Abstract
Genetic causes of hereditary hemochromatosis (HH) include mutations in the HFE gene, coding for a β2-microglobulin (β2m)-associated major histocompatibility complex class I-like protein. However, iron accumulation in patients with HH can be highly variable. Previously, analysis of β2mRag1-/- double-deficient mice, lacking all β2m-dependent molecules and lymphocytes, demonstrated increased iron accumulation in the pancreas and heart compared with β2m single knock-out mice. To evaluate whether the observed phenotype in β2mRag1-/- mice was due solely to the absence of Hfe or to other β2m-dependent molecules, we generated HfeRag1-/- double-deficient mice. Our studies revealed that introduction of Rag1 deficiency in Hfe knock-out mice leads to heightened iron overload, mainly in the liver, whereas the heart and pancreas are relatively spared compared with β2mRag1-/- mice. These results suggest that other β2m-interacting protein(s) may be involved in iron regulation and that in the absence of functional Hfe molecules lymphocyte numbers may influence iron overload severity. (Blood. 2004;103: 2847-2849)
Introduction
The protein involved in hereditary hemochromatosis (HH), HFE, has been identified as a β2-microglobulin (β2m)-dependent, major histocompatibility complex class I (MHC-I)-like molecule,1 complementing earlier findings that β2m deficiency in mice leads to iron overload.2 Significantly, knock-out of the Hfe gene clearly confers the HH phenotype to mice.3,4
Although HFE mutations are clearly associated with iron overload, the marked variability of the phenotype among homozygous individuals for the same HFE mutations indicates that other environmental and genetic factors modify disease severity.5 Earlier reports show that defective numbers of peripheral lymphocytes and liver lymphocyte populations are associated with a more severe clinical expression of iron overload and damage in HH.6,7
Previously, we investigated the effect of total lymphocyte absence in iron overload by crossing β2m-/- mice, lacking all β2m-dependent MHC-I molecules,2 with mice deficient in recombinase activator gene 1 (Rag1) required for normal B- and T-lymphocyte development.8 Immunodeficient β2mRag1-/- mice develop iron overload and, unlike their single knock-out counterparts, when challenged with dietary iron loading, iron accumulation occurs not only in the liver but also massively in the pancreas and heart myocytes.9
In this report, we further investigate the possible involvement of β2m-dependent molecules and lymphocytes in iron regulation. We examine changes in iron metabolism in compound mutant HfeRag-/- and β2mRag1-/- mice placed on a standard diet and after dietary iron challenge.
Study design
All procedures were performed in accordance with guidelines of Canadian Council on Animal Care and approved by the Animal Care Committee of the Centre Hospitalier de l'Université de Montréal. Hfe-/- mice have been described previously.3 β2m-/- and Rag1-/- mice were purchased from Jackson Laboratories (Bar Harbor, ME). All mutant mice have been back-crossed a minimum of 5 times onto the C57BL/6 (B6) background. Compound mutants β2mRag1-/- and HfeRag1-/- were obtained as described.9 Mouse peripheral blood cells were screened by flow cytometry in a Coulter Epics Elite counter (Coulter, Hialeah, FL) for the absence of T and B lymphocytes, using anti-αβTCR (T-cell marker) and anti-CD45R/B220 (B-cell marker) antibodies (PharMingen, San Diego, CA), and were genotyped by polymerase chain reaction (PCR) for β2m and Hfe mutations. All animals were 8 weeks old at the beginning of the experiments. They were given a commercial diet (Teklad Global 18% protein rodent diet; Harlan Teklad, Madison, WI), or, when indicated, the same standard diet supplemented with 2.5% (wt/wt) carbonyl iron (Sigma Immunochemicals, St Louis, MO). Iron levels were measured by acid digestion of tissue samples followed by iron quantification by atomic absorption spectroscopy.9 For histology, tissue sections were stained with Prussian blue for ferric iron detection (iron stain kit; Sigma Immunochemicals).
Results and discussion
To investigate whether the exacerbation of iron loading in β2mRag1-/- mice is due to the absence of only Hfe or to other β2m-dependent molecules, we generated mice lacking both Hfe and Rag1 (HfeRag1-/- mice). It was expected that if the only β2m-dependent molecule involved in iron regulation was Hfe, the β2mRag1-/- phenotype would be recapitulated in HfeRag1-/- double mutants. Surprisingly, hepatic iron rose dramatically in HfeRag1-/- double mutants, whereas iron levels in the heart and pancreas were not statistically different from Hfe-/- single knock-out mice (Table 1). The hepatic iron increment in HfeRag1-/- was significant (P < .01) when compared with Hfe-/- single knock-out mice. In contrast, liver iron stores remained similar in β2mRag1-/- compared with β2m-/- mice.
Tissue iron concentration in mice fed a standard or iron-enriched diet (2.5% wt/wt carbonyl iron) for 3 months
. | μg iron/g dry wt . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ . | B6 . | Rag1−/− . | Hfe−/− . | HfeRag1−/− . | β2m−/− . | β2mRag1−/− . | |||||
| Standard diet | |||||||||||
| n = 14 | n = 9 | n = 14 | n = 12 | n = 9 | n = 11 | ||||||
| Liver | 287 ± 63 | 298 ± 43 | 1034 ± 253* | 1607 ± 730†‡ | 788 ± 279* | 840 ± 292* | |||||
| Heart | 358 ± 52 | 371 ± 59 | 409 ± 45 | 412 ± 90 | 439 ± 57 | 586 ± 134§ | |||||
| Pancreas | 160 ± 35 | 151 ± 30 | 173 ± 45 | 185 ± 42 | 178 ± 38 | 676 ± 276∥ | |||||
| Iron-enriched diet | |||||||||||
| n = 10 | n = 10 | n = 10 | n = 10 | n = 9 | n = 11 | ||||||
| Liver | 4014 ± 1186 | 4279 ± 549 | 7276 ± 1247* | 12077 ± 4584‡¶ | 5742 ± 985*¶ | 6118 ± 1462* | |||||
| Heart | 467 ± 70 | 508 ± 72 | 500 ± 117 | 485 ± 84 | 501 ± 72 | 1181 ± 265∥ | |||||
| Pancreas | 252 ± 72 | 287 ± 83 | 253 ± 23 | 279 ± 106 | 283 ± 46 | 3844 ± 2010§ | |||||
. | μg iron/g dry wt . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Organ . | B6 . | Rag1−/− . | Hfe−/− . | HfeRag1−/− . | β2m−/− . | β2mRag1−/− . | |||||
| Standard diet | |||||||||||
| n = 14 | n = 9 | n = 14 | n = 12 | n = 9 | n = 11 | ||||||
| Liver | 287 ± 63 | 298 ± 43 | 1034 ± 253* | 1607 ± 730†‡ | 788 ± 279* | 840 ± 292* | |||||
| Heart | 358 ± 52 | 371 ± 59 | 409 ± 45 | 412 ± 90 | 439 ± 57 | 586 ± 134§ | |||||
| Pancreas | 160 ± 35 | 151 ± 30 | 173 ± 45 | 185 ± 42 | 178 ± 38 | 676 ± 276∥ | |||||
| Iron-enriched diet | |||||||||||
| n = 10 | n = 10 | n = 10 | n = 10 | n = 9 | n = 11 | ||||||
| Liver | 4014 ± 1186 | 4279 ± 549 | 7276 ± 1247* | 12077 ± 4584‡¶ | 5742 ± 985*¶ | 6118 ± 1462* | |||||
| Heart | 467 ± 70 | 508 ± 72 | 500 ± 117 | 485 ± 84 | 501 ± 72 | 1181 ± 265∥ | |||||
| Pancreas | 252 ± 72 | 287 ± 83 | 253 ± 23 | 279 ± 106 | 283 ± 46 | 3844 ± 2010§ | |||||
Mice (females) were 5 months old at the end of the experiment. Data are presented as mean ± SD.
P < .005 compared with B6 or Rag1−/− mice by Student t test.
P < .01 compared with Hfe−/− mice by Student t test.
P < .001 compared with β2mRag1−/− mice by Student t test.
P < .01 by Student t test compared with all other mouse strains.
P < .0001 by Student t test compared with all other mouse strains.
P < .05 compared with Hfe−/− mice by Student t test.
In the heart, iron concentration in HfeRag1-/- double mutants was similar to Hfe single knock-out mice (Table 1). In marked contrast, cardiac iron was significantly higher in β2mRag1-/- compared with β2m-/- mice (P < .01) or to the remaining mouse strains (P < .0005). Similarly, in the pancreas, HfeRag1-/- did not differ from Hfe-/- mice, whereas β2mRag1-/- mice showed a 3.7-fold increase in iron concentration over β2m-/- single knock-out mice (P < .0001) and all other mouse strains. Thus, on a standard diet, the phenotype of HfeRag1-/- mice is strikingly different from that of β2mRag1-/-, the main difference being the affected organs where introduction of the Rag1 mutation leads to augmented iron loading.
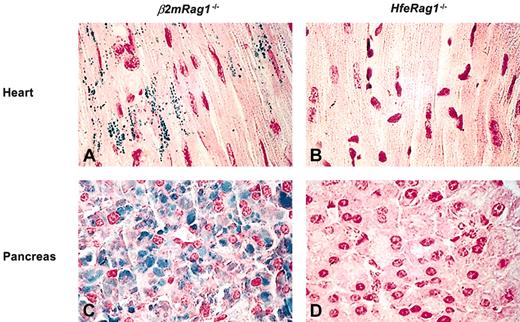
To further investigate the responses to dietary iron loading in compound mutants, mice were challenged with a 2.5% wt/wt carbonyl iron-supplemented diet for 3 months. In the liver, the highest iron concentration was found in HfeRag1-/- mice, followed by Hfe-/-, β2mRag1-/-, and β2m-/- mice (Table 1). In the heart and pancreas, β2mRag1-/- mice accumulated more iron than any other mouse strains. These differences were highly significant (P < .0001 and P < .01, respectively, in the heart and pancreas). Iron accumulation was visible in β2mRag1-/- cardiac myocytes and pancreas sections stained with Prussian blue but remained undetectable in HfeRag1-/- mice (Figure 1).
Storage of excess iron in the heart and pancreas of mice after 3 months on a diet supplemented with 2.5% wt/wt carbonyl iron (Prussian blue staining, n = 5). The animals were 5 months old at the end of the experiment. Heart section from representative β2mRag1-/- (A) and HfeRag1-/- mice (B). Pancreas section from representative β2mRag1-/- (C) andHfeRag1-/- mice (D). Original magnification, × 600.
Storage of excess iron in the heart and pancreas of mice after 3 months on a diet supplemented with 2.5% wt/wt carbonyl iron (Prussian blue staining, n = 5). The animals were 5 months old at the end of the experiment. Heart section from representative β2mRag1-/- (A) and HfeRag1-/- mice (B). Pancreas section from representative β2mRag1-/- (C) andHfeRag1-/- mice (D). Original magnification, × 600.
Thus, the observed phenotype differences between mice lacking both Hfe and Rag1 and mice lacking β2m and Rag1 persist and are even enhanced after dietary iron challenge. The differences in phenotype are likely due to the lack of other molecules that interact with β2m in β2m-deficient mice. Involvement of other β2m-dependent molecules in iron regulation has previously been suggested by the demonstration of increased hepatic iron in Hfeβ2m double-mutant mice compared with single knock outs10 and by the finding that MHC-I-deficient mice have elevated hepatic iron.11 Intriguingly, the phenotype of β2mRag1 double-deficient mice considerably resembles juvenile hemochromatosis (JH) in humans12 and hepcidin-deficient mice13 in the severity of iron overload and the involvement of the heart and pancreas. JH is an autosomal recessive disorder, which leads to early-onset, severe iron overload. Heart failure and endocrine manifestations are the most prominent clinical features of JH, whereas liver involvement, although present, is clinically less relevant. Iron accumulation in the liver of hepcidin-deficient mice is not particularly different from Hfe-/- or β2m-/- mice but rises dramatically in the pancreas and heart.13
In Hfe-deficient mice, hepatic hepcidin mRNA is inappropriately low, and the mice show a blunted response to iron loading.14-16 However, β2m-deficient mice have higher hepcidin levels than healthy mice, which is an appropriate, although insufficient response to iron loading.17 A possible explanation for this difference may reside in the fact that β2m-/- mice are known to be functionally “leaky,” as residual functional MHC-I expression can still be detected.18,19 By analogy, residual Hfe expression may occur in β2m-deficient mice, giving them limited control over iron-induced hepcidin expression. This finding may explain why Hfe-/- and HfeRag1-/- mice accumulate more iron in the liver than do β2m-/- and β2mRag1-/- mice, respectively.
Accumulating evidence suggests that iron does not directly regulate hepcidin expression in hepatocytes17,20 and that hepcidin levels may be indirectly regulated by other cells. In fact, hepcidin expression can be induced by interleukin 6 (IL-6),20 a cytokine synthesized predominantly by macrophages and lymphocytes. Thus, lymphocytes may interfere with hepcidin regulation either directly, by secreting IL-6, or indirectly, through effects on macrophage differentiation and function.21 Alternatively, the fact that phenotype differences between Hfe- and β2m-deficient mice are evident only after lymphocyte depletion may indicate that lymphocytes represent an important iron storage compartment.
Phenotype differences between HfeRag1-/- and β2mRag1-/- mice may be attributable to the existence of other β2m-dependent, MHC-I-like molecules involved in iron regulation. Hfe is predominantly expressed in the liver, with the highest levels of Hfe mRNA found in hepatocytes.22,23 This is consistent with the finding that Hfe deletion primarily affects the liver. Presumably other β2m-dependent molecules might be more relevant in the heart and pancreas, which also express hepcidin.24
In conclusion, the work presented here suggests that alterations in the expression of β2m-dependent, MHC-I-like molecules and lymphocyte numbers may relate to phenotype variation in HH patients.
Prepublished online as Blood First Edition Paper, December 4, 2003; DOI 10.1182/blood-2003-09-3300.
Supported by a grant from the Canadian Institutes of Health Research (CIHR; grant no. MOP44045), and the Fundação para a Ciência e a Tecnologia (FCT; grant no. CBO/33485/99-00). M.M.S. is the recipient of a CIHR New Investigator award. N.C.A. is an Associate Investigator of the Howard Hughes Medical Institute.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Christian Dallaire for his help with atomic absorption spectroscopy, F. Arosa and B. Fournier for valuable discussions, and Ovid Da Silva for his editorial assistance.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal