Abstract
Chronic transfusion of packed red blood cells, in addition to other ongoing treatment with warfarin, acetyl salicylic acid, desferrioxamine, and other supportive measures, was given to a splenectomized hemoglobin E/β-thalassemia woman with pulmonary arterial hypertension (PHT). Serial measurements of plasma thrombin-antithrombin III complex (TAT) levels and right-sided cardiac catheterization were used to monitor changes after treatment. Reduction of plasma TAT levels from 7.5 to 3.8 μg/L (normal, 3 ± 2.4 μg/L), pulmonary vascular resistance (PVR) from 553.8 to 238.6 dyne.sec.cm-5 (normal, 67 ± 30 dyne.sec.cm-5), and mean pulmonary arterial pressure from 51 to 32 mm Hg (normal, 9 to 19 mm Hg) occurred in tandem. Normalization of blood hypercoagulability as reflected in plasma TAT level by chronic blood transfusion was the likely basis for improvement of increased PVR, being secondary to thrombotic pulmonary arteriopathy and subsequently PHT. (Blood. 2004;103: 2844-2846)
Introduction
Pulmonary arterial hypertension (PHT), defined as mean pulmonary arterial (PA) pressure greater than 25 mm Hg at rest, can result from reductions in the caliber of the PA vessels and/or increases in pulmonary blood flow.1,2 Its incidence in thalassemia (Thal) is 10% in Thal major and more than 50% in Thal intermedia.3 Its pathogenesis is not yet completely understood. Normal left ventricular systolic function, increased cardiac output and pulmonary vascular resistance (PVR), and normal or slightly increased pulmonary capillary wedge pressure (PCWP) have been reported using echocardiography and right-sided cardiac catheterization.3,4 Severe PHT was found to be associated with a markedly increased PVR from thrombotic pulmonary arteriopathy,4 which is also found in primary PHT1 and sickle cell disease with PHT.5 Management is symptomatic and supportive to improve right ventricular function. Acetyl salicylic acid (ASA),6 with or without warfarin,7,8 is also prescribed by some. Response to treatment is poor and survival of patients with functional class PHT III to IV2 is about 1 to 2 years.
A role of phosphatidylserine (PS)-exposing red blood cells (RBCs) in hypercoagulability,7,8 platelet activation,9-12 and adhesion to the vascular endothelium13 leading to thrombotic pulmonary arteriopathy was recently suggested in splenectomized β-Thal.4 To test the hypothesis, we used chronic transfusion of RBCs to reduce the proportion of these pathologic RBCs in such a patient and evaluated plasma thrombin-antithrombin III complex (TAT) levels, PA pressure, and the above hemodynamic parameters by serial assessment.
Study design
A 31-year-old (born August 1972) single Thai woman with hemoglobin E/β-Thal underwent splenectomy and cholecystectomy at the age of 5 and 16 years, respectively. On presentation to us in June 1992, she was well developed and well nourished (weight 62 kg, height 165 cm) but pale and icteric. The liver edge was palpable 4 cm below the right costal margin, but there was no clinical or laboratory evidence of chronic liver, heart, or lung diseases. Hemoglobin (Hb) level was 76.0 g/L (7.6 g/dL), hematocrit 25%, nucleated RBCs 575:100 WBCs, and platelet count 984 × 109/L (984 × 103/μL). Serology for hepatitis B virus (HBV) surface antigen, anti-hepatitis C virus (HCV) antibody, and anti-human immunodeficiency virus (HIV) antibody were within normal limits. Indirect bilirubin concentration was 47.88 μM (2.8 mg/dL). Folic acid, vitamin E, ASA (75 mg), and packed RBC (PRC) transfusion were administered as indicated.
In May 1995 she developed shortness of breath on exertion with mild pretibial edema bilaterally. Chest radiograph showed cardiomegaly with a prominent PA trunk. A perfusion lung scan showed bilateral multiple peripheral subsegmental defects. However, both pulmonary angiogram and ultrafast computed tomography (CT) scan of the lungs were negative for findings of pulmonary embolism. She received PRCs and heparin followed by warfarin for 16 months. Prothrombin time (PT) international normalized ratio (INR) and Hb level ranged from 1.5 to 2 and 60 to 70 g/L (6-7 g/dL), respectively.
She was enrolled on a low-dose hydroxyurea program (10 mg/kg/d for 5 consecutive days per week in May 1998) after which she was minimally transfused. Hb level ranged from 45 to 65 g/L (4.5-6.5 g/dL). By November she had overt PHT. Echocardiogram showed normal left ventricular systolic function and increased PA pressure. Furosemide and lanoxin were added, and deferoxamine mesylate dosage was increased to 20 mg/kg/day subcutaneously in 10 hours for 6 days/week. Right-sided cardiac catheterization was performed (Table 1). To reduce the chronic low-grade hypercoagulability,7,8 warfarin was resumed in May 1999 with a targeted PT INR of 1.5. Hydroxyurea was discontinued in October 1999 because of progressive right-sided heart failure. A more aggressive PRC transfusion of 20 to 24 units per year (versus 0-7; median = 5 previously) was initiated to maintain the Hb level above 70 g/L (7 g/dL). Hydralazine was added. She responded well with improvement in functional class of PHT from III-IV to I-II2 and a smaller liver size.
Right-sided cardiac catheterization
. | . | Before transfusion . | After transfusion . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | 2/19/01 . | . | 1/21/03 . | . | |||
| Parameters . | Normal values . | 1/26/99 . | Before O2 . | After O2 . | Before O2 . | After O2 . | |||
| PA pressure, mm Hg; systolic/diastolic (mean) | 15-30/4-12 (9-19) | 74/37 (51) | 63/28 (44) | 49/23 (38) | 45/22 (32) | 39/19 (28) | |||
| PVR, dyne×s×cm−5 | 67 ± 30 | 553.8 | 454.9 | 347.2 | 238.6 | 176.3 | |||
| PVRI, dyne×s×cm−5×m2 | 123 ± 54 | 859.7 | 696.7 | 531.7 | 357.9 | 264.6 | |||
| CO, L/min | 5 | 5.2 | 5.1 | 5.3 | 5.7 | 5.9 | |||
| CI, L/min/m2 | 2.6-4.2 | 3.35 | 3.33 | 3.46 | 3.8 | 3.93 | |||
| PCWP, mm Hg | 2-10 | 15 | 15 | 15 | 15 | 15 | |||
| Arterial O2 saturation, % | 95-100 | — | 86.2 | 90.5 | 94.2 | 98.4 | |||
| Hemoglobin level, g/dL | 14 ± 2 | 6.5 | 8.8 | — | 7.6 | — | |||
. | . | Before transfusion . | After transfusion . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| . | . | . | 2/19/01 . | . | 1/21/03 . | . | |||
| Parameters . | Normal values . | 1/26/99 . | Before O2 . | After O2 . | Before O2 . | After O2 . | |||
| PA pressure, mm Hg; systolic/diastolic (mean) | 15-30/4-12 (9-19) | 74/37 (51) | 63/28 (44) | 49/23 (38) | 45/22 (32) | 39/19 (28) | |||
| PVR, dyne×s×cm−5 | 67 ± 30 | 553.8 | 454.9 | 347.2 | 238.6 | 176.3 | |||
| PVRI, dyne×s×cm−5×m2 | 123 ± 54 | 859.7 | 696.7 | 531.7 | 357.9 | 264.6 | |||
| CO, L/min | 5 | 5.2 | 5.1 | 5.3 | 5.7 | 5.9 | |||
| CI, L/min/m2 | 2.6-4.2 | 3.35 | 3.33 | 3.46 | 3.8 | 3.93 | |||
| PCWP, mm Hg | 2-10 | 15 | 15 | 15 | 15 | 15 | |||
| Arterial O2 saturation, % | 95-100 | — | 86.2 | 90.5 | 94.2 | 98.4 | |||
| Hemoglobin level, g/dL | 14 ± 2 | 6.5 | 8.8 | — | 7.6 | — | |||
Data from before and after chronic transfusion of packed red blood cells and other medical treatment are shown. Oxygen was inhaled for 10 minutes via a face mask with bag at 10 L/min flow rate.
PA indicates pulmonary arterial; PVR, pulmonary vascular resistance; PVRI, pulmonary vascular resistance index; CO, cardiac output; CI, cardiac index; PCWP, pulmonary capillary wedge pressure; and —, no data.
Complete blood count; hemoglobin typing; ferritin; TAT; serology for HBV, HCV, and HIV; and right-sided cardiac catheterization were performed as detailed elsewhere.4 Amount of PS-exposing RBCs was determined by flow cytometer as annexin V-positive cells as detailed elsewhere.8
All cardiac catheterizations were done electively during clinically stable periods. All treatment programs and interventions were clearly explained to the patient and signed written consents were obtained. Approval from the Ramathibodi Hospital institutional ethics committee was also obtained.
Results and discussion
Prior to the chronic transfusion program, PHT slowly progressed. There was no improvement with active iron chelation, which reduced serum ferritin levels from 3148 to 711 μg/L (normal, 10-130 μg/L). Chronic transfusion was initiated when the patient's anemia and clinical status worsened.
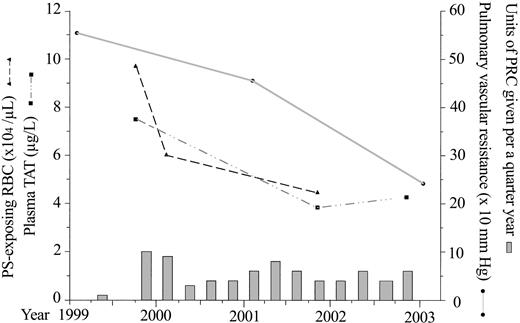
The amount of PS-exposing RBCs and plasma TAT levels were initially increased, reflecting the hypercoagulability of splenectomized hemoglobin E/β-Thal patients.8 The proportion of PS-exposing RBCs decreased from 4.24% of 2.3 × 106 to 1.7% of 2.94 × 106 RBCs/μL (normal, 1.25% ± 0.47% of 4.6 × 106 ± 0.28 × 106 RBCs/μL), suggesting suppression of the patient's erythropoiesis and dilution by transfusion. A concomitant decrease in plasma TAT levels from 7.5 to around 4 μg/L (normal, 3 ± 2.4 μg/L; Figure 1) were consistent with the decreased number of RBCs with high procoagulant activity.8 Decreased values of PVR and mean PA pressure in tandem suggested their direct relationship to the ameliorated hypercoagulable state.
Changes in the amount of phosphatidylserine (PS)-exposing red blood cells (RBCs), plasma thrombin-antithrombin III complex (TAT) levels, and pulmonary vascular resistance in relation to the units of packed red blood cell (PRC) transfusion given per each quarter year.
Changes in the amount of phosphatidylserine (PS)-exposing red blood cells (RBCs), plasma thrombin-antithrombin III complex (TAT) levels, and pulmonary vascular resistance in relation to the units of packed red blood cell (PRC) transfusion given per each quarter year.
Increased cardiac output, PCWP, and PVR initially were in keeping with a previous report.4 Both mean PA pressure and PVR progressively decreased, while PCWP was stable and cardiac output increased after treatment (Table 1). Marked drops in mean PA pressure from 51 to 32 mm Hg and PVR from 553.8 to 238.6 dyne.sec.cm-5 despite an increase in cardiac output and stable PCWP suggested that the major cause of PHT had been an increased PVR. This supports our hypothesis4 but differs from that of Aessopos et al,3 who stressed the role of increased cardiac output rather than increased PVR.
This marked reduction in PVR suggests an improvement in thrombotic pulmonary arteriopathy from the treatment regimen, the central feature of which was chronic transfusion. Improvement of oxygen saturation in the aorta from 86.2% to 94.2% supports the contention. This treatment is uniquely able to ameliorate or even reverse some pathologic lesions of thrombotic pulmonary arteriopathy.14
Continued drop in PA pressure after normalization of plasma TAT level suggests that the initial reduction of PVR was due to a decreased amount of intraluminal clot and that further reduction was due to improvement in vascular remodeling, a sequela of repeated in situ thrombosis.1 Drops in PA pressure after oxygen inhalation indicate an additional nonstructural component of PHT.
Although PHT improved after chronic transfusion, the adjunctive role of ASA,6 warfarin,7,8 and desferrioxamine15 in reducing hypercoagulability cannot be entirely excluded. However, warfarin dosage was less intense during chronic transfusion, which can itself also reduce RBC-vascular endothelial cell interaction.13 Chronic transfusion can also alleviate PHT in patients with sickle cell disease,16 which has many features in common with splenectomized β-Thal.7
Despite its beneficial effect, chronic transfusion and the accompanying iron chelation therapy are relatively inconvenient and costly. Nonmyeloablative progenitor cell transplantation to increase the amount of normal RBCs, as reported in hereditary spherocytotic mice with thrombotic tendency,17 is a possible alternative.
Compared with the dismal outcome of previous treatment approaches, the present report offers hope to such patients and should be explored further. The findings and results of treatment support and extend our understanding of the proposed mechanism and pathogenesis of PHT in splenectomized β-Thal patients.4
Prepublished online as Blood First Edition Paper, November 26, 2003; DOI 10.1182/blood-2003-09-3094.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal