mice may have overlapping phenotypes in hematopoiesis. Our studies have directly compared STAT5-deficient and c-Mpl–deficient HSCs and progenitors to determine whether there were functional overlaps in phenotype. Competitive engraftment of oligopotent progenitor (c-Kit+lin-Sca-1-; KL) and MPP/HSC (c-Kit+lin-Sca-1+; KLS) fractions was compared with the level of engraftment in multiple peripheral blood lineages. The KLS fraction of wild-type BM includes both HSCs and MPPs, whereas the KL fraction contains the common myeloid progenitor (CMP)39 and the common lymphoid progenitor (CLP).40 The CMP and CLP are responsible for myeloid-restricted and lymphoid-restricted hematopoietic differentiation, respectively. Furthermore, we determined the level of hematopoietic development that was most severely affected by loss of STAT5 or c-Mpl signaling.
Materials and methods
Mice
c-Mpl-/- mice were obtained from Genentech (South San Francisco, CA) where they were backcrossed at least 12 generations to the C57BL/6 background (Frederic de Sauvage [Genentech, South San Francisco, CA]; personal communication, October 2003).41 STAT5ab+/- mice were from Jim Ihle (St Jude Children's Research Hospital, Memphis, TN) where they were backcrossed at least 8 generations onto the C57BL/6 background as described previously.20 STAT5ab+/- mice were mated to generate homozygous mutants for experiments, whereas c-Mpl-/- mice were fertile and could be easily bred. Because of severe perinatal lethality on the C57BL/6 background, only about 1 of 4 STAT5ab-/- pups survived to weaning age (21 days), thereby reducing the overall yield of STAT5 mutant mice to 7% of all mice genotyped. Both sets of mice expressed the common CD45.2 (Ly-5.2) marker. A congenic strain of C57BL/6 mice expressing CD45.1 (B6.SJL-PtprcaPep3b/BoyJ; Ly-5.1) was used for competitive repopulation experiments and for serial transplantations. For some competitive repopulation experiments and serial transplantations, the STAT5ab-/- mutations were crossed onto the congenic B6.C-H1b/By (HW80) background, which differs from C57BL/6 in a small region of chromosome 7. C57BL/6 mice express the hemoglobin single (Hbs) and HW80 mice express the hemoglobin diffuse (Hbd). All congenic mouse strains were initially purchased from The Jackson Laboratory (Bar Harbor, ME) and were bred in the Holland Laboratory Vivarium.
Colony-forming unit in culture (CFU-C) assays
BM cells were harvested from both hind limbs, and total nucleated cell counts were performed with the use of a hemacytometer. BM cells were plated in methylcellulose medium (Stem Cell Technologies, Vancouver, BC, Canada) in the presence of growth factors. Growth factor combinations included either 10 ng/mL recombinant murine granulocyte-macrophage colony-stimulating factor (GM-CSF) alone or a cocktail of 20 ng/mL recombinant murine interleukin 3 (IL-3), 50 ng/mL recombinant human IL-6, 50 ng/mL recombinant murine SCF, and 3 U/mL recombinant human erythropoietin (EPO). On day 7 of culture, BM colonies of more than 50 cells were counted, and for a few experiments the distribution of granulocytic, macrophage, and mixed granulocytic/macrophage colonies were scored. All hematopoietic cytokines were from R&D Systems (Minneapolis, MN) except EPO (Stem Cell Technologies).
Competitive repopulation experiments
For experiments in Figure 2, BM cells were harvested from both hind limbs of donor mice and mixed with competitor Ly-5.1 cells at a donor equivalent ratio of 5:1. The mixed BM cells were injected into lethally irradiated (11 Gy [1100 rad]) recipient Ly-5.1 mice and followed for engraftment at times up to 17 weeks. The cell dose per recipient ranged from a minimum of 2 to 3 × 106 for STAT5ab-/- to a maximum 7 to 8 × 106 for wild-type donors. Secondary BM transplantations into lethally irradiated Ly-5.1 recipients were performed at times up to 26 weeks following the primary transplantation and analyzed 12 to 16 weeks later. Secondary transplantations were 1 donor equivalent of cells per 5 recipients. For some competitive repopulation experiments (Figures 3 and 4), mixing was done at a 1:1 ratio with congenic competitor cells from HW80 mice. Transplant recipients were then analyzed by hemoglobin electrophoresis 12 to 13 weeks later for the relative amounts of Hbs and Hbd present.
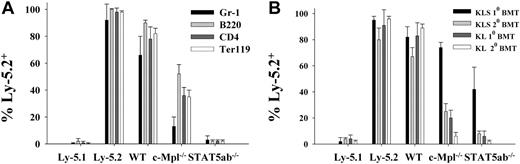
Competitive long-term repopulating ability of wild-type, c-Mpl-/-, and STAT5ab-/- BM cells in vivo. (A) BM cells from Ly-5.2 background wild-type, c-Mpl-/-, or STAT5ab-/- mice were collected from 1 donor mouse (experiment 1) or 2 donor mice (experiment 2) and mixed 5:1 with wild-type BM cells from congenic Ly-5.1 mice and injected into lethally irradiated hosts (Ly-5.1). Seventeen weeks later recipient mice were analyzed by FACS for Ly-5.2+ cells in multiple peripheral blood hematopoietic lineages (Gr-1, myeloid; B220, B-lymphocyte; CD4, T-lymphocyte; and Ter119, erythroid progenitor) from 3 recipients (experiment 1) and 3 recipients (experiment 2). The results of experiment 1 and experiment 2 (n = 6) were averaged. Shown is the percentage of Ly-5.2+ cells in multiple lineages of primary recipients, representing 3 donor mice from 2 separate experiments. The results of total Ly-5.2+ cells in all 10 primary recipients were similar to the multilineage analysis (data not shown). At 26 weeks (experiment 1) and 17 weeks (experiment 2), these mice were killed, and the BM cells were injected into secondary Ly-5.1 recipients, and after 12 to 16 additional weeks the engraftment in the total peripheral blood mononuclear fraction was determined by FACS (described in “Results”). (B) At 17 weeks after transplantation, a portion of the BM from the second of the 2 competitive repopulation experiments was harvested from the primary mice that received transplants and used for analysis of the relative engraftment in BM populations enriched for c-Kit expression. Ly-5.1–negative control and Ly-5.2–positive controls that did not receive transplants are shown on the left for both peripheral blood and BM analyses. Error bars indicate standard deviation.
Competitive long-term repopulating ability of wild-type, c-Mpl-/-, and STAT5ab-/- BM cells in vivo. (A) BM cells from Ly-5.2 background wild-type, c-Mpl-/-, or STAT5ab-/- mice were collected from 1 donor mouse (experiment 1) or 2 donor mice (experiment 2) and mixed 5:1 with wild-type BM cells from congenic Ly-5.1 mice and injected into lethally irradiated hosts (Ly-5.1). Seventeen weeks later recipient mice were analyzed by FACS for Ly-5.2+ cells in multiple peripheral blood hematopoietic lineages (Gr-1, myeloid; B220, B-lymphocyte; CD4, T-lymphocyte; and Ter119, erythroid progenitor) from 3 recipients (experiment 1) and 3 recipients (experiment 2). The results of experiment 1 and experiment 2 (n = 6) were averaged. Shown is the percentage of Ly-5.2+ cells in multiple lineages of primary recipients, representing 3 donor mice from 2 separate experiments. The results of total Ly-5.2+ cells in all 10 primary recipients were similar to the multilineage analysis (data not shown). At 26 weeks (experiment 1) and 17 weeks (experiment 2), these mice were killed, and the BM cells were injected into secondary Ly-5.1 recipients, and after 12 to 16 additional weeks the engraftment in the total peripheral blood mononuclear fraction was determined by FACS (described in “Results”). (B) At 17 weeks after transplantation, a portion of the BM from the second of the 2 competitive repopulation experiments was harvested from the primary mice that received transplants and used for analysis of the relative engraftment in BM populations enriched for c-Kit expression. Ly-5.1–negative control and Ly-5.2–positive controls that did not receive transplants are shown on the left for both peripheral blood and BM analyses. Error bars indicate standard deviation.
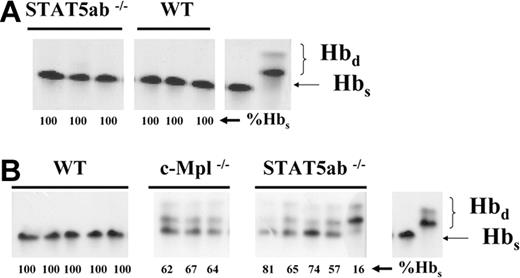
Competitive repopulation of congenic STAT5ab-/- mouse strains against each other. To generate mice that could be use for a direct head-to-head competitive repopulation assay against c-Mpl-/- BM cells, a congenic strain of the STAT5ab-/- mouse was first generated. The C57BL/6 background STAT5ab-/- mouse was crossed onto the HW80 congenic background. To determine whether the long-term repopulating HSC phenotype of the HW80 background mouse was the same as the original C57BL/6 background, head-to-head competitive repopulation experiments were set up by mixing 1:1 donor BM cells from each mouse strain. Shown are the results of 3 separate experiments in which mice were bled 16, 11, and 16 weeks, respectively, following transplantation and packed red blood cells were analyzed by hemoglobin electrophoresis for the relative contribution of Hbd and Hbs. Control samples were run with each experiment to demonstrate the typical bands for Hbd and Hbs. For experiment 2, competitions were also set up between STAT5ab-/- or c-Mpl-/- BM (Hbs) and wild-type BM (Hbd). For experiment 3 wild-type BM grafts (2) on either strain also were competed against each other. Each experiment was initiated from 1 to 2 donor mice and all recipient mice are shown. The %Hbs for each individual mouse is shown below each lane.
Competitive repopulation of congenic STAT5ab-/- mouse strains against each other. To generate mice that could be use for a direct head-to-head competitive repopulation assay against c-Mpl-/- BM cells, a congenic strain of the STAT5ab-/- mouse was first generated. The C57BL/6 background STAT5ab-/- mouse was crossed onto the HW80 congenic background. To determine whether the long-term repopulating HSC phenotype of the HW80 background mouse was the same as the original C57BL/6 background, head-to-head competitive repopulation experiments were set up by mixing 1:1 donor BM cells from each mouse strain. Shown are the results of 3 separate experiments in which mice were bled 16, 11, and 16 weeks, respectively, following transplantation and packed red blood cells were analyzed by hemoglobin electrophoresis for the relative contribution of Hbd and Hbs. Control samples were run with each experiment to demonstrate the typical bands for Hbd and Hbs. For experiment 2, competitions were also set up between STAT5ab-/- or c-Mpl-/- BM (Hbs) and wild-type BM (Hbd). For experiment 3 wild-type BM grafts (2) on either strain also were competed against each other. Each experiment was initiated from 1 to 2 donor mice and all recipient mice are shown. The %Hbs for each individual mouse is shown below each lane.
Direct head-to-head competitive repopulation of STAT5ab-/- and c-Mpl-/- BM grafts. (A) Because each STAT5ab-/- BM graft was equally competitive as shown in Figure 3, the HW80 background STAT5ab-/- BM graft was competed 1:1 with c-Mpl-/- BM in 3 separate competitive repopulation experiments. The results of 3 experiments are shown, with each experiment being initiated by using bone marrow cells from one donor STAT5ab-/- and one donor c-Mpl-/- mouse. Mice received transplants and were analyzed 13, 12, and 12 weeks later, respectively, for the relative contribution of Hbd and Hbs in circulating peripheral red blood cells. (B) Experiment 3 also included a second competitive repopulation of c-Mpl-/- BM with wild-type BM as was also shown in Figure 3. (C) Experiment 3 was also extended further by doing a secondary transplantation into lethally irradiated recipients and analysis of engraftment by hemoglobin electrophoresis 17 weeks later. Shown on the right of all sets of gels are positive controls for the Hbs and Hbd bands. The %Hbs for each individual mouse is shown below each lane.
Direct head-to-head competitive repopulation of STAT5ab-/- and c-Mpl-/- BM grafts. (A) Because each STAT5ab-/- BM graft was equally competitive as shown in Figure 3, the HW80 background STAT5ab-/- BM graft was competed 1:1 with c-Mpl-/- BM in 3 separate competitive repopulation experiments. The results of 3 experiments are shown, with each experiment being initiated by using bone marrow cells from one donor STAT5ab-/- and one donor c-Mpl-/- mouse. Mice received transplants and were analyzed 13, 12, and 12 weeks later, respectively, for the relative contribution of Hbd and Hbs in circulating peripheral red blood cells. (B) Experiment 3 also included a second competitive repopulation of c-Mpl-/- BM with wild-type BM as was also shown in Figure 3. (C) Experiment 3 was also extended further by doing a secondary transplantation into lethally irradiated recipients and analysis of engraftment by hemoglobin electrophoresis 17 weeks later. Shown on the right of all sets of gels are positive controls for the Hbs and Hbd bands. The %Hbs for each individual mouse is shown below each lane.
Flow cytometry
For competitive repopulation experiments, some BM cells were used for fluorescence activated cell sorting (FACS) analysis. Congenic mice that received transplants were analyzed as previously described.19,20 For 4-color analyses of BM fractions and Ly-5.2 on the BD LSR (BD Biosciences, San Jose, CA), the following conjugated antibodies were used: allophycocyanin (APC)–conjugated antibody to c-Kit, biotin-conjugated antibody to CD45.2 (Ly-5.2) followed by secondary streptavidin-phycoerythrin-Cy5 conjugate (SA-PE-Cy5), fluorescein isothiocyanate (FITC)–conjugated Ly-6A/E (Sca-1), PE-conjugated lineage antibodies.19 Lineage-negative cells were gated for c-Kit+Sca-1+ (KLS) or c-Kit+Sca-1- (KL) fractions.13 Events were collected for Ly-5.2 expression for each sample within the gates defined for KLS (range, 84-358 events; 0.06% ± 0.04% of total cells) or KL (range, 1210-6641 events; 4.5% ± 3.0% of total cells). BM cells from Ly-5.1 and Ly-5.2 control mice were included with each analysis, and the averages are reported. Other analyses for serial transplantation experiments were for Ly-5.2+ cells in the Ly-5.1+ host background. The percentage of peripheral blood Ly-5.2 donor engraftment in Ly-5.1 recipient mice was quantitated by using a FITC-conjugated CD45.2 (anti–Ly-5.2) antibody in combination with the PE-conjugated lineage antibodies. Sorting for Ly-5.2+ BM cells in one experiment was done by using a FACSVantage SE/FACSDiVa flow cytometer (BD Biosciences) equipped with an INNOVA 70C laser (BD Biosciences) providing 488 nm of excitation, at 70 mW output power. All antibodies for these studies were obtained from BD Pharmingen (San Diego, CA).
Serial transplantation experiments
For experiments in Figures 5, 6, 7, BM cells were harvested from both hind limbs of donor mice and transplanted into lethally irradiated primary hosts at a ratio of 1 donor equivalent to 5 recipients. All successive transplantations were also done into lethally irradiated recipients (11 Gy [1100 rad]) at the same 1:5 ratio. For experiment 1, successive transplantations were 16, 30, and 12 weeks apart. For experiment 2, successive transplantations were 12, 13, and 12 weeks apart. For experiment 3, successive transplantations were 17, 8, and 12 weeks apart. Survival of mice following each transplantation was recorded, and the relative level of engraftment was determined by either hemoglobin electrophoresis or FACS depending on the specific donor/recipient used. Experiment 1 used Ly-5.1 hosts, whereas experiments 2 and 3 used HW80 hosts. The results obtained were consistent for each set of experiments, regardless of background of the lethally irradiated host.
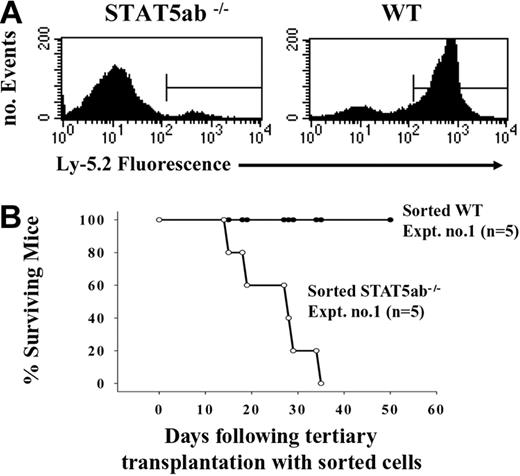
Analysis of engraftment and survival of Ly-5.1 hosts from experiment 1 receiving tertiary transplants of wild-type and STAT5ab-/- BM cells. (A) BM cells that were harvested from 2 Ly-5.2 wild-type and 2 STAT5ab-/- mice were serially transplanted into lethally irradiated Ly-5.1 recipients. Some of the mice that received secondary transplants were killed, and the BM was injected into lethally irradiated Ly-5.1 tertiary hosts. The mice were bled 30 weeks following tertiary transplantation, and the peripheral blood leukocytes were analyzed by FACS for the expression of Ly-5.2 or Ly-5.1. The percentage of donor Ly-5.2+ cells for representative recipients of wild-type (WT) or STAT5ab-/- BM cells is shown on the x-axis. Gates were defined on the basis of the forward and side scatter characteristics for total viable nucleated cells. (B) Because the engraftment levels were low in tertiary recipients of STAT5ab-/- BM cells, the BM cells from the remaining mice that received secondary transplants were collected and sorted for Ly-5.2+ cells. These cells were then injected into lethally irradiated Ly-5.1 hosts, and survival of recipient mice was determined by monitoring mice daily. Shown are the percentages of the total number of mice surviving (y-axis) at the particular time following tertiary transplantation (x-axis).
Analysis of engraftment and survival of Ly-5.1 hosts from experiment 1 receiving tertiary transplants of wild-type and STAT5ab-/- BM cells. (A) BM cells that were harvested from 2 Ly-5.2 wild-type and 2 STAT5ab-/- mice were serially transplanted into lethally irradiated Ly-5.1 recipients. Some of the mice that received secondary transplants were killed, and the BM was injected into lethally irradiated Ly-5.1 tertiary hosts. The mice were bled 30 weeks following tertiary transplantation, and the peripheral blood leukocytes were analyzed by FACS for the expression of Ly-5.2 or Ly-5.1. The percentage of donor Ly-5.2+ cells for representative recipients of wild-type (WT) or STAT5ab-/- BM cells is shown on the x-axis. Gates were defined on the basis of the forward and side scatter characteristics for total viable nucleated cells. (B) Because the engraftment levels were low in tertiary recipients of STAT5ab-/- BM cells, the BM cells from the remaining mice that received secondary transplants were collected and sorted for Ly-5.2+ cells. These cells were then injected into lethally irradiated Ly-5.1 hosts, and survival of recipient mice was determined by monitoring mice daily. Shown are the percentages of the total number of mice surviving (y-axis) at the particular time following tertiary transplantation (x-axis).
Analysis of engraftment of HW80 hosts from experiments 2 and 3 receiving tertiary transplants of wild-type, STAT5ab-/-, or c-Mpl-/- BM cells. BM cells harvested from 2 wild-type C57BL/6 (Hbs) and 2 STAT5ab-/- (Hbs) mice were serially transplanted into lethally irradiated HW80 recipients (Hbd) for both experiment 2 (A) and experiment 3 (B). Twelve weeks following tertiary transplantation, mice were bled, and the red blood cells were analyzed by hemoglobin electrophoresis for the relative contribution of Hbs and Hbd to the peripheral blood engraftment. In addition to the STAT5ab-/- and wild-type BM grafts, experiment 3 also included mice receiving c-Mpl-/- BM cells collected from 2 donor mice. Shown on the right of all sets of gels are positive controls for the Hbs and Hbd bands. The %Hbs for each individual mouse is shown below each lane.
Analysis of engraftment of HW80 hosts from experiments 2 and 3 receiving tertiary transplants of wild-type, STAT5ab-/-, or c-Mpl-/- BM cells. BM cells harvested from 2 wild-type C57BL/6 (Hbs) and 2 STAT5ab-/- (Hbs) mice were serially transplanted into lethally irradiated HW80 recipients (Hbd) for both experiment 2 (A) and experiment 3 (B). Twelve weeks following tertiary transplantation, mice were bled, and the red blood cells were analyzed by hemoglobin electrophoresis for the relative contribution of Hbs and Hbd to the peripheral blood engraftment. In addition to the STAT5ab-/- and wild-type BM grafts, experiment 3 also included mice receiving c-Mpl-/- BM cells collected from 2 donor mice. Shown on the right of all sets of gels are positive controls for the Hbs and Hbd bands. The %Hbs for each individual mouse is shown below each lane.
Analysis of survival of HW80 hosts from experiments 2 and 3 receiving transplants of wild-type, STAT5ab-/-, or c-Mpl-/- BM cells. For quaternary transplantations resulting from experiments 2 and 3, the survival of mice was not 100%. Shown are the results of daily monitoring of survival for mice from the quaternary transplantations. The percentage of survival (y-axis) is plotted against the number of days since injection into lethally irradiated quaternary hosts.
Analysis of survival of HW80 hosts from experiments 2 and 3 receiving transplants of wild-type, STAT5ab-/-, or c-Mpl-/- BM cells. For quaternary transplantations resulting from experiments 2 and 3, the survival of mice was not 100%. Shown are the results of daily monitoring of survival for mice from the quaternary transplantations. The percentage of survival (y-axis) is plotted against the number of days since injection into lethally irradiated quaternary hosts.
Densitometry
Dried transparent cellulose acetate hemoglobin electrophoresis gels were digitized by using a ScanJet IIcx/T scanner (Hewlett Packard, Palo Alto, CA) and analyzed by using ImageQuant software (Molecular Dynamics, Sunnyvale, CA). The relative percentage of Hbs and Hbd was determined.
Statistical analyses
P values were calculated by a paired t test by using either the statistical analysis function of Sigma Plot v8.0 (SPSS Science, Chicago, IL) or InStat for Windows version 1.5 (The University of Reading, United Kingdom).
Results
c-Mpl-/- and STAT5ab-/- mice have overlapping and nonoverlapping phenotypes in HSCs and progenitors
The roles of STAT5 and c-Mpl in hematopoietic function have previously been studied individually. However, the relative contribution of each to hematopoiesis at various stages of development has not been defined. To better characterize STAT5 and c-Mpl deficiency in mutant mice, we obtained backcrossed mice in which the mutations were both on the C57BL/6 background. This background was optimal for HSC studies requiring transplantation because of the availability of congenic strains for competitive repopulation, and the common background permitted direct comparison of STAT5ab-/- and c-Mpl-/- BM grafts. To initially characterize the c-Mpl-/- mouse for a typical phenotype, blood was collected for peripheral hematology analyses. Importantly, platelet numbers on the c-Mpl-/- C57BL/6 background were 114 000 ± 57 160/μL, which was 9% (P = .0002) of wild-type values of 1 246 800 ± 385 517/μL and consistent with published results.26
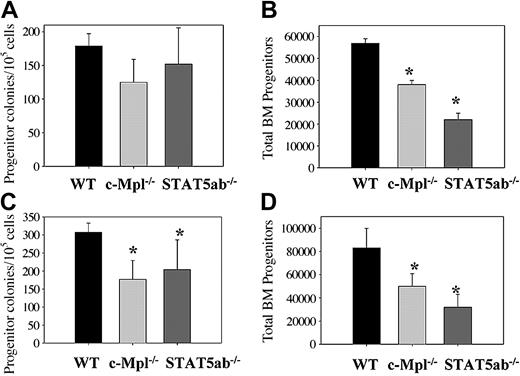
To assess committed myeloid progenitor proliferation and number, BM cells were plated into methylcellulose cultures, and the total number of colonies was determined 7 days later. Wild-type, c-Mpl-/-, and STAT5ab-/- BM cells were stimulated with GM-CSF (GM) (Figure 1A) or IL-3, IL-6, SCF, and EPO (IL36SE) (Figure 1C). The different cytokine cocktails were used to control for possible alterations in erythroid colony formation that could indirectly affect the number of myeloid colonies. Relative to wild-type BM cells, a significant reduction was observed in c-Mpl-/- colony frequency stimulated with IL36SE (1.7-fold; P = .0007) but not GM. A similar difference was also observed between wild-type and STAT5ab-/- myeloid progenitor frequency in IL36SE (1.5-fold; P = .03) but not GM. The absolute number of responsive progenitors per hind limb was also significantly decreased 1.5- to 1.7-fold for c-Mpl-/- (P = .004 for IL36SE, P = .0003 for GM) and 2.6-fold for STAT5ab-/- (P = .0005 for IL36SE, P = .0001 for GM) mice relative to wild-type controls irrespective of the cytokines used (Figure 1B,D). No difference in distribution between granulocytic, macrophage, and mixed colonies was observed (data not shown). Although the absolute number of STAT5ab-/- BM CFU-Cs was significantly lower than c-Mpl-/- CFU-Cs when stimulated with GM-CSF (1.7-fold; P = .0015), neither knockout had large defects in the absolute number of committed myeloid CFU-C progenitors relative to wild type. Progenitor level defects were also similar to the previously reported results on the 129/Sv background.12,26 The results of the colony-forming ability assays suggested that the primary defects were occurring at an earlier stage of hematopoietic development than the committed myeloid progenitor cell.
Colony-forming ability of wild-type, c-Mpl-/-, and STAT5ab-/- BM cells stimulated with hematopoietic cytokines in vitro. BM cells were harvested from both hind limbs of wild-type and mutant mouse strains. The total BM cellularity was determined to allow for comparison of the absolute number versus the frequency of colony-forming units in culture (CFU-Cs). The frequency of CFU-Cs assayed in methylcellulose was multiplied by the total BM cellularity to derive the absolute number of BM CFU-Cs per both hind limbs. (A) BM cells from wild-type (n = 3), c-Mpl-/- (n = 3), and STAT5ab-/- (n = 3) mice were stimulated with GM-CSF. The number of CFU-Cs per 1 × 105 cells plated is shown. (B) Shown is the absolute number of BM myeloid progenitors counted for the cells in panel A and normalized for the total BM cellularity. (C) BM cells from wild-type (n = 5), c-Mpl-/- (n = 6), and STAT5ab-/- (n = 5) mice were stimulated with IL-3, IL-6, SCF, and EPO. The number of CFU-Cs per 1 × 105 cells plated is shown. (D) Shown is the absolute number of BM myeloid progenitors counted for the cells in panel C and normalized for the total BM cellularity. *P ≤ .05 in comparison with wild-type BM cells. CFU-C counts from individual mice were derived from the average of duplicate plates, and the error bars represent the standard deviation.
Colony-forming ability of wild-type, c-Mpl-/-, and STAT5ab-/- BM cells stimulated with hematopoietic cytokines in vitro. BM cells were harvested from both hind limbs of wild-type and mutant mouse strains. The total BM cellularity was determined to allow for comparison of the absolute number versus the frequency of colony-forming units in culture (CFU-Cs). The frequency of CFU-Cs assayed in methylcellulose was multiplied by the total BM cellularity to derive the absolute number of BM CFU-Cs per both hind limbs. (A) BM cells from wild-type (n = 3), c-Mpl-/- (n = 3), and STAT5ab-/- (n = 3) mice were stimulated with GM-CSF. The number of CFU-Cs per 1 × 105 cells plated is shown. (B) Shown is the absolute number of BM myeloid progenitors counted for the cells in panel A and normalized for the total BM cellularity. (C) BM cells from wild-type (n = 5), c-Mpl-/- (n = 6), and STAT5ab-/- (n = 5) mice were stimulated with IL-3, IL-6, SCF, and EPO. The number of CFU-Cs per 1 × 105 cells plated is shown. (D) Shown is the absolute number of BM myeloid progenitors counted for the cells in panel C and normalized for the total BM cellularity. *P ≤ .05 in comparison with wild-type BM cells. CFU-C counts from individual mice were derived from the average of duplicate plates, and the error bars represent the standard deviation.
To examine whether defects in more primitive levels of hematopoiesis could be quantitated in defined populations of cells in the context of a competitive repopulation experiment, BM cells were harvested from donor mice and mixed 5:1 with competitor Ly-5.1 BM cells. Recipient mice were bled 17 weeks later, and nucleated peripheral blood cells were examined by FACS. Two independent experiments were performed, and a significant reduction in repopulating potential from the c-Mpl-/- and STAT5ab-/- BM was observed (Figure 2A). Ly-5.1/5.2 positive and negative controls that did not receive transplants were included in the FACS to demonstrate the specificity for the antibody staining in all groups. Interestingly, STAT5ab-/- BM showed much greater competitive repopulating defects than c-Mpl-/- BM in reconstitution of all peripheral blood lineages (Gr-1+, 4.4-fold, P = .0092; B220+, 23-fold, P < .0001; CD4+, 20-fold, P < .0001; Ter119+, 17-fold, P < .0001). The total Ly-5.2+ leukocyte engraftment of secondary recipients was 80% ± 5% for wild-type, 19% ± 13% for c-Mpl-/-, and 4% ± 2% for STAT5ab-/- BM recipients. To determine whether these defects were occurring in more primitive hematopoietic stem/progenitor fractions, BM cells were isolated from the mice that received transplants from experiment 2 and analyzed by FACS (Figure 2B). The c-Kit+lin-Sca-1+ (KLS) and the c-Kit+lin-Sca-1- (KL) fractions were examined by FACS for Ly-5.2+ wild-type (n = 4), c-Mpl-/- (n = 5), or STAT5ab-/- (n = 5) primary engraftment. Some of the BM cells from each experiment were transplanted at a 1:5 donor-to-recipient ratio into secondary recipients and analyzed for reconstitution 16 weeks later. The same analysis for wild-type (n = 3), c-Mpl-/- (n = 4), or STAT5ab-/- (n = 4) grafts was then performed on secondary recipients. Interestingly, the defects at the level of KLS precursors relative to wild-type were not as severe as that seen in peripheral blood and KL, suggesting that the most severe defects affect lymphomyeloid repopulating activity for either knock-out mouse was occurring in oligopotent KL progenitors. Although STAT5ab-/- BM was significantly more defective than c-Mpl-/- BM in reconstitution of primary KLS (1.8-fold, P = .004), primary KL (3.3-fold, P = .003), secondary KLS (3.1-fold, P = .0003), and secondary KL (3.0-fold, P = .022), the magnitude of these differences was much less than the difference observed in peripheral blood lineages, especially for lymphoid and erythroid precursors. Experiment 1 and experiment 2 were consistent, as reflected by the errors bars in Figure 2A, which combine data from both sets of primary and secondary transplantations.
c-Mpl-/- BM is dominant over STAT5ab-/- BM in head-to-head competitive repopulation experiments
The results of the 5:1 competitive repopulation experiments indicated that the multilineage hematopoietic repopulating defects were greater in STAT5ab-/- mice relative to c-Mpl-/- mice. To test this hypothesis through a direct comparison of repopulating potential, we chose to generate an HW80 congenic strain (Hbd) of the STAT5ab-/- mouse, so that we could track donor grafts in competitive repopulation experiments by using hemoglobin electrophoresis. The HW80 congenic mouse differs from the C57BL/6 parental strain only in a small region of chromosome 7 that contains 2 marker genes of interest, a hemoglobin polymorphism and an albino coat color gene (tyrosinase locus). As a result of this cross, the STAT5ab-/- mice obtained a white coat color and were Hbd compared with the original STAT5ab-/- mouse line that had black coat color and was Hbs. To confirm that this congenic strain was not different from the original strain in terms of reconstitution into lethally irradiated recipients, we set up competitive repopulation experiments between STAT5ab-/- C57BL/6 and STAT5ab-/- HW80 BM cells. As expected, both competed equally in a total of 3 separate competitive repopulation assays (Figure 3). The average engraftment with STAT5ab-/- Hbs was 52% ± 5% (n = 9 recipients) for 3 experiments. Control C57BL/6 wild-type (WT) versus HW80 WT competitions resulted in 48% ± 3% (n = 5 recipients). Additional controls showed that the C57BL/6 background STAT5ab-/- and c-Mpl-/- mice were defective in competition with wild-type HW80 BM cells.
Having established the proper controls, we next set up 3 separate direct head-to-head competitive repopulation experiments between STAT5ab-/- BM and c-Mpl-/- BM (Figure 4). In all 3 experiments, the contribution to engraftment by c-Mpl-/- BM was dominant over STAT5ab-/- BM, indicating less of a defect in long-term competitive reconstitution. Engraftment by STAT5ab-/- HW80 donor cells was only 2% ± 2% Hbd in experiment 1 and 3% ± 4% in experiment 2. Consistent with the results shown in Figure 3, c-Mpl-/- BM was again strikingly defective relative to wild-type BM. To determine whether there would be detectable differences in self-renewal capacity of 2 BM grafts being competed against each other, for experiment 3 in which greater STAT5ab-/- engraftment was observed, secondary transplantations were performed and analyzed. The contribution of STAT5ab-/- BM in experiment 3 significantly increased from 20% ± 1% Hbd to 47% ± 19% Hbd (P = .006). As a control, wild-type HW80 BM completely outcompeted the c-Mpl-/- BM cells in primary and secondary recipients of experiment 3. Although there was some variation in engraftment levels of individual mice in these experiments, this variation is not unexpected because competitive repopulation with limiting amounts of HSC activity for both grafts can be more variable than a competition with wild-type BM, which would be overwhelmingly dominant. These results showed a consistently greater deficiency for STAT5ab-/- donor engraftment, but that STAT5ab-/- donor graft competed more comparably with the c-Mpl-/- donor graft in secondary recipients. This result indicates that primary engraftment defects were strongly influenced by defects in primitive progenitor differentiation, which is consistent with the results shown in Figure 2.
c-Mpl-/- and STAT5ab-/- BM grafts are defective in serial transplantation ability
The defects observed in reconstitution of KLS cells in primary and secondary hosts, suggested that serial transplantation might lead to a limiting dilution situation for c-Mpl-/- and STAT5ab-/- BM grafts but not for wild-type C57BL/6 BM. A critical role for TPO/c-Mpl signaling in serial transplantation ability has previously been demonstrated by using c-Mpl-/- mice12 as well as TPO-/- mice.23 To formally test the possibility that defective STAT5 signaling reduces self-renewal capacity, we set up a series of 3 separate serial transplantation experiments. For experiment 1, the BM cells were serially transplanted into Ly-5.1 recipients, and engraftment was determined by FACS for Ly-5.2. High levels of engraftment were observed in all primary and secondary recipients of a 1:5 donor-to-recipient ratio as previously described.19 However, engraftment into tertiary recipients for this experiment was severely defective in STAT5ab-/- BM transplant recipients. Engraftment of tertiary recipients with wild-type BM cells was 82% ± 5% (n = 5) compared with 4% ± 3% (n = 5) for STAT5ab-/- BM cells. A representative FACS profile for each is shown (Figure 5A). To determine whether Ly-5.2+ BM cells from secondary hosts were intrinsically capable of reconstitution of tertiary hosts in the absence of any residual Ly-5.1 BM from secondary hosts, Ly-5.2+ BM cells were sorted by FACS. From 94% to 97% for wild-type and 89% to 92% for STATab-/- mice, purities of more than 99% sorted Ly-5.2+ cells following re-analysis were used for injection into tertiary recipients. The high purity of starting donor cells greatly reduced the likelihood that survival of the mice could be rescued by Ly-5.1+ host-derived cells from the primary transplantation. Consistent with the failure to engraft during tertiary transplantation using whole STAT5ab-/- BM cells, all recipients of sorted STAT5ab-/- BM cells died within 5 weeks. In contrast, all of the recipients of sorted wild-type Ly-5.2+ cells were radioprotected (Figure 5B) and showed long-term engraftment resulting in 76% ± 11% Ly-5.2+ peripheral blood leukocytes 3 months later. This experiment provided the first evidence supporting a self-renewal defect in STAT5ab-/- mice that was not strictly based on decreased reconstitution of KLS phenotype BM cells.
To follow up on this result, the experiment was repeated 2 more times, but in these experiments each successive transplantation was into HW80 hosts. In experiment 2, again wild-type and STAT5ab-/- BM cells underwent serial transplantation (Figure 6A), whereas experiment 3 was expanded to include a comparison of STAT5ab-/- with c-Mpl-/- BM (Figure 6B). Reconstitution of the hematopoietic system was variable at the tertiary transplantation stage with maintenance of engraftment ability in experiment 2 but partial loss of engraftment ability in experiment 3. Such fluctuation in engraftment ability among experiments and among individual recipient mice is consistent with engraftment at the tertiary transplantation stage being by a limiting dilution of HSCs. Notably, the failure to reconstitute tertiary hosts in experiment 3 was not different between the c-Mpl-/- (64% ± 3%) and STAT5ab-/- grafts (59% ± 25%). Despite the failed long-term donor chimerism in tertiary recipients, most recipient mice had survived the primary, secondary, and tertiary transplantations (Table 1). A few mice from experiment 3 died at the secondary transplantation stage, but this was observed equally in all 3 groups and was likely not indicative of graft failure because all tertiary transplant recipients survived. In all 3 experiments, no c-Mpl-/- or STAT5ab-/- BM grafts were able to radioprotect quaternary hosts, indicating loss of self-renewal potential (Figure 7). BM grafts from c-Mpl-/- mice (n = 4) and STAT5ab-/- mice (n = 4) from experiment 3 also failed to radioprotect quaternary hosts and resulted in similar survival curves. STAT5ab-/- BM grafts (n = 9) from experiment 2 gave longer-term survival, but all mice ultimately died by 8 weeks. In all experiments, only one death was observed from any wild-type BM grafts through all successive transplantations.
Survival of mice in experiments 2 and 3 following injection of serial transplanted BM cells
. | Primary . | Secondary . | Tertiary . | Quaternary . |
|---|---|---|---|---|
| WT, experiment 2 | 5/5 | 4/4 | 3/3 | 10/10 |
| WT, experiment 3 | 9/9 | 7/8 | 5/5 | 4/4 |
| STAT5ab−/−, experiment 2 | 5/5 | 5/5 | 3/3 | 0/9 |
| STAT5ab−/−, experiment 3 | 9/9 | 11/14 | 4/4 | 0/4 |
| c-Mpl−/−, experiment 3 | 8/8 | 11/12 | 3/3 | 0/4 |
. | Primary . | Secondary . | Tertiary . | Quaternary . |
|---|---|---|---|---|
| WT, experiment 2 | 5/5 | 4/4 | 3/3 | 10/10 |
| WT, experiment 3 | 9/9 | 7/8 | 5/5 | 4/4 |
| STAT5ab−/−, experiment 2 | 5/5 | 5/5 | 3/3 | 0/9 |
| STAT5ab−/−, experiment 3 | 9/9 | 11/14 | 4/4 | 0/4 |
| c-Mpl−/−, experiment 3 | 8/8 | 11/12 | 3/3 | 0/4 |
Wild-type C57BL/6 (Hbs; experiments 2 and 3), STAT5ab−/− (experiments 2 and 3), or c-Mpl−/− (experiment 3) BM cells were serially transplanted into lethally irradiated HW80 recipients (Hbd). At each transplantation, the survival of recipient mice was recorded. The ratio of surviving mice per number of transplant recipients is shown at the time of the primary, secondary, tertiary, and quaternary transplantations.
Discussion
The results presented here are the first to characterize the multilineage hematopoietic repopulating ability of c-Mpl-/- mice from the C57BL/6 background. The advantage of this background over the 129/Sv used in previous reports is that the engraftment level in multiple lymphomyeloid lineages could be determined by FACS, whereas previous studies used Southern blotting on BM to determine engraftment. The use of Southern blotting on BM to determine engraftment primarily characterized myeloid lineage engraftment and may have overestimated the multilineage repopulating defect. In our studies, the greatest defect from c-Mpl-/- BM relative to wild-type BM was in the myeloid Gr-1+ peripheral blood fraction (5-fold, P = .0042), whereas other lineages were less severely reduced (B220+, 2-fold, P = .0008; CD4+, 2-fold, P = .003; Ter119+, 2.4-fold, P = .0002). The modest defect in Ter119+ erythroid progenitor development from c-Mpl-/- BM was somewhat surprising, considering evidence for a bipotent Ter119+/4A5+ erythroid and megakaryocytic precursor cell42 and our observation of a 91% reduction in platelet count in the peripheral blood. However, a greater percentage of Ter119- mouse clonogenic megakaryocyte progenitors are derived directly from the CMP than from the myeloerythroid progenitor (MEP),43 indicating a more specific role for c-Mpl in megakaryocyte development. This finding is also consistent with recent unexpected results that c-Mpl is not highly expressed on triple-sorted CMP, granulocyte/macrophage progenitor (GMP), or MEP fractions of BM relative to HSC fractions.15
In contrast to the results obtained from c-Mpl-/- BM, all peripheral blood lineages of STAT5ab-/- BM grafts were highly defective (Gr-1+, 22-fold, P = .002; B220+, 45-fold, P < .0001; CD4+, 39-fold, P < .0001; Ter119+, 41-fold, P = .0002). The magnitude of defects in STAT5ab-/- competitive repopulating ability were comparable to previously reported results.20 The platelet counts of STAT5ab-/- mice, which we reported in the previous study,20 were about 4.4-fold higher than those reported here for c-Mpl-/- mice, indicating less of a defect in megakaryocyte progenitor differentiation. At the committed myeloid progenitor cell level, previous studies that used STAT5ab-/- mice on a mixed 129/Sv and C57BL/6 background also reported moderate effects on progenitor frequency but did not report absolute numbers.24 Our results extend the finding to show that absolute numbers are also low in these mice. The proliferative function, as indicated by CFU-C frequency, was only modestly affected by the STAT5 mutations, and this finding was consistent with previous observations.24 Similar results were also observed in CFU-C frequency and absolute number of embryonic day 14.5 wild-type and STAT5ab-/- fetal liver cells (data not shown). These findings indicate that the primary deficiency occurs in a cell type more primitive than the committed myeloid progenitor cell. Previous studies of c-Mpl-/- mice on the 129/Sv background reported moderate defects in absolute number of progenitors, similar to our results reported here by using mice on the C57BL/6 background. Therefore, both STAT5ab-/- and c-Mpl-/- mice had lower numbers of CFU-Cs relative to wild-type mice.
A significant deficiency in repopulation by both KLS and KL BM progenitor fractions was observed in these studies. However, the defects in primary KLS engraftment were less severe than that from KL and post-KL differentiation, leading to peripheral blood. Therefore, STAT5 activation was required for KL reconstitution and differentiation, whereas c-Mpl was required for KL reconstitution but less for differentiation. These differences also were apparent in head-to-head primary and secondary competitive transplantation experiments, in which the STAT5ab-/- BM was more competitive in secondary hosts than in primary hosts. Rescue of deficiencies in mature blood cell numbers by lineage-specific cytokines has been observed in TPO-/- mice.44 Our studies extend this observation further by demonstrating that post-KL differentiation is not as defective as the HSC level effects and suggests that STAT5-dependent signaling may compensate for post-KL defects in c-Mpl-/- mice. Although we have not defined the relative contributions of c-Mpl-/- or STAT5ab-/- BM to specific intermediary progenitors such as the GMP and MEP, we clearly demonstrate that the phenotypes of the 2 knock-out mice diverge between the oligopotent KL progenitor and the CFU-C.
Some interesting observations can be made about the role of cytokine receptors and signal transduction in hematopoiesis. c-Mpl is known to be expressed on the HSC population along with c-Kit and Sca-1, but c-Mpl expression is lower in the oligopotent KL progenitor and differentiated progeny such as the CFU-C and peripheral blood.15 In contrast, STAT5 expression is not enriched in HSC fractions.13,14 Defects in STAT5ab-/- BM cells appear to be most severe in oligopotent KL progenitors. On the basis of the overlapping phenotypes of these knockouts in the HSC/MPP and oligopotent progenitor fractions, we propose that the TPO-Mpl-STAT5 signaling axis plays a major role in primitive hematopoiesis. However, as the cells begin to differentiate from the post-KL fraction, the c-Mpl receptor expression level drops and STAT5 activation by other cytokine receptors becomes dominant for promoting long-term multilineage repopulating activity. This explains the much greater defects seen in peripheral blood engraftment of STAT5ab-/- BM compared with c-Mpl-/- BM in the competitive repopulation setting.
For these studies, we also set out to demonstrate unequivocally that STAT5ab-/- defects extended back to the self-renewing HSC. To assess the effects of STAT5 or c-Mpl mutations on more primitive cells, serial transplantations were performed. In secondary transplantations, a decline in the KLS reconstitution of both c-Mpl-/- (3-fold; P < .0001) and STAT5ab-/- (5.3-fold; P = .006) donor grafts was observed relative to primary transplanted KLS cells. This indicated that both mutant BM grafts were defective in competitive self-renewal of this c-Kit+ population that is also enriched for c-Mpl+ cells.45 In comparison, the drop in KLS engraftment of wild-type mice was only 1.2-fold (P = .05). Notably, the larger drop in KLS reconstitution of the mutant BM grafts was predictive of the type of hematopoietic defects that were observed in tertiary and quaternary transplantations. Although STAT5ab-/- and c-Mpl-/- BM grafts could rescue primary, secondary, and tertiary recipients, a failure to consistently fully engraft tertiary recipients was observed. Although this suggested that limiting dilution conditions were being achieved by serial transplantation, the strongest evidence supporting this came from the complete inability of either mutant BM graft to radioprotect quaternary recipients. It is important to note that we did not start out at limiting dilution in primary transplantations for these studies, because STAT5ab-/- BM cells show markedly reduced radioprotective capacity.21 However, it is predicted that if these experiments were initiated at limiting dilution, severe defects would have been observed in secondary hosts. We initiated all primary transplantations with donor equivalents that we have previously shown could reconstitute primary and secondary hosts in all lineages, except for the expected T-cell chimerism.20 This translated to a cell dose of approximately 2 to 3 × 106 per recipient. Previous studies only performed up to secondary transplantation by using the same transplantation conditions as reported here, and in all cases high levels of secondary reconstitution was observed.19 The results presented here are the first to definitively show a requirement for STAT5 activation in HSC self-renewal, demonstrating overlapping phenotypes for STAT5 and c-Mpl mutant mice in the most primitive self-renewing compartment of the BM.
In HSCs, STAT5 is likely to be a major signaling intermediate of c-Mpl, although c-Mpl can activate other signaling pathways such as the MAPK and PI3-K that may play important roles in HSC biology. TPO activation of p38 MAPK was recently shown to be important for HoxB4 up-regulation, with 2- to 5-fold lower HoxB4 levels in primitive TPO-/- BM cells.46 Therefore, other transcription factors can contribute to TPO-induced HSC self-renewal. It will be interesting to determine whether STAT5 directly signals HSC self-renewal or whether cross-talk with MAPK and HoxB4 is involved. In total, the experiments with secondary transplantations by using defined KLS populations, along with the serial transplantation experiments, are all consistent with self-renewal defects resulting from the absence of STAT5 signaling in HSCs. Although c-Mpl signaling defects can account for a substantial portion of the STAT5ab-/- self-renewal defect in KLS and KL fractions, a role for c-Kit activation of STAT5 could also be considered for defects at the HSC level. The role of STAT5 in c-Kit signaling has been suggested by recent studies, showing defective response of BM-derived mast cells from STAT5ab-/- mice to IL-3 and SCF stimulation.47 Also, KLS fractions from STAT5ab-/- BM were found to be less responsive to IL-3 and SCF.19
In summary, we show a major role for STAT5 in differentiation from primitive BM progenitor fractions, and we show defects in STAT5ab-/- mice cannot be accounted for simply by the absence of a response to TPO. Not only was STAT5 extremely important for multilineage hematopoietic differentiation, it was also required for HSC self-renewal, to a similar degree as c-Mpl. The extent to which STAT5 can be activated by early acting cytokines other than TPO or whether it is required specifically in GMP or MEP differentiation will require further study. Overall, these studies establish that STAT5 signals are broadly important for multiple levels of hematopoiesis, whereas c-Mpl signals are more specific for primitive HSCs and megakaryocyte progenitors.
Prepublished online as Blood First Edition Paper, December 30, 2003; DOI 10.1182/blood-2003-08-2963.
Supported by grants from the National Institutes of Health (NIHR01DK059380, NIHR21HL071171, and NIHR01HL073738) and the Lauri Strauss Leukemia Foundation (K.D.B.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Albert Forrero and Sheikha Tschand for providing technical assistance. We are also grateful to Teresa Hawley (Holland Laboratory Flow Cytometry Facility) for performing the cell sorting and to Bob Hawley for helpful comments and critical review of this manuscript. We also acknowledge Genentech, Inc, for generously providing the c-Mpl mutant mice for these studies.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal