Abstract
Cerebral hemorrhage associated with antithrombotic and thrombolytic therapy in acute stroke continues to present a major clinical problem. Rupture of the cerebral microvasculature involves the degradation and remodeling of extracellular matrix. Here we demonstrated that the delayed administration of heparin 3 hours after photothrombotic middle cerebral artery occlusion (MCAO) caused cerebral hemorrhage in wild-type (WT) mice but not in tissue plasminogen activator (tPA)–deficient knockout (KO) mice. Heparin administration increased tPA activity and its mRNA expression at 6 and 12 hours after MCAO in the ischemic hemispheres of WT mice. The expression of tPA was enhanced in microglial cells in the ischemic border zone. We also observed an exacerbation of matrix metalloproteinase (MMP) 9 expression at the mRNA level and its conversion to an active form after heparin administration in the ischemic hemisphere in WT mice but not in tPA KO mice. The increased MMP 9 expression was localized in microglial cells and endothelial cells. These findings suggest that endogenous tPA, through the enhancement of MMP 9 expression and proteolytic activation, plays an essential role in the pathogenesis of heparin-produced cerebral hemorrhage. Targeting tPA, MMP 9, or both may provide a new approach for preventing cerebral hemorrhage associated with antithrombotic therapy for stroke in humans.
Introduction
Cerebral hemorrhage associated with antithrombotic and thrombolytic therapy in acute stroke causes death and severe neurologic deficits.1 Injury to the coagulation system, the platelet-dependent process, and the cerebrovascular wall caused by the generation of free radicals is related to cerebral hemorrhage after an ischemic event.2,3 However, the mechanisms underlying hemorrhage after antithrombotic therapy are still unclear.
Plasminogen activators (PAs; tissue PA [tPA] and urokinase PA) are serine proteases that activate plasminogen into plasmin, a protease capable of degrading fibrin and most extracellular proteins directly and indirectly.4,5 tPA was reported to promote excitotoxic neuronal death after brain injury6 and to increase infarct size in focal ischemia,7 and tPA gene–deficient knockout (KO) mice are protected from cerebral ischemia.7,8 We also recently demonstrated that tPA has neurotoxic effects in the brain tissue after cerebral ischemia.9
Matrix metalloproteinases (MMPs) are a family of zinc- and calcium-dependent enzymes able to degrade extracellular matrix (ECM) components.10,11 In particular, 2 members of the MMP family, MMP 2 and MMP 9, are able to degrade collagens, laminins, and fibronectin, the major components of basal lamina around cerebral blood vessels.10 Previous studies have shown that MMP 2 and MMP 9 play major roles in the pathogenesis of blood-brain barrier (BBB) breakdown.12 Excessive expression of MMP 2 and MMP 9 is also correlated with cerebral ischemia reperfusion injury13 and cerebral hemorrhage.14
Evidence suggests that PAs and MMP family proteases interact with one another during ischemia15,16 ; however, little is known about this specific interaction. Furthermore, the nature and timing of the tPA and MMP protease expressions may vary with the species, timing, and models used.
In this study, we investigated the role of tPA in cerebral hemorrhage associated with cerebral ischemia in tPA KO and wild-type (WT) mice using the photothrombotic middle cerebral artery occlusion (MCAO) model. The photothrombotic model of MCAO was chosen because cerebral hemorrhage was produced after heparin administration in this model.17-19 In this model, the formation of a platelet- and fibrin-rich thrombus in the MCA was induced by a photochemical approach between rose bengal injection and green light irradiation. Photothrombotic occlusion of MCA is known to activate coagulation system and platelet processes and to generate free radicals.20,21 We demonstrated that cerebral hemorrhage associated with cerebral ischemia was exacerbated by heparin administration in WT mice. However, the exacerbation was not observed in tPA KO mice. Furthermore, we demonstrated that heparin administration increased the activity and mRNA expression of tPA and MMP 9 after cerebral ischemia.
Materials and methods
Mouse model of MCA photothrombotic occlusion
WT mice and tPA KO mice (gifts from the Center for Transgene Technology and Gene Therapy, KU Leuven, Belgium), 9 to 12 weeks old were used.22 The genetic background of these mice was a mixture of 75% C57B1/6 and 25% SV129. Photothrombotic occlusion of MCA was performed as described.9,17,23,24 In the MCA region, a 1.5-mm–diameter window was opened, and the dura mater was intact. Irradiation with green light (3.16 W/cm2 wavelength, 540 nm; L-4887, Hamamatsu Photonics, Japan) was directed using a 1-mm–diameter optic fiber. Rose bengal was injected through a catheter placed in the left jugular vein, and MCA was illuminated simultaneously. Infusion of rose bengal was continued for 1 minute, whereas irradiation was continued for 30 minutes. Porcine mucosal heparin (150 U/kg per hour; Hoechst Pharmaceutical, Japan) was continuously administered intraperitoneally with an osmotic pump (Alzet) for 24 hours, beginning 3 hours after the start of photoirradiation.
After 48 hours, the brain was removed and the infarct volume was determined by staining coronal sections (1 mm) with 1% triphenyltetrazolium chloride (TTC).17 For histologic examination, animals were perfused with phosphate-buffered saline (PBS) at 48 hours, and the cerebral sections (6 μm) were stained with hematoxylin-eosin. The Animal Care and Use Committee of Hamamatsu University School of Medicine approved all experimental protocols.
Determination of cerebral hemorrhage volume
Forty-eight hours after MCAO, mice were perfused transcardially with PBS. PBS (0.6 mL) was added to the ischemic hemispheric brain tissue of each mouse, followed by homogenization and centrifugation at 13 000 rpm. Drabkin reagent (0.8 mL, Sigma) was added to 200 μL aliquots, and optical density was measured at 540 nm. Hemorrhage volume was expressed in equivalent units by comparison with a reference curve generated using homologous blood.25
Analysis of tissue plasminogen activator activity
At 6, 12, 24, and 48 hours after MCAO, ischemic and contralateral hemispheres were obtained. Approximately 40 mg brain tissue from the ischemic border zone at 6 hours after MCAO in the untreated and the heparin-treated mice was also obtained. Brain tissues were homogenized and centrifuged. Each sample (5 μL) was placed in the well of the fibrin agarose plate containing 8 mg/mL agarose, 8 μg/mL plasminogen, 0.067 U/mL thrombin, and 2 mg/mL fibrinogen. The plate was incubated at 37°C, and the diameter of the lysis zone was measured. tPA activity was expressed in equivalent units by comparison with a reference curve constructed using purified tPA.
Primers and probes
Total RNA of brain (for tPA) and lung (for MMP 9) tissue was isolated,26 and DNA-free RNA was primed with random 9 mers. First-strand cDNA was amplified with oligonucleotide primer pairs complementary to tPA (forward, 5′-TGAGCCAACGCAGACAACTTA-3′ [213-233]; reverse, 5′-TGACAGCACCCAGCAGGAACT-3′ [1129-1149]) and to MMP 9 (forward, 5′-AAGACGACATAGACGGCATCC-3′ [903-923]; reverse, 5′-AAACTCACACGCCAGAAGAAT-3′ [1690-1710]) sequences. Polymerase chain reaction (PCR) products were ligated and cloned into PCR 2.1 vector (Invitrogen). Plasmid DNA was purified and digested by restriction enzyme. The cDNA subsequently was labeled with α-[32P] dCTP and was used as a probe in Northern blot and Southern blot analyses. The cDNA probe used for β-actin was a 443–base pair HinfI fragment.27 β-Actin primers used in reverse transcription-PCR (RT-PCR) were as follows: forward, 5′-CCACACTGTGCCCATCTACGA-3′ (560-580); reverse, 5′-ACATCTGCTGGAAGGTGGACA-3′ (1126-1146). tPA probes used for in situ hybridization were as follows: antisense, 5′-TGCTTGGCCTTTTAGGCGCATCTTCTGTAGAAGAGG-3′ (1823-1858); sense, 5′-CCTCTTCTACAGAAGATGCGCCTAAAAGGCCAAGCA-3′.
Northern blot analyses
Total RNA (10 μg) of hemispheric tissue was run on formaldehyde/agarose gels, transferred to a nylon membrane, and probed with α-[32P] dCTP-labeled cDNA for tPA, or β-actin at 42°C. The filter was washed in 2 × standard saline citrate (SSC) and 0.1% sodium dodecyl sulfate (SDS) and then washed in 0.1 × SSC and 0.1% SDS at 65°C. The filter was exposed to an imaging plate (Fuji), and the specific radioactivity was quantified by Bio-image analyzer BAS 2000 (Fuji).
In situ hybridization
The technique used for in situ hybridization was described previously.28 Serial cerebral sections (15 μm) were hybridized with a tPA or an MMP 9 α-[35S] dATP-labeled probe at 41°C. Slides were washed in a series of 1 × SSC washes at 55°C and were coated with Kodak NTB-2 emulsion (Eastman-Kodak). After exposure at 4°C in a dark box, the slides were developed in Kodak D-19 developer. Finally, the slides were counterstained with thionin solution to allow morphologic identification. As negative controls, α-[35S] dATP–labeled sense probes were used and showed only background levels.
Zymography analyses
Ischemic and contralateral hemispheric brain samples were homogenized and centrifuged. Gelatinase activity in the supernatants was extracted and purified with gelatin-Sepharose 4B (Pharmacia, Uppsala, Sweden).29 Samples normalized for protein concentration were mixed with sample buffer and loaded onto 10% SDS-polyacrylamide gel containing 1 mg/mL gelatin. After electrophoresis, the gel was incubated in 150 mL of 50 mM Tris-HCl (pH 7.6) containing 10 mM CaCl2 and 0.02% NaN3 at 37°C for 24 hours. Gels were stained with 0.5% Coomassie blue R-250 and destained appropriately.
RT-PCR Southern blot analyses
Total RNA (0.5 μg) isolated from the ischemic and contralateral hemispheres was amplified by RT-PCR for 35 cycles for MMP 9 and for 18 cycles for β-actin. Amplification products were run on 2% agarose gel (Seakman). DNA fixed to a filter was hybridized with a randomly labeled α-[32P] dCTP MMP 9 and β-actin cDNA probe at 65°C. The filter was washed in 2 × SSC and 0.1% SDS and then washed twice in 0.1 × SSC and 0.1% SDS. A control experiment showed the radiointensity of DNA was proportional to the PCR cycles within 30, 35, and 40 cycles for MMP 9 and 15, 18, 21, and 24 cycles for β-actin. Specific radioactivity was linear within 0.25, 0.5, and 1.0 μg of the input total RNA for MMP 9 (35-cycle amplification) and β-actin (18-cycle amplification).
Immunofluorescence double labeling
Fresh-frozen cerebral tissue cryosections (15 μm) were blocked with 1% bovine serum albumin (BSA) and 10% normal goat serum or normal donkey serum in PBS and were incubated with a combination of antibodies of rabbit antimouse tPA (RabtPA) (1:100) (Table 1) and F4/80 (1:5), or rat antiglial fibrillary acidic protein (GFAP) (1:50) goat polyclonal antibody against tPA (GoatPA) (1:100) and rabbit antineurofilament polyclonal antibody (NEURO) (1:100), or von Willebrand factor (VWF) (1:5) or anti-MMP 9 (1:100), and VWF (1:5) or F4/80 (1:5) overnight at 4°C. For RabtPA and F4/80 or GFAP, the sections were then rinsed and incubated with a mixture of Alexa Fluor 546 goat antirabbit immunoglobulin G (IgG) (1:2000; Molecular Probes) and Alexa Fluor 488 goat antirat IgG (1:1000; Molecular Probes). For GoatPA and VWF or NEURO, or MMP 9 and VWF, a mixture of Alexa Fluor 488 donkey antigoat IgG (1:400; Molecular Probes) and rhodamine donkey antirabbit IgG (1:1000; Chemicon) was used. For MMP 9 and F4/80, a mixture of Alexa Fluor 488 donkey antigoat IgG (1:400) and rhodamine donkey antirat IgG (1:200; Chemicon) was used. Sections were observed with a fluorescence microscope.
Primary antibodies used in immunofluorescence double staining
Staining . | Primary antibody . | Source . |
|---|---|---|
| tPA/microglia | RabtPA | American Diagnostica, Greenwich, CT |
| Rat antimouse F4/80 | Serotec, Oxford, United Kingdom | |
| tPA/astrocyte | RabtPA | American Diagnostica |
| GFAP | Zymed Laboratories, South San Francisco, CA | |
| tPA/neuron | GoatPA | Santa Cruz Biotechnology, Santa Cruz, CA |
| NEURO | Chemicon, Temecula, CA | |
| tPA/endothelial | GoatPA | Santa Cruz Biotechnology |
| Polyclonal rabbit antihuman VWF | DAKO, Carpinteria, CA | |
| MMP 9/endothelial | Goat antimouse MMP 9 | R&D Systems, Minneapolis, MN |
| Polyclonal rabbit antihuman VWF | DAKO | |
| MMP 9/microglia | Goat antimouse MMP 9 | R&D Systems |
| Rat antimouse F4/80 | Serotec |
Staining . | Primary antibody . | Source . |
|---|---|---|
| tPA/microglia | RabtPA | American Diagnostica, Greenwich, CT |
| Rat antimouse F4/80 | Serotec, Oxford, United Kingdom | |
| tPA/astrocyte | RabtPA | American Diagnostica |
| GFAP | Zymed Laboratories, South San Francisco, CA | |
| tPA/neuron | GoatPA | Santa Cruz Biotechnology, Santa Cruz, CA |
| NEURO | Chemicon, Temecula, CA | |
| tPA/endothelial | GoatPA | Santa Cruz Biotechnology |
| Polyclonal rabbit antihuman VWF | DAKO, Carpinteria, CA | |
| MMP 9/endothelial | Goat antimouse MMP 9 | R&D Systems, Minneapolis, MN |
| Polyclonal rabbit antihuman VWF | DAKO | |
| MMP 9/microglia | Goat antimouse MMP 9 | R&D Systems |
| Rat antimouse F4/80 | Serotec |
Statistical analysis
Values are represented as mean ± SD. Statistical comparisons were performed using 1-way analysis of variance and Fisher PLSD test. P values less than .05 were considered statistically significant.
Results
Heparin administration increased cerebral hemorrhage in WT mice but not in tPA KO mice after photothrombotic MCAO
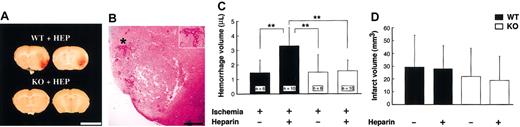
In WT mice, heparin administration produced gross cerebral hemorrhage in the MCA territories 48 hours after MCAO (Figure 1A). Figure 1B shows the microscopic hemorrhage. Cerebral hemorrhage produced by heparin in WT mice gradually grew in a time-dependent manner up to 48 hours after MCAO (data not shown) and was significantly larger than it was in untreated WT and tPA KO mice at 48 hours (the latter had no evident hemorrhage). By using a spectrophotometric hemoglobin assay, we found that hemorrhage volume in heparin-treated WT mice was significantly (P < .01) larger than in untreated mice (Figure 1C) or in heparin-treated tPA KO mice (P < .01). There was no significant difference in infarct volume among the 4 groups (D). The aim of this study was to investigate the mechanisms of heparin-produced cerebral hemorrhage. In preliminary studies, we examined different doses of heparin. For example, a low dose of heparin (75 U/kg per hour) also produced a small cerebral hemorrhage in WT mice, but this hemorrhage could not be detected by hemoglobin quantification. To produce detectable cerebral hemorrhage in WT mice, a higher dose of heparin was used in the present study. However, this dose of heparin caused a slight reduction in cerebral infarct (Figure 1D) without enhancing the hemorrhage in tPA KO mice (Figure 1C).
Heparin-produced cerebral hemorrhage. (A) Representative photographs of cerebral sections (1 mm) from heparin-treated WT and tPA KO mice. At 48 hours after MCA occlusion, gross cerebral hemorrhage was observed only in WT mice with heparin administration. Scale bars equal 5 mm. (B) Photomicrograph of cerebral hemorrhage from WT mice. Inset is 1.7-fold magnified from the area denoted by the asterisk. Scale bar equals 2 mm. (C) Effect of heparin on hemorrhage volume. Infusion of heparin 3 hours after MCAO significantly increased hemorrhage volume in WT mice but not in tPA KO mice. (D) No significant differences in infarct volumes were observed among any of the groups (n = 6). (C-D) Values are means ± SD. **P < .01.
Heparin-produced cerebral hemorrhage. (A) Representative photographs of cerebral sections (1 mm) from heparin-treated WT and tPA KO mice. At 48 hours after MCA occlusion, gross cerebral hemorrhage was observed only in WT mice with heparin administration. Scale bars equal 5 mm. (B) Photomicrograph of cerebral hemorrhage from WT mice. Inset is 1.7-fold magnified from the area denoted by the asterisk. Scale bar equals 2 mm. (C) Effect of heparin on hemorrhage volume. Infusion of heparin 3 hours after MCAO significantly increased hemorrhage volume in WT mice but not in tPA KO mice. (D) No significant differences in infarct volumes were observed among any of the groups (n = 6). (C-D) Values are means ± SD. **P < .01.
Heparin administration increased tPA activity and mRNA level in ischemic brains of WT mice
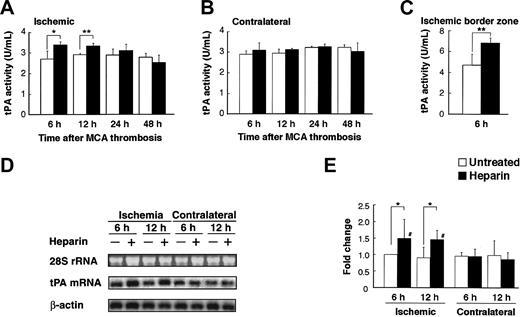
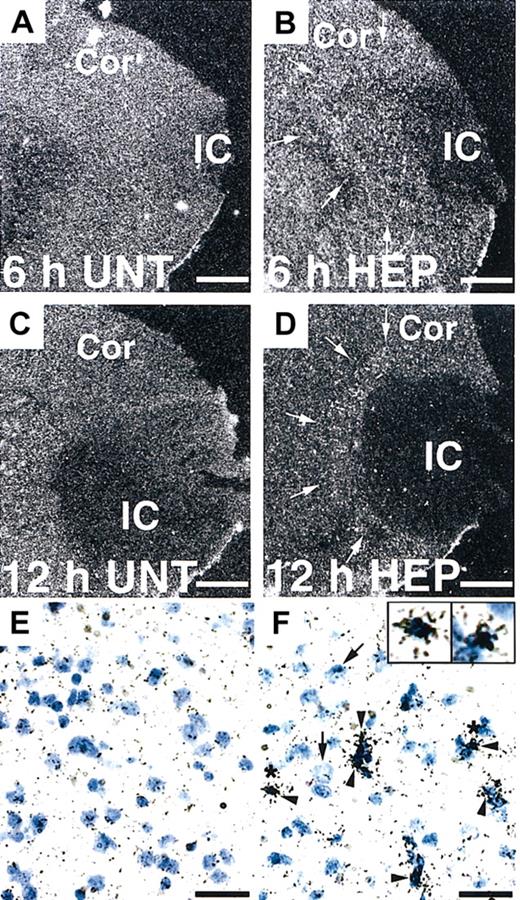
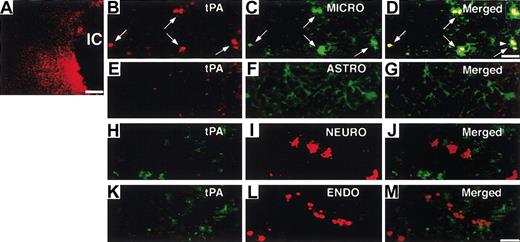
Next, we investigated the potential effect of heparin administration after cerebral ischemia on tPA activity in WT mice. Compared with untreated mice, mice treated with heparin starting from 3 hours after MCAO showed significantly (P < .05 and P < .01, respectively) increased tPA activity in the ischemic hemisphere at 6 and 12 hours after MCAO (Figure 2A). In the ischemic border zone, tPA activity was further increased by heparin administration at 6 hours (Figure 2C). In contrast, heparin did not affect tPA activity in the contralateral hemisphere (Figure 2B). At 24 and 48 hours, tPA activity appeared to return to the baseline, suggesting that the increase in tPA activity was early and transient. This finding indicates that a heparin-mediated increase in tPA activity is associated with the appearance of hemorrhage in the ischemic hemisphere of WT mice in this model. Then, we investigated the effect of heparin on mRNA expression of tPA. Northern blot analysis of RNA extracts prepared from mice at 6 and 12 hours after MCAO demonstrated that tPA mRNA expression was significantly (P < .05) elevated in the ipsilateral hemisphere but not in the contralateral hemisphere in heparin-treated WT mice, compared with those in untreated mice (Figure 2D-E). Based on these findings, the increase in tPA activity was associated with the increase in tPA mRNA level. As shown by in situ hybridization, tPA mRNA expression increased in the area surrounding the ischemic core at 6 hours after ischemia and remained elevated at 12 hours in heparin-treated mice (Figure 3B, D). Ischemia alone showed no significant effect on tPA mRNA expression (Figure 3A, C, E). Sections counterstained with thionin suggested that increased tPA mRNA was expressed in microglial cells (Figure 3F) but not in neurons. In parallel, immunofluorescence staining showed that increased immunofluorescence of tPA was observed in the ischemic border zone in heparin-treated WT mice 6 hours after MCAO (Figure 4A). Double labeling of brain sections indicated that increased tPA was expressed in microglial cells (Figure 4B-D) and that tPA was not absent in astrocytes (Figure 4E-G), neurons (Figure 4H-J), or endothelial cells (Figure 4K-M).
Heparin administration induced tPA activity and mRNA expression. (A-B) Time course of tPA activity after MCAO in ischemic and nonischemic hemispheres in WT mice. Compared with the untreated mice, heparin-treated mice had significantly higher tPA activity in the ischemic hemisphere at 6 and 12 hours (A). No significant difference in tPA activity in the nonischemic hemisphere was noted between heparin-treated and untreated mice (B). Values are means ± SD (n = 4 or 5). *P < .05; **P < .01. (C) In the ischemic border zone, tPA activity was further increased by heparin administration at 6 hours. Values are means ± SD (n = 5). (D) Northern blot analyses of tPA mRNA expression 6 and 12 hours after MCAO in ipsilateral and contralateral cerebral hemispheres of ischemia in WT mice, which was normalized by the expression of β-actin (E). Compared with the untreated mice, heparin-treated mice had significantly higher tPA mRNA levels in ischemic hemispheres at 6 and 12 hours. Ethidium bromide staining of the 28S ribosomal RNA revealed equal RNA loading in all lanes. Values are means ± SD (n = 4). *P < .05; #P < .05 compared with contralateral hemispheres.
Heparin administration induced tPA activity and mRNA expression. (A-B) Time course of tPA activity after MCAO in ischemic and nonischemic hemispheres in WT mice. Compared with the untreated mice, heparin-treated mice had significantly higher tPA activity in the ischemic hemisphere at 6 and 12 hours (A). No significant difference in tPA activity in the nonischemic hemisphere was noted between heparin-treated and untreated mice (B). Values are means ± SD (n = 4 or 5). *P < .05; **P < .01. (C) In the ischemic border zone, tPA activity was further increased by heparin administration at 6 hours. Values are means ± SD (n = 5). (D) Northern blot analyses of tPA mRNA expression 6 and 12 hours after MCAO in ipsilateral and contralateral cerebral hemispheres of ischemia in WT mice, which was normalized by the expression of β-actin (E). Compared with the untreated mice, heparin-treated mice had significantly higher tPA mRNA levels in ischemic hemispheres at 6 and 12 hours. Ethidium bromide staining of the 28S ribosomal RNA revealed equal RNA loading in all lanes. Values are means ± SD (n = 4). *P < .05; #P < .05 compared with contralateral hemispheres.
In situ hybridization of tPA mRNA expression in the mouse brain. (A-D) Dark-field photomicrographs show significant up-regulation of tPA mRNA in the border of the ischemic core (IC) at 6 and 12 hours after MCAO in heparin (HEP)–treated WT mice (arrows in B, D) compared with untreated (UNT) WT mice (A, C). Cor indicates cerebral cortex. Bright-field photomicrographs show that increased tPA mRNA levels were expressed in microglial cells (F, arrowheads) but not neurons (F, arrows). Insets are 2.5-fold magnified cells from the area denoted by the asterisk. Scale bars equal 500 μm (A-D) and 50 μm (E-F).
In situ hybridization of tPA mRNA expression in the mouse brain. (A-D) Dark-field photomicrographs show significant up-regulation of tPA mRNA in the border of the ischemic core (IC) at 6 and 12 hours after MCAO in heparin (HEP)–treated WT mice (arrows in B, D) compared with untreated (UNT) WT mice (A, C). Cor indicates cerebral cortex. Bright-field photomicrographs show that increased tPA mRNA levels were expressed in microglial cells (F, arrowheads) but not neurons (F, arrows). Insets are 2.5-fold magnified cells from the area denoted by the asterisk. Scale bars equal 500 μm (A-D) and 50 μm (E-F).
Localization of increased tPA by heparin administration. (A) Immunofluorescence staining shows tPA increased at the border of the ischemic core (IC) at 6 hours after MCAO administration in heparin-treated WT mice. Double labeling of tPA (red; B, arrows) with rabbit anti-tPA and microglial (MICRO) cells (green; C, arrows) with rat antiF4/80, tPA (red; E), and astrocytes (ASTRO; green) (F) with rat anti-GFAP, tPA (green; H) with goat polyclonal antibody, and neuron (NEURO; I) with rabbit antineurofilament polyclonal antibody, tPA (green; K), and endothelial cells (ENDO; red) (L) with polyclonal rabbit anti-VWF in the ischemic border zone. Merged images indicate tPA was expressed in microglial cells (D, arrows) but not in astrocytes (G), neurons (J), or endothelial cells (M). Inset in panel D is 1.4-fold magnified cells from the area denoted by the arrowhead. Scale bars equal 300 μm (A), 5 μm (B-D), and 12.5 μm (E-M).
Localization of increased tPA by heparin administration. (A) Immunofluorescence staining shows tPA increased at the border of the ischemic core (IC) at 6 hours after MCAO administration in heparin-treated WT mice. Double labeling of tPA (red; B, arrows) with rabbit anti-tPA and microglial (MICRO) cells (green; C, arrows) with rat antiF4/80, tPA (red; E), and astrocytes (ASTRO; green) (F) with rat anti-GFAP, tPA (green; H) with goat polyclonal antibody, and neuron (NEURO; I) with rabbit antineurofilament polyclonal antibody, tPA (green; K), and endothelial cells (ENDO; red) (L) with polyclonal rabbit anti-VWF in the ischemic border zone. Merged images indicate tPA was expressed in microglial cells (D, arrows) but not in astrocytes (G), neurons (J), or endothelial cells (M). Inset in panel D is 1.4-fold magnified cells from the area denoted by the arrowhead. Scale bars equal 300 μm (A), 5 μm (B-D), and 12.5 μm (E-M).
Heparin administration increased MMP9 mRNA and induced its proteolytic activation in the ischemic brains of WT mice but not of tPA KO mice
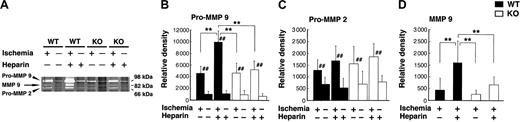
To clarify the possibility that tPA participates in the hemorrhage through MMP activation, we investigated the effect of heparin on MMP levels after cerebral ischemia using zymography. Increased MMP 9 levels were observed 6 hours after MCAO (data not shown) and continued up to 48 hours. Increases in pro–MMP 9 and the active form of MMP 9 were significantly (P < .01) higher at 48 hours after MCAO in heparin-treated WT mice than in untreated mice and heparin-treated tPA KO mice (Figure 5A-B, D). MMP 2 levels, which were much lower than MMP 9 levels, also increased in the ipsilateral ischemic hemisphere, whereas no significant differences appeared in those between heparin-treated WT mice and untreated mice or in heparin-treated tPA KO mice up to 48 hours (Figure 4C). These results suggested an involvement of MMP 9 but not of MMP 2 in heparin-produced cerebral hemorrhage after cerebral ischemia. We also investigated changes in MMP mRNA expression in ischemic brains, measured using RT-PCR; quantitative analysis was performed using Southern blot. MMP 9 mRNA expression increased in the ischemic hemisphere at 24 hours after MCAO in WT and tPA KO mice (Figure 6A-B). In heparin-treated WT mice, increased MMP 9 mRNA expression in the ischemic hemisphere was significantly (P < .05 and P < .01, respectively) higher than in untreated WT mice or heparin-treated tPA KO mice at 24 hours. At 48 hours, MMP 9 mRNA expression was still elevated in the ischemic hemisphere, but there were no significant differences between heparin-treated mice and untreated mice (Figure 6C). Increased MMP 9 mRNA expression with heparin administration could be identified using RT-PCR but not in situ hybridization (data not shown); this may be attributed to the higher sensitivity of RT-PCR. This result also suggests that the increase in MMP 9 mRNA expression with heparin administration was mild. To further identify the cellular sites of increased MMP 9 expression, immunofluorescence staining was performed. MMP 9 expression was seen in the ischemic area, but more MMP 9 expression was detected in the ischemic border zone in heparin-treated WT mice 48 hours after MCAO (Figure 6D). In the ischemic border zone, MMP 9 expression was localized in endothelial cells (Figure 6E-G) and microglial cells (Figure 6H-J).
Zymogram shows that heparin administration induced MMP 9 up-regulation. (A) Zymographic analysis of protein extract from ischemic and nonischemic brains of WT and tPA KO mice at 48 hours after MCAO. (B-D) Densitometric analysis of zymographic gels. Pro–MMP 9 (B) and pro–MMP 2 (C) levels were significantly increased in the ischemic hemispheres in WT and tPA KO mice. The increase in pro–MMP 9 was exacerbated by heparin administration in WT mice but not in tPA KO mice (B). The active form of MMP 9 was detected only in the ischemic hemispheres and was significantly increased in heparin-treated WT mice (D). Values are means ± SD (n = 5). **P < .01; ##P < .01 compared with contralateral hemispheres.
Zymogram shows that heparin administration induced MMP 9 up-regulation. (A) Zymographic analysis of protein extract from ischemic and nonischemic brains of WT and tPA KO mice at 48 hours after MCAO. (B-D) Densitometric analysis of zymographic gels. Pro–MMP 9 (B) and pro–MMP 2 (C) levels were significantly increased in the ischemic hemispheres in WT and tPA KO mice. The increase in pro–MMP 9 was exacerbated by heparin administration in WT mice but not in tPA KO mice (B). The active form of MMP 9 was detected only in the ischemic hemispheres and was significantly increased in heparin-treated WT mice (D). Values are means ± SD (n = 5). **P < .01; ##P < .01 compared with contralateral hemispheres.
Heparin administration induced MMP 9 expression. (A) RT-PCR Southern blot analysis of MMP 9 mRNA. PCR products were subjected to Southern blot analyses with α-[32P] dCTP–labeled cDNA probes. Specific radioactivity was normalized to the expression of β-actin (B-C). Twenty-four hours after MCAO, MMP 9 mRNA levels were increased in the ischemic brains of WT and tPA KO mice. MMP 9 mRNA levels were exacerbated significantly by heparin administration in WT mice but not in tPA KO mice. Values are means ± SD (n = 3). *P < .05, **P < .01, #P < .05, and ##P < .01 compared with contralateral hemispheres. (D) Immunofluorescence staining shows MMP 9 expression was observed in the ischemic area, whereas more MMP 9 expression was detected in the border of the ischemic core (IC) at 48 hours after MCAO in heparin-treated WT mice. (E-J) Brain sections were double stained for MMP 9 (green; E, arrows) and endothelial (ENDO) cells (red; F, arrows) or MMP 9 (green; H, arrows) and microglial (MICRO) cells (red; I, arrows). Merged images from the ischemic border zone indicate the increased MMP 9 expression was localized in endothelial cells (G, arrows) and microglial cells (J, arrows). Scale bars, 300 μm (D), 50 μm (E-G), and 3 μm (H-J).
Heparin administration induced MMP 9 expression. (A) RT-PCR Southern blot analysis of MMP 9 mRNA. PCR products were subjected to Southern blot analyses with α-[32P] dCTP–labeled cDNA probes. Specific radioactivity was normalized to the expression of β-actin (B-C). Twenty-four hours after MCAO, MMP 9 mRNA levels were increased in the ischemic brains of WT and tPA KO mice. MMP 9 mRNA levels were exacerbated significantly by heparin administration in WT mice but not in tPA KO mice. Values are means ± SD (n = 3). *P < .05, **P < .01, #P < .05, and ##P < .01 compared with contralateral hemispheres. (D) Immunofluorescence staining shows MMP 9 expression was observed in the ischemic area, whereas more MMP 9 expression was detected in the border of the ischemic core (IC) at 48 hours after MCAO in heparin-treated WT mice. (E-J) Brain sections were double stained for MMP 9 (green; E, arrows) and endothelial (ENDO) cells (red; F, arrows) or MMP 9 (green; H, arrows) and microglial (MICRO) cells (red; I, arrows). Merged images from the ischemic border zone indicate the increased MMP 9 expression was localized in endothelial cells (G, arrows) and microglial cells (J, arrows). Scale bars, 300 μm (D), 50 μm (E-G), and 3 μm (H-J).
Discussion
Cerebral hemorrhage is the major complication associated with antithrombotic and thrombolytic therapy.1 In this study, using the photothrombotic technique in mice, we demonstrated that cerebral hemorrhage after delayed heparin administration was increased in WT mice but not in tPA KO mice. These results suggested the essential involvement of endogenous tPA in increased hemorrhage with heparin administration in the mouse stroke model. We also demonstrated that the cooperation of ischemia and heparin administration increased the activity and the mRNA levels of tPA during the early stages of ischemia in the mouse brain. In contrast, ischemia alone did not increase tPA expression, which is consistent with previous results that reported no change in tPA activity in primates30 and mice31 after cerebral ischemia. However, tPA activity after ischemia was also reported to be increased32 or decreased.33 Because we used the mouse model of photothrombotic MCAO, in which spontaneous reperfusion of MCA after thrombotic occlusion following cyclic reductions of blood flow was observed,17,19 a difference in the species and models used in the present study could have affected the expression of tPA. In addition, because high-dose heparin was chosen to produce detectable hemorrhage, we could not rule out the possibility that some of the results might have been attributed to non-anticoagulant effects of heparin.
Although the location of tPA in the normal brain has been described,4 the cellular origin of tPA in the brain parenchyma after injury to the brain is not well characterized. Using in situ hybridization and immunofluorescence staining, we demonstrated that tPA mRNA and protein were induced in microglial cells at the ischemic border zone after heparin administration. These results suggested that newly synthesized tPA in microglial cells at the border zone of the ischemic area after heparin administration resulted in the disruption of brain vessels.
Matrix metalloproteinases are able to degrade ECM proteins and to activate inflammatory mediators,34 leading to a damaged BBB and an amplified ischemic injury. In addition, the inflammatory response that occurs within the ischemic tissue might play a major role in the control of MMP activity. Recently, the roles of MMP 2 and MMP 9 on cerebral ischemia and hemorrhage have been addressed in several studies.13,14 Heo et al14 suggested that the early potential role of MMP 2 was in the degradation of basal lamina and that the association of MMP 9 was with hemorrhagic transformation. ECM degradation assessed through Evans Blue leakage was significantly attenuated in MMP 9 KO mice compared with WT mice after transient focal ischemia.12 In experiments with rats with hemorrhagic injury, increases in MMP 9 were seen after 16 to 24 hours.35 However, it was not demonstrated that antithrombotic agents modulate MMP activities in acute stroke. Here we found that after cerebral ischemia, MMP 2 and MMP 9 were activated in WT and tPA KO mice. However, the increased MMP 9 levels and mRNA expression by heparin administration were limited in WT mice, indicating that MMP 9 is involved in the progression of heparin-produced hemorrhagic transformation following stroke. Previous studies demonstrated that MMP inhibitors effectively attenuated the rate of tPA-produced hemorrhage,36 suggesting that increases in MMP 9 expression may contribute to tPA and heparin-mediated hemorrhage. However, further studies will be important to determine the exact role of MMP 9 expression in cerebral hemorrhage.
In the present study, we showed that increased levels of tPA and MMP 9 were involved in heparin-produced hemorrhage, suggesting that the plasminogen-plasmin and MMP systems may cooperate in achieving ECM degradation. The lack of increase in the MMP 9 mRNA level in tPA KO mice by heparin administration after cerebral ischemia suggests that endogenous tPA is involved not only in the protease cascade but also in the cell-mediating process.
In this study, the mechanism of heparin-induced MMP 9 generation is unclear. It is possible that the up-regulation of tPA after heparin administration may increase MMP 9 expression. In fact, a recent report showed that tPA can directly induce MMP 9 up-regulation at the transcriptional level in vitro and that it may be mediated by the low-density lipoprotein–related protein receptor.37 Moreover, tPA treatment increases MMP 9 levels after cerebral ischemia in vivo.25 Further study would be important to address the relationship between tPA and MMP 9. Tsirka et al6 reported that the activation of microglial cells in tPA KO mice was less than that in WT mice after the administration of glutamate analog. Here we demonstrated that tPA and MMP 9 were expressed in microglial cells at the ischemic border zone in heparin-treated WT mice, suggesting that less activation of microglial cells may result in lower induction of tPA and MMP 9 and in decreased hemorrhage.
In summary, the findings of the present study suggest that inducing endogenous tPA and MMP 9 is essential for heparin-produced cerebral hemorrhage after cerebral ischemia, implying that inhibiting increased tPA or MMP levels may preserve microvascular integrity and thus may be useful for preventing cerebral hemorrhage during stroke treatment with antithrombotic agents. Targeting these molecules in human stroke may provide a new approach to prevent cerebral hemorrhage associated with antithrombotic therapy.
Prepublished online as Blood First Edition Paper, November 20, 2003; DOI 10.1182/blood-2003-03-0835.
Supported in part by the Japan China Medical Association and by grants 12672211 and 15390173 from the Ministry of Education, Science and Culture in Japan.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 6. Heparin administration induced MMP 9 expression. (A) RT-PCR Southern blot analysis of MMP 9 mRNA. PCR products were subjected to Southern blot analyses with α-[32P] dCTP–labeled cDNA probes. Specific radioactivity was normalized to the expression of β-actin (B-C). Twenty-four hours after MCAO, MMP 9 mRNA levels were increased in the ischemic brains of WT and tPA KO mice. MMP 9 mRNA levels were exacerbated significantly by heparin administration in WT mice but not in tPA KO mice. Values are means ± SD (n = 3). *P < .05, **P < .01, #P < .05, and ##P < .01 compared with contralateral hemispheres. (D) Immunofluorescence staining shows MMP 9 expression was observed in the ischemic area, whereas more MMP 9 expression was detected in the border of the ischemic core (IC) at 48 hours after MCAO in heparin-treated WT mice. (E-J) Brain sections were double stained for MMP 9 (green; E, arrows) and endothelial (ENDO) cells (red; F, arrows) or MMP 9 (green; H, arrows) and microglial (MICRO) cells (red; I, arrows). Merged images from the ischemic border zone indicate the increased MMP 9 expression was localized in endothelial cells (G, arrows) and microglial cells (J, arrows). Scale bars, 300 μm (D), 50 μm (E-G), and 3 μm (H-J).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/103/7/10.1182_blood-2003-03-0835/6/m_zh80070459390006.jpeg?Expires=1770119108&Signature=OEpXdsD3OQO3RQkOjtcDZEtadx5yrM2qk2EjZ0voB8Fji-6pEyWl9xRnfTI45cex31OMfYDtE29Xto6B3uLGITtp7F7wDItA4CqYPNOQdXf8Cjf7ACsNhAIvfeE438Lh8qzLzvPiP6OstC~J5t5xz2OoaNRI8SPSFzI7iWd2rItvtCECtd-ufLZkCVbCd~-dJkSSHzs1UMHyoQ3dbKM~2bexdvFm7qis4n2FUkxIIuNi60qbWMqstbiBbRX2mPGRh7K8CqD5~vY3ETbE2Xl3LVJKjSB4VbYVQw75TFykQxWIYMuF5olj2zUHo46vIw-D3rvZFZgVcgUeC5amUYXgZQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal